Abstract
Callous-unemotional (CU) traits are characterized by deficits in guilt/empathy, shallow affect, and the callous and manipulative use of others. Individuals showing CU traits have increased risk for behavior problems and reduced responses to displays of distress in others. To explore how deficits in emotion-processing are associated with CU traits, the current study examined the association between callous-unemotionality and a neural index of facial emotion processing, using the event-related potential technique in a group of 3–5 year olds. Children viewed a series of static emotional faces, depicting either fear or happiness, while electroencephalography data were collected. The N170 component, thought to index the neural processes associated with face perception, was examined along with CU traits. Findings suggest that the unemotional dimension of CU traits is associated with diminished emotion-processing responses to fearful faces. Reduced neural responses to facial depictions of fear could be a biomarker for unemotional traits in early childhood.
Keywords: CU traits, unemotional traits, event-related potential, early childhood
Callous-unemotional (CU) traits are defined by a pattern of interpersonal and affective features including deficits in guilt and empathy, shallow affect, and a tendency toward the callous and manipulative use of others [1]. Early occurring CU traits have been associated with a heightened risk for developing psychopathy, a disorder that encompasses a constellation of affective (e.g., callousness), interpersonal (e.g., manipulativeness), and behavioral (e.g., impulsiveness) features in adulthood [2]. Evidence suggests that high levels of callous-unemotionality are associated with increased conduct problems in both early [3] and later childhood [1,4]. Indeed, the presence of CU traits was recently added to the Diagnostic and Statistical Manual of Mental Disorders– 5th edition [DSM; 5] as a specifier of conduct disorder, suggesting agreement among many clinicians and researchers about the diagnostic utility of callous-unemotionality.
Research has identified several characteristic features of children showing CU traits, including more persistent and severe behavior problems, poor response to intervention, insensitivity to punishment cues, and atypical processing of emotional displays of distress [3,6,7]. The current study focuses specifically on this latter feature. Difficulties processing emotional stimuli may be a core, stable feature of callous-unemotionality across ages. Indeed, such difficulties in emotion processing may describe a key process through which callous-unemotionality develops and is maintained. However, little is known about emotion processing difficulties in young children displaying elevated levels of CU traits. To address this gap in the literature, we examined the association between difficulties in emotion processing and CU traits in a sample of young children. More specifically, the current study focuses on facial emotion processing difficulties, as facial expressions are a primary avenue through which emotional information is communicated from individual to individual [8].
Facial emotion processing in adults with CU/psychopathic traits
Most previous research on facial emotion processing and callous-unemotionality has focused on adults. In the majority of these laboratory-based, experimental studies, individuals view a stream of static or dynamic faces depicting various emotions and complete an emotion identification task. To date, three meta-analyses have considered studies focusing on the association between CU/psychopathic traits and facial emotion processing [9,10,11]. Wilson et al. [11] found that individuals with CU/psychopathic traits show a deficit in processing a range of emotional facial stimuli, with the largest effect sizes observed for fearful and sad emotions. Similarly, the findings of Dawel et al. [9] suggest that emotion-processing deficits in individuals with CU/psychopathic traits span multiple emotion categories, including fear and sadness, but also happiness and surprise. However, studies focused particularly on the affective features of CU/psychopathic traits (but not the impulsive features) found more robust deficits in identifying fearful and sad facial expressions than other emotion categories. Additionally, Marsh and Blair [10] suggest that adults displaying antisocial behavior, both with and without concurrent CU/psychopathic traits, show poorer emotion identification capacities to fearful and sad facial stimuli. However, these results do not focus solely on CU traits, therefore it is difficult to determine whether or not CU traits are responsible for this association [10].
Overall, there is evidence to suggest that individuals with high levels of CU/psychopathic traits show more robust deficits in processing facial displays of distress than individuals with low levels of CU/psychopathic traits. Such deficits have been the focus of theories examining the development of CU/psychopathic traits and antisocial behavior. According to Blair’s Violence Inhibition Mechanism (VIM) hypothesis [12], children who have difficulty processing emotional expressions, particularly those emotions communicating distress (i.e., fear or sadness), may not experience this distress in others as aversive. Therefore, key efforts at socialization, which are often based on teaching children to act in ways that do not cause distress to others, are likely to be ineffective. Ineffective socialization efforts may result in abnormally low levels of prosocial behavior and high levels of conduct problems [12]. High levels of conduct problems can prompt a developmental cascade in which the child’s behavior problems are further exacerbated by negative responses and expectations from caregivers and peers.
Facial emotion processing in children with CU traits
Impairments in processing distressed facial expressions may also be present in children and adolescents with elevated levels of callous-unemotionality. Findings from several research groups suggest that children and adolescents displaying high levels of CU traits have difficulty recognizing and identifying faces depicting fearful and sad emotions [13,14,15,16]. The vast majority of this research has focused on school-aged children and adolescents.
Given that CU traits have been identified in very early childhood [17,18], it is plausible that the emotion recognition deficits associated with CU traits are also present early in development. Indeed, it may even be the case that the presence of emotion recognition deficits in young children confers a specific risk for developing CU traits. Early childhood is a sensitive period for socialization, and children who fail to process distress in others are thought to be at heightened risk for poor socialization outcomes [12]. However, to our knowledge, few studies have examined difficulties processing emotions among very young children. Those few studies support the interpretation that young children with elevated levels of CU traits show facial emotion processing deficits. Kimonis et al. [18] showed, in a community sample of children between 3 and 5 years old, that high levels of CU traits were associated with deficits in recognizing fearful static and dynamic facial stimuli. Additionally, preschoolers with low concern for others (e.g., acted like he/she did not care when someone felt bad or sad; a feature of CU traits in early childhood) were found to have deficits in recognizing fearful facial stimuli [19]. Previous work from our lab focusing on preschoolers suggests that higher levels of CU traits, more specifically callous-uncaring traits, were associated with reduced responses to fearful vocal stimuli [20].
Neural correlates of facial emotion processing in children.
The capacity to recognize and identify facial emotional expressions improves substantially during early childhood [21] with developments in specific brain regions (e.g., the amygdala) thought to underlie these improvements. Research using fMRI has demonstrated that children and adolescents displaying high levels of CU traits show diminished activation patterns during emotion-viewing tasks in the brain regions thought to underlie emotion processing. In particular, children and adolescents with high levels of CU traits show significantly less activation in the left and right amygdala than controls, both when viewing emotional facial stimuli [22,23,24] and during an affective theory of mind task [25]. Additionally, children with high levels of CU traits show a pattern of diminished functional connectivity between the ventromedial prefrontal cortex and the amygdala, suggesting a potentially poor network for regulation of emotional responses in children with CU traits [22]. Of note, these findings with children and adolescents with CU traits correspond with the findings of similar research with adults [26].
Although fMRI is a useful tool for investigating the activation patterns underlying processing emotional facial expressions, it is difficult to use this methodology with awake children under the age of four. Additionally, given the rapid time course with which emotion processing occurs [21], methodologies with higher temporal resolution may be especially well-suited for the study of emotion processing. Electroencephalography (EEG), which is feasible for use with very young children and provides highly accurate temporal information on the order of milliseconds (ms), can complement existing research using fMRI.
Research on the event-related potential (ERP) correlates of emotional face processing in children frequently focuses on the N170. The N170 is a negative-going component that is maximal at posterior-lateral electrodes and peaks between 130 to 200 ms post-stimulus (in adults). The N170 is thought to reflect the neural processes associated with encoding structural information about faces [27], and it has been localized to the superior temporal sulcus [28] and the fusiform gyrus [29], regions thought to underlie face-specific processing mechanisms. The neural generators of the N170 include a widespread network of visual areas that are sensitive to visual shapes and representations of faces [27]. Moreover, recent meta-analytic evidence also suggests that the N170 may be sensitive to emotional expressions, such that N170 amplitudes are larger (more negative) to fearful, happy, and angry expressions than to neutral expressions [30]. Therefore, N170 amplitudes may be a useful tool for investigating facial emotion processing capacities.
The N170 has been used to investigate disorders in which emotion processing capacities may be affected, including autism spectrum disorders [31,32], major depressive disorder [33], and anxiety disorders [34]. Given that individuals with CU/psychopathic traits also tend to show alterations in their capacity to process emotional faces, this ERP component may be useful for examining the neural correlates of emotional face processing deficits in these individuals. There are indeed studies on the association between the N170 and CU/psychopathic traits in adults. Brislin et al. [35] demonstrated that adults with higher scores on the callousness-aggression subfactor of the Externalizing Spectrum Inventory [36] showed reduced N170s specifically to fearful faces. Almeida et al. [37] demonstrated that higher scores on the fearless-dominance scale of the Psychopathic Personality Inventory – Revised [PPI-R; 38] were associated with diminished N170 amplitudes to facial stimuli across emotion categories (including fear, anger, and happiness). This finding suggests that adults displaying higher levels of fearless dominance, a trait indexing characteristically low levels of anxiety or inhibition, show poorer processing capacities for facial emotional stimuli [37]. Through manipulating the spatial frequencies of static, facial emotion stimuli, this association between fearless dominance and the N170 was found to emerge only for images with low spatial frequencies. Facial stimuli depicted with low spatial frequencies are coarse and shadowy, displaying the general structure of the face without a high level of detail. Facial stimuli depicted with high spatial frequencies include more detail in terms of both shape and texture. Almeida’s finding was interpreted to suggest that the association between fearless dominance and the N170 may stem from reduced input from the amygdala to the visual cortex (i.e., through effects on the tectopulvinar pathway). Notably, Almeida et al. also found that higher scores on the coldheartedness scale of the PPI-R, a scale assessing meanness and low levels of empathy, were associated with an enhanced N170 to fearful and happy stimuli, across both high and low spatial frequencies. Almeida et al. proposed that this enhanced N170 may indicate that adults displaying higher levels of coldheartedness may have to devote additional effort and attention to facial emotion processing, potentially through increasing activity in areas dedicated to effortful control or detailed visual analysis (i.e., through effects on geniculostriate pathway). However, without additional fMRI recordings or spatial localization techniques, it is difficult to confirm this interpretation. Despite this finding with coldheartedness, generally, the findings with adults suggest that individuals displaying higher levels of CU/psychopathic traits show diminished N170 amplitudes characteristic of poorer facial processing capacities. To our knowledge, no studies have examined the association between CU traits and the neural correlates of facial emotion processing in preschoolers.
The Current Study
The current study examined the association between CU traits in early childhood and the N170, a well-studied ERP correlate of facial processing that is likely sensitive to emotion. Based on prior findings with adults [35,37] and findings indicating that children displaying CU traits may show difficulties in processing emotional stimuli associated with distress [18,19,20,22,23,24], it was expected that young children displaying higher levels of CU traits would show smaller N170 amplitudes, suggesting diminished processing of facial emotion stimuli. Additionally, given that diminished emotion processing capacities in children with CU traits may be specific to emotions associated with distress, the association between CU traits and N170 amplitudes was expected to emerge for facial stimuli depicting fear, but not for facial stimuli depicting happiness.
Methods
Participants
Participants in the current study included 40 preschool-aged children (23 female) between the ages of 3 and 5 years. Each child’s primary caregiver completed a screening measure that assessed the child’s eligibility to participate in the laboratory portion of the study. Children were ineligible for the study if they 1) spoke a language other than English, 2) had significant developmental delays or, 3) had ever been referred to social services, as this may indicate maltreatment or severe family adversity, suggesting the possibility that environmental adversity may have contributed to the development of CU traits. Children exposed to severe environmental adversity may have key differences in the mechanisms through which CU traits develop, as well as in the neural responses to emotional stimuli [39,40]. Primary caregivers of eligible children were then contacted to schedule a follow-up laboratory visit in which the child participated in two tasks while electroencephalography (EEG) data were recorded. Children were recruited from local preschools (n = 36) as well as a University-based clinic for children with disruptive behavior disorders (n = 4). We opted to recruit from both a community as well as a clinical setting in order to ensure that our sample contained children displaying a range of externalizing problem severity.
Of those children whose caregivers completed the screening measure (n = 40), 29 children (17 female, M age = 49.55 months, SD = 8.40 months) participated in the EEG portion of the study. The 11 children who did not complete the lab portion of the experiment were excluded because 1) they met one of the exclusion criteria (n = 3), 2) caregivers were no longer interested in participating (n = 6), or 3) caregivers were interested in participating, but were unable to schedule a lab visit (n = 2). The children who were and were not included in the final sample did not differ significantly from one another in terms of CU traits or demographic variables. Three of these children were unable to be included in the final sample because their EEG data contained excessive movement artifacts (described below).
For the final sample with usable EEG data (n = 26, 14 female), the family’s socioeconomic status (SES), based on the Hollingshead Four Factor Index [41], which takes into account parent marital status, employment status, education level, and occupational prestige, ranged from 23.5 to 66 (M = 49.73, SD = 13.61), indicating that the sample was predominantly middle class. The sample included children who were Caucasian (70%) and Mixed ethnocultural groups (30%). Children were primarily from two-parent households (76%), and primary caregivers were typically college educated (76%). Child performance on a standardized measure of receptive vocabulary [Peabody Picture Vocabulary Test, Fourth Edition; PPVT-4; 42] was within the normal range for preschool-aged children (standardized score: M = 112.16, SD = 12.37, range = 89–131). None of the children had any known visual or hearing impairments, per primary caregiver report.
Procedures
Eligible children were scheduled for an hour-long lab visit accompanied by their primary caregiver. During the lab visit, children participated in two passive tasks, an auditory oddball task and an emotional face-viewing paradigm, while EEG data were collected. The current study focuses exclusively on the passive emotional face-viewing paradigm. After the child completed the EEG tasks, a research assistant administered the PPVT-4 and a behavioral emotion-identification task (using the stimuli from the passive emotion face-viewing paradigm) to the child. Before and during the lab visit, the child’s primary caregiver completed a series of questionnaires, including measures of child behavior problems. Families received monetary compensation, and children received a small toy to thank them for their participation. The current study complied with all the ethical standards of the American Psychological Association, and all procedures were approved by the institutional review board at Indiana University.
Measures
ERP measures of facial emotion processing.
During the emotional face-viewing task, the child was presented with a series of static facial stimuli depicting various emotional expressions. Neural responses during this task served as an index of the child’s capacity to process emotional facial affect. To retain the maximum amount of data, we opted to use a passive task, because of the known variability in behavioral response capacities of preschool-aged children. Through using a passive task, children across a range of ability levels were able to participate and provide usable data. During the 6-minute task, a research assistant sat with the child to ensure compliance. Children were instructed to sit as still as possible, while watching a TV monitor located approximately 2 meters in front of them. If the child looked away from the monitor, talked, or made excessive movements, the task was paused, and the research assistant gently redirected the child.
Task stimuli included a series of static, upright and inverted emotional faces. The faces, which were taken from the NimStim stimulus collection [43], displayed one of two emotional expressions, happiness or fear, each portrayed by six different female actors. To ensure that each participating child could correctly identify the emotions displayed on the upright faces, children were asked to behaviorally identify what faces displayed what emotion after the task was completed. All children included in the final sample were able to identify correctly each emotional face. The task was divided into four blocks containing 48 trials, which included a mix of upright and inverted, happy and fearful faces. Inverted faces were used as a control condition for the upright emotional faces, because face inversion is known to disrupt the recognition of expressions of emotions [44,45]. Each of the four stimulus types (upright happy, inverted happy, upright fearful, and inverted fearful) was presented with equal probability (48 trials each) for a total of 192 trials. A checkerboard pattern, which served as a pre-trial fixation point, appeared for 350 ms prior to the presentation of each facial stimulus. Facial stimuli appeared on screen for approximately 1000 ms. The resulting ERP waveforms were time locked to the presentation of each facial stimulus.
CU traits.
Growing evidence suggests that CU traits in childhood are unlikely to be a unidimensional construct. Three dimensions of CU traits have been proposed, including callous, uncaring, and unemotional traits. The callous dimension indexes traits indicative of a lack of empathy or remorse for others. The uncaring dimension includes traits indicative of a lack of concern for the feelings of others. The unemotional dimension indexes traits indicative of impoverished affect and abnormal emotional experiences and expressions. Both the callous and uncaring dimensions of CU traits have been shown to converge and diverge with the external correlates of psychopathy (e.g., aggression and delinquency vs prosocial development) in expected ways [46,47,48]. However, the unemotional dimension, despite demonstrating adequate reliability, typically shows low correlations with aggression and the other two CU trait dimensions [46,47,48]. For this reason, the unemotional scale is thought to index traits that are different from those encompassed within the callous and uncaring dimension [46,48]. In the current study, each child’s primary caregiver completed the Inventory of Callous-Unemotional Traits (ICU) preschool-version, a widely used measure of CU traits [49]. The items of the ICU were assessed using a four-point scale ranging from not at all true (0) to definitely true (3). We used the two-factor structure proposed by Henry et al. [48]: (1) callousness-uncaring dimension (17 items, e.g., “seems very cold and uncaring”), and (2) unemotional dimension (5 items, e.g., “does not show his/her emotions to others”). Cronbach α values for the callousness-uncaring and the unemotional dimensions were .90 and .78, respectively. Additionally, we examined a higher order factor that took into account all of the items on the ICU, the ICU Total Score [50].
Control variables.
Child age in months and sex were also included as control variables in analysis, as these variables have been shown to be associated with emotion processing capacities [21].
Recordings and data processing
Netstation Acquisition Software 4.4.2 (Electrical Geodesic, Inc.: Eugene, OR) was used to collect and process the continuous EEG data recorded during the face-viewing paradigm. Data were collected using a 128-electrode Hydrocel Geodesic Sensor Net with a sampling rate of 250 hertz. Throughout the recording session, all electrode impedences were adjusted to be at or below 50 kΩ. After collection, the continuous waveform was band-pass filtered from 0.3 to 30 Hz, and then segmented into 1200 ms epochs that began 200 ms prior to presentation of each facial stimulus. Epochs were manually inspected for artifacts, and then automatically examined for artifacts. The automatic artifact detection procedure included the identification and removal of channels that contained a voltage shift greater than 150 μV during a given segment of length 80 ms, and the removal of epochs that contained 20 or more bad channels. For a child’s ERP data to be included in analysis, he or she had to meet the criterion of having at least 9 artifact-free trials in each of the four stimulus conditions. Of the 29 children who participated in the passive emotional face viewing paradigm, 26 met the criterion, with 3 children unable to be included in the final sample because their data contained excessive movement artifacts. There were no significant differences between the children who did and did not provide usable EEG data on any of the variables examined in the analysis. Each individual’s epoched data were then re-referenced to an average reference (the average of all scalp electrodes), and baseline corrected by subtracting the average activity from each epoch’s 200 ms baseline.
After processing, the ERP waveform was statistically decomposed using a sequential, temporo-spatial principal components analysis (tsPCA), which objectively and empirically determines the regions of electrodes and time frames that parsimoniously account for the majority of the variance in the waveforms [51]. The factors identified by the tsPCA are thought to correspond with traditionally-defined ERP components [51]. Given our use of high-density ERPs, this approach allowed us to effectively reduce and describe our data in an empirical, data-driven way [52], that did not rely solely on expectations about component morphology and topography based on research with older children and adults. Our use of tsPCA joins several other studies that were based on this technique to examine the ERP correlations of emotion processing [e.g., 53,54]. Sequential tsPCA was conducted using the ERP PCA toolkit [55], a toolkit that is publicly available for use within the Matlab software package. The initial tsPCA identified 12 temporal factors that accounted for 96% of the variance, and the subsequent tsPCA identified 5 temporo-spatial factors that accounted for 85% of the variance in the waveform. The temporo-spatial factor thought to correspond with the N170 was selected based on a priori expectations about the latency and topography of the component. The chosen temporo-spatial factor peaked around 160 – 260 ms post-stimulus and was characterized by a posterior negativity.
Analysis Plan
The association between N170 amplitudes and CU traits was first examined using Pearson correlations. Significant correlations were then further examined using multiple regression analysis to examine the association between ERP amplitudes and CU traits while controlling for covariates (i.e., child age and sex). All models were fitted using the R statistical software package [56].
Results
Grand-averaged waveforms
The grand-averaged waveform for the N170, across the cluster of posterior electrodes, is presented in Figure 1. Corresponding to previous research on the N170, the amplitudes to fearful faces were larger (i.e., more negative/less positive, as the N170 is a negative-going component) than the amplitudes to inverted fearful faces (amplitudes to fearful faces: M = 3.14 μV vs amplitudes to inverted fearful faces: M = 5.39 μV). However, this difference did not surpass the threshold for significance of p > .05 (t[25] = −1.13, p = .27). This finding could potentially be due to the variability that characterizes the ERPs in young children, along with the relatively small sample size of the current study. The N170 amplitudes to happy faces were somewhat more positive than the N170 amplitudes to inverted happy faces (amplitudes to happy faces M: 3.44 μV vs inverted happy faces M amplitudes: 2.08 μV), but this difference was not significant (t[25] = 0.81, p = .43). Descriptive values for all variables included in analysis are provided in Table 1.
Figure 1.
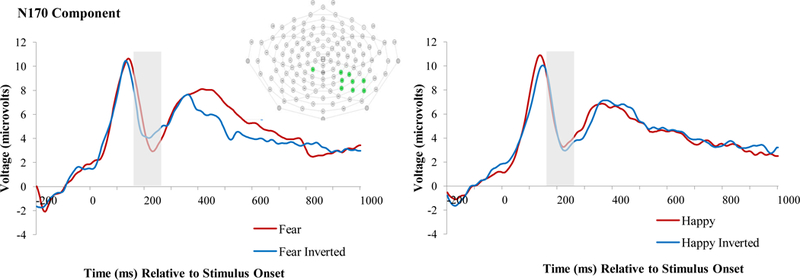
Grand-averaged waveform across the depicted posterior cluster of electrodes. Fear (with fear inverted) and happy (with happy inverted) conditions are presented separately. Gray boxes indicate the time frame over which the N170 was calculated.
Table 1.
Descriptive values for all study variables for the final sample
| N | M | SD | ||
|---|---|---|---|---|
| N170 amplitudes | Happy amplitudes | 26 | 3.44 | 3.92 |
| Happy inverted amplitudes | 26 | 2.08 | 9.19 | |
| Fear amplitudes | 26 | 3.14 | 6.38 | |
| Fear inverted amplitudes | 26 | 5.39 | 7.03 | |
| CU traits | ICU unemotional a | 25 | 2.44 | 2.10 |
| ICU callousness-uncaring a | 25 | 13.80 | 5.66 | |
| ICU total ab | 25 | 17.04 | 6.56 | |
Note.
Data on CU traits were missing for one child.
The ICU total score was calculated by summing all of the items on the ICU, not just those items included in the Henry et al. (2016) unemotional and callousness-uncaring scales.
ICU: Inventory of Callous-unemotional Traits
The Fear N170 and CU Traits
Correlations between the variables included in the analysis are presented in Table 2. There was a significant, positive association between unemotional traits as reported on the ICU and Fear N170 amplitudes (r = .46, p < .05), such that more positive N170 amplitudes were associated with higher levels of unemotional traits. As the N170 is a negative-going component, this finding indicates that smaller (more positive) N170 amplitudes to fearful faces are associated with higher levels of unemotional traits. The N170 is thought to index processing capacities related to facial stimuli, with smaller (more positive) amplitude indicative of poorer facial processing capacities. The current findings indicate that children showing higher levels of unemotional traits also show impaired processing of fearful faces. Notably, N170 amplitudes to inverted fearful, inverted happy, and happy faces were not associated with unemotional traits. The N170 (elicited across each task block) was not associated with callousness-uncaring traits or the total score on the ICU.
Table 2.
Correlations between variables included in analysis.
| N170 amplitudes | CU traits | Covariates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Happy amp |
2. Happy inverted amp |
3. Fear amp |
4. Fear inverted amp |
5. ICU unemotional |
6.
ICU callousness- uncaring |
7. ICU total |
8. Age | 9. Sex | |||
|
N170 Amplitudes |
1. Happy amp | 1 | |||||||||
| 2. Happy inverted amp | .36† | 1 | |||||||||
| 3. Fear amp | .46* | .05 | 1 | ||||||||
| 4. Fear inverted amp | .23 | .58** | -.15 | 1 | |||||||
|
CU traits |
5. ICU unemotional | .10 | -.04 | .46* | -.11 | 1 | |||||
| 6. ICU callousness- uncaring |
-.13 |
.12 |
-.05 |
.13 |
.18 |
1 |
|||||
| 7. ICU total | -.11 | .14 | .09 | .11 | .47* | .95*** | 1 | ||||
|
Covariates |
8. Age | .41* | .39* | .55** | .46* | .19 | -.06 | .05 | 1 | ||
| 9. Sex | -.17 | -.33† | .01 | -.26 | .07 | -.38† | -.33 | .01 | 1 | ||
Note:
p ≤ .05,
p ≤ .01,
p ≤ .001,
p < .10
ICU: Inventory of Callous-unemotional Traits
Amp: Amplitudes
Female = 1, Male = 0
Control variables.
Using multiple regression analysis, we examined if the significant association between unemotional traits and the N170 to fearful faces (Part A; Table 3) was maintained after controlling for child age and sex (Part B; Table 3). The inclusion of these control variables did not change the overall pattern of association between unemotional traits and the N170. In this model, the control variable age also significantly predicted fear N170 amplitudes (β = 0.47, p < .05), such that older children had smaller (more positive) N170 amplitudes to fearful faces.
Table 3.
Multiple Regression Analyses Predicting N170 Amplitudes to Upright, Fear Stimuli, including Control Variables
| β | p-value | |
|---|---|---|
| Part A | ||
| ICU unemotional traits | 0.46 | .02 |
| F(1,23) = 6.05, p = .02, R2 = .21 | ||
| Part B | ||
| ICU unemotional traits | 0.37 | .04 |
| Control variables: | ||
| Age (months) | 0.47 | .01 |
| Sex* | 0.01 | .94 |
| F(3,21) = 5.11, p = .008, R2 = .42 | ||
Note:
Female = 1, Male = 0
ICU: Inventory of Callous-unemotional Traits
The Happy N170 and CU Traits
As the difficulties processing emotional facial stimuli shown by children displaying CU traits are thought to be more specific to distress stimuli (e.g., fear and sadness), we did not anticipate finding an association between CU traits and N170 amplitudes in the happy or happy inverted conditions. Aligning with expectations, no significant associations were observed between CU traits and happy or happy inverted N170 amplitudes (see Table 2).
Discussion
The current study examined the association between CU traits, as reported by primary caregivers, and the N170, an ERP component associated with face processing that is likely sensitive to emotional expressions. As expected, we found an association between CU traits and the N170 component to fearful faces, such that children who were rated as more unemotional showed a pattern of neural activity indicative of diminished facial emotion-processing for fearful faces. These findings are consistent with previous research indicating that children displaying CU traits may have difficulties processing emotional stimuli. Additionally, these emotion processing difficulties, at least in the context of facial stimuli, are specific to fear (but not happy) stimuli. This provides further support for research indicating that deficits in emotion processing associated with callous-unemotionality are specific to distress-related emotional stimuli.
Our previous research using the same sample suggests that preschoolers displaying higher levels of callousness-uncaring traits show neural activation patterns indicative of less sensitive processing of vocal fear [20]. However, in the current study, the association between the neural correlates of facial affect processing and CU traits was limited to the unemotional scale, a scale that is typically uncorrelated with scores on the callous and uncaring dimension of CU traits [46]. The possible reasons for these different associations are discussed below; however, when considered together, the current study and Hoyniak et al. [20] provide converging evidence that the difficulties in processing fear that have been noted for older children, adolescents, and adults with high levels of CU traits are present in very early childhood. These results also support the interpretation that the emotion-processing difficulties of children with CU traits extend across both auditory and visual sensory modalities.
Neither study found an association between CU traits and neural responses to happy stimuli (although Hoyniak et al. [20] did find a trending, positive association between CU traits and happy mismatch negativity [MMN] ERP amplitudes, such that children with higher levels of callousness-uncaring traits showed better capacity to differentiate auditory tones with happy valence). Coupled with a growing literature that suggests that emotion processing difficulties in individuals displaying CU traits may be specific to emotions associated with distress, especially fear [10,57], our findings support Blair’s hypothesis [12] that emotion-processing deficits in early childhood could be the foundation for the development of later CU traits.
It is important to note that, in the current study, the association between CU traits and the N170 was specific to the unemotional subscale of the preschool ICU. Previous work, including our own, has typically focused on the callousness-uncaring subscale of the ICU. Traditionally, the callous and uncaring dimensions of CU traits are more highly correlated with each other and relevant external correlates (e.g., externalizing and antisocial behavior), whereas the unemotional scale is relatively distinct from both the callous and uncaring dimensions as well as relevant external correlates. Such findings have led researchers to question the utility of the unemotional scale to capture traits conferring specific risk for callous-unemotionality, arguing that the low levels of emotionality could be indicative of numerous phenotypic expressions of psychopathology, including autism spectrum disorders and depression/anhedonia. Explanations for these findings have been proposed, including that the low number of items of the unemotional scale might influence the utility of this trait dimension [46]. However, despite concerns regarding the specificity of the unemotional scale, this dimension captures a meaningful construct of developmental psychopathology: the propensity for impoverished affective expressions. Indeed, the unemotional dimension is an important symptom dimension worth investigating because of its association with a broad dimension of psychopathology, as well as its association with diminished empathy skills [46]. Our findings suggest that the presence of elevated unemotional traits is associated with diminished neural processing of fearful faces, but neither unemotional traits nor neural facial processing deficits (e.g., a diminished N170) were associated with callous-uncaring traits. Although additional research is needed to understand the utility of the unemotional scale as an index of CU traits, the findings of the current study suggest that parent-reported child unemotionality is associated with a biomarker of diminished facial emotion processing.
When controlling for age and sex, the current study’s main findings (i.e., the association between unemotional traits and the fear N170) were unchanged. However, the multiple regression analysis indicated that, when included in the same multiple regression equation, both unemotional traits and age significantly predicted fear N170 amplitudes. Research describing the development of the face-sensitive N170 component from childhood to adulthood suggests that the N170 stabilizes in the preschool years, and shows little change in amplitude or latency with age [58]. Based on these findings, we did not expect to see age-related changes in the N170 during this age window, especially since we limited our sample to children between the ages of 3 and 5. However, the findings of our study seem to suggest that there may be age-related changes in the N170 over the preschool era. Age was positively associated with N170 amplitudes across task conditions, such that older children showed reduced N170 amplitudes to both emotional and inverted facial stimuli. This finding generally corresponds with previous research, across ERP components, that suggests that ERP amplitudes decrease with age, potentially reflecting that cortical activations become more focalized across development. However, it is difficult to reconcile this developmental finding with other robust findings that smaller components index poorer processing capacities. Although reconciling this issue is beyond the scope of the current study, we found that the association of interest (CU traits and the fear N170) held even when accounting for the effect of age on the N170. Additionally, unemotionality was not associated with age, suggesting that our findings were not simply an artifact of the age-related association. However, there is no doubt that additional research addressing the influence of normative developmental changes on ERP findings in childhood is necessary. While the current study lacks the power or the design to do this, larger cross-sectional studies and longitudinal studies will be necessary to fully explore the effects of development on the N170 in early childhood.
Strengths and Limitations
This study had strengths worth noting. First, our focus on the neural correlates of emotion processing, rather than merely the behavioral indexes of emotion processing, makes this study an important and novel contribution to this literature. The current study adds to a literature demonstrating the association between the neural correlates of facial emotion processing and CU traits [10,57], extending these findings to a sample of preschoolers. Additionally, the current study joins a growing literature exploring CU traits, and related correlates, in infancy and early childhood [59]. This increased focus on describing the early behavioral [e.g., preferences for faces in infancy; 59] and neural features of young children displaying high levels of CU traits enables us to better understand mechanisms in the development of CU traits. As this literature matures, novel targets for early intervention and prevention efforts may be identified.
Additionally, the current study uses ERPs, a tool that is ideally suited for use with young children, who may struggle to comply with other forms of neural data collection (e.g., fMRI). Additionally, through focusing on ERPs, rather than explicit behavior, passive tasks can be used. Passive tasks are useful tools in early childhood, a developmental era when behavioral response capacities are still developing and thus less stable, potentially complicating interpretations of behavioral task performance.
This study also had limitations worth noting. The sample size of the current study is relatively modest. Because of this, we may have lacked the power to detect more nuanced, smaller-sized effects, and may have overestimated the size of the effects we did detect. This sample size is within the typical range of comparable ERP studies focusing on developmental samples (average of 15–27 children per study [60]), but eventually, it will be important for larger studies focusing on preschool-aged children to replicate and extend these findings. Additionally, despite a concerted effort to recruit families from a variety of ethnocultural groups and income backgrounds, the sample was predominantly middle class, and reflected the population of a small Midwestern city. As children from low SES backgrounds are more likely to show increased antisocial behavior, in general, and increased CU traits, in particular [61], our sample’s small percentage of lower SES families may have influenced our results. Future studies should seek to not only replicate these results in higher risk samples, but also with in more diverse samples.
Conclusion
Given increased interest in the utility of CU traits to explain heterogeneity in children with conduct problems (i.e., the newly-added limited prosocial emotions specifier of conduct disorder in the DSM; 5), the current study provides important information about the neural correlates that potentially underlie these traits in early childhood. Our findings suggest that facial emotion processing deficits are associated with unemotionality in early childhood, suggesting that a diminished N170 may be a biomarker for unemotionality. Although additional research is needed to understand the utility of the unemotional dimension of the ICU [49], the current study identifies a target neural system involved in this symptom dimension in early childhood.
Summary
The current study examined the association between parent-reported CU traits and the N170, a well-studied ERP index of facial emotion processing, in a sample of preschool-aged children. We found an association between CU traits and the N170 elicited to fearful faces, such that children who were rated as more unemotional showed a pattern of neural activity indicative of diminished facial emotion-processing abilities for fearful faces. These findings support a growing literature indicating that children displaying CU traits may have difficulties processing distress-related emotional stimuli, and extends this literature by indicating that such emotion processing deficits are present in early childhood.
Acknowledgments
Acknowledgements: This project was supported by a Project Development Team within the ICTSI NIH/NCRR Grant Number TR000006 and the Department of Criminal Justice, Indiana University Bloomington. Caroline Hoyniak is supported by a Graduate Research Fellowship from the National Science Foundation Grant Number 1342962. Dr. Nathalie Fontaine is a Research Scholar, Junior 1, Fonds de recherche du Québec–Santé. We thank Professors Dennis Molfese, Aina Puce and Bennett Bertenthal for their support, and gratefully acknowledge the contribution of the research assistants, families, and children.
Footnotes
Conflicts of interest: None.
References
- 1.Frick PJ, Ray JV, Thornton LC, Kahn RE (2014) Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol Bull 140: 1–57 [DOI] [PubMed] [Google Scholar]
- 2.Lynam DR, Caspi A, Moffitt TE, Loeber R, Stouthamer-Loeber M (2007) Longitudinal evidence that psychopathy scores in early adolescence predict adult psychopathy. J Abnorm Psychol 116: 155–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longman T, Hawes DJ, Kohlhoff J (2015) Callous–unemotional traits as markers for conduct problem severity in early childhood: A meta-analysis. Child Psychiatry Hum Dev 47: 326–334 [DOI] [PubMed] [Google Scholar]
- 4.Fontaine NM, Rijsdijk FV, McCrory EJ, Viding E (2010) Etiology of different developmental trajectories of callous-unemotional traits. J Am Acad Child Adolesc Psychiatry 49: 656–664 [DOI] [PubMed] [Google Scholar]
- 5.American Psychological Association (2013) Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC. [Google Scholar]
- 6.Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT, Loney BR (2003) Callous-unemotional traits and developmental pathways to severe conduct problems. Dev Psychol 39: 246–260 [DOI] [PubMed] [Google Scholar]
- 7.Waller R, Hyde LW (2017) Callous–unemotional behaviors in early childhood: Measurement, meaning, and the influence of parenting. Child Dev Perspectives 11: 120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis H, Young AW (1998) Faces in their social and biological context. In Young AW (Ed.), Face and Mind (67–96). London: Oxford University Press [Google Scholar]
- 9.Dawel A, O’Kearney R, McKone E, Palermo R (2012) Not just fear and sadness: Meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neurosci Biobehav Rev 36: 2288–2304 [DOI] [PubMed] [Google Scholar]
- 10.Marsh AA, Blair RJR (2008) Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neurosci Biobehav Rev 32: 454–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson K, Juodis M, Porter S (2011) Fear and loathing in psychopaths: A meta-analytic investigation of the facial affect recognition deficit. Crim Justice Behav : 38: 659–668 [Google Scholar]
- 12.Blair RJR (1995) A cognitive developmental approach to morality: Investigating the psychopath. Cognition 57: 1–29 [DOI] [PubMed] [Google Scholar]
- 13.Blair RJR, Coles M (2000) Expression recognition and behavioural problems in early adolescence. Cogn Dev 15: 421–434 [Google Scholar]
- 14.Blair RJR, Colledge E, Murray L, Mitchell D (2001) A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J Abnorm Child Psychol 29: 491–498 [DOI] [PubMed] [Google Scholar]
- 15.Stevens D, Charman T, Blair RJR (2001) Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. J Genet Psychol 162: 201–211 [DOI] [PubMed] [Google Scholar]
- 16.Dadds M, Perry Y, Hawes D, Merz S, Riddell A, Haines D (2006) Attention to the eyes reverses fear recognition deficits in psychopathy. Br J Psychiatry 189: 280–281 [DOI] [PubMed] [Google Scholar]
- 17.Hyde LW, Shaw DS, Gardner F, Cheong J, Dishion TJ, Wilson M (2013) Dimensions of callousness in early childhood: Links to problem behavior and family intervention effectiveness. Dev Psychopathol 25: 347–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimonis ER, Fanti KA, Anastassiou-Hadjicharalambous X, Mertan B, Goulter N, Katsimicha, E (2016) Can callous-unemotional traits be reliably measured in preschoolers? J Abnorm Child Psychol 44: 625–638 [DOI] [PubMed] [Google Scholar]
- 19.White SF, Briggs-Gowan MJ, Voss JL, Petitclerc A, McCarthy KR, Blair RJR, et al. (2016). Can the fear recognition deficits associated with callous-unemotional traits be identified in early childhood? J Clin Exp Neuropsychol 38: 672–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoyniak CP, Bates JE, Petersen IT, Yang C, Darcy I, Fontaine NMG (2017) Reduced neural response to vocal fear: A potential biomarker for callous-uncaring traits in early childhood. Dev Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herba C, Phillips M (2004) Annotation: Development of facial expression recognition from childhood to adolescence: Behavioural and neurological perspectives. J Child Psychol Psychiatry 45: 1185–1198 [DOI] [PubMed] [Google Scholar]
- 22.Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS et al. (2008) Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry 165: 712–720 [DOI] [PubMed] [Google Scholar]
- 23.Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E (2009) Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry 166: 95–102 [DOI] [PubMed] [Google Scholar]
- 24.Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA et al. (2012) Amygdala response to preattentive masked fear in children with conduct problems: The role of callous-unemotional traits. Am J Psychiatry 169: 1109–1116 [DOI] [PubMed] [Google Scholar]
- 25.Sebastian CL, McCrory EJ, Cecil CA, Lockwood PL, De Brito SA, Fontaine NM et al. (2012) Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch Gen Psychiatry 69: 814–822 [DOI] [PubMed] [Google Scholar]
- 26.Decety J, Skelly L, Yoder KJ, Kiehl KA (2014) Neural processing of dynamic emotional facial expressions in psychopaths. Soc Neurosci 9: 36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossion B, Jacques C (2011) The N170: understanding the time-course of face perception in the human brain. In Luck S, Kappenman E (Eds.), The Oxford handbook of event-related potential components 115–142 [Google Scholar]
- 28.Itier RJ, Taylor MJ (2004) Source analysis of the N170 to faces and objects. Neuroreport 15: 1261–1265 [DOI] [PubMed] [Google Scholar]
- 29.Sadeh B, Podlipsky I, Zhdanov A, Yovel G (2010) Event‐related potential and functional MRI measures of face‐selectivity are highly correlated: A simultaneous ERP‐fMRI investigation. Hum Brain Map 31: 1490–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinojosa J, Mercado F, Carretié L (2015) N170 sensitivity to facial expression: A meta-analysis. Neurosci Biobehav Rev 55: 498–509 [DOI] [PubMed] [Google Scholar]
- 31.Hileman CM, Henderson H, Mundy P, Newell L, Jaime M (2011) Developmental and individual differences on the P1 and N170 ERP components in children with and without autism. Dev Neuropsychol 36: 214–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ (2004) Event‐related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J Child Psychol Psychiatry 45: 1235–1245 [DOI] [PubMed] [Google Scholar]
- 33.Proudfit GH, Bress JN, Foti D, Kujawa A, Klein DN (2015) Depression and event-related potentials: Emotional disengagement and reward insensitivity. Curr Opin Psychol 4: 110–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, Phan KL (2015) Enhanced neural reactivity to threatening faces in anxious youth: Evidence from event-related potentials. J Abnorm Child 43: 1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brislin SJ, Yancey JR, Perkins ER, Palumbo IM, Drislane LE, Salekin RT et al. (in press) Callousness and affective face processing in adults: Behavioral and brain-potential indicators. Personal Disord [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD (2007) Linking antisocial behavior, substance use, and personality: An integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol 116: 645–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almeida PR, Ferreira‐Santos F, Vieira JB, Moreira PS, Barbosa F, Marques‐Teixeira J (2014) Dissociable effects of psychopathic traits on cortical and subcortical visual pathways during facial emotion processing: An ERP study on the N170. Psychophysiology 51: 645–657 [DOI] [PubMed] [Google Scholar]
- 38.Lilienfeld S, Widows M (2005) Psychopathic Personality Inventory-Revised: Professional Manual. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- 39.Fanti KA, Demetriou CA, Kimonis ER (2013) Variants of callous-unemotional conduct problems in a community sample of adolescents. J Youth Adolesc 42: 964–979 [DOI] [PubMed] [Google Scholar]
- 40.McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA et al. (2011) Heightened neural reactivity to threat in child victims of family violence. Curr Biol 21: R947-R948 [DOI] [PubMed] [Google Scholar]
- 41.Hollingshead AB (1975) Four factor index of social status. Unpublished work. [Google Scholar]
- 42.Dunn LM, Dunn DM (2007) PPVT-4: Peabody picture vocabulary test. Minneapolis, MN.: Pearson Assessments. [Google Scholar]
- 43.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA et al. (2009) The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res 168: 242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eimer M, Holmes A (2002) An ERP study on the time course of emotional face processing. Neuroreport 13: 427–431 [DOI] [PubMed] [Google Scholar]
- 45.Searcy JH, Bartlett JC (1996) Inversion and processing of component and spatial–relational information in faces. J Exp Psychol Hum Percept Perform 22: 904–915 [DOI] [PubMed] [Google Scholar]
- 46.Cardinale EM, Marsh AA (in press) The reliability and validity of the Inventory of Callous Unemotional Traits: A meta-analytic review. Assessment [DOI] [PubMed] [Google Scholar]
- 47.Hawes SW, Byrd AL, Henderson CE, Gazda RL, Burke JD, Loeber R et al. (2014). Refining the parent-reported Inventory of Callous–Unemotional Traits in boys with conduct problems. Psychol Assess 26: 256–266 [DOI] [PubMed] [Google Scholar]
- 48.Henry J, Pingault JB, Boivin M, Rijsdijk F, Viding E (2016) Genetic and environmental aetiology of the dimensions of Callous-Unemotional traits. Psychol Med 46: 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frick PJ (2004) The Inventory of Callous-Unemotional traits. Unpublished rating scale. University of New Orleans [Google Scholar]
- 50.Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC et al. (2008) Assessing callous–unemotional traits in adolescent offenders: Validation of the Inventory of Callous–Unemotional traits. Int J Law Psychiatry 31: 241–252 [DOI] [PubMed] [Google Scholar]
- 51.Dien J, Frishkoff GA (2005) Introduction to principal components analysis of event-related potentials. In Handy TC (Ed.), Event related potentials: A methods handbook (189 – 207). Cambridge, Massachussetts: MIT Press [Google Scholar]
- 52.Dien J (2010) Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. Psychophysiology 47: 170–183 [DOI] [PubMed] [Google Scholar]
- 53.Foti D, Hajcak G, Dien J (2009) Differentiating neural responses to emotional pictures: evidence from temporal ‐ spatial PCA. Psychophysiology 46: 521–530 [DOI] [PubMed] [Google Scholar]
- 54.Kujawa A, Weinberg A, Hajcak G, Klein D (2013) Differentiating event-related potential components sensitive to emotion in middle childhood: Evidence from temporal–spatial PCA. Dev Psychobiol 55: 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dien J (2010) The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. J Neurosci Methods 187: 138–145 [DOI] [PubMed] [Google Scholar]
- 56.R Core Team (2012) R: A language and environment for statistical computing. [Google Scholar]
- 57.White SF, Williams WC, Brislin SJ, Sinclair S, Blair KS, Fowler KA et al. (2012) Reduced activity within the dorsal endogenous orienting of attention network to fearful expressions in youth with disruptive behavior disorders and psychopathic traits. Dev Psychopathol 24: 1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuefner D, de Heering A, Jacques C, Palmero-Soler E, Rossion B (2010) Early visually evoked electrophysiological responses over the human brain (P1, N170) show stable patterns of face-sensitivity from 4 years to adulthood. Front Hum Neurosci 3:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bedford R, Pickles A, Sharp H, Wright N, Hill J (2014) Reduced face preference in infancy: A developmental precursor to callous-unemotional traits? Biol Psychiatry 78: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoyniak C (2017) Changes in the NoGo N2 Event-Related Potential component across childhood: A systematic review and meta-analysis. Dev Neuropsychol 42: 1–24 [DOI] [PubMed] [Google Scholar]
- 61.Piotrowska P, Stride C, Croft S, Rowe R (2015) Socioeconomic status and antisocial behaviour among children and adolescents: A systematic review and meta-analysis. Clin Psychol Rev 35: 47–55. [DOI] [PubMed] [Google Scholar]


