1. Introduction
One third to one half of patients with sarcoidosis have a chronic, unremitting course with persistent inflammation causing progressive organ impairment that prompts treatment [1]. Corticosteroid therapy is the cornerstone of therapy but with chronic disease, adverse effects of corticosteroids are unavoidable. Thus, expert consensus recommendations include the use of steroid sparing therapies, though their exact role remains uncertain given a lack of comparison studies [2]. Commonly used therapies include hydroxychloroquine, methotrexate, azathioprine, mycophenolate and anti-tumor necrosis factor (TNF) therapies [3–5]. These therapies are not effective or poorly tolerated in 20–40% of patients, [3,6] all have potential serious adverse effects and none are FDA approved for sarcoidosis. Thus, additional steroid sparing therapies are needed for chronic sarcoidosis.
Our research has previously demonstrated a potential key role for the protein serum amyloid A (SAA) in the pathogenesis of sarcoidosis [7,8]. SAA is an amyloid precursor protein and acute phase reactant that is known to be upregulated in the blood of sarcoidosis patients, and we have shown that SAA is concentrated within sarcoidosis granulomas where it can promote Thl-mediated granulomatous inflammation [7]. A potent upregulator of SAA is interleukin(IL)-6, a pleomorphic pro-inflammatory cytokine previously shown to be highly expressed in sarcoidosis-affected tissues [9]. Accordingly, inhibition of the IL-6 pathway presents an intriguing approach for the treatment of sarcoidosis.
Tocilizumab is a recombinant, humanized, anti-human IL-6 receptor monoclonal antibody and powerful inhibitor of the IL-6 pathway. Tocilizumab is FDA-approved for treatment of moderate-to-severe rheumatoid arthritis, polyarticular or systemic juvenile idiopathic arthritis, and giant cell arteritis [10] and has also shown promise in treating amyloid A amyloidosis and Castleman disease [11,12]. We hypothesize that tocilizumab could serve as a novel corticosteroid-sparing therapy in chronic sarcoidosis.
2. Materials and methods
We performed a chart review of 4 patients over a six-year time course (2012–2018) seen in the Johns Hopkins Sarcoidosis Clinic that were treated with tocilizumab. Data was extracted from the medical records with IRB approval.
3. Results
3.1. Patient 1
Patient 1 is a white woman (now in her 50s), never smoker, who was diagnosed with biopsy-proven pulmonary sarcoidosis and hypercalcemia in her 20s. She had an initial chest x-ray showing mediastinal and hilar lymphadenopathy and interstitial infiltrates (Scadding stage II). She was treated for progressive respiratory symptoms and reduced pulmonary function with prednisone. Multiple attempts to taper prednisone below 15 mg/day resulted in recurrent symptoms and reduced lung function. She was continuously treated with prednisone for approximately 28 years with cumulative adverse effects from corticosteroids including osteopenia, hypertension, diabetes, and osteonecrosis requiring bilateral hip replacements. Multiple steroid sparing therapies were tried including hydroxychloroquine, methotrexate, azathioprine, minocycline, mycophenolate, adalimumab, infliximab, and leflunomide. These medications were ineffective in allowing steroid tapering or had adverse effects such as leukopenia with mycophenolate. With these ineffective trials, lung function gradually decreased and chest radiographs showed progressive pulmonary fibrosis. With informed consent, the patient was initiated on 4 mg/kg tocilizumab monthly infusions. Within days the patient reported improvement in her symptoms with reduced dyspnea and improved well-being with gradual improvement in her pulmonary function over several months. She continued to have further improvement over the following several months and her prednisone was tapered slowly to a dose of 5–7 mg daily with improved symptoms and pulmonary function (Table 1). Tocilizumab was increased to 8 mg/kg after 6 months of treatment in an attempt to taper off prednisone completely, however tapering of prednisone resulted in worsening pulmonary symptoms and function. She continued on a monthly dose of tocilizumab with low dose prednisone for 32 months without worsening. She developed an episode of bronchitis which was treated with antibiotics and tocilizumab therapy was temporarily discontinued. Her prednisone dose was increased back to prednisone 15mg/day. After her infection resolved, tocilizumab subcutaneous injections were initiated at 162 mg per week in addition to methotrexate 5 mg weekly. Tocilizumab subcutaneous injections were chosen because of a recent trial which showed effectiveness of subcutaneous tocilizumab in giant cell arteritis [10]. The prednisone dose was reduced again to prednisone 5 mg/day with stable symptoms and pulmonary function.
Table 1.
Summary of outcomes with tocilizumab in sarcoidosis.
| Primary Manifestationa |
Prednisone Dose | Symptoms | Pulmonary Function Tests (Phenotype and Response) | |
|---|---|---|---|---|
| Patient 1 | Pulmonary | 15 mg-5 mg (66% reduction) | Improved | Restrictive FVC% predicted 49 to 55 Obstructive FEV1/FVC stable FEV1% predicted: 38 to 42 |
| Patient 2 | Lupus Pernio Sinus Disease Pulmonary |
15 mg-5 mg (66% reduction) | Improved | Normal pattern |
| Patient 3 | Pulmonary Arthralgias Abdominal |
15 mg-10 mg (33% reduction) | Improved | Gas Transfer Defect DLCO% predicted: 62 to 80 |
| Patient 4 | Pulmonary Intractable Chest Pain |
20 mg-10 mg (50% reduction) | Improved | Restrictive FVC% predicted: 78 to 84 |
Primary manifestation refers to organ involvement that directed steroid sparing therapy.
3.2. Patient 2
Patient 2 is a black woman (now in her 50s), never smoker, who was diagnosed with pulmonary sarcoidosis, biopsy-proven disfiguring lupus pernio, and severe sinus disease with septal perforation in her 20s. She had a chest x-ray Scadding stage II at initial presentation. She was treated continuously with prednisone for 10 years with a minimum effective dose of 15 mg daily that was only partially effective in controlling her facial nodules and erythema. Adverse steroid effects included worsening diabetes and osteopenia. Steroid sparing agents including hydroxychloroquine, methotrexate, minocycline, and adalimumab were ineffective for the lupus pernio and sinus disease. Infliximab therapy was initiated with improvement in her skin, sinus and pulmonary manifestations; she continued on this therapy for 2 years until it was discontinued due to reduced effectiveness. With informed consent, the patient was then initiated on monthly infusions of 4 mg/kg tocilizumab. She developed improvement in her respiratory symptoms, cutaneous lesions, and well-being within 1–2 months of treatment. On clinical exam, she had significant reduction in the size, nodularity, and erythema of her skin eruptions. Her prednisone dose was tapered ultimately to 5 mg daily with persistent improvement in her cutaneous manifestation and stable lung function (Table 1). Unfortunately, during her 3rd year of treatment with tocilizumab, the patient developed breast cancer and tocilizumab was discontinued.
3.3. Patient 3
Patient 3 is a white man (now in his 50s), never smoker, who was diagnosed with biopsy-proven pulmonary sarcoidosis, severe constitutional manifestations including fevers, sweats and severe fatigue, joint pains and diarrhea in his 40s. He had a chest x-ray Scadding stage II at initial presentation. He was treated with prednisone for approximately 4 years with a minimum dose of 15 mg daily with incomplete control of his multiple symptoms, suggesting his minimum effective dose of prednisone was likely higher. He underwent an extensive GI evaluation and was found to have abdominal lymphadenopathy, but no additional specific pathology. His C-reactive protein (CRP) was consistently elevated above 100mg/l (normal < 5 mg/l). Steroid adverse effects included hyperlipidemia and cushingoid facial and skin features. Methotrexate and adalimumab were not effective as steroid sparing therapy in controlling his pulmonary, joint or systemic symptoms. With informed consent, the patient was treated with tocilizumab 162 mg subcutaneous injections every 2 weeks. He experienced significant improvement with reduced shortness of breath, improved pulmonary function and resolution of fevers and sweats, improved energy, reduced joint pains, and resolution of his GI symptoms; his CRP decreased to less than 5 mg/l within 1–2 months (Table 1). His prednisone has been tapered to 10 mg/day and he continues on a slow steroid taper.
3.4. Patient 4
Patient 4 is a white man (now in his 60s), never smoker, who was diagnosed with biopsy-proven pulmonary sarcoidosis in his 30s complicated by intractable chest pain and cutaneous sarcoidosis. He had a chest x-ray Scadding stage III (interstitial infiltrates without lymphadenopathy) at initial presentation. He was treated with prednisone initially for 6 months at the time of diagnosis, then remained off treatment for 15 years. He subsequently had a return of dyspnea and chest pain and he was placed back on prednisone. He was treated with prednisone for approximately 8 years. A minimum dose of prednisone 20 mg daily was needed to control his respiratory symptoms and chest pains. Steroid adverse effects included weight gain and cushingoid features. Methotrexate and infliximab were not effective at controlling his manifestations. With informed consent, the patient was treated with monthly infusions of 4 mg/kg of tocilizumab. He experienced improvement in dyspnea and significant resolution of his chest pains allowing his prednisone to be tapered to 10 mg daily within 1–2 months (Table 1). Tocilizumab was recently discontinued after 6 years due to the development of peripheral neuropathy and he is undergoing an evaluation for this problem.
4. Discussion
This case series briefly summarizes the outcome of tocilizumab treatment in four sarcoidosis patients with chronic, severe manifestations who had failed multiple steroid sparing therapies. All four patients had a positive, and in 3 cases, a dramatic response to tocilizumab with improved symptoms and improved organ function while allowing a significant reduction of corticosteroid dosing. These anecdotal cases suggest that targeting the IL6 pathway may be beneficial in multisystem chronic sarcoidosis.
We postulate that the effect of IL6 receptor inhibition in sarcoidosis is to reduce SAA precursor protein, which is concentrated within sarcoidosis granulomas [8]. In other diseases involving progressive protein aggregation such as in amyloidosis, prion or Alzheimer disease, therapies that lower precursor production of the accumulated protein may be therapeutic [13]. Based on our studies and the known biology of SAA as an amyloid precursor protein which will self-aggregate at low concentrations, we hypothesized that IL6 receptor blockade would reduce SAA aggregation at sites of granuloma formation, thus inhibiting the feed-forward amplification of local proinflammatory Th1 responses necessary for the chronic granulomatous inflammation in sarcoidosis [7].
There are other mechanisms that likely play a role in the potential effectiveness of tocilizumab in sarcoidosis (Fig. 1). IL6 is a key cytokine in promoting the differentiation of Th17 effector cells. Both Th17 and more recently the subset of Th17.1 effector T cells that express INFγ have been implicated in sarcoidosis pathobiology [14,15]. Thus, IL6 could be a key regulator of the Th17 and Th17.1 local immune responses in sarcoidosis. A third potential mechanism involves the link between IL6 and activation of the metabolic checkpoint kinase mammalian target of rapamycin complex 1 (mTORC1) in macrophages based a recent study by Linke and colleagues that implicates this pathway as a key regulator of epithelioid cell differentiation in sarcoidosis [16]. IL6 activates the mTOR pathway through Janus tyrosine Kinase (JAK) [17,18]. Of note, there has been a recent description of a patient with cutaneous sarcoidosis being treated with tofacitinib, a JAK inhibitor, and having clinical improvement, supporting a role for JAK signaling in sarcoidosis [19]. A fourth possible mechanism involves the finding that IL6 may inhibit differentiation of regulatory T cells; this effect may allow enhanced effector T-cell responses including those involving Th1/Th17/Th17.1 immune responses at sites of disease [20].
Fig. 1.
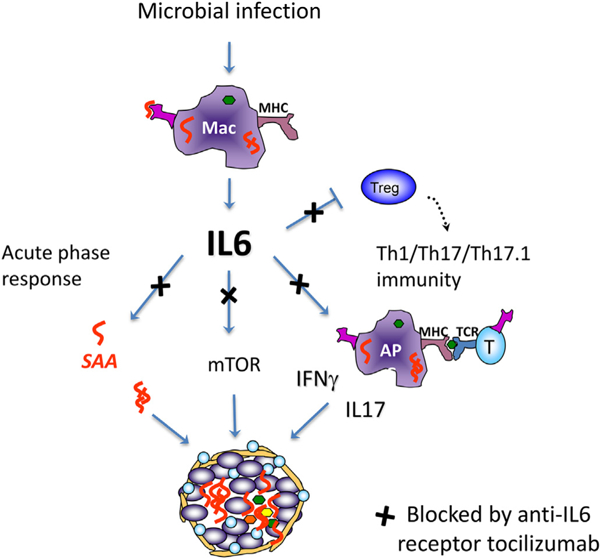
Hypothesized therapeutic role of blocking IL6 pathway in Sarcoidosis. Innate response to microbial infection induces IL6 by macrophages and dendritic cells leading to 1. Activation of the acute phase response with induction of serum amyloid A (SAA) that aggregates within granulomas and stimulates local T cell immunity; 2. induction of mTOR with metabolic changes and promoting epithelioid cell differentiation; 3. promotion of Th17 cell differentiation; 4. inhibition of regulatory T cell differentiation enhancing local Th1/ Th17/Th17.1 immunity. Blocking IL6 receptor with tocilizumab would potentially inhibit these responses.
There are two case reports of sarcoidosis being associated with the use of tocilizumab as treatment for rheumatoid arthritis [21,22].Whether these are examples of IL6 blockade being ineffective in the development of sarcoidosis or that in some cases, alteration of the immune system with IL6 blockade may predispose to sarcoidosis, remains uncertain [23].
Although tocilizumab appears beneficial in at least a subset of sarcoidosis patients, the overall safety profile of tocilizumab appears to be similar to other biologics such as anti-TNF agents. Tocilizumab has reported adverse effects including increased risk of infection and cancer. The increased rate of infection appears to be similar to other biologics such as anti-TNF agents [24]. The risk of malignancy with tocilizumab is uncertain; one study reported the risk of malignancy with tocilizumab to be similar to biologic drug-naive patients in a national register-based cohort [25]. We report one patient who developed breast cancer after receiving tocilizumab, however she was also treated with anti-TNF agents and other immunosuppressive therapies in the preceding years and it is impossible to know if any of these agents played a role in this cancer. Tocilizumab has been associated with adverse GI events such as bowel perforation. Given that intestinal involvement in sarcoidosis is rare, [26] it is uncertain whether there is any increased disease-specific risk of bowel perforation in sarcoidosis.
These case reports suggest tocilizumab shows promise in the treatment of sarcoidosis that have indications for potent steroid sparing therapy. Tocilizumab or direct inhibition of IL6 could be considered for other serious manifestations of sarcoidosis such as severe ocular or neurological involvement that have indications for potent steroid sparing therapy. Currently, a role for tocilizumab in sarcoidosis remains uncertain given a lack of clinical trials in this disease. We propose that formal clinical trials of IL6 inhibitory therapies are warranted in order to rigorously determine the effectiveness IL6 pathway inhibition for those sarcoidosis patients with indications for steroid sparing therapies.
Acknowledgements
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number T32HL007534. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- [1].Statement on sarcoidosis. Joint statement of the American thoracic society (ATS), the European respiratory society (ERS) and the world association of sarcoidosis and other granulomatous disorders (WASOG) adopted by the ATS board of directors and by the ERS executive committee, February 1999, Am. J. Respir. Crit. Care Med. 160 (1999) 736–755. [DOI] [PubMed] [Google Scholar]
- [2].Bradley B, et al. , Interstitial lung disease guideline: the British thoracic society in collaboration with the thoracic society of Australia and New Zealand and the Irish thoracic society, Thorax 63 (Suppl 5) (2008) v1–58. [DOI] [PubMed] [Google Scholar]
- [3].Vorselaars ADM, et al. , Methotrexate vs azathioprine in second-line therapy of sarcoidosis, Chest 144 (2013) 805–812. [DOI] [PubMed] [Google Scholar]
- [4].Beegle SH, Barba K, Gobunsuy R, Judson MA, Current and emerging pharmacological treatments for sarcoidosis: a review, Drug Des. Dev. Ther. 7 (2013) 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baughman RP, et al. , Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement, Am. J. Respir. Crit. Care Med. 174 (2006) 795–802. [DOI] [PubMed] [Google Scholar]
- [6].Lower EE, Baughman RP, Prolonged use of methotrexate for sarcoidosis, Arch. Intern. Med. 155 (1995) 846–851. [PubMed] [Google Scholar]
- [7].Chen ES, et al. , Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2, Am. J. Respir. Crit. Care Med. 181 (2010) 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen ES, Moller DR, Sarcoidosis-scientific progress and clinical challenges, Nat. Rev. Rheumatol. 7 (2011) 457–467. [DOI] [PubMed] [Google Scholar]
- [9].Girgis RE, Basha MA, Maliarik M, Popovich J Jr., Iannuzzi MC, Cytokines in the bronchoalveolar lavage fluid of patients with active pulmonary sarcoidosis, Am. J. Respir. Crit. Care Med. 152 (1995) 71–75. [DOI] [PubMed] [Google Scholar]
- [10].Stone JH, et al. , Trial of tocilizumab in giant-cell arteritis, N. Engl. J. Med. 377 (2017) 317–328. [DOI] [PubMed] [Google Scholar]
- [11].Okuda Y, Takasugi K, Successful use of a humanized anti-interleukin-6 receptor antibody, tocilizumab, to treat amyloid A amyloidosis complicating juvenile idiopathic arthritis, Arthritis Rheum. 54 (2006) 2997–3000. [DOI] [PubMed] [Google Scholar]
- [12].van Rhee F, et al. , International, evidence-based consensus treatment guidelines for idiopathic multicentric Castleman disease, Blood 132 (2018) 2115–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prusiner SB, Cell biology. A unifying role for prions in neurodegenerative diseases, Science 336 (2012) 1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Facco M, et al. , Sarcoidosis is a Th1/Th17 multisystem disorder, Thorax 66 (2011) 144–150. [DOI] [PubMed] [Google Scholar]
- [15].Ramstein J, et al. , IFN-gamma-Producing T-Helper 17.1 cells are increased in sarcoidosis and are more Prevalent than T-Helper type 1 cells, Am. J. Respir. Crit. Care Med. 193 (2016) 1281–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Linke M, et al. , Chronic signaling via the metabolic checkpoint kinase mTORC1 induces macrophage granuloma formation and marks sarcoidosis progression, Nat. Immunol. 18 (2017) 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sansone P, Bromberg J, Targeting the interleukin-6/Jak/stat pathway in human malignancies, J. Clin. Oncol.: Offic. J. Am. Soc. Clin. Oncol. 30 (2012) 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vainchenker W, Constantinescu SN, JAK/STAT signaling in hematological malignancies, Oncogene 32 (2013) 2601–2613. [DOI] [PubMed] [Google Scholar]
- [19].Damsky W, Thakral D, Emeagwali N, Galan A, King B, Tofacitinib treatment and molecular analysis of cutaneous sarcoidosis, N. Engl. J. Med. 379 (2018) 2540–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dienz O, Rincon M, The effects of IL-6 on CD4 T cell responses, Clin. Immunol. (Orlando, Fla.) 130 (2009) 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nutz A, Pernet C, Combe B, Cohen JD, Sarcoidosis induced by tocilizumab: a paradoxical event? J. Rheumatol. 40 (2013) 1773–1774. [DOI] [PubMed] [Google Scholar]
- [22].Shono Y, et al. , Cutaneous sarcoidosis in a patient with rheumatoid arthritis receiving tocilizumab, J. Dermatol. 45 (8) (2018. August) e217–e218. [DOI] [PubMed] [Google Scholar]
- [23].Bustamente L, Buscot M, Marquette CH, Roux C, Sarcoidosis and tocilizumab: is there a link? Clin. Exp. Rheumatol. 35 (2017) 716. [PubMed] [Google Scholar]
- [24].Mori S, et al. , Comparative risk of hospitalized infection between biological agents in rheumatoid arthritis patients: a multicenter retrospective cohort study in Japan, PLoS One 12 (2017) e0179179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wadstrom H, Frisell T, Askling J, Malignant neoplasms in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors, tocilizumab, abatacept, or rituximab in clinical Practice: a nationwide cohort study from Sweden, JAMA Intern. Med. 177 (2017) 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baughman RP, et al. , Clinical characteristics of patients in a case control study of sarcoidosis, Am. J. Respir. Crit. Care Med. 164 (2001) 1885–1889. [DOI] [PubMed] [Google Scholar]


