Abstract
Objectives:
To assess the impact of undisclosed HIV infection and antiretroviral (ARV) therapy (ART) on national estimates of diagnosed HIV and ART coverage in Kenya.
Methods:
HIV-positive dried blood spot samples from Kenya’s second AIDS Indicator Survey were tested for an ARV biomarker by liquid chromatography-tandem mass spectrometry. Estimates of diagnosed HIV and ART use based on self-report were compared with those corrected for undisclosed HIV infection and ART use based on ARV testing. Multivariate analysis determined factors associated with undisclosed HIV infection and ART use among persons on ART.
Results:
Among 559 HIV-positive samples, the ARV biomarker was detected in 42.5% (CI 37.4–47.7). ARV drugs were present in 90.7% (CI 86.1–95.2) reporting HIV-positive status and receiving ART, 66.7% (CI 59.9–73.4) reporting HIV-positive status irrespective of ART use, 21.0% (CI 13.4–28.6) reporting HIV-negative status, and 19.3% (CI 9.0–29.5) reporting no previous HIV test. After correcting for undisclosed HIV infection and ART use, diagnosed HIV increased from 46.9% to 57.2% and ART coverage increased from 31.8% to 42.8%. Undisclosed HIV infection on ART was associated with being aged 25–39 years and not visiting a health provider in the past year, while younger age and higher wealth was associated with undisclosed ART use.
Conclusion:
Substantial levels of undisclosed HIV infection and ART use while on ART were observed, resulting in diagnosed HIV underestimated by 112,000 persons and ART coverage by 131,000 persons. Supplementing self-reported ART status with objective measures of ART use in national population-based sero-surveys can improve monitoring of treatment targets in countries.
Keywords: HIV diagnosis, antiretroviral therapy, viral load, self-reported data, HIV surveillance, sub-Saharan Africa, Kenya
Introduction
Reliable estimates of HIV diagnosis and access to antiretroviral (ARV) therapy (ART) among persons living with HIV (PLHIV) are needed to monitor progress towards fast-track targets set by the Joint United Nations Programme on HIV/AIDS (UNAIDS) to control the HIV pandemic by 2030 [1]. Nationally representative HIV sero-surveys provide insight on progress through collection of population-based information on HIV status and coverage of HIV services among HIV-infected persons. In combination with biomarkers, this allows for the assessment of trends in the continuum of care among PLHIV, from HIV diagnosis, linkage to care, ART initiation, to viral suppression. Robust estimates of these indicators, however, rely on participants answering questions related to HIV status and access to services accurately during an interview.
The Kenya AIDS Indicator Survey (KAIS) 2012 was a national sero-survey that linked information on demographics, behavior, and access to health services to HIV-related biomarkers to monitor the impact of the national HIV response. The criteria for ART initiation at the time of the survey included persons with CD4 counts ≤350 cells/mm3, or, irrespective of CD4 count, persons with WHO clinical stage 3/4 disease, persons with active tuberculosis co-infection, or persons with Hepatitis B virus co-infection with evidence of liver disease [3]. In 2012, the national ART program reported that 549,000 HIV-infected adults were receiving ART, representing 78% of ART-eligible adults living with HIV infection in Kenya [4].
In KAIS 2012, 23% of persons who reported HIV-positive status with no history of ART use were virally suppressed (unpublished data). This finding was surprising given that the control of viral replication in the absence of treatment is considered rare [5,6]. This phenomenon was documented in other epidemiological surveys and clinical trials that later confirmed that underreporting of ART use in these studies resulted in higher than expected levels of viral suppression among PLHIV who reported being treatment naïve [7–11]. The parallel finding in KAIS 2012 raised concerns that some respondents may have misreported their ART status resulting in an underestimate of ART coverage measured from the survey.
Testing HIV-positive blood for the presence of ARV metabolites provides a direct biomedical measure to improve estimates of ART coverage, and, by extension, estimates of diagnosed HIV among PLHIV. Such data remain important for clinical trials that enroll participants based on no prior history of HIV treatment and population-based surveys that are designed to generalize levels of undiagnosed HIV, ART access, and viral suppression in the population. In this analysis we assess the level of undisclosed HIV infection and ART use among persons with the ARV biomarker in KAIS 2012 and impact on national estimates of coverage of diagnosed HIV and ART use.
Methods
KAIS 2012 was a nationally representative household survey of persons aged 18 months to 64 years [12,13]. Survey respondents were administered face-to-face interviews that collected information on demographics, sexual behavior, HIV testing history, HIV status, and receipt of health interventions, including ART for persons who reported HIV-positive status. Venous blood samples were collected in CD4 stabilization tubes which were used to prepare dried blood spot (DBS) cards in a field laboratory. Respondents were offered on-site rapid HIV testing based on national guidelines [14], and results were returned immediately with counselling by trained staff in a private area in the home.
Blood samples were transported to a central laboratory where DBS were tested for HIV-1 antibodies using Vironostika HIV-1/2 UNIF II Plus O enzyme immunoassay (EIA) (sensitivity/specificity=100%) and Murex HIV.1.2.0 EIA (sensitivity=100%; specificity=99.5%) [5–16]. CD4 counts were measured using the BD FACSCalibur flow cytometer (Becton Dickinson Biosciences, San Jose, CA). HIV-positive DBS were tested for HIV-1 RNA concentration (Abbott M2000 Real-Time HIV-1 Assay, Abbott Laboratories, Abbott Park, IL). Undetectable viral load was defined as HIV-1 RNA concentration <1,000 copies/mL. Remnant DBS samples were stored in −70°C freezers for future testing. Two years after survey completion, HIV-positive DBS were shipped to the University of Cape Town in South Africa where a qualitative ARV assay was applied to test for nevirapine (NVP), efavirenz (EFV), lamivudine (3TC), and lopinavir (LPV) using liquid chromatography-tandem mass spectrometry (LC/MS/MS) [17]. The assay’s lower limit of detection (LLD) was 0.02 μg/mL. The number of days post ingestion to the LLD was 12–28 days for EFV, 8–9 days for NVP, 1.5 days for 3TC, and 1.5–2.5 days for LPV. Samples that fell above this threshold for any of the ARV drugs tested were classified as having the ARV biomarker present. Though validations studies assessing the performance of DBS on the qualitative assay have yet to be conducted, a validation study that compared the performance of quantitative measurement of ARV drug levels in plasma and DBS using LC/MS/MS found no major differences [18]. In 2012, the first-line standardized ARV regimen for Kenyan adults was tenofovir (TDF) + lamivudine (3TC) + efavirenz (EFV) or nevirapine (NVP) and second-line regimen was zidovudine (AZT) + 3TC + lopinavir/ritonavir (LPV/r) [3]. For pregnant women or patients intolerant to TDF, the recommended first-line regimen was AZT + 3TC + EFV/NVP.
Measures
The primary outcomes of interest were undisclosed HIV infection and undisclosed ART use while on ART. We categorized self-reported HIV status into five categories: HIV-positive, HIV-negative, HIV-indeterminate, never tested for HIV, and unknown self-reported HIV status (i.e., persons who reported testing for HIV but did not provide a result). HIV-infected persons who reported HIV-positive status were classified as having disclosed HIV infection while HIV-infected persons with the ARV biomarker who reported HIV-negative, HIV-indeterminate, never tested for HIV, or had unknown self-reported status were classified as having undisclosed HIV infection while on ART. Persons who reported ART use, irrespective of ARV test results, were classified as having disclosed ART use. Respondents with the ARV biomarker who: 1) reported HIV-positive status with no history of ART use or 2) reported HIV-negative, HIV-indeterminate, never tested for HIV, or unknown self-reported status were classified as having undisclosed ART use while on ART.
Explanatory variables tested for potential associations with the outcomes of interest included demographic variables: sex, age, marital status, education, wealth, urban/rural residence, and region; behavioral variables: number of sexual partners in the past year, condom use, and knowledge of partner HIV status; and clinical variables: history of visiting a health provider in the past year, history of tuberculosis disease, undetectable viral load, and median CD4 count.
Analysis
We restricted the analysis to respondents aged 15–64 years who tested HIV-positive in the survey with sufficient volume of blood available in DBS for ARV testing. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of self-reported ART status compared to ARV biomarker results was assessed. The Rao-Scott χ2 test was used to measure associations between categorical variables and outcomes of interest in bivariate analysis. Medians and interquartile ranges (IQR) were reported for continuous variables. The Wilcoxon rank sum test was used to measure the association between continuous variables and the outcome variables. We used PROC SURVEYLOGISTIC in SAS version 9.3 (SAS Institute, Inc., Cary NC) to determine independent and significant correlates of undisclosed HIV infection and ART use among persons on ART in multivariate logistic regression. The model included variables that were associated with the outcomes of interest in bivariate analysis at a p-value <0.2. Associations were considered statistically significant if the 95% confidence interval (CI) for the adjusted odds ratio (AOR) did not include 1.0. All estimates presented were weighted to account for sampling probability and survey nonresponse.
In a sub-analysis we compared national estimates of diagnosed HIV and ART coverage based on: 1) self-report; 2) self-report corrected for undisclosed HIV and ART use among persons on ART; and, 3) for comparison of ART coverage estimates only, treatment coverage based on national ART program data. We extrapolated national population counts of the number of diagnosed PLHIV and the number of PLHIV on ART by applying non-normalized survey weights based on the 2012 projected population data in the 2009 Kenya Population and Housing Census to the outcome variables [19].
For programmatic estimates of ART coverage, Spectrum version 5.31 (Avenir Health, Glastonbury, Connecticut) was used to project estimates of the number of PLHIV aged 15 years and older from 1970 to 2020 [2]. The number of adult ART patients in 2012 was based on ARV drug dispensing data from Kenya’s Logistics Management Information System. Programmatic ART coverage was calculated by dividing the number of adult ART patients by the projected number of adult PLHIV from Spectrum. Plausibility bounds around programmatic ART coverage were calculated using an uncertainty analysis which applied Monte Carlo simulations to estimate the impact of uncertainty in modeled HIV incidence and model assumptions [20,21]. A z-test was conducted to test for differences in ART coverage by estimation method.
Results
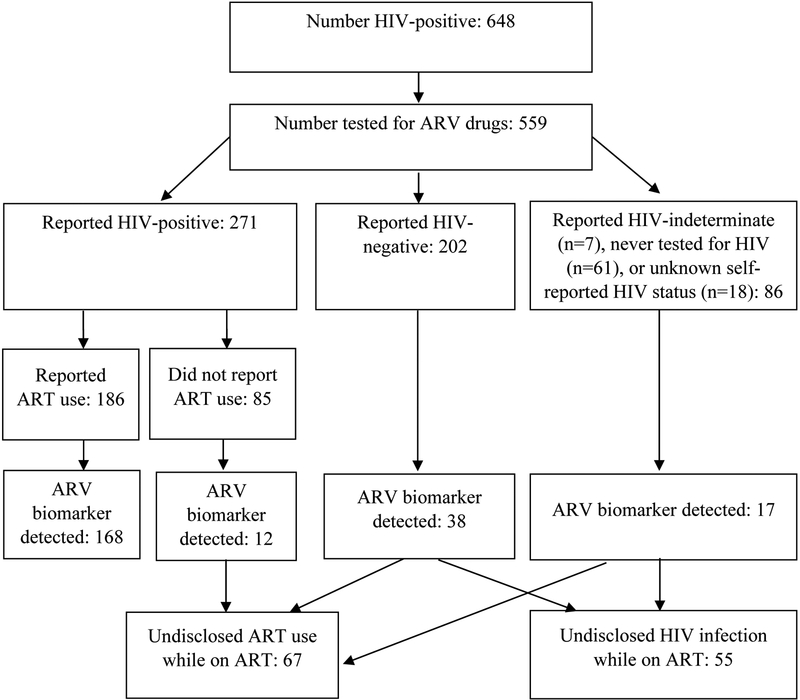
Overall, 648 of 11,626 respondents tested HIV-positive (Figure 1). Of those, 559 (86.3%) had sufficient volume available in stored DBS for ARV testing. No statistically significant differences in sex, age, urban/rural residence, region, marital status, education, or wealth were found among HIV-positive respondents with ARV test results compared to HIV-positive respondents without these results. Among the 559 indivdiuals with ARV test results, 271 (47.7%; CI 41.8–53.6) reported HIV-positive status, 202 (35.9%; CI 31.0–40.9) reported HIV-negative status, 7 (1.0%; CI 0–2.0) reported HIV-indeterminate status, 18 (3.1%; CI 1.5–4.8) had unknown self-reported status, and 61 (12.2%; CI 8.9–15.6) reported that they had never tested for HIV. Among respondents who disclosed HIV infection (n=271), 186 (69.1%; CI 62.3–76.0) reported receiving ART.
Figure 1.
HIV-infected survey respondents aged 15–64 years by self-reported HIV status and detection of ARV biomarker, Kenya AIDS Indicator Survey 2012
Presence of the ARV biomarker
Among the 559 HIV-positive DBS tested for ARV drugs, at least one drug was detected in 235 specimens, representing 42.5% (CI 37.4–47.7) of HIV-positive persons. Among those, 3TC was detected in 94.5% (CI 91.3–97.8), NVP in 62.9% (CI 55.9–69.9), EFV in 34.0% (CI 27.1–40.9), and LPV in 3.9% (CI 0.8–7.0).
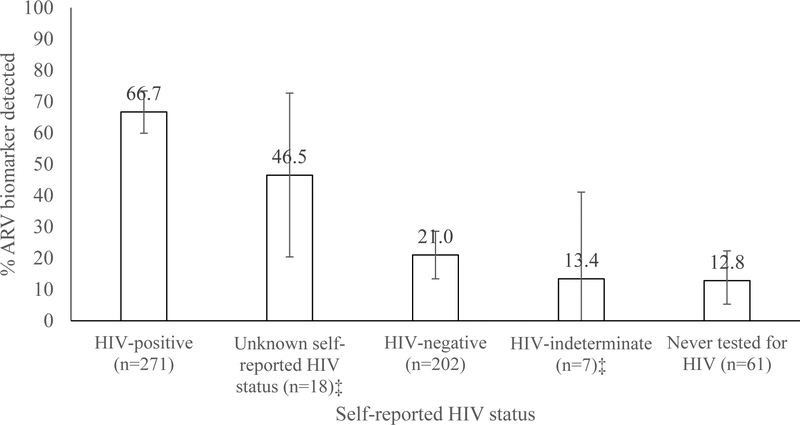
Among persons who disclosed HIV infection (n=271), 180 (66.7%; CI 59.9–73.4) had detectable ARVs (Figure 2) while those who disclosed both HIV infection and ART use (n=186) had ARVs detected in 168 (90.7%; CI 86.1–95.2). Among persons who disclosed HIV infection but did not disclose using ART (n=85), 12 (12.9%; CI 5.2–20.6) had ARVs detected.
Figure 2.
Percentage of HIV-infected respondents aged 15–64 years with the ARV biomarker by self-reported HIV status, Kenya AIDS Indicator Survey 2012†
†Analysis restricted to 559 HIV-positive dried blood spot samples available for ARV testing. Presented estimates include 95% confidence intervals.
‡Estimates unreliable due to denominator <25 observations and should be interpreted cautiously.
A total of 202 HIV-infected persons reported HIV-negative status. Of those, 38 (21.0%; CI 13.4–28.6) had the ARV biomarker, and 26 (61.7%; CI 43.0–80.5) of those reported receiving their last HIV-negative result within the year preceding the survey. Among HIV-infected persons who reported never testing for HIV (n=61), 8 (12.8%; CI 3.3–22.3) had the ARV biomarker present. A minority of persons reported that their last HIV test was indeterminate (n=7) or reported an unknown HIV serostatus (n=18). Of those, the ARV biomarker was present in 13.4% (CI 0–41.1) and 46.5% (CI 20.4–72.7), respsectively. Among all persons with the ARV biomarker (n=235), 18.3% (CI 12.7–24.0) did not disclose their HIV infection (n=55) and 6.0% (CI 2.5–9.5) did not disclose ART use despite disclosing HIV infection (n=12). Compared to results from ARV testing, the sensitivity of self-reported ART use was 71%, the specificity was 94%, the PPV was 90%, and the NPV was 82%.
Characteristics of persons on ART with undisclosed HIV infection
In bivariate analysis, male sex, younger age (15–24 years), living in urban residences, higher wealth, not visitng a health provider in the past year, and being virally suppressed were associated with undisclosed HIV infection while on ART at a p-value <0.1 (Table 1). In multivariate analysis, compared to HIV-infected persons who disclosed their HIV infection, persons aged 25–39 years (compared to aged 40–64 years: AOR 5.0; CI 1.1–22.0) and not visiting a health provider in the past year (AOR 6.1; CI 1.4–26.1) were associated with significnatly higher adjusted odds of having undisclosed HIV infection while on ART.
Table 1.
Demographic, behavioral, and clinical characteristics of HIV-infected persons aged 15–64 years by HIV disclosure status, Kenya 2012
| Disclosed HIV infection (n=271) | Undisclosed HIV infection on ART (n=55) | Unadjusted odds ratio (95% CI) |
p-value | Adjusted odds ratio (95% CI) | |||
|---|---|---|---|---|---|---|---|
|
Demographic variables |
|||||||
| Sex | |||||||
| Men | 64 | 74.6 (63.1 – 86.0) | 19 | 25.4 (14.0 – 36.9) | 2.0 (1.0 – 4.0) | 0.057 | 2.9 (0.8 – 10.5) |
| Women | 207 | 85.2 (79.8 – 90.6) | 36 | 14.8 (9.4 – 20.2) | 1.0 | 1.0 | |
| Age category (years) | |||||||
| 15 – 24 | 15 | 55.3 (29.7 – 80.9) | 8 | 44.7 (19.1 – 70.3) | 4.1 (1.2 – 13.4) | 0.051 | 8.8 (0.5 – 150.9) |
| 25 – 39 | 131 | 84.0 (75.2 – 92.8) | 23 | 16.0 (7.2 – 24.8) | 1.0 (0.4 – 2.1) | 5.0 (1.1 – 22.0) | |
| 40 – 64 | 125 | 83.4 (76.5 – 90.4) | 24 | 16.6 (9.6 – 23.5) | 1.0 | 1.0 | |
| Region | |||||||
| Nairobi | 24 | 79.1 (64.1 – 94.0) | 6 | 20.9 (6.0 – 35.9) | 1.0 | 0.139 | 1.0 |
| Central | 26 | 79.9 (62.0 – 97.8) | 5 | 20.1 (2.2 – 38.0) | 1.0 (0.2 – 4.0) | 2.2 (0.1 – 40.0) | |
| Coast | 22 | 76.3 (59.9 – 92.7) | 9 | 23.7 (7.3 – 40.1) | 1.2 (0.3 – 4.2) | 1.1 (0.03 – 40.0) | |
| Eastern | 33 | 71.1 (52.4 – 89.9) | 12 | 28.9 (10.1 – 47.6) | 1.5 (0.4 – 5.5) | 5.2 (0.3 – 100.0) | |
| Nyanza | 111 | 89.0 (83.1 – 94.8) | 13 | 11.0 (5.2 – 16.9) | 0.5 (0.2 – 1.4) | 0.7 (0.06 – 7.8) | |
| Rift Valley | 28 | 71.8 (51.1 – 92.5) | 9 | 28.2 (7.5 – 48.9) | 1.5 (0.4 – 5.8) | 2.5 (0.3 – 22.5) | |
| Western | 27 | 95.7 (87.5 – 100) | 1 | 4.3 (0.0 – 12.5) | 0.2 (0.02 – 1.5) | 0.8 (0.04 – 16.7) | |
| Residence | |||||||
| Rural | 160 | 86.0 (79.7 – 92.2) | 25 | 14.0 (7.8 – 20.3) | 1.0 | 0.091 | 0.6 (0.1 – 2.7) |
| Urban | 111 | 76.2 (66.1 – 86.3) | 30 | 23.8 (13.7 – 33.9) | 1.9 (0.9 – 4.1) | 1.0 | |
| Educational level | |||||||
| No primary | 14 | 74.8 (50.2 – 99.4) | 6 | 25.2 (0.6 – 49.8) | 1.5 (0.4 – 5.9) | 0.825 | -- |
| Incomplete primary | 22 | 83.2 (67.1 – 99.4) | 4 | 16.8 (0.6 – 32.9) | 0.9 (0.3 – 2.9) | -- | |
| Primary and higher | 235 | 81.8 (75.9 – 87.8) | 45 | 18.2 (12.2 – 24.1) | 1.0 | -- | |
| Wealth index | |||||||
| Lowest | 42 | 90.4 (81.0 – 99.9) | 7 | 9.6 (0.1 – 19.0) | 0.8 (0.2 – 2.9) | 0.003 | 0.8 (0.1 – 8.1) |
| Middle | 133 | 88.5 (82.3 – 94.8) | 17 | 11.5 (5.2 – 17.7) | 1.0 | 1.0 | |
| Highest | 96 | 70.1 (59.4 – 80.8) | 31 | 29.9 (19.2 – 40.6) | 3.3 (1.5 – 7.2) | 4.6 (0.8 – 27.2) | |
| Current Marital status | |||||||
| Never married/cohabited | 23 | 79.7 (57.3 – 100) | 4 | 20.3 (0.0 – 42.7) | 1.0 | 0.857 | -- |
| Ever married/cohabited | 248 | 81.9 (75.7 – 88.0) | 51 | 18.1 (12.0 – 24.3) | 0.9 (0.2 – 3.9) | -- | |
| Behavioral variables | |||||||
| Number of sex partners in past year | |||||||
| 0 | 86 | 85.8 (76.2 – 95.4) | 11 | 14.2 (4.6 – 23.8) | 1.0 | 0.559 | -- |
| 1 | 156 | 79.5 (71.5 – 87.6) | 37 | 20.5 (12.4 – 28.5) | 1.6 (0.6 – 4.1) | -- | |
| 2 or more | 28 | 85.0 (71.8 – 98.3) | 6 | 15.0 (1.7 – 28.2) | 1.1 (0.3 – 3.8) | -- | |
| Used a condom with all partners in past year | |||||||
| Yes | 79 | 82.4 (73.3 – 91.5) | 12 | 17.6 (8.5 – 26.7) | 0.8 (0.4 – 1.8) | 0.604 | -- |
| No | 105 | 79.0 (69.2 – 88.9) | 31 | 21.0 (11.1 – 30.8) | 1.0 | -- | |
| Knows HIV status of all partners in past year | |||||||
| Yes | 119 | 80.4 (70.7 – 90.2) | 26 | 19.6 (9.8 – 29.3) | 1.0 (0.4 – 2.3) | 0.981 | -- |
| No | 65 | 80.3 (71.1 – 89.5) | 17 | 19.7 (10.5 – 28.9) | 1.0 | -- | |
| Clinical variables | |||||||
| Visited health provider in past 12 months | |||||||
| Yes | 182 | 86.9 (81.2 – 92.5) | 26 | 13.1 (7.5 – 18.8) | 1.0 | 1.0 | |
| No | 88 | 73.1 (61.9 – 84.4) | 28 | 26.9 (15.6 – 38.1) | 2.4 (1.2 – 5.1) | 0.018 | 6.1 (1.4 – 26.1) |
| Diagnosed with tuberculosis (ever) | |||||||
| Yes | 55 | 84.6 (72.7 – 96.5) | 7 | 15.4 (3.5 – 27.3) | 0.8 (0.3 – 2.0) | 0.614 | -- |
| No | 209 | 81.2 (75.3 – 87.1) | 46 | 18.8 (12.9 – 24.7) | 1.0 | -- | |
| HIV-1 RNA concentration† | |||||||
| Detectable RNA | 107 | 87.9 (81.2 – 94.6) | 15 | 12.1 (5.4 – 18.8) | 1.0 | 0.039 | 1.0 |
| Undetectable RNA | 162 | 78.0 (70.7 – 85.2) | 40 | 22.0 (14.8 – 29.3) | 2.1 (1.0 – 4.1) | 2.9 (0.6 – 14.7) | |
| Median CD4+ T-cell count (IQR) (cells/mm3) | 145 | 550 (337 – 848) | 27 | 437 (276 – 654) | -- | 0.345 | -- |
Detectable RNA defined as HIV-1 RNA concentration ≥1,000 copies/mL. Undetectable RNA defined as HIV-1 RNA <1,000 copies/mL.
IQR = interquartile range.
Bolded adjusted odds ratios and 95% confidence intervals are those that are statistically significant.
Characteristics of persons on ART with undisclosed ART use and relevance to viral suppression
In bivariate analysis, undisclosed ART use was associated with younger age (aged 15–24 years), higher wealth, and not visiting a health provider in the past year at a p-value <1.0. In multivariate analysis, younger age (compared to aged 40–64 years: AOR 5.3; CI 1.4–19.8) and having higher wealth compared to lower wealth (AOR 3.1; CI 1.1–9.2) remained significantly associated with higher adjusted odds of undisclosed ART use while on ART.
Viral suppression ranged from 22.6% (CI 17.1–28.1) among HIV-infected persons who reported no prior history of ART use to 76.2% (CI 69.4–83.0) among those who reported taking ART (data not shown). After accounting for undisclosed ART use based on results of ARV testing, viral suppression decreased to 10.4% (CI 6.4–14.4) among persons not on ART and increased to 80.4% (CI 74.7–86.1) among those on ART.
National estimates HIV diagnosis and ART coverage
The percentage of persons with diagnosed HIV increased from 46.9% (CI 41.3–52.4) (305/648) based on self-report (population estimate: 558,000; CI 457,000–660,000) to 56.2% (CI 50.7–61.7) (360/648) (population estimate: 670,000; CI 561,000–779,000) after correcting for undisclosed HIV infection among persons on ART, representing an increase of 112,000 persons with diagnosed HIV. ART coverage increased from 31.8% (CI 27.1–36.5) (205/648) (population estimate: 379,000; CI 304,000–454,000) based on self-report to 42.8% (272/648, CI 37.9–47.8) (population estimate: 510,000; CI 425,000–596,000) after correcting for undisclosed ART use among persons on ART, equivalent to an additional 131,000 persons on ART. In 2012, the national ART program reported that programmatic ART coverage was 47% (CI 40–53) based on a numerator of 549,000 adult patients who received ART in health facilities that year and a projected denominator of 1,157,000 adult PLHIV based on the Spectrum model. Programmatic ART coverage was statistically different from ART coverage based on self-report (p<0.001) but similar to ART coverage adjusted for the ARV biomarker (p=0.315).
Discussion
We confirm that substantial misreporting occurred when HIV-infected respondents were asked to report their HIV and ART status in KAIS 2012. Only 71% of respondents with detectable ARVs reported a history of ART use, resulting in an underestimation of ART use by eleven percentage points and diagnosed HIV by nine percentage points. The impact of this bias on population estimates was substantial, resulting in 131,000 persons on ART and 112,000 persons with diagnosed HIV who were left unaccounted for in national estimates of coverage based on self-report [22].
Over 90% of persons who reported ART use in the interview had evidence of at least one ARV drug in their blood, suggestive of high levels of treatment adherence among HIV-infected persons who acknowledged receiving ART. The percentage of individual ARV drugs that were detected were aligned with the expected coverage of these drugs in the national ART program. Approximately 95% of individuals with the ARV biomarker had evidence of 3TC, a drug recommended in both first- and second-line ART regimens for adults. Additionally, 96% of persons with the ARV biomarker were receiving NVP or EVF, also recommended as first-line therapy for adults. Approximately 16% of ART patients were expected to experience treatment failure after one year on treatment, requiring switch to second-line ART [22]. Though this estimate is higher than the percentage of second-line drug (LPV) detected in our sample, KAIS 2012 did not collect information on duration of ART use, treatment failure, and access to second-line therapy to confirm whether this level was lower than expected.
Programmatic ART coverage were robust when compared to ART coverage that accounted for undisclosed ART based on ARV testing in a national survey. This finding is reassuring considering that routine program data are often criticized for poor data quality, though they remain the main source of information used by LMIC to plan, monitor, and evaluate ART access.
Compared to the ARV biomarker, self-reported ART use had modest sensitivity and negative predictive value, which was likely influenced by social desirability bias. Our findings also confirmed that self-reported serostatus was impacted by this bias. The odds of undisclosed HIV infection while on ART was highest among younger persons and individuals who had not visited a health provider in the past year while the odds of undisclosed ART use while on ART was highest among persons who were young or wealthy. Interestingly, 6% of persons with detectable ARVs accurately disclosed their HIV infection but did not disclose ART use. These results were unexpected given that the perceived level of stigma associated with acknowledging HIV infection is assumed to be higher than that of acknowledging ART use, particularly in settings where treatment is heavily promoted and readily accessible for PLHIV.
Disclosure of sensitive health-related information is complex and may be influenced by a number of factors related to the respondent, interviewer, interview mode, and interview setting. Respondents may feel embarrassed or fear judgement when asked sensitive questions; they may want to please the interviewer and report behaviors they perceive to be more socially acceptable; they may feel that their responses will not be kept private and respond inaccurately to protect their anonymity; they may believe what they report; or they may have misunderstood the question. Additionally, the interviewer may have re-worded questions inaccurately or incorrectly coded responses. Self-administered questionnaires or computer-assisted self-interviews that remove the interviewer could yield higher rates of accurate responses to sensitive questions, though measurement of these indicators may need to be simplified before considering this option. Nonetheless, every effort should be made to reduce reporting biases by ensuring that high-quality interviews that promote accurate reporting are delivered and that the mode of interview ensures that participants to feel at ease in their responses.
Estimated viral suppression in the absence of treatment decreased by over 50%, from 23% based on self-report to 10% after correction through ARV testing. A similar rate was reported in rural Uganda during a population-based health campaign in 2011, where 10% of persons who were not on ART were virally suppressed [23]. Though these rates are high for elite controllers [5, 6], they are not implausible given that the HIV epidemic in East Africa has existed for a long period resulting in more pressure to select those who have naturally lower viral loads as the duration of the HIV epidemic increases [24].
This analysis had the following limitations. Although estimates of ART coverage improved with inclusion of an ARV biomarker, true population ART coverage may still be underestimated. The parent drugs selected for ARV testing were based on the standardized treatment regimens available in public health facilities in Kenya. If a patient was on a regimen that did not include any of the four drugs tested, evidence of ART use would not have been captured. Additionally, given the short half-life of the ARV drugs tested, the ARV biomarker is an indicator of recent exposure to ARVs and could underestimate ART use if adherence was poor. While we found that ARV testing improved estimates of diagnosed HIV, a downward bias is still present by missing individuals who may be aware of their infection (but do not report it) and have not yet accessed ART. To understand the extent of this bias, 7% of persons with an HIV diagnosis in Kenya had not accessed care services in 2012, and of those in care, 22% had not yet accessed treatment. This limitation should be lessened in the future as treatment for all PLHIV is introduced irrespective of CD4 count [25]. Finally, we were unable to compare reported ART use with ARV biomarker results for HIV-infected persons who did not disclose HIV-positive status given that a history of ART use was collected only for persons who acknowledged that they were HIV-positive during the interview.
In conclusion, ARV testing in a national population-based sero-survey in Kenya confirmed that self-reported information on HIV-positive status and ART use should not be used alone for monitoring population-based trends in diagnosed HIV and ART coverage [26]. The change in viral suppression by ART status illustrates a further limitation of reliance on self-reported ART status. Not only did it result in an underestimate of diagnosed HIV and ART coverage at the population-level, the resulting misclassification at the individual-level can potentially bias associations between known status, ART use, and other explanatory factors. We were encouraged to find that routine ART program monitoring data used to estimate national ART coverage were valid. However, current program monitoring systems do not yet have the ability to directly link diagnosed HIV cases to their sentinel events in the cascade of care, limiting our understanding of the full impact of HIV prevention and treatment interventions in reducing HIV transmission on a population-level [27]. Until these systems are established and validated, population-based sero-surveys that include objective measures of ART use and biomarkers to measure viral suppression, serve as the best source of data for countries to monitor the reach and effectiveness of testing and treatment interventions on achieving a successful treatment continuum. In settings where ARV testing is not feasible, self-reported data on ART use should continue to be collected but supplemented with information that can confirm treatment status, such as documentation of ART use in a health record. Triangulation of biological, epidemiological, and programmatic data remains an essential approach to generate best-supported estimates of treatment targets in a country.
Table 2.
Demographic, behavioral, and clinical characteristics of HIV-infected persons aged 15–64 years on ART by ART disclosure status, Kenya 2012
| Disclosed ART use (n=168) | Did not disclose ART use (n=67) | Unadjusted odds ratio (95% CI) | p-value | Adjusted odds ratio (95% CI) |
|||
|---|---|---|---|---|---|---|---|
| Demographic variables | |||||||
| Sex | |||||||
| Male | 45 | 65.0 (51.5 – 78.5) | 23 | 35.0 (21.5 – 48.5) | 1.7 (0.9 – 3.4) | 0.107 | 1.8 (0.7 – 4.5) |
| Female | 141 | 76.3 (68.9 – 83.7) | 44 | 23.7 (16.3 – 31.1) | 1.0 | 1.0 | |
| Age category (years) | 0.016 | ||||||
| 15–24 | 6 | 34.1 (9.0 – 59.2) | 8 | 65.9 (40.8 – 91.0) | 6.4 (1.8 – 22.8) | 5.3 (1.4 – 19.8) | |
| 25–39 | 84 | 72.7 (61.0 – 84.4) | 29 | 27.3 (15.6 – 39.0) | 1.2 (0.6 – 2.5) | 1.0 (0.4 – 2.4) | |
| 40–64 | 96 | 76.7 (68.5 – 85.0) | 30 | 23.3 (15.0 – 31.5) | 1.0 | ||
| Region | |||||||
| Nairobi | 18 | 71.9 (52.6 – 91.2) | 7 | 28.1 (8.8 – 47.4) | 1.0 | 0.420 | -- |
| Central | 19 | 74.6 (52.6 – 96.7) | 5 | 25.4 (3.3 – 47.4) | 0.9 (0.2 – 3.9) | -- | |
| Coast | 17 | 63.0 (47.9 – 78.1) | 11 | 37.0 (21.9 – 52.1) | 1.5 (0.5 – 4.8) | -- | |
| Eastern | 27 | 64.2 (45.1 – 83.3) | 13 | 35.8 (16.7 – 54.9) | 1.4 (0.4 – 5.1) | -- | |
| Nyanza | 71 | 80.2 (71.0 – 89.4) | 17 | 19.8 (10.6 – 29.0) | 0.6 (0.2 – 1.9) | -- | |
| Rift Valley | 18 | 62.8 (36.0 – 89.7) | 9 | 37.2 (10.3 – 64.0) | 1.5 (0.3 – 6.8) | -- | |
| Western | 16 | 81.4 (64.0 – 98.9) | 5 | 18.6 (1.1 – 36.0) | 0.6 (0.1 – 2.6) | -- | |
| Residence | |||||||
| Rural | 105 | 76.5 (67.8 – 85.3) | 33 | 23.5 (14.7 – 32.2) | 1.0 | 0.206 | -- |
| Urban | 81 | 67.2 (55.3 – 79.1) | 34 | 32.8 (20.9 – 44.7) | 1.6 (0.8 – 3.3) | -- | |
| Educational level | |||||||
| No primary | 12 | 72.0 (45.3 – 98.7) | 6 | 28.0 (1.3 – 54.7) | 1.0 (0.3 – 4.0) | 0.999 | -- |
| Incomplete primary | 14 | 72.9 (51.3 – 94.5) | 6 | 27.1 (5.5 – 48.7) | 1.0 (0.3 – 2.9) | -- | |
| Primary and higher | 160 | 72.3 (64.6 – 80.1) | 55 | 27.7 (19.9 – 35.4) | 1.0 | -- | |
| Wealth index | |||||||
| Low | 29 | 81.2 (68.6 – 93.7) | 10 | 18.8 (6.3 – 31.4) | 1.0 (0.3 – 2.7) | 0.015 | 0.9 (0.2 – 3.7) |
| Middle | 87 | 80.5 (71.1 – 90.0) | 22 | 19.5 (10.0 – 28.9) | 1.0 | 1.0 | |
| High | 70 | 60.0 (47.4 – 72.7) | 35 | 40.0 (27.3 – 52.6) | 2.8 (1.3 – 6.0) | 3.1 (1.1 – 9.2) | |
| Current marital status | |||||||
| Never married/cohabited | 15 | 68.9 (42.4 – 95.3) | 6 | 31.1 (4.7 – 57.6) | 1.0 | 0.787 | -- |
| Ever married/cohabiting | 171 | 72.7 (64.8 – 80.5) | 61 | 27.3 (19.5 – 35.2) | 0.8 (0.2 – 3.2) | -- | |
| Behavioral variables | |||||||
| Number of sex partners in past year | |||||||
| 0 | 65 | 80.2 (68.4 – 92.1) | 13 | 19.8 (7.9 – 31.6) | 1.0 | 0.431 | -- |
| 1 | 103 | 68.9 (58.8 – 79.0) | 46 | 31.1 (21.0 – 41.2) | 1.83 (0.7 – 4.6) | -- | |
| 2 or more | 17 | 73.2 (53.6 – 92.8) | 7 | 26.8 (7.2 – 46.4) | 1.5 (0.4 – 5.0) | -- | |
| Used a condom with all partners in past year | |||||||
| Yes | 61 | 76.7 (65.9 – 87.6) | 14 | 23.3 (12.4 – 34.1) | 0.5 (0.2 – 1.2) | 0.117 | 0.4 (0.2 – 1.2) |
| No | 59 | 63.7 (50.0 – 77.3) | 39 | 36.3 (22.7 – 50.0) | 1.0 | ||
| Knows HIV status of all partners in past year | |||||||
| Yes | 85 | 72.3 (60.5 – 84.2) | 32 | 27.7 (15.8 – 39.5) | 0.7 (0.3 – 1.4) | 0.278 | -- |
| No | 35 | 62.9 (49.5 – 76.3) | 21 | 37.1 (23.7 – 50.5) | 1.0 | -- | |
| Clinical variables | |||||||
| Visited health provider in past 12 months | |||||||
| Yes | 128 | 78.7 (70.9 – 86.5) | 34 | 21.3 (13.5 – 29.1) | 1.0 | 0.029 | 1.0 |
| No | 58 | 62.8 (49.7 – 75.9) | 31 | 37.2 (24.1 – 50.3) | 2.2 (1.1 – 4.4) | 1.8 (0.8 – 4.4) | |
| Diagnosed with tuberculosis (ever) | |||||||
| Yes | 52 | 81.8 (69.3 – 94.3) | 9 | 18.2 (5.7 – 30.7) | 0.5 (0.2 – 1.2) | 0.123 | 0.7 (0.3 – 1.7) |
| No | 129 | 69.4 (61.2 – 77.6) | 56 | 30.6 (22.4 – 38.8) | 1.0 | 1.0 | |
| HIV-1 RNA concentration† | |||||||
| Detectable RNA | 45 | 72.4 (59.8 – 85.0) | 18 | 27.6 (15.0 – 40.2) | 1.0 (0.5 – 2.0) | 0.998 | -- |
| Undetectable RNA | 140 | 72.4 (64.4 – 80.4) | 48 | 27.6 (19.6 – 35.6) | 1.0 | -- | |
| Median CD4+ T-cell count (IQR) (cells/mm3) | 104 | 533 (309– 754) | 33 | 506 (299 – 654) | -- | 0.981 | -- |
Detectable RNA defined as HIV-1 RNA concentration ≥1,000 copies/mL. Undetectable RNA defined as HIV-1 RNA <1,000 copies/mL.
IQR = interquartile range.
Bolded adjusted odds ratios and 95% confidence intervals are those that are statistically significant.
Acknowledgements
AAK, IM, JW, LN, and KDC conceived and designed the experiments. PWY, SM, and LW performed the experiments. AAK analyzed the data and wrote the paper. AAK, IM, PWY, JM, SM, JW, NB, LW, LN, and KDC reviewed and approved the manuscript.
We would like to thank Wanjiru Waruiru from the University of California, San Francisco and Jennifer Norman and Karen Cohen from the University of Cape Town, South Africa for their support in facilitating ARV testing of KAIS 2012 specimens used in this analysis. We would also like to thank the KAIS 2012 survey participants for their willingness to participate in the survey and the KAIS Study Group for their contribution to the design of the survey and collection of the data set. Member of the KAIS Study Group are: Willis Akhwale, Sehin Birhanu, John Bore, Angela Broad, Robert Buluma, Thomas Gachuki, Jennifer Galbraith, Anthony Gichangi, Beth Gikonyo, Margaret Gitau, Joshua Gitonga, Mike Grasso, Andrew Imbwaga, Malayah Harper, Muthoni Junghae, William Maina, Nicolas Muraguri, Mutua Kakinyi, Samuel Mwangi Kamiru, Nicholas Owenje Kandege, Lucy Kanyara, Yasuyo Kawamura, Timothy Kellogg, George Kichamu, Andrea Kim, Lucy Kimondo, Davies Kimanga, Elija Kinyanjui, Stephen Kipkerich, Dan Koros, Danson Kimutai Koske, Agneta Mbithi, Veronica Lee, Serenita Lewis, Ernest Makokha, Agneta Mbithi, Joy Mirjahangir, Ibrahim Mohamed, Rex Mpazanje, Nicolas Muraguri, Patrick Mureithi, Lilly Muthoni, James Muttunga, Jane Mwangi, Mary Mwangi, Sophie Mwanyumba, Francis Ndichu, Anne Ng’ang’a, James Ng’ang’a, John Gitahi Ng’ang’a, Lucy Ng’ang’a, Carol Ngare, Bernadette Ng’eno, Inviolata Njeri, David Njogu, Caleb Obada, Bernard Obasi, Macdonald Obudho, Edwin Ochieng, Linus Odawo, James Odek, Jacob Odhiambo, Samuel Ogola, David Ojakaa, James Kwach Ojwang, George Okumu, Patricia Oluoch, Tom Oluoch, Osborn Otieno, Kennedy Ochieng Omondi, Yakubu Owolabi, Boniface O.K’Oyugi, Bharat Parekh, George Rutherford, Sandra Schwarcz, Shahnaaz Sharrif, Victor Ssempijja, Lydia Tabuke, Yuko Takenaka, Mamo Umuro, Brian Eugene Wakhutu, Wanjiru Waruiru, Celia Wandera, John Wanyungu, Anthony Waruru, Paul Waweru, Larry Westerman, and Kelly Winter.
Conflicts of Interest and Source of Funding: The second Kenya AIDS Indicator Survey (KAIS 2012) was conducted by National AIDS and STI Control Programme (NASCOP), Kenya National Bureau of Statistics, (KNBS), National Public Health Laboratory Services (NPHLS), National AIDS Control Council (NACC), National Council for Population and Development (NCPD), Kenya Medical Research Institute (KEMRI), U.S. Centers for Disease Control and Prevention (CDC/Kenya, CDC/Atlanta), United States Agency for International Development (USAID/Kenya), University of California, San Francisco (UCSF), the United States Agency for International Development (USAID/Kenya), Joint United Nations Team on HIV/AIDS, Japan International Cooperation Agency (JICA), the Elizabeth Glaser Paediatric AIDS Foundation (EGPAF), Liverpool Voluntary Counselling and Testing (LVCT), the African Medical and Research Foundation (AMREF), the World Bank, and the Global Fund.
Attribution of support: This publication was made possible by support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through cooperative agreements [#PS001805, GH000069, and PS001814] through the U.S. Centers for Disease Control and Prevention (CDC), Division of Global HIV and Tuberculosis (DGHT). This work was also funded in part by support from the Global Fund, World Bank, and the Joint United Nations Team for HIV/AIDS. Antiretroviral drug analysis reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number UM1 AI068634, UM1 AI068636 and UM1 AI106701. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or National Institutes of Health
A portion of this analysis was presented at the 2015 International AIDS Society Meeting in Vancouver, Canada, July 2015.
References
- 1.Joint United National Programme on HIV/AIDS (UNAIDS). Ambitious Treatment Targets: Writing the Final Chapter of the AIDS Epidemic. Geneva: UNAIDS; 2014. [Google Scholar]
- 2.Stover J, Adreev K, Slaymaker E, Gopalappa C, Sabin K, Velasquez C, et al. Updates to the spectrum model to estimate key HIV indicators for adults and children. AIDS 2014; suppl4: S427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National AIDS and STI Control Programme (NASCOP). Guidelines for antiretroviral therapy in Kenya: 4th edition 2011. Nairobi: NASCOP; 2011. [Google Scholar]
- 4.National AIDS Control Council (NACC) and National AIDS and STI Control Programme (NASCOP). Kenya HIV Estimates 2014. Nairobi: NACC; 2014. [Google Scholar]
- 5.Walker BD. Elite control of HIV infection: Implications for vaccines and treatment. Top HIV Med 2007; 15:134–136. [PubMed] [Google Scholar]
- 6.Olson AD, Meyer L, Prins M, Thiebaut R, Gurdasani D, Guiguet M, et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS ONE 2014; 9(1): e86719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan AK, Savage EJ, Lowndes CM, Paul G, Murphy, Carne S, et al. Nondisclosure of HIV status in UK sexual health clinics – a pilot study to identify nondisclosure within a national unlinked anonymous seroprevalence survey. Sex Transm Infect 2013; 89:120–121. [DOI] [PubMed] [Google Scholar]
- 8.Das M, Fisher Raymond H, Chu P, Nieves-Rivera I, Pandori M, Louie B, et al. Measuring the unknown: calculating community viral load among HIV-infected MSM unaware of their HIV status in San Francisco from national HIV behavioral surveillance, 2004–2011. J Acquir Immune Defic Syndr 2013; 63(2):e84–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marzinke MA, Clarke W, Wang L, Cummings V, Liu T, Piwowar-Manning E, Breaud A, et al. Nondisclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly diagnosed and previously diagnosed HIV infection. Clin Infect Dis 2014; 58(1):117–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogel JM, Wang L, Parson TL, Ou SS, Piwowar-Manning E, Chen Y, et al. Undisclosed antiretroviral drug use in a multinational clinical trial (HIV prevention trials network 052). Journal of Infectious Diseases 2013; 208:1624–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lingappa JR, Kahle E, Mugo N, Mujugira A, Magaret A, Baeten J, et al. Characteristics of HIV-1 discordant couples enrolled in a trial of HSV-2 suppression to reduce HIV-1 transmission: the Partners study. PLoS ONE 2009; 4(4): e5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National AIDS and STI Control Programme (NASCOP). The Kenya AIDS Indicator Survey 2012: Final Report. Nairobi: NASCOP;2014. [Google Scholar]
- 13.Waruiru W, Kim AA, Kimanga DO, Ng’ang’a J, Schwarcz Kimondo L, et al. The Kenya AIDS Indicator Survey 2012: rationale, methods, description of participants, and response rates. J Acquir Immune Defic Synd 2014; 66(Suppl1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National AIDS and STI Control Programme (NASCOP). National Guidelines for HIV Testing and Counseling in Kenya. 2nd ed Nairobi, Kenya: NASCOP; 2010. [Google Scholar]
- 15.Vironostika HIV-1 Plus O Microelisa System [package insert]. Marcy-l’Etoile, France: bioMérieux; 2000. [Google Scholar]
- 16.Murex HIV-1.2.0 enzyme immunoassay [package insert], Dartford, UK: Abbott Laboratories; 2009. [Google Scholar]
- 17.Rehle T, Johnson L, Hallett T, Mahy M, Kim A, Odido H, et al. A comparison of South African national HIV incidence estimates: A critical appraisal of different methods. PLoS ONE. 2015; 10(7):e0133255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koal T, Burhenne H, Romling R, Svoboda M, Resh K, Kaever V. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun Mass spectrum. 2005; 19: 2995–3001. [DOI] [PubMed] [Google Scholar]
- 19.Kenya National Bureau of Statistics. 2009 Kenya population and housing census results: Population distribution by age, sex and administrative units: Kenya National Bureau of Statistics [Internet]. August 2010. Cited December 10, 2015 Available from: http://www.knbs.or.ke/docs/PresentationbyMinisterforPlanningrevised.pdf [Google Scholar]
- 20.Stover J, Johnson P, Zaba B, Zwahlen M, Dabis F, Ekpini RE. The Spectrum projection package: improvements in estimating mortality, ART needs, PMTCT impact and uncertainty bounds. Sex Transm Inf 2008; 84:i24–i30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stover J, Brown T, Marston M, et al. Updates to the Spectrum/Estimation and Projection Package (EPP) model to estimate HIV trends for adults and children. Sex Transm Infect 2012; 88:i11–i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Odhiambo JO, Kellogg TA, Kim AA, Ng’ang’a L, Mukui I, Umuro M, Mohammed I, et al. Antiretroviral treatment scale-up among persons living with HIV in Kenya: results from a nationally-representative survey. J Acquir Immune Defic Synd 2014; 66(Suppl 1):116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahon JH, Elliott JH, Bertagnolio S, Kubiak R, Jordan MR. Viral suppression after 12 months of antiretroviral therapy in low- and middle-income countries: a systematic review. Bull World Health Organ. 2013; 91(5):377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain V, Liegler T, Kabami J, Chamie G, Clark TD, Black D, et al. Assessment of population-based HIV RNA levels in a rural east African setting using a fingerprick-based blood collection method. Clin Infect Dis 2013; 56:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallett TB, Ghys P, Barnighausen T, Yan P, Garnett GP. Errors in ‘BED’-derived estimates of HIV incidence will vary by place, time and age. PloS ONE. 2009; 4(5):e5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health; Organization (WHO). Guidelines on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: WHO: 2015. [PubMed] [Google Scholar]
- 27.Johnston LG, Sabin ML, McFarland W, Prybylski D, Sabin K, Baral S, et al. Asking self-reported HIV serostatus in bio-behavioral surveys is crucial in the era of 90-90-90. Bulletin of the World Health Organization (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Cock KM. Plus ça change … antiretroviral therapy, HIV prevention, and the HIV treatment cascade. Clin Infect Dis 2014; 58(7):1012–4. [DOI] [PubMed] [Google Scholar]