Abstract
Transport of ADP and ATP across mitochondria is one of the primary points of regulation to maintain cellular energy homeostasis. This process is mainly mediated by adenine nucleotide translocase (ANT) located on the mitochondrial inner membrane. There are four human ANT isoforms, each having a unique tissue-specific expression pattern and biological function, highlighting their potential as drug targets for diverse clinical indications, including male contraception and cancer. In this study, we present a novel yeast-based high-throughput screening (HTS) strategy to identify compounds inhibiting the function of ANT. Yeast strains generated by deletion of endogenous proteins with ANT activity followed by insertion of individual human ANT isoforms are sensitive to cell-permeable ANT inhibitors, which reduce proliferation. Screening hits identified in the yeast proliferation assay were characterized in ADP/ATP exchange as says employing recombinant ANT isoforms expressed in isolated yeast mitochondria and Lactococcus lactis as well as by oxygen consumption rate in mammalian cells. Using this approach, closantel and CD437 were identified as broad-spectrum ANT inhibitors, whereas leelamine was found to be a modulator of ANT function. This yeast “knock-out/knock-in” screening strategy is applicable to a broad range of essential molecular targets that are required for yeast survival.
Keywords: adenine nucleotide translocase, high throughput screening, yeast mitochondria, Lactococcus lactis, ADP/ATP exchange
Introduction
Despite the tight regulation and critical importance of energy homeostasis mechanisms for survival, it has recently become clear that modulators targeting specific proteins playing key roles in energy metabolism can provide therapeutic benefit with an acceptable safety margin.1 Energy generation by cells is mainly through production of ATP from two primary sources: glycolysis in the cytosol and oxidative phosphorylation (OXPHOS) localized to mitochondria. ATP flux across the mitochondrial membrane plays a key role in maintaining cellular energy levels. This ATP transport between the mitochondrial and cytosolic compartments is primarily mediated by adenine nucleotide translocase (ANT).2
ANT is a well-conserved protein located on the mitochondrial inner membrane that mediates the exchange of ADP for ATP between the mitochondria and cytosol. In humans, there are four ANT isoforms (ANT1–4) with tissue-specific expression patterns. ANT1 is mainly expressed in muscle and brain tissue, ANT2 is preferentially expressed in proliferating tissue, ANT3 has a ubiquitous pattern of expression, and ANT4 is localized exclusively to the testis.3 Recently, ANT isoforms have been targeted for several therapeutic indications, including cancer,4 diabetes,5 cardiovascular disease,6 and obesity.7 Notably, ANT4 has been proposed as a target for male contraception supported by at least three lines of evidence: (1) ANT4 is exclusively expressed in germ cells,8 (2) male ANT4 knockout mice were infertile,8 and (3) ATP generated in mitochondria by OXPHOS contributes to the motility of mature sperm.9 We hypothesized that a specific ANT4 inhibitor would target sperm motility and would be efficacious as a male contraceptive, without adverse side effects on other tissues.10,11
The natural products atractyloside (ATR), carboxyatrac-tyloside (cATR), and bongkrekic acid (BKA) (Suppl. Fig. S1) have been extensively studied as ANT inhibitors.12 They are potent inhibitors with high specificity for ANT, but all of them are broad-spectrum ANT inhibitors lacking isoform selectivity and none of them possess drug-like properties. These compounds are large with a molecular weight close to 1000 Dalton, are difficult to prepare synthetically, and either have low cell membrane permeability (BKA) or are impermeable (ATR and cATR). New chemical scaffolds are needed to develop potent and selective drug-like molecules suitable for therapeutic use.
In this study, we present a novel strategy to identify ANT-specific inhibitors by high-throughput screening (HTS) using yeast strains expressing individual human ANT isoforms to identify ANT inhibitors that reduce yeast proliferation. A combination of yeast and Lactococcus lactis ADP/ATP exchange assays and mammalian cell oxygen consumption rate (OCR) assays were employed to characterize the hits identified in the yeast cell survival assays. Novel ANT inhibitors and modulators were identified, demonstrating the utility of this “knock-out/knock-in” yeast survival screening strategy.
Materials and Methods
Chemical Libraries, Repurchased Compounds, Hit Triage, and Expression of Human ANT Isoforms in Yeast
A screening library of ~65,000 compounds obtained from commercial sources was used for primary HTS. HTS triage involved substructure filters and cluster analysis. The Saccharomyces cerevisiae strains expressing hANT isoforms were previously described13 (see Suppl. Materials and Methods for details).
HTS
Individual colonies of the hANT4 strain were inoculated into liquid complete ethanol-glycerol medium (YPEG medium) and cultured overnight at 30 °C in 50 mL YPEG medium in 250-mL flasks in a shaker at 200 rpm. The yeast cells were harvested the next morning by centrifugation at 3000 rpm for 5 min. Yeast cell density was adjusted to 1.0 × 107/mL in YPEG medium. Following addition of 20 μL YPEG medium into 384-well plates using a Biomek FX (Beckman Coulter, Brea, CA), 40 nL compound from the screening collection (final concentration 10 μM) was dispensed using an ECHO550 acoustic dispenser (Labcyte, Sunnyvale, CA), with the first two and last two columns reserved for controls. Finally, 20 μL 1 × 107/mL yeast was plated using a BioMek FX liquid handler (Beckman Coulter), and the absorbance at 600 nm was read at 0 h to determine if the compound was colored or cloudy and again at 48 h following incubation at 30 °C to measure yeast growth. To reduce interference by colored compounds, the OD values of compound wells at t = 0 that were greater than yeast control wells were subtracted from the OD at 48 h. Percent inhibition relative to control wells containing 0.1% DMSO was then calculated for each compound well following subtraction of the average OD value of blank wells containing media alone.
A pilot screen of the Library of Pharmacological Active Compounds (LOPAC) was conducted using hANT1, −2, −3, or −4 yeast strains grown in nonfermentable YPEG medium at a yeast cell density of 0.5 × 107/mL. DMSO was used as vehicle control, and the known ANT inhibitor BKA (final 50 μM) was used as positive control. Amiodarone was identified as a hit in the pilot screen. As BKA was weakly active, amiodarone was used as positive control for the remainder of the HTS campaign.
Hit Confirmation
Primary screening hits selected for concentration-response experiments were tested in the primary screening hANT4 activity assay in YPEG medium at eight concentrations using 5-fold serial dilutions from the highest concentration of 60 μM, and IC50 values were calculated using Prism (GraphPad, San Diego, CA). Selectivity for hANT4 was determined in concentration-response experiments using yeast strains individually expressing hANT1, −2, or −3 in an identical manner to that described for hANT4. A cytotoxicity assay was run in the same manner as the activity assay, except the strains were inoculated into and grown in complete glucose (YPD) medium. Hits that were more potent in the activity assay than the cytotoxicity assay for the hANT4 isoform were repurchased and tested from solid samples in activity and cytotoxicity assays using all four hANT isoforms. Hits exhibiting activity in the hANT yeast growth assay from solid samples were tested in hANT1, −2, −3, or −4 yeast mitochondrial and hANT1, −2, or −3 bacterial ADP/ ATP exchange assays to confirm direct inhibition of ANT.
ADP/ATP Exchange with Isolated Yeast Mitochondria Expressing Human ANT
Mitochondria were isolated according to a previous report13 with some modifications (see Suppl. Materials and Methods). The ADP/ATP exchange assay was carried out in a filter plate format using a 96-well MultiScreenHTS FC filter plate (Millipore, Billerica, MA). Compound or DMSO diluted into mitochondria isolation buffer (MIB) (20 mM HEPES-KOH, 0.6 M mannitol, pH 7.4) was transferred into the precooled plate (50 μL/well). Mitochondria were added in 25 μL MIB (50 μg/well) and incubated for 10 min. ADP/ ATP exchange was initiated by adding 25 μL of 20 μM [3H] ADP (5 μCi/mL). The exchange reaction was then stopped after 1 min by the addition of 25 μL cATR (5 μM final). The reaction mix in the filter plate was then through on a vacuum manifold, and the plate was washed four times with 300 μL/well MIB buffer. Supermix scintillation cocktail (PerkinElmer, Waltham, MA) (100 μL) was transferred into each well and incubated overnight, and the [3H]ADP taken up by mitochondria was measured with a MicroBeta scintillation counter (PerkinElmer).
Expression of Human ANT in Lactococcus lactis
To express human ANT protein in L. lactis, the nisin-controlled gene expression system from MoBiTec (Goettingen, Germany) was used. Briefly, the DNA fragments encoding human ANT1, −2, −3, or −4 were cloned into the pNZ8148L plasmid and transformed into NZ9700 cells. Positive clones were picked from M17 agar plates (supplemented with 1% glucose and 10 μg/mL chloramphenicol) and sequenced to confirm the identity of the expression construct. To express hANT protein, L. lactis cells were grown at 30 °C in M17 medium (supplemented with 1% glucose and 10 μg/mL chloramphenicol) until OD600 reached 0.5. Then, nisin A was added at 2 ng/mL to induce gene expression for 3 h. Before the ADP/ATP exchange assay, cells were washed twice with Dulbecco’s phosphate-buffered saline (DPBS) and resuspended in DPBS at 20 OD/mL.
ADP/ATP Exchange with Lactococcus lactis Expressing Human ANT
The ADP/ATP exchange assay with ANT expressed in L. lactis was conducted in a similar manner to mitochondria except that the entire assay was conducted at room temperature in DPBS without Ca2+ and Mg2+, the exchange reaction was initiated by adding 25 μL of 20 μM [3H]ADP (2 μCi/ mL) into each well containing 0.5 OD of L. lactis cells, the incubation time was 1 h, and cells were retained on 0.2-μm PVDF membrane Filtrex filter plates (Corning, Tewksbury, MA) washed with DPBS.
Oxygen Consumption Rate
HCT116 (human colon cancer) and MiaPaCa-2 (human pancreatic carcinoma) cell lines obtained from ATCC (Manassas, VA) were maintained in RPMI1640 medium supplemented with 10% fetal bovine serum. Briefly, 20,000 cells in 80 μL were plated into each well of an XFe96 Cell Culture Microplate (Seahorse Bioscience, North Billerica, MA) and incubated in a 37 °C incubator with 5% CO2 overnight. Prior to the assay, the medium was changed to MAS buffer (110 mM mannitol, 70 mM sucrose, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA, 0.2% fatty acid free bovine serum albumin, pH 7.4) containing 10 mM pyruvate and maleate. Compounds and other reagents were programmed to be added sequentially, and OCR was measured with an XFe96 Extracellular Flux Analyzer (Seahorse Bioscience).
Results
Yeast Growth Assays
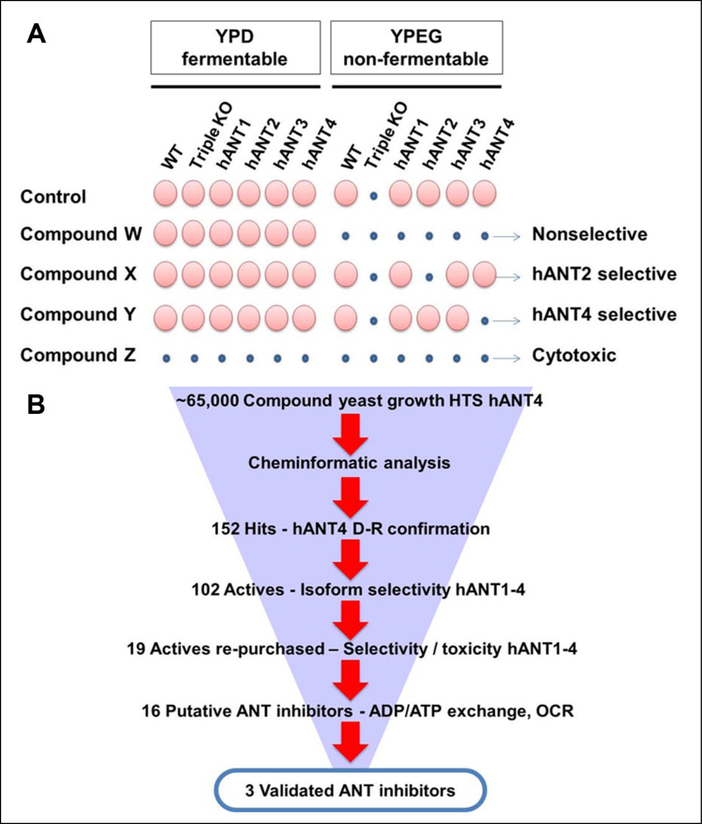
As the initial goal was to identify selective hANT4 inhibitors, the primary screen was conducted with yeast expressing hANT4 in nonfermentable medium containing glycerol and ethanol (YPEG). In this medium, net ATP can only be generated in mitochondria through OXPHOS. A cell-permeable ANT inhibitor is expected to block the growth of hANT4 yeast cell by blocking ATP transport. As shown in Figure 1A, all strains grow in the control condition, except the triple knock-out strain, which does not have mitochondrial ATP/ADP exchange activity. A hypothetical selective ANT4 inhibitor (compound Y) is expected to inhibit the growth of hANT4 yeast but not other hANT yeast in YPEG medium. A broad-spectrum hANT inhibitor (compound W) would inhibit the growth of all four strains. In contrast, hANT inhibitors would not block the growth of any of these strains in fermentable glucose-containing medium (YPD), as yeast can generate sufficient amount of ATP in the cytosol via glycolysis. However, a compound that is cytotoxic by a mechanism unrelated to ANT inhibition (compound Z) would block cell growth of all strains in both fermentable and nonfermentable media.
Figure 1.
Human adenine nucleotide translocase (ANT) high-throughput screening (HTS) and hit confirmation, characterization, and validation (see Results for description). (A) HTS assay principle. (B) Overview of human ANT yeast HTS and hit follow-up.
An overview of the HTS and follow-up is shown in Figure 1B. During the course of this HTS, about 65,000 compounds were screened in the hANT4 yeast cell growth assay. As a measure of assay robustness, the Z’ value was calculated to be 0.74 ± 0.07, indicative of a robust screen (Suppl. Fig. S2A). Most compounds (99.6%) screened at 10 μM produced an inhibition between −50% and +50% (Suppl. Fig. S2B). A total of 238 of these compounds were identified as primary hits as they produced >50% inhibition of hANT4 yeast growth (hit rate = 0.34%). Compounds (152) that produced either >50% inhibition or selected by cheminformatic analysis were cherrypicked for initial confirmation in dose-response assays with hANT4 yeast. Hits confirmed using hANT4 (102 compounds) were further tested with hANT1, −2, and −3 yeast cell lines in dose response assays to determine hANT isoform selectivity. The 19 hits that showed some selectivity for hANT4 were repurchased and tested again for isoform selectivity with the four yeast strains. These repurchased hits were also evaluated for their cytotoxic effect using the four hANT strains grown in glucose-containing medium.
The potency, selectivity, and cytotoxicity of reference compounds and repurchased HTS hits in the yeast growth assay are shown in Table 1. The known ANT inhibitor BKA14 inhibited cell growth with IC50 values between 33 and 46 μM in the four hANT yeast strains, indicating it is a broad-spectrum ANT inhibitor. The potent ANT inhibitor cATR2 was inactive up to 100 μM for all four strains. The low potency of BKA and the inactivity of cATR are likely due to poor cell penetration, as both are highly charged, anionic ligands. Although minocycline was reported to be an ANT inhibitor based on its interaction with cATR in mitochondrial respiration studies,15 this antibiotic was inactive up to 100 μM in the yeast growth assay. As significant effects on mitochondrial respiration were reported only at 100 to 200 μM,15 it may be that concentrations of minocycline >100 μM would be required to reduce growth of yeast expressing hANT, particularly if it has poor yeast cell penetration. Of the 19 repurchased hit compounds, 15 inhibited yeast growth with potencies ranging from ~0.5 to ~50 μM (Table 1). None of these active repurchased hit compounds displayed selectivity for hANT4 yeast over other isoforms. Most of the hit compounds did not inhibit yeast growth in fermentable, glucose-containing medium, suggesting that they primarily target mitochondrial energy pathways. However, a few of the compounds (amiodarone, IMD 0354, and CD437) exhibited cytotoxic effect in certain strains, and leelamine was only 2- to 3-fold more potent in yeast grown in YPEG medium compared to YPD medium. To confirm that these hit compounds are indeed ANT inhibitors, they were further evaluated in ATP/ADP exchange assays with isolated yeast mitochondria and L. lactis expressing hANT isoforms.
Table 1.
Potency, Selectivity, and Toxicity of HTS Hits and Control Compounds in Yeast Strains Expressing Human Adenine Nucleotide Translocases (hANTs).
| Activity |
Toxicity |
|||||||
|---|---|---|---|---|---|---|---|---|
| Compound | hANT1 | hANT2 | hANT3 | hANT4 | hANTI | hANT2 | hANT3 | hANT4 |
|
IC50 (μM) | ||||||||
| BKA | 38 ± 6 | 41 ± 4 | 46 ± 10 | 33 ± 9 | >100 | >100 | >100 | >100 |
| cATR | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| Minocycline | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| Amiodarone | 31 ± 7 | 20 ± 9 | 16 ± 10 | 22 ± 10 | >100 | >100 | 37 ± 12 | >100 |
| IMD 0354 | 0.58 ± 0.10 | 0.45 ± 0.1 | 0.52 ± 0.08 | 0.85 ± 0.07 | >100 | >100 | 34 ± 18 | 54 ± 21 |
| P132–0333 | 1.1 ± 0.8 | 0.72 ± 0.59 | 1.4 ± 0.7 | 1.0 ± 0.6 | >100 | >100 | >100 | >100 |
| P132–0289 | 2.4 ± 0.5 | 2.5 ± 1.9 | 3.3 ± 1.4 | 1.6 ± 1.3 | >100 | >100 | >100 | >100 |
| Nonactin | 4.4 ± 1.3 | 3.3 ± 1.1 | 4.7 ± 1.5 | 3.3 ± 0.4 | >100 | >100 | >100 | >100 |
| S838462 | 3.1 ± 0.7 | 3.1 ± 0.5 | 5.6 ± 2.0 | 5.4 ± 2.0 | >100 | >100 | >100 | >100 |
| Leelamine | 10 ± 4 | 5.1 ± 1.8 | 9.6 ± 4.8 | 8.6 ± 2.9 | 26 ± 8 | I2 ± 5 | 19 ± 5 | 22 ± 10 |
| Closantel | 4.7 ± 0.8 | 6.1 ± 2.4 | 6.3 ± 2.3 | 9.6 ± 8.7 | >100 | >100 | >100 | >100 |
| Tyrphostin A9 | 42 ± 20 | 35 ± 15 | 24 ± 10 | 13 ± 4 | >100 | >100 | >100 | >100 |
| L923–0673 | 13 ± 6 | 6.0 ± 3.1 | 12 ± 6 | 29 ± 8 | >100 | >100 | >100 | >100 |
| CD437 | 20 ± 6 | 15 ± 2.1 | 16 ± 3 | 18 ± 3 | >100 | 52 ± 34 | >100 | 53 ± 4 |
| 7252511 | 3.9 ± 1.0 | 20 ± 16 | 19 ± 13 | 18 ± 19 | >100 | >100 | >100 | >100 |
| P132–0615 | 0.34 ± 0.28 | 23 ± 17 | 38 ± 17 | 22 ± 11 | >100 | >100 | >100 | >100 |
| S899542 | 25 ± 15 | 11 ± 2 | 32 ± 11 | 29 ± 8 | >100 | >100 | >100 | >100 |
| P132–0394 | 1.7 ± 1.4 | 15 ± 6 | 19 ± 1 | 56 ± 41 | >100 | >100 | >100 | >100 |
Activity was measured as a reduction in yeast strain growth in nonfermentable (YPEG) medium, whereas toxicity was determined under identical conditions except that yeast cells were cultured in fermentable (YPD) medium. IC50 values are means ± SEM of at least three independent experiments. BKA, bongkrekic acid; cATR, carboxyatractyloside.
ADP/ATP Exchange Assays
To verify that the compounds active in the yeast proliferation assay are hANT inhibitors, we developed an ADP/ATP exchange assay with isolated mitochondria from the four yeast strains expressing human ANT1, −2, −3, and −4. This approach has the advantage that a direct measure of the function of the ANT can be established for all of the human ANT isoforms. In this forward exchange assay,16 [3H]ADP is taken up by isolated mitochondria, which exchange endogenous ATP to the extra-mitochondria space (Fig. 2A). This exchange process is rapid and is complete within 2 min (data not shown) due to abundant hANT expression on the inner membrane. In the assay, 5 μM cATR was added to stop the reaction after a 1-min incubation, and the [3H]ADP taken up by the mitochondria was measured. Natural product ANT inhibitors, such as cATR, BKA, and ATR at 10 μM, completely inhibited ADP/ATP exchange activity.
Figure 2.
ADP/ATP exchange assays with human ANT isoforms expressed in isolated yeast mitochondria and Lactococcus lactis. (A) Assay principle (see Results for description). (B) Assay validation with known compounds (10 μM) in isolated yeast mitochondria (left panel) and L. lactis (right panel) using the hANT2 isoform. The ADP/ATP exchange activity was expressed as the percent relative to the DMSO control. Mean ± SD from a single experiment. ANT, adenine nucleotide translocase; TTFA, thenoyltrifluoroacetone; FCCP, carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone; MPTP, mitochondrial permeability transition pore
To validate the specificity of this assay, a number of known compounds with defined mode of action were tested, which include mitochondrial complex inhibitors, membrane permeability transition pore modulators, and ionophores (Fig. 2B, left). Most of the compounds at 10 μM did not show an inhibitory effect, but antimycin, chelerythrine, and lonidamine reduced ADP uptake in yeast mitochondria expressing hANT2. These three compounds are known to impair mitochondrial function by inhibiting complex III, BCL-2, protein kinase C,17 or hexokinase18 and also by generation of reactive oxygen species (ROS) (chelerythrine),19 thereby only indirectly affecting ADP/ATP exchange. Thus, the yeast ADP/ATP exchange assay is capable of eliminating many false-positive hits from the yeast proliferation assay that block growth by mechanisms unrelated to inhibition of ANT, but it may not eliminate all such compounds.
To avoid the indirect effects on ADP transport observed in isolated mitochondria, an independent ADP/ATP exchange assay for compound characterization was developed in the Gram-positive bacterium L. lactis. Using the same set of reference compounds examined in isolated mitochondria, only known ANT inhibitors reduced [3H] ADP uptake in L. lactis expressing the human ANT2 isoform (Fig. 2B, right), indicating that this bacterial exchange assay has higher fidelity than the isolated yeast mitochondrial assay to detect direct ANT inhibitors.
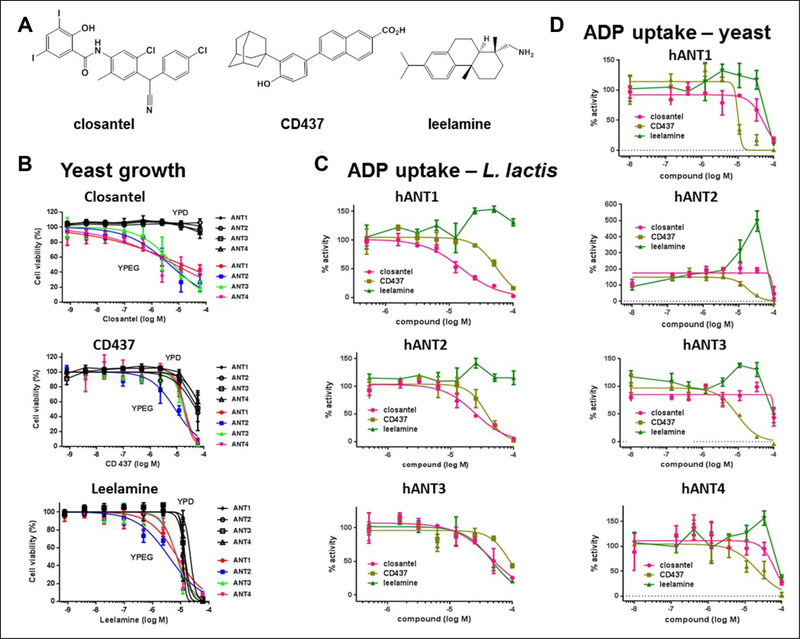
Sixteen compounds selected from the yeast growth assay were evaluated for their effects on ADP/ATP exchange by individual human ANT isoforms expressed in both isolated yeast mitochondria and L. lactis. In the mitochondrial assay, 11 of 16 compounds (30 μM) demonstrated more than 50% inhibition of exchange activity in at least one yeast strain (Suppl. Table S1). However, only three of these compounds inhibited [3H]ADP uptake by L. lactis: closantel, CD437, and leelamine. Minocycline showed strong inhibition in the yeast assay but was completely inactive in the L. lactis assay. Although previously reported to be an ANT inhibitor based on its interaction with cATR in mitochondrial respiration studies,15 minocycline is unlikely to be a direct inhibitor of ANT because it did not reduce ADP/ATP exchange by human ANT isoforms expressed in L. lactis. Some compounds, such as IMD 0354, nonactin, S838462, leelamine, closantel, L923–0673, and S899542, appeared to favor a particular hANT isoform in the yeast mitochondrial assay, although none were hANT4 selective. For this reason, we focused further efforts on identifying ANT inhibitors that possess novel selectivity profiles, rather than focusing exclusively on ANT4 selectivity.
Considering the high specificity of the L. lactis exchange assay demonstrated with a broad array of compounds with known mechanisms of action, it is likely that closantel, CD437, and leelamine directly inhibit hANT. Therefore, these compounds were selected for further profiling in concentration-response ADP/ATP exchange assays (Table 2, Fig. 3). Whereas the highly potent ANT inhibitor cATR showed some selectivity for hANT1 in the yeast mitochondrial assays, this selectivity was not observed in the L. lactis assay (Table 2), indicating that cATR is a nonselective hANT inhibitor consistent with previous reports.12 The much weaker inhibitor closantel was generally more potent in the L. lactis assay than the mitochondrial assay, although by only 2- to 4-fold (Table 2, Fig. 3C,D). Closantel was more potent in the yeast growth assay than either of the translocase assays, possibly because it has activity at other molecular targets, such as the known activity at the Na+/H+ antiporter.20 In contrast, CD437 was 2- to 10-fold more potent in the yeast mitochondrial than the L. lactis assay and had similar potency in the yeast growth and mitochondrial assays. Although closantel and CD437 showed ~3-fold selectivity for hANT1 and hANT2, respectively, over hANT3 in the L. lactis assay, little or no selectivity for the hANT isoforms was observed in the mitochondrial assay, suggesting that these compounds have little or no ANT selectivity.
Table 2.
Confirmed Hits Inhibit ADP/ATP Exchange Mediated by Human Adenine Nucleotide Translocase (ANT) Expressed in Isolated Yeast Mitochondria and Lactococcus lactis.
| Yeast Mitochondria |
L. lactis |
||||||
|---|---|---|---|---|---|---|---|
| hANT1 | hANT2 | hANT3 | hANT4 | hANT1 | hANT2 | hANT3 | |
|
IC50 (μM) | |||||||
| Closantel | 52 ± 15 | 92 ± 32 | 100 ± 32 | 64 ± 25 | 15 ± 2.8 | 24 ± 4.2 | 47 ± 11 |
| CD437 | 10 ± 1.5 | 18 ± 6.4 | 9.5 ± 2.7 | 22 ± 12 | 54 ± 9 | 36 ± 6.3 | 94 ± 19 |
| Leelamine | 87 ± 25 | >100 | 98 ± 25 | 95 ± 42 | >100 | >100 | 47 ± 11 |
| cATR | 0.077 ± 0.041 | 0.41 ± 12 | 0.42 ± 0.12 | 0.77 ± 0.47 | 0.0024 ± 0.0005 | 0.0017 ± 0.0004 | 0.0023 ± 0.0007 |
Carboxyatractyloside (cATR) was used as a positive control. IC50 values are means ± SEM of at least three independent experiments.
Figure 3.
Activity of the three validated hits, leelamine, closantel, and CD437, in yeast growth and ADP/ATP exchange assays. (A) Chemical structures. (B) Growth inhibition in yeast expressing human adenine nucleotide translocase (ANT). Inhibition of hANTI, −2, −3, or −4 yeast strain growth in nonfermentable YPEG (– glucose) and fermentable YPD (+ glucose) media. Cell growth was measured at OD600 and expressed as percent of DMSO control from three independent experiments. (C) Modulation of ADP/ATP exchange in Lactococcus lactis expressing hANTI, −2, or −3. (D) Modulation of ADP/ATP exchange in isolated yeast mitochondria expressing hANTI, −2, −3, or −4. For C and D, ANT activity is expressed as [3H]ADP uptake from three independent experiments.
Leelamine displays a more complex behavior that can best be described as a modulator of hANT activity, whose activity depends on the exact context of the translocase. Leelamine was active in the yeast growth assay in nonfermentable medium but was cytotoxic at 2- to 3-fold higher concentrations measured in fermentable medium (Table 1, Fig. 3B). Leelamine inhibited hANT activity at 100 μM in mitochondrial assays expressing all isoforms, although at lower concentrations (10 and 30 μM), leelamine paradoxically increased translocase activity in mitochondria from the hANT4, hANT3, and particularly hANT2 strains (Fig. 3D). Similarly leelamine increased translocase activity in L. lactis expressing hANTl and hANT2 but inhibited the activity of hANT3 (Fig. 3C).
Oxygen Consumption Rate
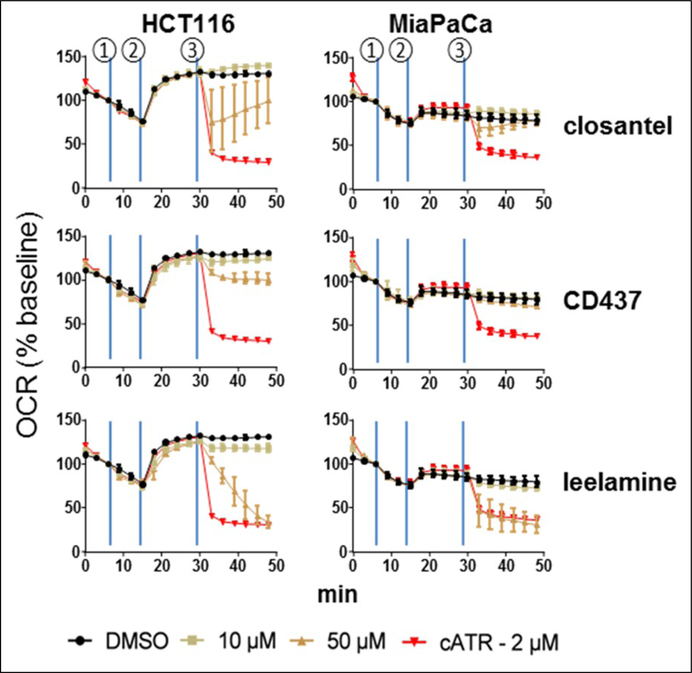
cATR, closantel, CD437, and leelamine decreased OCR in two cell lines, consistent with inhibition of ANT-mediated ADP/ATP exchange (Fig. 4). Upon addition of ADP (②), a rapid increase of OCR was observed, which was maintained at a high level for at least 30 min. Addition of leelamine (50 pM) (③) decreased the OCR to a similar extent as cATR. Both CD437 and closantel inhibited OCR in HCT116 cells, although they were not as effective as leelamine and cATR. Neither CD437 nor closantel produced an appreciable level of reduction of OCR in MiaPaCa cells.
Figure 4.
Leelamine, closantel, and CD437 reduce oxygen consumption rate (OCR) in human cells. HCTII6 and MiaPaCa adherent cells were treated sequentially by 10 pM atpenin A5 (①), 3.5 mM ADP, 0.375 nM XF Plasma Membrane Permeabilizer (③), and hit compounds or with cATR as positive control (©). OCR was measured using the XFe96 Extracellular Flux Analyzer. OCR is expressed as percent of baseline from three independent experiments with the third measurement point (①) defined as 100% of baseline.
Discussion
In this study, we present a novel yeast-based HTS strategy to identify compounds that reduce the cellular energy flow between mitochondria and cytosol by inhibiting ANT. The initial focus of this approach was to identify selective inhibitors of hANT4, which is exclusively expressed in testis. Selective hANT4 inhibitors have the potential to be optimized for use as male contraceptive agents, as deletion of the ANT4 gene leads to early meiotic arrest of murine male germ cells in knock-out mice.8
An HTS campaign of ~65,000 compounds to identify inhibitors of the proliferation of the hANT4 yeast strain was followed by evaluation of 102 confirmed hits for selectivity in the hANT1, −2, and −3 proliferation assays and for cytotoxicity in glucose-containing medium. The yeast cell growth assay was used as the primary screen not only because it is relatively inexpensive but also because it is likely to detect cell-permeable ANT inhibitors, a property not shared by ANT probe molecules such as cATR. Using compounds cherrypicked from DMSO stocks, 19 hits were identified that showed activity in the hANT4 yeast strain and apparent selectivity for hANT4 over other hANT isoforms in the yeast survival assay. The selectivity and cytotoxicity profiles determined with repurchased samples resulted in 16 compounds that were active in the hANT isoform survival assays, although none were selective for hANT4 over the other hANT isoforms.
HTS hits that selectivity blocked hANT4 yeast strain growth in nonfermentable (YPEG) medium relative to fermentable (YPD) medium are expected to inhibit mitochondrial function. However, these compounds can inhibit ADP/ATP exchange by either direct or indirect mechanisms. We therefore employed ADP/ATP exchange assays using human ANT isoforms expressed in both isolated mitochondria and L. lactis to characterize compounds identified as hits in the yeast growth assay. The L. lactis assay had higher fidelity for differentiating between direct and indirect inhibitors of ADP/ATP exchange than the isolated mitochondria assay. The higher incidence of detecting indirect ADP transport inhibitors in the isolated yeast mitochondria assay compared to L. lactis assay may be due to the relative fragility of mitochondria compared with bacteria and the susceptibility of yeast mitochondria to functional impairment by compounds that interfere with yeast growth by mechanisms other than ANT inhibition. In addition, the L. lactis assay is substantially more reproducible between experiments, has considerably lower variability between replicates, and is very convenient to prepare in large quantities. cATR was 30- to >300-fold more potent in the L. lactis than the mitochondrial assay for hANT1, −2, and −3, possibly due to greater access to the translocase expressed in the bacterial cell membrane and/or abundant functional hANT protein in mitochondria, requiring concentrations of cATR approaching that of the hANT protein to detect inhibition. Somewhat surprisingly, L. lactis expresses hANT molecules with the same orientation in the membrane as in mitochondria indicated by the complete inhibition of exchange by the membrane-impermeable inhibitor cATR, which binds only to the surface of ANT facing the mitochondrial intermembrane space. The correct insertion of hANT isoforms into L. lactis may be a reflection of the theory that mitochondria originated from bacterial cells and share a common ancestor. Several mitochondrial inner membrane carriers have been successfully expressed in L. lactis.21,22
Of the 16 compounds identified as inhibitors in the yeast growth assay with low toxicity, three confirmed in the ADP/ ATP exchange assays in both yeast mitochondria and L. lactis. An additional eight hit compounds that inhibited [3H] ADP uptake ≥50% in at least one human ANT isoform expressed in yeast mitochondria did not produce appreciable inhibition in the L. lactis assay. Importantly, the three hit compounds active in both the mitochondrial and L. lactis exchange assays (CD437, closantel, and leelamine) also showed inhibition of mitochondrial OCR in mammalian cells similar to the natural ANT inhibitor cATR. Although the OCR data are consistent with ANT inhibition, inhibitors of the electron transport chain and ATP synthase can also decrease OCR in this cellular assay. However, modulation of ADP/ATP exchange in mitochondria and particularly L. lactis by these compounds indicates a direct interaction with ANT. Taken together, these data strongly support the hypothesis that closantel, CD437, and leelamine directly inhibit or modulate human ANT activity. Confirmation that these hits are active in a variety of functional assays demonstrates that the yeast growth screening strategy is a valid, practical, and inexpensive approach to identify novel ANT inhibitors.
CD437 is a synthetic retinoid that induces tumor growth arrest and apoptosis. The exact molecular basis of the underlying mechanism is not yet well understood, but it displays both RARγ-dependent and RARγ-independent effects.23 CD437 was previously reported to interact with hANT by modulating its pore-forming function.24 Our findings for the first time show that CD437 inhibits hANT-mediated ADP/ATP exchange, suggesting a direct interaction between CD437 with ANT. Closantel is an anthelmintic that is a proton ionophore, although its mechanism has not been completely elucidated. Closantel was reported to disrupt the proton gradient across the mitochondrial inner membrane and to be an uncoupler of OXPHOS.20 However, it is unlikely that these activities explain the inhibition of ADP/ATP exchange activity in L. lactis, as the potent proton ionophore FCCP was inactive in this assay. Closantel appears to be a direct hANT inhibitor in the micromolar concentration range.
Leelamine is a natural product derived from the bark of pine trees. Its mechanism is poorly understood as it has been reported to have several unrelated activities, including weak affinity for the CB1 and CB2 cannabinoid receptors,25 inhibition of pyruvate dehydrogenase kinase,26 and disruption of cholesterol transport.27 In the current study, we provide evidence that leelamine modulates the functional activity of ANT. In contrast to the ANT inhibitors cATR, closantel, and CD437, leelamine stimulates or inhibits translocase activity in a concentration-, isoform-, and expression system-dependent manner. The modulatory activity of leelamine may depend on the conformation of the ANT protein influenced by the isoform, membrane insertion environment, and/or composition of the surrounding milieu on both faces of the membrane. In addition, it is possible that differences in the level of expression of the ANT isoforms may affect the modulatory activity by leelamine. Indeed, hANT2 and hANT4 expression in yeast was much higher than hANTl and hANT3 (preliminary observations), which may reflect toxic effects of ANT1 and ANT3 overexpression. Each hANT strain likely expresses the optimal levels of each hANT for cell survival.
To our knowledge, this is the first report demonstrating a practical and valid HTS strategy to identify compounds specifically inhibiting the energy flux between mitochondria and cytosol. The human ANT inhibitors identified in the screen may be useful probes to aid in understanding the biological consequences of interruption of energy homeostasis. Although these probes lack high potency and isoform selectivity, medicinal chemistry optimization may increase their affinity and selectivity as well as conferring drug-like properties. Potent, isoform-specific inhibitors of human ANT have potential clinical utility in diverse clinical indications.
Supplementary Material
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work conducted at the University of Minnesota was funded by National Institutes of Health (NIH) NICHD U01 HD076542 and HHSN275201300017C. This work was carried out in part using computing resources at the University of Minnesota Supercomputing Institute. Support for effort at the University of Florida was provided by NIH NICHD U01 HD060474. This work was partly supported by Otsuka Pharmaceuticals, Inc.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Flight MH Drug Screening: Shifting Energy Metabolism. Nat. Rev. Drug Discov 2010, 9, 272. [DOI] [PubMed] [Google Scholar]
- 2.Pebay-Peyroula E; Dahout-Gonzalez C; Kahn R; et al. Structure of Mitochondrial ADP/ATP Carrier in Complex with Carboxyatractyloside. Nature 2003, 426, 39–44. [DOI] [PubMed] [Google Scholar]
- 3.Clemencon B; Babot M; Trezeguet V The Mitochondrial ADP/ATP Carrier (SLC25 Family): Pathological Implications of Its Dysfunction. Mol. Aspects Med 2013, 34, 485–493. [DOI] [PubMed] [Google Scholar]
- 4.Sharaf el dein O; Mayola E; Chopineau J; et al. The Adenine Nucleotide Translocase 2, a Mitochondrial Target for Anticancer Biotherapy. Curr. Drug Targets 2011, 12, 894–901. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y; Ebermann L; Sterner-Kock A; et al. Myocardial Overexpression of Adenine Nucleotide Translocase 1 Ameliorates Diabetic Cardiomyopathy in Mice. Exp. Physiol 2009, 94, 220–227. [DOI] [PubMed] [Google Scholar]
- 6.Nadtochiy SM; Zhu Q; Urciuoli W; et al. Nitroalkenes Confer Acute Cardioprotection via Adenine Nucleotide Translocase 1. J. Biol. Chem 2012, 287, 3573–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YS; Kim JW; Osborne O; et al. Increased Adipocyte O2 Consumption Triggers HIF-l alpha, Causing Inflammation and Insulin Resistance in Obesity. Cell 2014, 157, 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brower JV; Rodic N; Seki T; et al. Evolutionarily Conserved Mammalian Adenine Nucleotide Translocase 4 Is Essential for Spermatogenesis. J. Biol. Chem 2007, 282, 29658–29666. [DOI] [PubMed] [Google Scholar]
- 9.Ford WC Glycolysis and Sperm Motility: Does a Spoonful of Sugar Help the Flagellum Go Round? Hum. Reprod. Update 2006, 12, 269–274. [DOI] [PubMed] [Google Scholar]
- 10.Murdoch FE; Goldberg E Male Contraception: Another Holy Grail. Bioorg. Med. Chem. Lett 2014, 24, 419–424. [DOI] [PubMed] [Google Scholar]
- 11.Leung WY; Hamazaki T; Ostrov DA; et al. Identification of Adenine Nucleotide Translocase 4 Inhibitors by Molecular Docking. J. Mol. Graph. Model 2013, 45, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingenberg M The ADP and ATP Transport in Mitochondria and Its Carrier. Biochim. Biophys. Acta 2008, 1778, 1978–2021. [DOI] [PubMed] [Google Scholar]
- 13.Hamazaki T; Leung WY; Cain BD; et al. Functional Expression of Human Adenine Nucleotide Translocase 4 in Saccharomyces cerevisiae. PLoS One 2011, 6, e19250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson PJ; Lardy HA Bongkrekic Acid: An Inhibitor of the Adenine Nucleotide Translocase of Mitochondria. J. Biol. Chem 1970, 245, 1319–1326. [PubMed] [Google Scholar]
- 15.Cuenca-Lopez MD; Karachitos A; Massarotto L; et al. Minocycline Exerts Uncoupling and Inhibiting Effects on Mitochondrial Respiration through Adenine Nucleotide Translocase Inhibition. Pharmacol. Res 2012, 65, 120–128. [DOI] [PubMed] [Google Scholar]
- 16.Pfaff E; Klingenberg M Adenine Nucleotide Translocation of Mitochondria: 1. Specificity and Control. Eur. J. Biochem. FEBS 1968, 6, 66–79. [DOI] [PubMed] [Google Scholar]
- 17.Herbert JM; Augereau JM; Gleye J; et al. Chelerythrine Is a Potent and Specific Inhibitor of Protein Kinase C. Biochem. Biophys. Res. Commun 1990, 172, 993–999. [DOI] [PubMed] [Google Scholar]
- 18.Paggi MG; Fanciulli M; Perrotti N; et al. The Role of Mitochondrial Hexokinase in Neoplastic Phenotype and Its Sensitivity to Lonidamine. Ann. N. Y. Acad. Sci 1988, 551, 358–360. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto S; Seta K; Morisco C; et al. Chelerythrine Rapidly Induces Apoptosis through Generation of Reactive Oxygen Species in Cardiac Myocytes. J. Mol. Cell. Cardiol 2001, 33, 1829–1848. [DOI] [PubMed] [Google Scholar]
- 20.Martin RJ Modes of Action of Anthelmintic Drugs. Vet. J. 1997, 154, 11–34. [DOI] [PubMed] [Google Scholar]
- 21.Monne M; Chan KW; Slotboom DJ; et al. Functional Expression of Eukaryotic Membrane Proteins in Lactococcus lactis. Protein Sci. 2005, 14, 3048–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booty LM; King MS; Thangaratnarajah C; et al. The Mitochondrial Dicarboxylate and 2-Oxoglutarate Carriers Do Not Transport Glutathione. FEBS Lett. 2015, 589, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao X; Spanjaard RA The Apoptotic Action of the Retinoid CD437/AHPN: Diverse Effects, Common Basis. J. Biomed. Sci 2003, 10, 44–49. [DOI] [PubMed] [Google Scholar]
- 24.Belzacq AS; El Hamel C; Vieira HL; et al. Adenine Nucleotide Translocator Mediates the Mitochondrial Membrane Permeabilization Induced by Lonidamine, Arsenite and CD437. Oncogene 2001, 20, 7579–7587. [DOI] [PubMed] [Google Scholar]
- 25.Lovinger DM Presynaptic Modulation by Endocannabinoids. Handb. Exp. Pharmacol 2008, 184, 435–477. [DOI] [PubMed] [Google Scholar]
- 26.Cadoudal T; Distel E; Durant S; et al. Pyruvate Dehydrogenase Kinase 4: Regulation by Thiazolidinediones and Implication in Glyceroneogenesis in Adipose Tissue. Diabetes 2008, 57, 2272–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuzu OF; Gowda R; Sharma A; et al. Leelamine Mediates Cancer Cell Death through Inhibition of Intracellular Cholesterol Transport. Mol. Cancer Ther 2014, 13, 1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.