Abstract
Objective.
Most patients with rheumatoid arthritis (RA) harbor antibodies to citrullinated autoantigens, such as citrullinated fibrinogen. Two isoforms of peptidyl arginine deiminase (PAD), PAD2 and PAD4, which catalyze citrullination with different substrate specificities, can be detected in RA synovium. This study was undertaken to determine whether RA patient antibodies preferentially bind PAD2 or PAD4 citrullinated fibrinogen.
Methods.
RA patient and normal donor plasma was tested for binding to PAD2 or PAD4 citrullinated fibrinogen, native fibrinogen, or citrullinated fibrinogen peptides in various dilutions by ELISA and Western blot. Bands corresponding to masses demonstrating RA patient reactivity by Western blot were excised and analyzed by mass spectrometry.
Results.
At low titer, there was no significant difference between RA patient antibody reactivity to PAD2 and PAD4 citrullinated fibrinogen. When plasma was further diluted to 1:250 and 1:1000, RA patient plasma bound PAD4 citrullinated fibrinogen significantly more than PAD2 citrullinated fibrinogen, as measured by ELISA and Western blot. Increased antibody titer was associated with increased avidity for both PAD2 or PAD4 citrullinated fibrinogen. Both enzymes hypercitrullinated fibrinogen, but PAD4 citrullinates arginines more intermittently, generating a mix of citrullinated and nonmodifed arginines. Peptide ELISA and preadsorption assays confirmed that the region of intermittent citrullination accounts for the majority of RA antibody binding to the beta chain of citrullinated fibrinogen.
Conclusion.
At high titer, RA patient antibodies preferentially bind fibrinogen modified by PAD4 because intermittent citrullination offers a more diverse assortment of citrullinated epitopes.
The majority of rheumatoid arthritis (RA) patients harbor autoantibodies to citrullinated isoforms of self-proteins, called anti-citrullinated protein antibodies (ACPA), which are highly specific for the disease(1). Citrullinated protein targets of ACPA include filaggrin, type II collagen, alpha-enolase, vimentin, aggrecan, histone and fibrinogen(2–4). Anti-citrullinated fibrinogen antibodies are more sensitive for RA(5) than other ACPA such as antibodies that bind citrullinated type II collagen and alpha-enolase(2, 4) and have similar diagnostic performance to the clinical version of the ACPA assay, anti-cyclic citrullinated peptides 2 (CCP2), suggesting citrullinated fibrinogen may be particularly important in RA.
Proteins can become citrullinated as a result of enzymatic modification by peptidyl arginine deiminase (PAD) enzymes, which catalyze conversion of the positively charged amino acid, arginine, to a neutral citrulline. Treatment with a pan-PAD inhibitor reduced the severity of arthritis and was associated with lower autoantibody production in an animal model of arthritis(6). It is hypothesized that PAD inhibitors may alleviate RA via prevention of further activation of the immune system by limiting autoantigen production. There are five PAD family members (PAD1–4 and 6), each with unique tissue distribution. It is not known which of the PAD family members are most important for generating the antigens that drive RA immune responses and therefore, it is not known which PADs should to be targeted for this treatment approach to be most effective. PAD2 and PAD4 are expressed in immune cells and are overexpressed in RA synovium(7). PAD2 is broadly expressed in sites including skin, intestine, brain, muscle and hematopoietic cells while PAD4 expression is restricted to certain cell types including neurons and neutrophils. PAD4 is unique among PAD family members because it harbors a nuclear localization sequence and is required for neutrophil NETosis(8–11). Our group recently showed that neutrophils undergoing necrotic or NETotic death release both PAD2 and PAD4 and this leads to citrullination of extracellular fibrinogen(12). Surface plasmon resonance imaging of PAD2 and PAD4 citrullinated fibrinogen have demonstrated that the two isoforms citrullinate different arginine residues within fibrinogen(13), with PAD2 causing more extensive arginine citrullination than PAD4. Since RA related autoantibody responses to citrullinated fibrin are closely restricted(14), we hypothesized that immunoreactivity to PAD2 and PAD4 citrullinated fibrinogen may differ. We therefore compared RA patient antibody levels to PAD2 and PAD4 citrullinated fibrinogen and report that RA patient plasma harbors increased levels of antibody to fibrinogen when it is citrullinated by PAD4. Mass spectrometry based analysis of our samples revealed that though both enzymes heavily citrullinated arginines in a hotspot region between position 44 and 74, PAD2 citrullinates more consistently while PAD4 citrullinates intermittently and this provides a more diverse collection of target citrullinated epitopes against which antibodies are produced.
Patients and Methods
Patients and healthy subjects
Plasma samples were collected from 12 patients with established, anti-CCP antibody positive RA who met the American College of Rheumatology 2010 classification criteria(15). Plasma samples were also collected from healthy subjects, matched by sex and age within 5 years. The ethics review board of The Rockefeller University Hospital approved this study, and all study subjects gave written informed consent.
ELISA
PAD2 (Cayman Chemical #10785, Ann Arbor, MI) or PAD4 (Cayman Chemical #10500, Ann Arbor, MI) were auto-citrullinated by incubating 10ug/ml in 100mM Tris buffer (Sigma-Aldrich, St. Louis, MO), 10mM calcium chloride and 5mM dithiothreitol (DTT) (Affymetrix, Santa Clara, CA) at 37°C overnight. Effective citrullination was verified by using Citrulline-specific probe (Cayman Chemical #16172, Ann Arbor, MI) (data not shown). Microtiter plates (Nunc, Roskilde, Denmark) were coated for 1 hour at room temperature with fibrinogen, PAD4 citrullinated fibrinogen (Cayman Chemical #400076, Ann Arbor, MI), PAD2 citrullinated fibrinogen (Cayman Chemical #18473, Ann Arbor, MI), citrullinated PAD4, citrullinated PAD2 or peptide antigens (synthesized by Fmoc chemistry) at 5 μg/ml in 0.05M carbonate-bicarbonate buffer (pH 9.6). Plates were washed with 0.05% Tween-20 (Sigma-Aldrich, St. Louis, MO) in PBS (Cellgro, Manassas, VA), and blocked for 1 hour with 1% bovine plasma albumin (BSA) in PBS. Plasma, diluted at various concentrations in 1% BSA/PBS, was added for 1 hour. Horseradish peroxidase–conjugated goat anti-human IgG (Jackson ImmunoResearch, West Grove, PA) was added for detection. Bound antibodies were revealed using the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich, St. Louis, MO) and stopped with 2M H2SO4. Plates were read at 450 nm.
Avidity was measured by adding various concentrations of sodium thiocyanate (Sigma-Aldrich, St. Louis, MO) to ELISA plates. Avidity is presented as the avidity index, which was calculated by dividing the amount of residual antibodies bound to the antigen-coated plate by the amount of antibodies bound in the absence of chaotropic agent.
Western blot
1ug per lane of either PAD4 citrullinated fibrinogen or PAD2 citrullinated fibrinogen were resolved by polyacrylamide gel electrophoresis and transferred to PVDF membranes (Millipore, Billerica, MA). The membranes were blocked in 5% non-fat milk in TBS containing 0.1% Tween 20 for 1 hour at room temperature. Membranes were probed with antibodies: monoclonal anti-citrullinated fibrinogen (1:1000) (Cayman Chemical, Ann Arbor, MI), monoclonal anti-PAD2 antibody (1:500) (16) (Abnova, #H00011240-M01, Taipei City, Taiwan), anti-PAD4 antibody (1:1000) (17) (Abcam #ab128086, Cambridge, MA), monoclonal anti-fibrinogen (1:500) (Abcam #ab10066, Cambridge, MA), or patient plasma diluted 1:1000 in 5% non-fat milk in TBS containing 0.1% Tween 20. Bound immunoglobulin was detected with horse-radish peroxidase-linked secondary antibodies (Life Technologies, Grand Island, NY) and visualized with ECL (Western Lightning® Plus-ECL, Enhanced Chemilluminescence Substrate, Perkin Elmer, Waltham, MA) system.
For preadsorption assays, patient plasma, diluted 1:1000 in TBS containing 0.1% Tween 20 and 5% non-fat milk, were pre-incubated with PAD2 citrullinated fibrinogen protein or peptide (10ug/ml) for 1 hour at 37°C prior to being used to probe membranes for 2 hours at room temperature. Density of the bands was evaluated using ImageJ 1.46r software (Wayne Rasband, National Institutes of Health, USA).
Plasmin cleavage assay
0.2 mg/ml of PAD4 citrullinated fibrinogen was incubated with 0.006 mg/ml plasmin (Sigma-Aldrich, Saint Louis, MO) in 20 mM TRIS-HCl, 2 mM CaCl2 and 5 mM DTT for 1 hour at 37°C. 1ug of PAD4 citrullinated fibrinogen and 5ug of plasmin digested PAD4 citrullinated fibrinogen were resolved using 12% SDS-PAGE. Proteins were transferred to PVDF membrane and probed with patient plasma 1:1000 as described above.
Liquid chromatography-mass spectrometry (LC-MS)
1μg of each sample, unmodified, PAD2-modified, or PAD4-modified fibrinogen was separated by SDS-PAGE and stained with Coomasie Brilliant Blue. Gel bands corresponding to masses demonstrating reactivity against patient antiplasma by Western blot were excised for in-gel proteolytical digestion(18, 19). In short, following reduction of cysteines with dithiothreitol and alkylation with iodoacetamide (Sigma-Aldrich, Steinheim, Germany), digestion was conducted with either trypsin (Promega, Fitchburg, WI) and LysC (Waco Chemicals, Richmond, VA) or chymotrypsin (Promega, Fitchburg, WI). Samples were processed and analyzed in triplicate to provide additional confidence of assignment. Following overnight incubation, peptides were extracted from gel pieces with acetonitrile, dried, and resuspended in 2% acetonitrile, 0.1% trifluoroacetic acid. Each sample was analyzed by nano LC-MS/MS (Dionex 3000 HPLC coupled to Q Exactive mass spectrometer, Thermo Scientific, San Jose, CA). Peptides were separated at 200 nL/min using a gradient increasing from 5% B to 45% B in 60 minutes (A: 0.1% formic acid, B: acetonitrile/0.1% formic acid). Peptides were loaded onto a trap column prior to separation on a packed-in-emitter C18 column (75 μm by 12 cm, 3 μm particles - Nikkyo Technos Co., Ltd. Japan). For data-dependent analysis of peptide/modification identification, the mass spectrometer was operated in “preferred mode”: fragmentation of up to 20 ions per cycle using an under fill ratio of 1%. For quantitation and specific site assignment of arginine citrullination in the identified ‘hot spot’ (R44, R47, R53, R60, R72 and R74), parallel reaction monitoring (PRM)(20) was used to quantify using parent and fragment ions for distinct modification sites. MS spectra (m/z range: 300–1400) were recorded at a resolution of 70,000 (AGC: 5e5) and MS/MS spectra at 17,500 (AGC: 1e5) with a lowest m/z of 100. Generated LC-MS/MS data were queried against Uniprot’s complete Human Proteome (March 2016) using Mascot, with carbamidomethylation of cysteine (C) as a static modification and the following variable modifications: oxidation of methionine (M), acetylation of protein N-termini, deamidation of aspargine (N) and glutamine (Q), and citrullination of arginine (R). Identifications were filtered to include only precursor masses <5 ppm and highest PSM ranking, and false discovery rates <1% were controlled by Percolator(21). Deamidation was included to allow consideration of de facto N/Q deamidation and the isobaric citrullination modification. Up to 4 missed cleavages were allowed for the chymotrypsin searches, while up to 7 missed cleavages were allowed for the trypsin searches (since citrullination will lead to missed cleavage of modified arginines). For the trypsin-digested samples, peptides identified as citrullinated were required to contain a missed cleavage following a modified arginine. Skyline(22) was used for quantitation of PRM data for relative modification site comparison, by summing precursor peak areas. In the case of isobaric modification variants, the parent signal was distributed proportionally to the discriminating fragment ion areas. A spectral library generated from the Mascot searches were used to assign peak identity.
Statistical analysis
Unpaired t-test was used for comparing OD of ELISA results between normal and RA donor plasma. Paired t-test was used to compare plasma ELISA OD from individual donors to PAD4 citrullinated fibrinogen and PAD2 citrullinated fibrinogen. Wilcoxon matched-pairs signed rank test was used to compare normalized band intensity of Western blots. One-way analysis of the variance (ANOVA) and the Tukey test for multiple comparisons was used to evaluate statistical differences between three different titers in the avidity ELISA at the concentration of 3N sodium thiocyanate. P values less than 0.05 were considered significant. Statistical analysis was performed using Prism software (GraphPad Software, San Diego, CA, U.S.A.).
Results
RA patient plasma contains higher levels of antibody to PAD4 than PAD2 citrullinated fibrinogen as measured by ELISA.
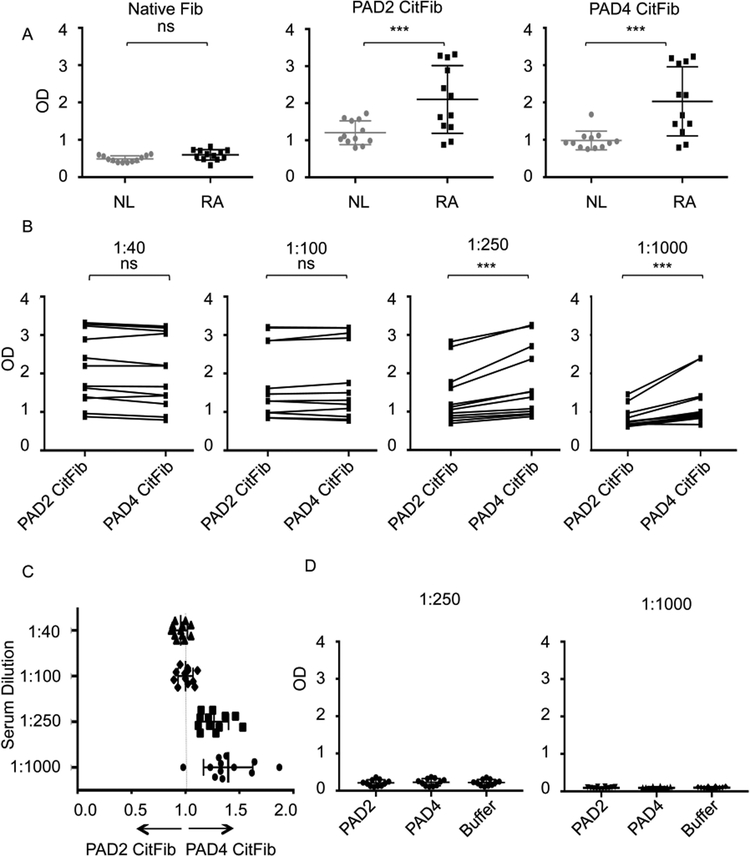
We tested responses of normal donor and RA patient plasma to fibrinogen as well as PAD2 and PAD4 citrullinated fibrinogen. RA patient characteristics are outlined in the Supplemental Table. Notable features of this cohort are that 100% of patients harbored high titer CCP antibodies and 66% of patients had rheumatoid nodules. Otherwise the patients had varying durations of disease, levels of disease activity and exposures to medications. As expected, at low dilutions neither normal donors nor RA patients harbored antibodies to unreactive (native) plasma derived fibrinogen while RA patients harbored significantly elevated antibody binding to both PAD2 and PAD4 citrullinated fibrinogen (Figure 1A). To determine the relative reactivity of RA plasma to PAD4 versus PAD2 citrullinated fibrinogen, we tested a range of plasma dilutions. Binding to PAD4 citrullinated fibrinogen was significantly greater than PAD2 citrullinated fibrinogen at high dilutions (1:250 and 1:1000), though similar at low dilutions (1:40 and 1:100), likely due to assay saturation at low dilution (Figure 1B). The ratio of ELISA OD of PAD4 citrullinated fibrinogen divided by PAD2 citrullinated fibrinogen was calculated for each patient. The mean ratios of PAD4/PAD2 citrullinated fibrinogen at the 1:250 and 1:1000 dilutions were 1.26 (95% confidence interval 1.18,1.35) and 1.39 (95% confidence interval 1.24,1.53) respectively (Figure 1C). To test whether the increased recognition of PAD4 citrullinated fibrinogen was attributable to antibodies against PAD4 itself, we also measured reactivity against PAD2 and PAD4. We did not detect significant reactivity to PAD4 or PAD2 above background levels to buffer alone at either 1:250 or 1:1000 (Figure 1D).
Figure 1:
RA patient plasma preferentially binds PAD4 citrullinated fibrinogen as measured by ELISA. (A) Normal donor and RA patient antibody levels, as measured by ELISA, to unmodified fibrinogen at 1:20 dilution, PAD2 citrullinated fibrinogen at 1:40 dilution and PAD4 citrullinated fibrinogen at 1:40 dilution. (B) Individual RA patient antibody levels to PAD4 citrullinated fibrinogen and PAD2 citrullinated fibrinogen at various dilutions. (C) Ratio of individual RA patient plasma binding to PAD4 or PAD2 citrullinated fibrinogen. Ratio calculated as (OD of PAD4 citrullinated fibrinogen)/(OD of PAD2 citrullinated fibrinogen) of individual patients from (B) at various dilutions. (D) Individual RA antibody levels to autocitrullinated PAD2 and PAD4 at various dilutions. Data represent 1 of 3 independent experiments. CitFib= citrullinated fibrinogen OD= optical density. *** P<0.005, ns=not significant.
RA patient antibodies bind PAD4 citrullinated fibrinogen better than PAD2 citrullinated fibrinogen on Western blot.
One limitation of measuring RA patient antibody responses to citrullinated fibrinogen by ELISA is that the relative importance of the three polypeptide chains (alpha, beta, and gamma) cannot be distinguished. To address this question, we resolved equal amounts of PAD4 citrullinated fibrinogen and PAD2 citrullinated fibrinogen on adjacent lanes on multiple Western blots and probed with monoclonal anti-citrullinated beta fibrinogen antibody (Supplemental Figure 1A), PAD2 antibody (Supplemental Figure 1B), PAD4 antibody (Supplemental Figure 1C), and anti-fibrinogen antibody (Supplemental Figure 1D). A monoclonal anti-citrullinated fibrinogen antibody binds at the predicted molecular weight of the beta chain of fibrinogen (fibrinogen beta), 56 kD, while PAD2 and PAD4 antibodies binds at molecular weight closer to 76 kD, consistent with their established molecular weights of 76 and 74 kD respectively. A polyclonal anti-fibrinogen antibody recognizes several bands consistent with the various molecular weights of the three polypeptide chains of fibrinogen: alpha, beta and gamma. We next compared 12 individual RA patient plasma samples, diluted 1:1000, binding to the PAD2 or PAD4 citrullinated fibrinogen separated on adjacent lanes of Western blots (Supplemental Figure 1E). At this dilution, the individual RA patient plasma most consistently recognized a band just above 52 kD, consistent with the known molecular weight of fibrinogen beta, 56 kD. Some patients’ plasma also recognized a larger molecular weight band, just above 76 kD, which likely represents recognition of the alpha chain of fibrinogen (fibrinogen alpha), given there was no recognition of PAD2 or PAD4 themselves on ELISA. Band intensity of the 56 kD region was quantitated using densitometric analysis. RA patient antibodies generated significantly more intense bands to the beta chain of fibrinogen when citrullinated by PAD4 compared to PAD2 (Figure 2).
Figure 2:
RA patient plasma preferentially recognizes PAD4 citrullinated fibrinogen on Western blot. Comparison of normalized band intensities at 76 kD and 56 kD of PAD2 citrullinated fibrinogen and PAD4 citrullinated fibrinogen resolved on SDS-page and probed with individual RA patient plasma diluted 1:1000. Normalized band intensity indicates band intensity of RA patient plasma divided by load control anti-fibrinogen antibody. Data represent 1 of 2 independent experiments. ** P<0.01
There is no difference in RA patient antibody avidity to PAD4 or PAD2 citrullinated fibrinogen.
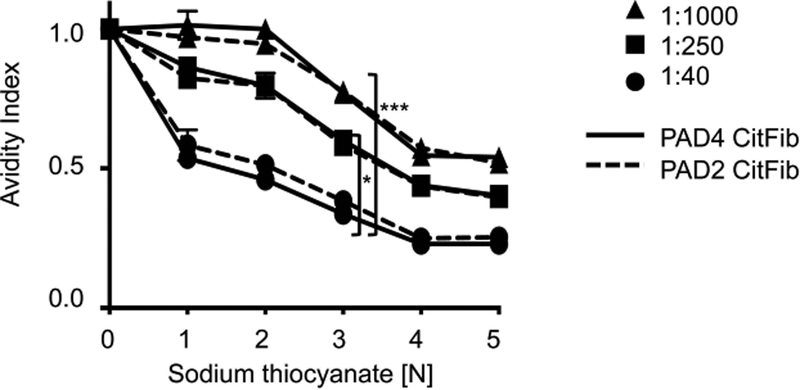
RA patient antibodies could display increased binding to PAD4 citrullinated fibrinogen on ELISA and Western blot due to differences in avidity (increased cumulative binding affinity to PAD4 citrullinated fibrinogen) or specificity (more relevant epitopes on PAD4 citrullinated fibrinogen). To compare the avidity of RA antibodies, we compared the ELISA OD of RA patient plasma incubated with either PAD4 or PAD2 citrullinated fibrinogen and various concentrations of a chaotropic agent, sodium thiocyanate. Three patient plasma samples that produced 56 kD bands but not 76 kD bands on Western blot were chosen for this assay to specifically measure binding avidity to citrullinated fibrinogen beta, since it is more commonly recognized than citrullinated fibrinogen alpha in our very high titer studies. Significantly more chaotropic agent was required to inhibit binding for increasing antibody titers, indicating a significant increase in avidity with increased titer (Figure 3). Avidity index and antibody titer were correlated in both PAD4 and PAD2 citrullinated fibrinogen samples. There was no difference, however, in the avidity of RA patient antibodies to PAD4 or PAD2 citrullinated fibrinogen at high titers (1:250, 1:1000), which produced a robust difference in ELISA and Western blot (Figure 3). While it is possible that there is a subtle difference in avidity that is below the limit of detection of this assay, this potential difference is less significant than the difference conferred by increasing titer. There was a trend towards increased avidity index for PAD2 citrullinated fibrinogen relative to PAD4 citrullinated fibrinogen at low titers (1:40) but this was not significant. For the three samples tested, differential avidity cannot explain the increased RA patient antibody binding of PAD4 citrullinated fibrinogen.
Figure 3:
There is no difference in RA patient antibody avidity to PAD4 or PAD2 citrullinated fibrinogen. Avidity ELISA of RA patient plasma at various titers, incubated with either PAD2 citrullinated fibrinogen or PAD4 citrullinated fibrinogen with increasing concentrations of sodium thiocyanate. Avidity index represents OD of RA patient plasma for a given concentration of sodium thiocyanate divided by the OD without sodium thiocyanate. * P<0.05, *** P<0.001. Data presented are from 1 RA patient and are representative of 3 patients tested.
Fibrinogen beta 44–74 is a hotspot of citrullination that is uniformly modified by PAD2 and intermittently modified by PAD4.
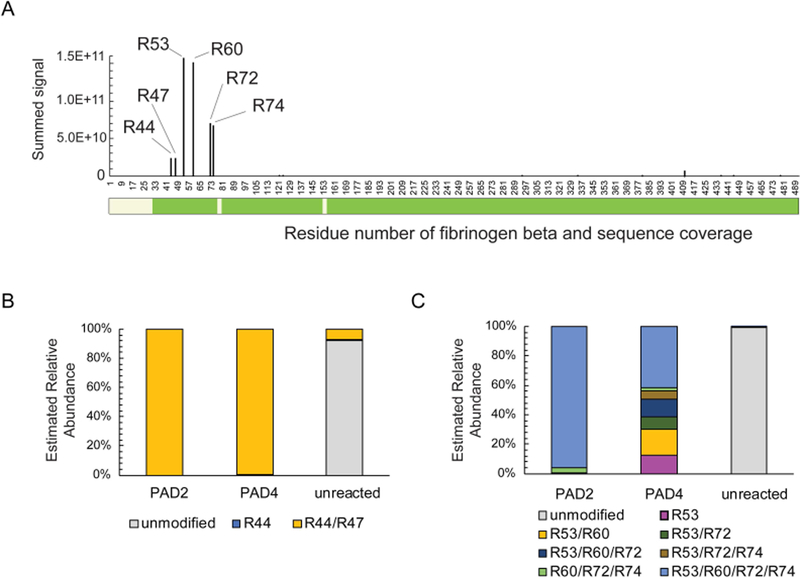
It is possible that the difference in RA antibody recognition of PAD2 and PAD4 citrullinated fibrinogen is due to a difference in the level of citrullination in the samples. To quantify the extent of citrullination of either unmodified, PAD2, or PAD4 citrullinated fibrinogen, we performed bottom-up mass spectrometry of the 56 kD band (fibrinogen beta) to identify and quantify modified versus non-modified peptides. Fibrinogen beta was chosen for this quantitative analysis because RA patient plasma diluted 1:1000 bound it more frequently than the alpha chain. Distal to the signal peptide, starting at position 31, protein sequence coverage was nearly complete, with 98% sequence coverage. This coverage accounted for all arginines. We identified a “hotspot” of citrullination of arginines at positions R44, R47, R53, R60, R72 and R74 in both PAD2 and PAD4 modified fibrinogen beta (Figure 4A). At these sites, citrullination was strikingly more abundant than other downstream citrullination sites. The citrullination hotspot region between positions 44 and 74 was analyzed in depth to identify differences in citrullination between PAD2 and PAD4 modified samples. PAD2 and PAD4 citrullinated both R44 and R47 fully (>99%) (Figure 4B). Interestingly, these positions were also citrullinated, but at a lower level (8%) in unreacted (native) fibrinogen samples. While PAD2 resulted in a 95% citrullination at all four of the remaining hotspot residues (R53, R60, R72, R74), PAD4 treatment resulted in complete citrullination of all 4 residues in only 42% of peptides identified (Figure 4C). In the majority of peptides, R53, R60, R72 and R74 were citrullinated intermittently. The next most commonly identified combination of citrullination by PAD4 was at R53 and R60, followed by R53, R60 and R72. Besides this hotspot region between position R44 and R74, citrullination was detected at low level at several sites in the C-terminal region of the fibrinogen beta protein. While initial analysis detected putative citrullination sites at low levels throughout the fibrinogen beta protein, this low level of citrullination can be difficult to discriminate from artifactual deamidation of asparagine and glutamine since these modifications are isobaric. These sites were manually validated to confirm that fragmentation spectral coverage could differentiate from any adjacent asparagine or glutamine residues. A list of validated citrullination sites and their relative quantitation by parallel reaction monitoring are presented in Table 1. Taken together, these data indicate that while both PAD2 and PAD4 hypercitrullinate a hotspot region between R44 and R74 on fibrinogen beta, PAD4 mediated citrullination is more intermittent at residues R53, R60, R72 and R74.
Figure 4:
Fibrinogen beta arginine sites 44–74 are a hotspot of citrullination that are uniformly modified by PAD2 and intermittently modified by PAD4. Unmodified, PAD2, or PAD4 citrullinated fibrinogen were enzymatically digested and analyzed by mass spectrometry. (A) Summed signal (PAD2 and PAD4) of peptides containing a citrullinated arginine are plotted as a function of amino acid residue number for fibrinogen beta chain. Green bar below depicts residues covered based on peptides matched with a false discovery of <1%. (B) Relative abundance of identified peptide signals carrying modifications at R44 and R47 in PAD2 and PAD4 citrullinated fibrinogen. Unreacted fibrinogen beta is presented as a control. (C) Relative abundance of identified peptide signals carrying modifications at sites R53, R60, R72 and R74 in PAD2 and PAD4 citrullinated fibrinogen. Unreacted fibrinogen beta is presented as a control.
Table 1:
Intensity based mass spectrometry quantitation of citrullination of PAD2 and PAD4 modified fibrinogen at various arginine sites.
| SITE | PAD2 | PAD4 |
|---|---|---|
| 44 | 1.44 × 1010 | 9.39 × 109 |
| 47 | 1.44 × 1010 | 9.39 × 109 |
| 53 | 8.12 × 1010 | 7.59 × 1010 |
| 60 | 8.42 × 1010 | 6.65 × 1010 |
| 72 | 4.55 × 1010 | 2.41 × 1010 |
| 74 | 4.55 × 1010 | 2.16 × 1010 |
| 334 | 0.00 | 2.76 × 108 |
| 436 | 1.40 × 108 | 1.11 × 108 |
| 445 | 7.78 × 107 | 8.39 × 107 |
| 478 | 0.00 | 2.22 × 107 |
Data represent parallel reaction monitoring quantification = PAD2 or PAD4 modified fibrinogen, requiring missed cleavage by trypsin, present in at least two of three technical replicates.
RA antibodies bind PAD4 citrullinated fibrinogen more than PAD2 citrullinated fibrinogen because intermittent citrullination of the hotspot region includes a broader array of citrullinated epitopes.
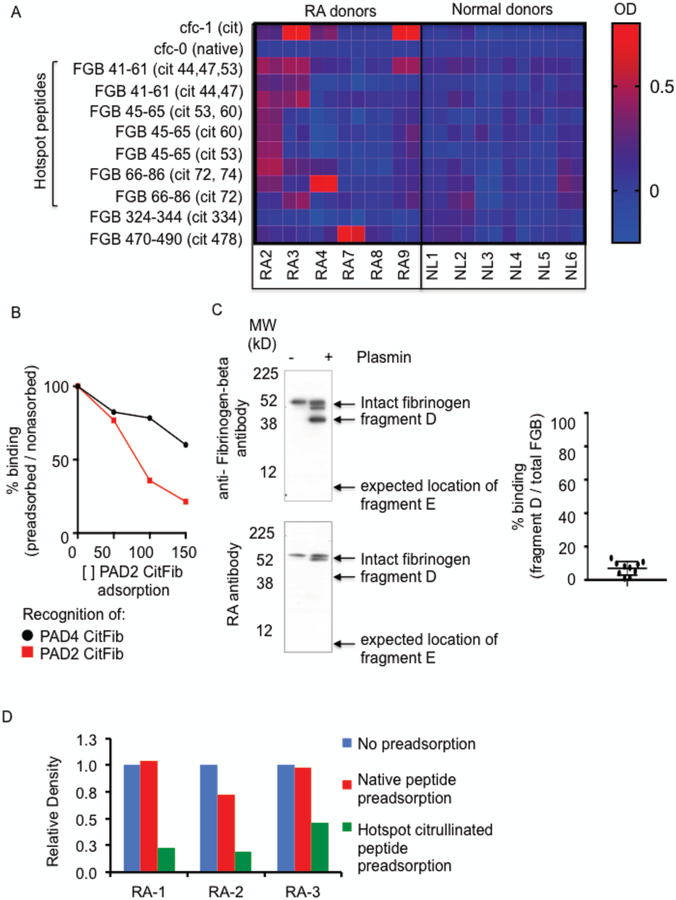
To determine whether the preferential binding of PAD4 citrullinated fibrinogen was due to recognition of partially citrullinated peptides in the hotspot region (spanning 44–74) or PAD4 uniquely citrullinated peptides (334 and 478) we compared RA and normal donor antibody responses to citrullinated peptides by ELISA. cfc-1, cyclized citrullinated fillaggrin peptide-1 was used as a positive control in this experiment, while cfc-0 (the non-citrullinated isoform) was used as a negative control. Half of the RA patients tested recognized the positive control peptide cfc-1, while no patients recognized the negative control peptide cfc-0 (Figure 5A). We did not identify a single citrullinated fibrinogen beta peptide that was consistently more immunogenic when only partially citrullinated. Instead, there was a diverse array of peptide recognition. This variability in RA patient antibody responses to various citrullinated isoforms of the same peptide region is reminiscent of the original work mapping epitopes of citrullinated filaggrin(23). The clinically useful test for antibodies to citrullinated filaggrin peptides include an array of peptides derived from filaggrin spanning position 306–324. This region includes five arginine residues, and while only 36% of RA patient antibodies bind the peptide when the first arginine residue is citrullinated, the sensitivity of the assay is improved considerably to 76% when it includes a mix of nine peptides spanning this same region but with different citrullination sites(23). Since PAD4 variably citrullinates arginines in the hotspot region, it stands to reason that the improved RA antibody recognition of PAD4 citrullinated fibrinogen reflects that RA antibodies bind an assortment of citrullinated epitopes and that citrullination by PAD4 results in a broader array of potential target epitopes.
Figure 5:
RA antibodies preferentially bind PAD4 citrullinated fibrinogen because it contains a broader array of citrullinated epitopes. (A) ELISA ODs of RA patient and normal donor (NL) antibodies to citrullinated peptides (1:40). Data represent test peptide minus irrelevant beta-galactosidase peptide. Technical duplicates are presented. cfc-1 = citrullinated filaggrin peptide-1, cfc-0 = noncitrullinated filaggrin peptide-1, FGB= fibrinogen peptides (B) Band intensity of PAD2 or PAD4 citrullinated fibrinogen on Western blot of RA plasma preadsorbed with increasing concentrations of PAD2 citrullinated fibrinogen. Data represent 1 of 3 experiments. (C) Western blots of citrullinated fibrinogen or plasmin cleaved citrullinated fibrinogen. Top panel: probed with polyclonal anti-fibrinogen antibody. Bottom panel: probed with RA patient plasma. Data represent 1 of 3 experiments. Right Panel: Summary of Western blot results presented in (C). Data represents relative band intensity of fragment D divided by relative band intensity of total fibrinogen beta from 10 RA patient samples. (D) Band intensity of citrullinated fibrinogen beta probed with RA patient plasma samples preadsorbed with native peptides or citrullinated peptides from the hotspot region of FGB. Native peptides: FBG34–54, FBG41–61, FBG45–65, FBG66–86, FBG285–305, Citrullinated peptides: FBG41–61(cit 44,47,53), FBG41–61(cit 44, 47), FBG45–65(cit 53, 60), FBG45–65 (cit 53), FBG45–65(cit 60), FBG66–86(cit 72, 74), FBG66–86(cit 72)
To confirm that the increased binding of RA patient plasma antibodies to PAD4 citrullinated fibrinogen was due to the availability of epitopes not present in PAD2 citrullinated fibrinogen, we preadsorbed RA plasma with PAD2 citrullinated fibrinogen at various concentrations and tested whether there was remaining antibody capable of binding PAD4 citrullinated fibrinogen. Preadsorption with increasing concentrations of PAD2 citrullinated fibrinogen led to a sharp decrease in recognition of PAD2 citrullinated fibrinogen but relatively preserved binding of PAD4 citrullinated fibrinogen (Figure 5B). Though RA patient antibodies bind both PAD2 and PAD4 citrullinated fibrinogen, this result establishes that PAD4 citrullinated fibrinogen includes additional epitopes that can be recognized by RA patient plasma.
To compare the relative importance of the N-terminal hotspot region and the C-terminal citrullination sites (R334 and R478), we treated PAD4 citrullinated fibrinogen with plasmin, which cleaves fibrinogen beta at positions 152 and 163. This results in the generation of two main fibrinogen-beta fragments(24). The C-terminal, 38kD fragment D spans residues 164–491, and therefore includes the PAD4 specific, low abundant citrullination sites R334 and R478. The smaller N-terminal, 8kD, fragment E spans residues 30–153, which includes the hotspot region of citrullination. Citrullinated fibrinogen was incubated with plasmin in conditions sufficient to cause partial digestion of the protein and resolved by SDS-PAGE. A positive control polyclonal anti-fibrinogen beta antibody detected intact fibrinogen beta (56kD) and the beta chain fragment D (38kD), but did not detect the beta chain fragment E (Figure 5C-left panel) even when increasing the amount 5 fold and detecting this fragment at it’s expected molecular weight of 8kD by Coumassie stain (supplemental figure 2). Loading an increased amount of cleaved fibrinogen revealed the presence of heavy chain antibodies at 52kD (fibrinogen was purified from human plasma). All RA patient antibodies tested in this manner produced markedly less recognition of the fragment D compared to intact fibrinogen (the relative band intensity of RA patient antibody recognition of fragment D relative to intact fibrinogen ranged from 1–13%, Figure 5C- right panel). The consistently weaker recognition of fragment D, relative to intact fibrinogen, indicates that RA patient antibodies likely bind to the N-terminal region, lower than residue 153. It should be noted that plasmin cleavage could have directly disrupted an important site of antibody recognition, however there is only one arginine residue in proximity to a plasmin cleavage site, R158, and careful re-analysis of mass spectrometry data failed to detect modification of this arginine in our PAD modified fibrinogen samples.
To test whether citrullinated peptides from the hotspot region encompass the critical antibody recognition sites, we compared the effect of preadsorbing RA plasma with native or citrullinated isoforms of peptides spanning 41–77. Preadsorption of three RA patient plasma samples with citrullinated hotspot peptide pools decreased recognition of fibrinogen beta by 77%, 53% and 80%, while preadsorption with native peptide pools decreased recognition between 0 and 20% (Figure 5D). We therefore conclude that the key site of RA antibody recognition of citrullinated fibrinogen resides in the hotspot region spanning R44 to R74. Taken together this data demonstrates that RA antibodies bind PAD4 citrullinated fibrinogen more than PAD2 citrullinated fibrinogen because intermittent citrullination of the hotspot region offers a more diverse assortment of citrullinated epitopes.
Discussion
“High titer CCP” contributes three points toward the 2010 ACR/EULAR classification criteria for RA. According to this criteria, high titer refers to values >3 times the upper limit of normal (ULN). Few commercially available assays extend titers beyond 10 times ULN so relatively little is known about very high titer CCP. The likelihood of undifferentiated arthritis progressing to persistent arthritis further increases in patients with very high (>10 times ULN) relative to those with high (4–10 times ULN) titer antibodies(25), suggesting there may also be a clinically important distinction between high and very high titer CCP antibodies. Considering very high titer antibodies are also very high avidity, it is conceivable that they play a more important role in immune targeting and merit further exploration. In this study, we report that at very high titers, RA patient antibodies demonstrate preferential binding of PAD4 compared to PAD2 citrullinated fibrinogen. Bearing in mind the broad repertoire of citrullinated antigens targeted by RA antibodies, one might have predicted that decreased antibody binding would be due to less efficient citrullination in the PAD2 citrullinated fibrinogen samples, but mass spectrometry of our samples demonstrated less efficient citrullination in PAD4 citrullinated fibrinogen.
An important limitation of this report is that it describes antibody binding to citrullinated fibrinogen generated in vitro, and there may be differences in the activity of PAD2 and PAD4 in vivo. The citrullinated fibrinogen used in this work was generated by adding either PAD2 or PAD4 in buffer containing 10mM CaCl2, and incubated overnight at 37°C (as per personal communication with Cayman Chemical), which likely represents higher calcium concentration and longer duration of exposure than occurs in vivo. Interestingly, despite the use of conditions that were optimized to maximize citrullination rather than model conditions in vivo, the validated citrullination sites detected were restricted to two domains in the fibrinogen beta protein: the N-terminal central domain and the C-terminal globular domain. We could not confirm citrullination at arginine sites in the coiled-coil region.
While the reaction conditions for citrullinating fibrinogen used in these assays make it difficult to conclude that PAD4 play a more important role in generating autoantigen targets of RA antibodies, it does not detract from the observation that RA patient antibodies distinguish fibrinogen beta with subtle differences in citrullination profiles. In support of the in vivo relevance of the hotspot of citrullination identified here, citrullinated fibrinogen beta epitopes derived from the hotspot region have been identified in RA synovial tissue(26), fluid(27), as well as circulating RA immune complexes(28).
In summary, the work presented here demonstrates that very high titer antibodies bind PAD4 citrullinated fibrinogen more than PAD2 citrullinated fibrinogen and that this could not be attributed to increased citrullination in PAD4 reacted samples. Instead, while both PAD2 and PAD4 heavily citrullinate a hotspot region between R44 and R74, the PAD4 citrullination profile is more intermittent. Other studies of ACPA have largely used PAD2 modified target proteins or peptides with citrullination of all possible arginines. The work presented here indicates that high titer RA patient antibodies preferentially bind PAD4 citrullinated fibrinogen because it produces an assortment of citrullinated isoforms from a hotspot region of citrullinated fibrinogen beta. In the future, it would be useful to compare the relative binding of high titer RA antibodies to other known citrullinated target antigens modified by PAD2 or PAD4 to evaluate the generalizability of this result.
Supplementary Material
Acknowledgments
Supported in part by funding from grant #8 UL1 TR000043 from the National Center for Advancing Translational Sciences, National Institutes of Health Clinical and Translational Science Award program (DEO) and The Arthritis Foundation (DEO).
We are grateful to the research participants and the research staff of The Rockefeller University Hospital for facilitating research studies. We thank Michael Moore and Sergio Schwartzman for helpful discussions and review of the article.
DEO was supported by Arthritis Foundation Clinical to Research Award Grant, Rockefeller University Pilot Award and grant # UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program.
References
- 1.van Venrooij WJ, Vossenaar ER, and Zendman AJ, Anti-CCP antibodies: the new rheumatoid factor in the serology of rheumatoid arthritis. Autoimmun Rev, 2004. 3 Suppl 1: p. S17–9. [PubMed] [Google Scholar]
- 2.Snir O, Widhe M, von Spee C, Lindberg J, Padyukov L, Lundberg K, et al. , Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: association with HLA-DRB1 alleles. Ann Rheum Dis, 2009. 68(5): p. 736–43. [DOI] [PubMed] [Google Scholar]
- 3.Ioan-Facsinay A, Willemze A, Robinson DB, Peschken CA, Markland J, van der Woude D, et al. , Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum, 2008. 58(10): p. 3000–8. [DOI] [PubMed] [Google Scholar]
- 4.Snir O, Widhe M, Hermansson M, von Spee C, Lindberg J, Hensen S, et al. , Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum, 2010. 62(1): p. 44–52. [DOI] [PubMed] [Google Scholar]
- 5.Vander Cruyssen B, Cantaert T, Nogueira L, Clavel C, De Rycke L, Dendoven A, et al. , Diagnostic value of anti-human citrullinated fibrinogen ELISA and comparison with four other anti-citrullinated protein assays. Arthritis Res Ther, 2006. 8(4): p. R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, et al. , N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol, 2011. 186(7): p. 4396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foulquier C, Sebbag M, Clavel C, Chapuy-Regaud S, Al Badine R, Mechin MC, et al. , Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis Rheum, 2007. 56(11): p. 3541–53. [DOI] [PubMed] [Google Scholar]
- 8.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. , NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med, 2013. 5(178): p. 178ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spengler J, Lugonja B, Jimmy Ytterberg A, Zubarev RA, Creese AJ, Pearson MJ, et al. , Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid. Arthritis Rheumatol, 2015. 67(12): p. 3135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, and Wang Y, PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med, 2010. 207(9): p. 1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones JE, Causey CP, Knuckley B, Slack-Noyes JL, and Thompson PR, Protein arginine deiminase 4 (PAD4): Current understanding and future therapeutic potential. Curr Opin Drug Discov Devel, 2009. 12(5): p. 616–27. [PMC free article] [PubMed] [Google Scholar]
- 12.Blachere NE, Parveen S, Fak J, Frank MO, and Orange DE, Inflammatory but not apoptotic death of granulocytes citrullinates fibrinogen. Arthritis Res Ther, 2015. 17(1): p. 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raijmakers R, van Beers JJ, El-Azzouny M, Visser NF, Bozic B, Pruijn GJ, et al. , Elevated levels of fibrinogen-derived endogenous citrullinated peptides in synovial fluid of rheumatoid arthritis patients. Arthritis Res Ther, 2012. 14(3): p. R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iobagiu C, Magyar A, Nogueira L, Cornillet M, Sebbag M, Arnaud J, et al. , The antigen specificity of the rheumatoid arthritis-associated ACPA directed to citrullinated fibrin is very closely restricted. J Autoimmun, 2011. 37(4): p. 263–72. [DOI] [PubMed] [Google Scholar]
- 15.Villeneuve E, Nam J, and Emery P, 2010 ACR-EULAR classification criteria for rheumatoid arthritis. Rev Bras Reumatol, 2010. 50(5): p. 481–3. [PubMed] [Google Scholar]
- 16.Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, et al. , Suppression of colitis in mice by Cl-amidine: a novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol, 2011. 300(6): p. G929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horikoshi N, Tachiwana H, Saito K, Osakabe A, Sato M, Yamada M, et al. , Structural and biochemical analyses of the human PAD4 variant encoded by a functional haplotype gene. Acta Crystallogr D Biol Crystallogr, 2011. 67(Pt 2): p. 112–8. [DOI] [PubMed] [Google Scholar]
- 18.Shevchenko A, Wilm M, Vorm O, and Mann M, Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem, 1996. 68(5): p. 850–8. [DOI] [PubMed] [Google Scholar]
- 19.Shevchenko A, Wilm M, Vorm O, and Mann M, Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels. Analytical Chemistry, 1996. 68: p. 850–858. [DOI] [PubMed] [Google Scholar]
- 20.Peterson AC, Russell JD, Bailey DJ, Westphall MS, and Coon JJ, Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics. Mol Cell Proteomics, 2012. 11(11): p. 1475–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kall L, Canterbury JD, Weston J, Noble WS, and MacCoss MJ, Semi-supervised learning for peptide identification from shotgun proteomics datasets. Nat Methods, 2007. 4(11): p. 923–5. [DOI] [PubMed] [Google Scholar]
- 22.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. , Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics, 2010. 26(7): p. 966–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, and van Venrooij WJ, Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest, 1998. 101(1): p. 273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budzynski AZ, Marder VJ, and Shainoff JR, Structure of plasmic degradation products of human fibrinogen. Fibrinopeptide and polypeptide chain analysis. J Biol Chem, 1974. 249(7): p. 2294–302. [PubMed] [Google Scholar]
- 25.Mjaavatten MD, van der Heijde D, Uhlig T, Haugen AJ, Nygaard H, Sidenvall G, et al. , The likelihood of persistent arthritis increases with the level of anti-citrullinated peptide antibody and immunoglobulin M rheumatoid factor: a longitudinal study of 376 patients with very early undifferentiated arthritis. Arthritis Res Ther, 2010. 12(3): p. R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermansson M, Artemenko K, Ossipova E, Eriksson H, Lengqvist J, Makrygiannakis D, et al. , MS analysis of rheumatoid arthritic synovial tissue identifies specific citrullination sites on fibrinogen. Proteomics Clin Appl, 2010. 4(5): p. 511–8. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Chen FF, Gao WB, Wang HY, Zhao NW, Xu M, et al. , Identification of citrullinated peptides in the synovial fluid of patients with rheumatoid arthritis using LC-MALDI-TOF/TOF. Clin Rheumatol, 2016. 35(9): p. 2185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X, Okeke NL, Sharpe O, Batliwalla FM, Lee AT, Ho PP, et al. , Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther, 2008. 10(4): p. R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.