Abstract
Purpose: Osteoarthritis is the single most common cause of disability in older adults with an estimated 10% to 15% prevalence in individuals above 60 years. The contemporary medications include nonsteroidal anti-inflammatory drugs acetaminophen, cyclooxygenase-2 inhibitors, and surgical interventions. In view of safety issues regarding their longterm use, necessitating search for effective and safe alternatives, we evaluated Capsule Longvida® Optimized Curcumin prepared using solid lipid curcumin particles (SLCP) technology in patients with knee osteoarthritis.
Patients and methods: Eligible patients fulfilling American College of Rheumatology Criteria were randomized to SLCP group (400 mg twice daily delivering 80 mg of curcumin per capsule) or Ibuprofen with placebo group (400 mg each once daily) for 90 days. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the Visual Analog Scale (VAS) were used for clinical assessment of knee pain and function. Degree of knee flexion and swelling were also noted. Blood biochemistry included hemogram, blood urea, creatinine, Random blood sugar and inflammatory markers viz. PGE2, TNF α, IL6, IL1β and LTB4 while urine examination included degenerative marker CTX II. The parametric data was analyzed using unpaired t test while non-parametric data was analyzed using Friedman’s test or Mann Whitney t test as applicable. A level of p<0.05 was considered as statistically significant.
Results: Out of 50 recruitments, 25 from the Ibuprofen group and 17 from the SLCP group completed the study with significant improvements in VAS and WOMAC scores indicating comparable efficacy of SLCP in alleviating pain with Ibuprofen. None of the markers displayed significant changes. Except one withdrawal in the study group due to rash and itching, the study drug was found safe.
Conclusions: SLCP in a dose of 160 mg daily was found to be effective and safe in alleviating symptoms in patients suffering from knee osteoarthritis when administered for 90 days.
Keywords: inflammation, Ibuprofen, solid lipid curcumin particles (SLCP), WOMAC score
Introduction
Osteoarthritis (OA) is estimated to have affected more than 100 million people globally and is one of the top five most disabling conditions affecting more than one third of persons above 65 years of age.1 OA of knee is a major cause of morbidity, physical limitation, and increased health care utilization,2 and it is a leading cause of disability in older adults.3 Since OA normally progresses with age, its economic burden may increase with the aging human population. It is now recognized that inflammation in joints affected by OA contributes to not only the development of symptoms, including pain and stiffness, but also the progression of structural damage, including cartilage degradation.2,3 The demand for arthritis pain control has resulted in the widespread use of palliative drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs), acetaminophen and cyclooxygenase-2 (COX-2) inhibitors.4 However, their long-term use is found to be associated with several safety concerns, such as gastrointestinal erosions with or without bleeding, cardiovascular risks and hypertension especially in older patients. This warrants need for effective yet safe options to treat this condition.
Curcuma longa (Turmeric) is a widely used Ayurvedic medicine for the management of various diseases such as inflammatory skin and joint diseases, diabetes, febrile illness etc.5 Curcumin is a polyphenol extracted from turmeric with a promising nutraceutical potential because of its anti-inflammatory and anti-oxidative functions.6 It has demonstrated inhibitory effect on substances involved in the inflammatory pathway, including lipoxygenase, cyclooxygenase (COX), phospholipase, collagenase, elastase, and hyaluronidase.7–10 Furthermore, it was found to inhibit the activation of free radical activated transcription factors such as nuclear factor kappa B and nitric oxide synthase.7 It has also shown reduction in the pro-inflammatory cytokines viz. tumor necrosis factor alpha, interleukin (IL)-1 beta, IL-8, and matrix metalloproteinase.7,11,12 Curcumin is proposed to be a potent inhibitor of the production of inflammatory and catabolic mediators by chondrocytes.13 It was demonstrated to be safe, even after high-dose ingestion of up to 8,000 mg/day for 3 months.14,15
However, in spite of its appreciated pharmacological effects and safety, poor bioavailability of curcumin has limited complete translation of its in vitro benefits into clinical conditions and its beneficial health effects, because only a small amount of curcumin is absorbed with oral administration. The major limiting factors of curcumin absorption are rapid glucuronidation/sulfation of curcumin’s phenolic hydroxyl groups and high “first pass” clearance, it’s instability in aqueous solution at pH 7 and above; and it is very hydrophobic and not water soluble at acidic pH and when delivered as a dry powder in existing supplements. Hence, it would be advantageous if a composition or delivery device which improves the stability, solubility and permeability of certain types of biologically active compounds in the gut after oral consumption, resulting in parent (native) compound levels that are therapeutic (as opposed to inactive metabolites such as glucuronides), could be formulated.
Several drug delivery strategies have been tested to improve the bioavailability of curcumin including solid dispersions, complexation with cyclodextrins, copolymeric micelles, polymeric nanoparticles, lipid-based nanoparticles, liposomes, and micro-emulsions but few have made commercial success and been able to deliver efficacious levels of free curcumin opposed to its inactive metabolites such as glucuronides. The Solid Lipid Curcumin Particle (SLCP) Technology patented as Capsule Longvida® encapsulates the free curcumin in a tri-lipid matrix, enhancing its solubility, allowing it to survive digestion and enter the bloodstream, target tissues.16,17
A recently carried out systematic review and meta-analysis of randomized clinical trials evaluating the efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis has highlighted the efficacy of turmeric extract (about 1000 mg/day of curcumin) in the treatment of arthritis, but has suggested the need for more rigorous clinical studies.18
With this background, the present study was planned to evaluate the efficacy and safety of SLCP (160 mg/day optimized curcumin) in patients with knee osteoarthritis using potent biomarkers for efficacy monitoring.
Material and methods
Ethical considerations
The patients were screened at Kayachikitsa (General Medicine) Out Patient Department of Bharati Ayurved Hospital, Bharati Vidyapeeth Deemed University, Pune over a period of 11 months (November 2015 to October 2016) after obtaining ethics approval from Bharati Vidyapeeth Deemed University College of Ayurveda Ethics Committee- (BVDUCOA/EC/127/15-16). The study was performed in compliance with the Helsinki Declaration and ICH-GCP guidelines.
Eligibility criteria
Patients of either sex between 40 and 65 years of age, suffering from osteoarthritis of the knee joint based on American College of Rheumatology (ACR) criteria like knee pain, stiffness/crepitus and osteophytes in X-ray and those who were willing to comply with the study protocol were recruited in the study. Written informed consent was obtained from all patients before participation in the study.
Patients with any other joint pathology except osteoarthritis (eg congenital arthropathy, rheumatoid arthritis, active gout, other types of arthritis with/without inflammation like fibromyalgia or collagen vascular disease) were excluded from the study. Patients with known history of coagulopathies, uncontrolled diabetes and/or hypertension, severe cardiac, renal or hepatic disease, trauma/surgery on knee joint, BMI>40 kg/m2, osteoarthritis of other joints except knee were also not considered eligible. The patients who had participated in any other clinical study within 30 days’ prior enrolment into the study were not included. Pregnant and lactating women were also not considered eligible to participate in the study.
After thorough physical and systemic examination, the study coordinator administered the study interventions to the patients as per the study number assigned to them.
Sample size
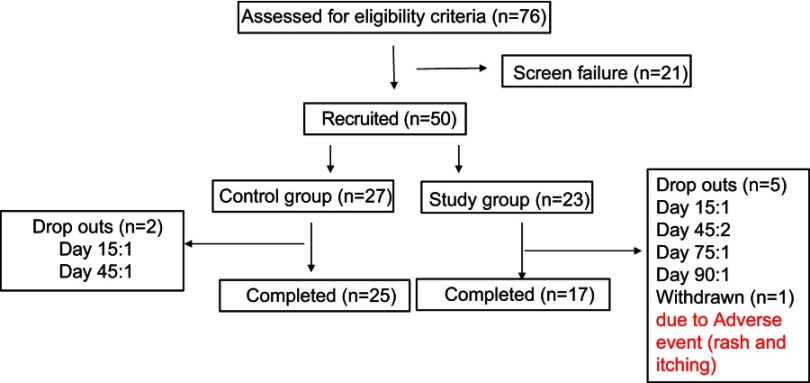
Since this was a pilot study, sample size calculation was not done. A sample of 42 completed patients, 25 in the control group and 17 in the study group was considered for study analysis. There were two dropouts in the control group and five in the study group.
Study interventions
The study group received SLCP in the form of capsules twice daily (80 mg/capsule) post meals with water for a period of 90 days. Each capsule is a 400 mg patented lipophilic matrix delivering 80 mg curcumin, made with SLCP™ Technology. Longvida® is a trademark of Verdure Sciences, Noblesville, IN, USA.
The control group received Capsule Ibuprofen (400 mg) once in the morning followed by Placebo (dextrin) in the evening post meals for the same duration of 90 days.
All the study interventions viz. curcumin, ibuprofen and placebo were similar in color and size. The sponsor packaged them in the containers as per the randomization list and sent them to the study site with only the number of the patient, printed on the label. The randomization code was provided to the Principal Investigator, which was opened only after completion of the study.
Outcome measures
At baseline, demographic details and baseline health characteristics such as age, sex, Body Mass Index (BMI), duration of pain, radiological findings and concomitant diseases were recorded.
During the course of treatment, the patients were asked to report at the study site after every 15 days. Only the second follow-up visit was scheduled after 7 days post recruitment. The Western Ontario and McMaster Universities’ Arthritis Index (WOMAC) was calculated at baseline and days 30, 60, and 90. Patients’ perception about pain was documented on the Visual Analogue Scale (VAS) at baseline, days 7, 15, 45, 75, and 90. Besides VAS and WOMAC assessment, other signs and symptoms such as crepitus, swelling, rise in temperature (indicating acute inflammation) and degree of flexion (using goniometer) were also noted.
From every patient, approximately 10 ml blood was collected at baseline and then on days 7, 45, and 90. Both, the inflammatory markers (viz. PGE2, TNF-α, LTB4, IL-6 and IL-1β) and other blood investigations (viz. hemogram, ESR, serum urea, creatinine, and random blood sugar) were carried out at baseline and on day 90. In addition, the inflammatory markers were evaluated on day 7 to assess if the study drug reduces the inflammatory markers faster than the comparator, while the other blood parameters were estimated on day 45 as well to assess safety of the study drug.
Fresh morning urine samples were collected at baseline, day 45 and day 90 for routine examination, while C-Terminal telopeptides of type II Collagen (U-CTX-II), a degenerative marker was estimated from urine only on day 0 and 90. The inflammatory markers and U-CTX-II were analyzed using the ELISA method.
The primary outcome measure was reduction in pain while the secondary measure was change in the inflammatory and degenerative markers.
Statistical analysis
The data was analyzed Per Protocol. The parametric data was presented as mean ± standard deviation (SD). The control group was compared with the study group using unpaired t test. The non-parametric data was presented as median (range). The baseline values were compared with follow up-values using Friedman’s test while the control group was compared with the study group using the Mann Whitney t test. In the case of biomarkers, the percentage change from baseline in each group was also calculated to compare treatment effects. A sub-group analysis of patients was also carried out according to presence of concomitant conditions, different age and BMI groups and a comparison of the efficacy of curcumin with ibuprofen. A level of p<0.05 was considered as statistically significant.
Results
A total of 50 patients were recruited in the study of which 42 completed the study. There were a total of seven drop outs throughout the study, two patients from the control group dropped out due to no improvement in the pain. Out of five drop outs in the study group, three patients were lost to follow-up, while two patients complained of heartburn and nausea after taking the drug. One patient was withdrawn from the study group since the patient complained of rash and itching all over the body after two doses of drug intake (Figure 1).
Figure 1.
Patients' flow in the study.
The demographic details and baseline health characteristics of the completed patients are shown in Table 1.
Table 1.
Demographics and health characteristics
| Control group | Study group | p-value | |
|---|---|---|---|
| Age (years) | 54±8 | 57±7.5 | |
| 41–45 | 6 (24%) | 1 (6%) | 0.3012 |
| 46–50 | 3 (12%) | 1 (6%) | |
| 51–55 | 3 (12%) | 4 (23.5%) | |
| 56–60 | 3 (12%) | 5 (29.4%) | |
| 61–65 | 10 (40%) | 6 (35%) | |
| Sex (M: F) | 2 (8%): 23 (92%) | 6 (35%): 11 (64.7%) | 0.0270 |
| BMI (kg/m2) | 30.45±5.09 | 28.22±5.81 | |
| Normal | 3 (12%) | 8 (47%) | 0.0375 |
| Overweight | 9 (36%) | 3 (17.64%) | |
| Obese | 13 (52%) | 6 (35.29%) | |
| Concomitant diseases (Present: Absent) | 5 (20%):20 (80%) | 9 (52.9%):8 (47%) | 0.0262 |
| Duration (years)* | |||
| <1 | 3 (8%) | 1 (7.14%) | 0.1880 |
| 1–5 | 19 (76%) | 12 (70.58%) | |
| 6–10 | 2 (8%) | 3 (17.64%) | |
| >11 | 1(4%) | 1 (7.14%) | |
| Severity of disease | |||
| Early | 4 (16%) | 2 (11.76%) | 0.2480 |
| Mild | 4 (16%) | 3 (17.64%) | |
| Moderate | 12 (48%) | 4 23.52%) | |
| Severe | 5 (20%) | 8 (47.05%) |
Notes: All qualitative variables have been expressed as number (percentages), while quantitative variables have been expressed as Mean ± SD.
It was observed that both the groups were comparable in terms of age and duration of years. However, there were significantly more females in the control group. The number of normal weight patients and patients with concomitant diseases was significantly higher in the study group.
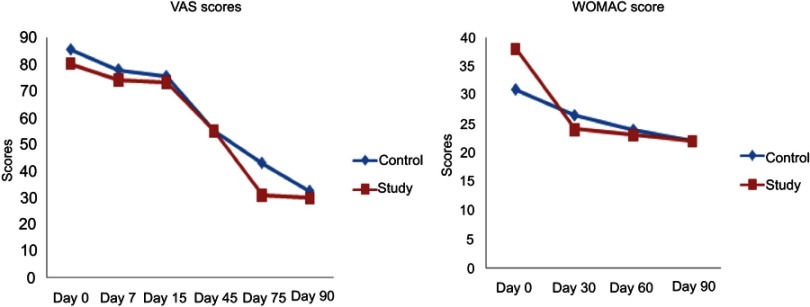
The WOMAC and VAS score showed a gradual decrease in both the groups. There was significant improvement in VAS scores in both the groups from day 45 onwards. In the case of WOMAC scores also a gradual decrease was seen. The decrease was statistically significant as compared to baseline scores in both the groups from day 60. There was no significant difference between both the groups (Figure 2).
Figure 2.
Effect of SLCP on clinical pain scores.
Abbreviations: VAS, visual analog scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; SLCP, solid lipid curcumin particles.
There was no difference in both the groups in terms of other signs and symptoms such as crepitus, swelling, and rise in temperature. Also, the degree of flexion showed no difference in the pre- and post-treatment stages in either of the groups.
The inflammatory markers showed a decrease in the control group, while in the study group there was an increase in the markers. These changes were however not statistically significant as compared to baseline. There was also no difference between the groups (Table 2).
Table 2.
Effect of SLCP on inflammatory markers (pg/ml)
| Inflammatory markers | Groups | Day 0 | Day 7 | Day 90 |
|---|---|---|---|---|
| PGE2 | Control | 4.27 (0.88–6.37)* | 3.98 (0.76–8.69) | 3.12 (0.05–8.69) |
| Study | 3.20 (0.15–7.85) | 3.92 (1.60–7.12) | 4.12 (0.58–6.75) | |
| LTB4 | Control | 6.24 (0.06–6.44) | 5.98 (0.07–6.44) | 5.55 (0.07–6.44) |
| Study | 6.04 (0.09–6.44) | 5.85 (0.08–6.40) | 5.97 (0.06–6.45) | |
| IL-6 | Control | 11.16 (2.25–74.13) | 8.43 (1.0–27.03) | 8.18 (0.50–25.29) |
| Study | 7.44 (1.75–46.36) | 10.17 (2.48–61.24) | 10.91 (5.20–148.26) | |
| IL-1β | Control | 12.33 (3.67–42) | 13 (3.67–20.67) | 11.67 (3.67–33) |
| Study | 13.67 (11.40–30) | 14.67 (10.50–29.10) | 15.30 (0–28.20) | |
| TNF-α | Control | 60 (39.09–107.27) | 58.18 (35.45–100.91) | 50 (34.55–108.18) |
| Study | 63.64 (32.73–208.18) | 64.55 (31.82–177.27) | 66.36 (30.91–212.73) |
Notes: Values expressed as Median (Range).
*p<0.05 as compared to Day 0 of group treated with SLCP.
Abbreviation: SLCP, solid lipid curcumin particles.
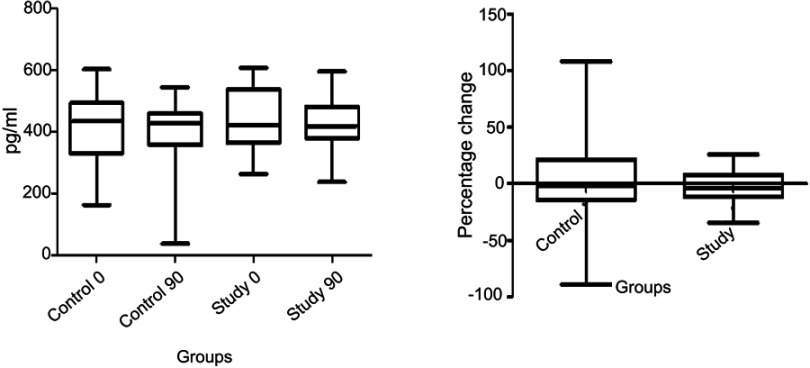
The levels of urine CTX-II were maintained in both the groups with no significant increase or decrease within or between the groups. The percentage change in the levels was less in the study group as compared to the control group though the difference was not significant statistically (Figure 3).
Figure 3.
Effect of SLCP on CTX-II.
Abbreviation: SLCP, solid lipid curcumin particles.
There were no serious adverse events reported in both the groups. In study group, two patients reported heartburn and nausea after taking the medicine. Both these patients dropped out from the study. One patient from the study group was withdrawn due to rash and itching all over the body after two doses of drug intake. The drug was de-challenged and re-challenged in this patient, but the symptoms reappeared. No significant changes in the blood and urine parameters were seen.
Discussion
The present study showed that curcumin significantly decreased the VAS and WOMAC as compared to baseline from day 45 onwards. This effect was comparable to the low dose of ibuprofen that was used in the present study. In an earlier study, Curcumin Phosphatidylcholine Complex (CPC) has shown better disease control, a decreased use of NSAIDs and an overall improvement in Quality of Life of the OA patients. However, this was an add-on study where CPC was used along with NSAIDs in the study group and was compared with only NSAIDs as control group.19 Therefore, the efficacy of SLCP as a standalone drug for OA was not completely established in this study. Our study was an ambitious effort to compare the efficacy of SLCP vs NSAID.
The study and control groups were comparable in terms of age and disease duration but not with respect to sex, BMI and concomitant disease profile, probably because they were not considered as criteria for inclusion. The drop outs were apparently higher in the study group, but it was primarily due to inability of patients to report for follow-up at the study site on the scheduled dates. The sub-group analysis for efficacy revealed no difference between the groups. In the study group, of the 23 patients recruited, only one patient was withdrawn due to skin rash and itching, while two patients reported adverse events. These reactions cannot be explained on the basis of the available literature on curcumin and therefore it can be no more than an idiosyncratic effect. Apart from this, there was no change in hemogram and the biochemical markers indicating safety of the study drug. We could not replace the dropped out or withdrawn patients due to time and budgetary constraints.
Of various biomarkers available for OA,20 we selected the ones reflecting cartilage degeneration (U-CTX-II) along with the markers for synovial or systemic inflammation and associated pain. U-CTX-II is commonly used as a surrogate outcome in clinical trials on OA as it performs consistently.21 Interestingly U-CTX-II did not show any deterioration, rather maintained levels in both the groups. When the percentage change was calculated, the levels in study group showed less dispersion or in other words more consistent results. These findings suggest that though the study drug could not prevent cartilage destruction completely, it may have some effects on joint tissue metabolism.
Of the inflammatory markers, PGE2 and LTB4 represent lipoxygenase (LOX) and cyclo-oxygenase (COX) pathways in membrane arachidonic acid metabolism.22 Both these markers did not show positive correlation with clinical scores (WOMAC and VAS scores) indicating the possibility of other degenerative pathologies like meniscal tear contributing to the pain. However, we did not have serial radiographic or MRI-detected structural abnormalities findings to substantiate these pathologies.
Amongst other markers, IL-6 has been shown to inhibit the production of type II collagen in animal models. IL-1β is another important pro-inflammatory cytokine that suppresses type II collagen and aggrecan synthesis, the key constituents of cartilage. In animal models, IL-1β is reported to play an important role in pain sensitivity and severity of joint inflammation. Furthermore, it induces production of a number of pro-inflammatory cytokines and chemokines including IL-6. TNF-α is produced predominantly by activated macrophages which affects the production of cytokines including IL-6 and IL-8. Soluble TNF receptors in serum samples from OA patients have been shown to have a positive correlation with pain, joint stiffness and higher radiographic severity of disease.23 We did not observe statistically significant changes in any of the inflammatory markers or the degenerative marker between the groups and within the groups.
One of the major drawbacks of our study was the use of low doses of both the drugs; SLCP and ibuprofen. Although curcumin is one of the most investigated natural products and is preferred for diverse inflammatory conditions including OA, there are hardly any reported clinical studies available. Since the bioavailability of curcumin is very poor, when administered orally, unrealistically high doses (>10 g/day) are required to achieve the plasma concentrations suggested by the preclinical studies.24 As an attempt to improve the bioavailability, in the present study free curcumin was encapsulated in a tri-lipid matrix. It was used in a dose (160 mg/day) comparable to doses used in earlier studies employing novel delivery systems of curcumin. To compare this dose of SLCP, we selected a lower dose of ibuprofen (400 mg/day) as well. It is interesting to note that, both the drugs reduced the pain though no effect was seen on biomarkers. However, this poses a question whether the symptom improvement has occurred spontaneously or due to the placebo effect. This needs to be further investigated in dose finding studies.
Conclusion
Our study has proven that there was improvement in symptoms in patients suffering from knee OA after administration of SLCP (~160 mg) which was not substantiated by inflammatory markers over 90 days. This improvement is comparable to sub-therapeutic doses of ibuprofen.
Acknowledgments
Authors acknowledge support from Director, IRSHA and Superintendent, Bharati Ayurved Hospital. This study has been funded by Pharmanza Herbals Pvt. Ltd. Dr Lal Lachhmandas Hingorani and Dr Amol Panjabrao Deshmukh are listed as co-authors in this study and they represent the sponsor Pharmanza Herbals Pvt. Ltd.
Ethics approval and consent to participate
The study was approved by Bharati Vidyapeeth Deemed University College of Ayurveda Ethics Committee (BVDUCOA-EC)/127 in the year 2015 and performed in compliance with the Helsinki Declaration and ICH-GCP guidelines. Trial registration number: CTRI/2015/07/006061 registered retrospectively. Registry- Clinical Trial Registry of India. Registered on July 31, 2015.
Abbreviations list
WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; VAS, Visual Analog Scale; OA, osteoarthritis; NSAIDs, nonsteroidal anti-inflammatory drugs; COX, cyclooxygenase; TNF α, tumor necrosis factor alpha; IL, interleukin; SLCP, solid lipid curcumin particle; ACR, American College of Rheumatology; BMI, body mass index; PGE2, prostaglandin E2; LTB4, leukotriene B4; CTX-II, C terminal telopeptides of type II collagen; LOX, lipoxygenase.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bhatia D, Bejarano T, Novo M. Current interventions in the management of knee osteoarthritis. J Pharm Bio-Allied Sci. 2013;5(1):30–38. doi: 10.4103/0975-7406.106561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samuels J, Krasnokutsky S, Abramson SB. Osteoarthritis: a tale of three tissues. Bull NYU Hosp Jt Dis. 2008;66(3):244–250. [PubMed] [Google Scholar]
- 3.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi: 10.1016/j.bone.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed S, Anuntiyo J, Malemud CJ, Haqqi TM. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evid Based Complement Alternat Med. 2005;2(3):301–308. doi: 10.1093/ecam/neh117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chunekar KC, Hota NP. Plants of Bhavprakash. Sharma SK, Ed. New Delhi: Director, Rashtriya Ayurved Vidyapeeth, National Academy of Ayurveda; 1999:107. [Google Scholar]
- 6.Nakagawa Y, Mukai S, Yamada S. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J Orthop Sci. 2014;19:933–939. doi: 10.1007/s0077-014-0633-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengmark S. Curcumin, an atoxic antioxidant and natural NF kappa B, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. J Parenter Enteral Nutr. 2006;30(1):45–51. doi: 10.1177/014860710603000145 [DOI] [PubMed] [Google Scholar]
- 8.Oyagbemi AA, Saba AB, Ibraheem AO. Curcumin: from food spice to cancer prevention. Asian Pac J Cancer Prev. 2009;10(6):963–967. [PubMed] [Google Scholar]
- 9.Khanna D, Sethi G, Ahn KS, et al. Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol. 2007;7(3):344–351. doi: 10.1016/j.coph.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Saja K, Babu MS, Karunagaran D, Sudhakaran PR. Anti-inflammatory effect of curcumin involves downregulation of MMP-9 in blood mononuclear cells. IntImmunopharmacol. 2007;7(13):1659–1667. doi: 10.1016/j.intimp.2007.08.018 [DOI] [PubMed] [Google Scholar]
- 11.Kim KH, Lee EN, Park JK, et al. Curcumin attenuates TNF-α-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother Res. 2012;26(7):1037–1047. doi: 10.1002/ptr.3694 [DOI] [PubMed] [Google Scholar]
- 12.Yang Q, Wu S, Mao X, Wang W, Tai H. Inhibition effect of curcumin on TNF-α and MMP-13 expression induced by advanced glycation end products in chondrocytes. Pharmacology. 2013;91(1–2):77–85. doi: 10.1159/000345345 [DOI] [PubMed] [Google Scholar]
- 13.Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58:899–908. doi: 10.1007/s00011-009-0063-1 [DOI] [PubMed] [Google Scholar]
- 14.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumuric (Curcuma longa). J Altern Complement Med. 2003;9(1):161–168. doi: 10.1089/107555303321223035 [DOI] [PubMed] [Google Scholar]
- 15.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 16.Nahar PP, Slitt AL, Seeram NP. Anti-inflammatory effects of novel standardized solid lipid Curcumin formulations. J Med Food. 2015;18(7):786–792. doi: 10.1089/jmf.2014.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gota VS, Maru GB, Soni TG, Gandhi TR, Kochar N, Agarwal MG. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J Agric Food Chem. 2010;58(4):2095–2099. doi: 10.1021/jf9024807 [DOI] [PubMed] [Google Scholar]
- 18.Daily JW, Yang M, Park S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J Med Food. 2016;19(8):717–729. doi: 10.1089/jmf.2016.3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belcaro G, Cesarone M, Dugall M, et al. Efficacy and safety of Meriva, a curcumin-phosphatidylcholine complex during extended administration in osteoarthritis patients. Altern Med Rev. 2010;15(4):337–344. [PubMed] [Google Scholar]
- 20.Jordan J, Kraus V. Biomarkers in osteoarthritis: a clinical trial perspective. Future Rheumatol. 2006;1(5):587–596. doi: 10.2217/17460816.1.5.587 [DOI] [Google Scholar]
- 21.Lotz M, Martel-Pelletier J, Christiansen C, et al. Value of biomarkers in osteoarthritis: current status and perspectives. Ann Rheum Dis. 2013;72(11):1756–1763. doi: 10.1136/annrheumdis-2013-203726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guipu L, Yuanqing F, Zheng J, Duo L. Anti-inflammatory activity and mechanism of a lipid extract from hard-shelled mussel (Mytilus coruscus) on chronic arthritis in rats. Mar Drugs. 2014;12(2):568–588. doi: 10.3390/md12020568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6(1):95–105. doi: 10.5312/wjo.v6.i1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belcaro G, Cesarone MR, Dugall M, et al. Efficacy and safety of Meriva®, a curcumin- phosphatidylcholine complex during extended administration in osteoarthritis patients. Alt Med Rev. 2010;15(4):337–344. [PubMed] [Google Scholar]