Abstract
Introduction:
Few data exist examining the effects of whole grain (WG) versus refined grain (RG) diets on glucose-stimulated insulin secretion (GSIS) and β-cell function.
Methods:
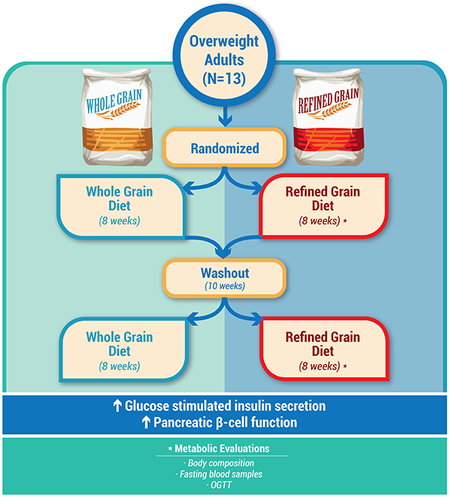
In a double-blind crossover randomized controlled trial, 13 sedentary prediabetic adults (37.2±1.8 y, BMI: 33.6±1.4 kg/m2, 2h glucose: 146.9±11.6 mg/dl) were provided isocaloric-matched WG and RG diets for 8-weeks each, with an 8–10 week washout between diets. Glucose, insulin and C-peptide metabolism was studied over 240 min following a 75g OGTT. Incretins (GLP-1 and GIP), PYY, and total ghrelin were assessed at 0, 30 and 60 min. Mixed-meal diets for carbohydrate (54%), fat (28%), and protein (18%) contained either WG (50g/1000 kcal) or equivalent RG.
Results:
Both diets induced fat loss (~2 kg). While neither diet impacted early phase GSIS, the WG diet increased total GSIS (iAUC of C-peptide0–240/Glc0–240, P=0.02) and β-cell function (disposition index; GSIS × insulin sensitivity, P=0.02). GIP and PYY were unaltered by either diet, but GLP-1 was higher at 30 min following RG versus WG (P=0.04). Ghrelin levels were higher at 60 min of the OGTT following both diet interventions (P=0.01).
Conclusion:
A WG-rich diet increases β-cell function independent of gut hormone responses in adults with prediabetes.
Keywords: alkylresorcinols, low-glycemic diet, glucose tolerance, obesity, insulin
Graphical Abstract
Whole grains are reported to reduce type 2 diabetes risk, but there are few randomized controlled trials and the mechanism is unclear. Herein, we show that whole grains increase pancreatic insulin secretion independent of weight/body fat loss or gut hormones (e.g., GLP-1, GIP, PYY and ghrelin). Together, these findings are clinically relevant since pancreatic function is the primary determinant of type 2 diabetes development.
INTRODUCTION
Nearly 35 million adults in the United States have prediabetes and are at increased risk for type 2 diabetes. Although insulin resistance is considered a key etiological factor in the deterioration of glucose homeostasis, failure to secrete adequate amounts of insulin causes hyperglycemia [1]. Subsequently, identifying strategies that prevent β-cell decompensation and/or improve insulin secretion are paramount to the management and prevention of type 2 diabetes.
Major health organizations, including the American Diabetes Association and the American Association of Clinical Endocrinologists, encourage replacing refined grain foods with whole-grain foods since whole-grains are associated with a lower incidence of type 2 diabetes [2–5]. However, the evidence that whole-grain consumption improves glycemic control in adults with predisposing factors for type 2 diabetes is mixed [6–12] and the majority of these studies have focused solely on insulin sensitivity as an etiological factor [9]. This is clinically problematic since glucose stimulated-insulin secretion (GSIS) corrected for the ambient insulin sensitivity (i.e. the disposition index or pancreatic function) is a better predictor of future diabetes development than insulin sensitivity alone [13]. In the few studies that have tested the effect of whole-grains on insulin secretion in clinical populations, the reports suggest that dietary carbohydrate modification with whole-grains enhance early phase insulin secretion using intravenous or oral glucose tolerance tests [10, 14]. Using this approach limits physiologic understanding of how whole-grains may modulate insulin secretion since no study has examined the total phase insulin secretion response to understand mechanistically if whole-grains impact release of insulin available in secretory granules during the first, or early phase of glucose stimulated secretion or the synthesis of new insulin in the β-cell during the late phase of secretion following post-prandial glucose ingestion. This is metabolically relevant as defects in early compared with later phase insulin secretion is linked to the development of diabetes [15]. Interestingly, the only prior work on this topic suggests that whole-kernel rye bread decreases glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) in healthy people [11]. The ability of more commonly consumed whole-grains such as wheat and rice to affect GLP-1 or GIP, or other gut hormone derived peptides (e.g., PYY or ghrelin) known to impact glucose homeostasis in obese individuals at risk for type 2 diabetes remains unclear. This knowledge gap is of great interest since we recently found that whole-grains improved blood pressure, maintained adiponectin concentrations, and improved insulin sensitivity while refined grain intake did not [16, 17]. Whether this insulin sensitizing benefit of whole-grains extends to insulin secretion remains unknown. Therefore, we tested the hypothesis that whole-grains would improve β-cell function and that gut hormone responses would correlate with the enhanced metabolic outcomes in a clinically relevant population of adults at risk for developing type 2 diabetes.
METHODS
Subjects and Design:
We performed a randomized, double-blind, controlled, crossover trial in thirty-three middle-aged, overweight/obese adults at risk for type 2 diabetes. Subjects included in the present report were part of a larger study examining dietary whole-grain regulation of body composition and CVD [16]. The first group of subjects recruited underwent more extensive testing for effects on glucose metabolism (n=14) [16, 17], and only subjects with available C-peptide data (n=13) were included for assessment of GSIS (Table 1). Randomization to diet treatment occurred prior to metabolic testing, and only the study dietitian and statistical consultant were aware of subject assignments. Subjects were included if < 50 y, weight stable (< 2 kg in prior 6 months), and physically inactive (< 60 min/wk) with a BMI 28–40 kg/m2. Women were pre-menopausal and studied during the mid-follicular phase (i.e., 5–10 d post menses). All subjects were non-smoking and underwent medical history as well as a physical examination that included a resting electrocardiogram. Blood and urine chemistry analysis was also conducted to exclude people with known type 2 diabetes, liver disease, cardiac dysfunction, pulmonary abnormalities and renal/liver complications or anti-diabetic medication use (e.g., metformin, GLP-1 agonists, etc.). Individuals were verbally briefed about the study and signed informed consent documents approved by the Cleveland Clinic Institutional Review Board (ClinicalTrials.gov registration # NCT01411540).
Table 1.
Effects of Whole-Grain and Refined-Grain Diets on body composition and glucose regulation.
| WG | RG | Paired t-Test | Paired t-Test | ANCOVA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ | Pre | Post | Δ | Pre vs. Pre | Post vs. Post | Δ vs. Δ | |
| Demographics | |||||||||
| n (M,F) | 13 (10F, 3M) | - | - | - | - | - | - | ||
| Age (years) | 37.2 ± 1.8 | - | - | - | - | - | |||
| Weight (kg) | 97.1 ± 4.1 | 94.6 ± 4.1* | −2.4 ± 0.7 | 95.4 ± 3.2 | 94.6 ± 4.1* | −2.3 ± 0.6 | 0.84 | 0.91 | 0.61 |
| BMI (kg/m2) | 33.6 ± 1.4 | 32.8 ± 1.3* | −0.9 ± 0.2 | 33.6 ± 0.9 | 32.9 ± 0.9* | −0.8 ± 0.2 | 0.88 | 0.93 | 0.72 |
| Fat Mass (kg) | 40.7 ± 2.7 | 38.8 ± 2.6* | −1.9 ± 0.5 | 40.9 ± 2.0 | 38.5 ± 1.9 | −2.4 ± 0.5 | 0.44 | 0.95 | 0.02 |
| Body Fat (%) | 43.1 ± 2.0 | 42.4 ± 2.1* | −0.6 ± 0.2 | 43.3 ± 1.6 | 42.8 ± 1.5* | −1.4 ± 0.3 | 0.26 | 0.90 | <0.01 |
| FFM (kg) | 56.4 ± 3.2 | 55.3 ± 3.2* | −1.1 ± 0.2† | 54.7 ± 2.6 | 54.5 ± 2.7 | −0.2 ± 0.3 | 0.12 | 0.31 | 0.05 |
| Glucose Metabolism | |||||||||
| FPG (mg/dl) | 93.0 ± 2.8 | 91.2 ± 2.1 | −2.4 ± 2.4 | 94.4 ± 2.6 | 92.4 ± 2.3 | −1.8 ± 1.2 | 0.15 | 0.46 | 0.85 |
| 2-hr PG (mg/dl) | 146.9 ± 11.6 | 148.8 ± 11.3 | 2.0 ± 6.2 | 150.1 ± 10.6 | 152.5 ± 9.8 | 2.5 ± 5.2 | 0.55 | 0.48 | 0.94 |
| PG iAUC (mg/dl*30min) | 975.0 ± 104.2 | 824.9 ± 112.0 | −150.1 ± 76.5 | 1028.5 ± 81.7 | 1009.8 ± 81.7 | −18.7 ± 64.1 | 0.43 | 0.05 | 0.20 |
| PG iAUC (mg/dl*240min) | 9980.3 ± 1394.7 | 9664.1 ± 1057.5 | −316.2 ± 666.1 | 9528.1 ± 1477.1 | 10688.8 ± 1275.8 | 1160.7 ± 798.2 | 0.51 | 0.06 | 0.08 |
| FPI (μU/ml) | 23.0 ± 2.7 | 15.9 ± 1.8 | −3.5 ± 2.2 | 21.6 ± 3.6 | 17.1 ± 2.7* | −4.5 ± 1.5 | 0.53 | 0.36 | 0.53 |
| 2-hr PI (μU/ml) | 150.9 ± 22.2 | 122.0 ± 20.8 | −31.7 ± 15.5† | 136.2 ± 20.3 | 164.5 ± 22.5 | 28.3 ± 12.1 | 0.44 | <0.01 | <0.01 |
| PI iAUC (μU/ml*30min) | 2128.9 ± 334.3 | 1796.9 ± 273.2* | −332 ± 151.5 | 2061.6 ± 274.9 | 2113.3 ± 248.9 | 51.7 ± 202.2 | 0.78 | 0.08 | 0.23 |
| PI iAUC (μU/ml*240min) | 18980 ± 2364.3 | 17982 ± 2567 | −997.5 ± 1025.2 | 18690 ± 2152.4 | 18980 ± 2364.3 | 1708.2 ± 1607.0 | 0.89 | 0.22 | 0.22 |
| FPCpep (ng/ml) | 1.7 ± 0.2 | 1.4 ± 0.2* | −0.3 ± 0.1 | 1.5 ± 0.2 | 1.3 ± 0.2 | −0.2 ± 0.2 | 0.13 | 0.36 | 0.67 |
| 2-hr PCpep (ng/ml) | 4.8 ± 0.3 | 4.5 ± 0.3 | −0.3 ± 0.1 | 4.7 ± 0.3 | 4.5 ± 0.3 | 0.0 ± 0.3 | 0.46 | 0.82 | 0.45 |
| PCpep iAUC (ng/ml*30min) | 37.5 ± 3.7 | 36.7 ± 4.7 | −0.8 ± 3.3 | 41.9 ± 7.7 | 37.5 ± 3.7 | 48.1 ± 7.3 | 0.64 | 0.38 | 0.28 |
| PCpep iAUC (ng/ml*240min) | 516.8 ± 50.4 | 631.0 ± 50.4* | 114.3 ± 32.1 | 515.4 ± 42.4 | 695.4 ± 65.9* | 180.0 ± 43.6 | 0.93 | 0.09 | 0.20 |
Data are reported as mean ± SEM. Compared to Pre,
P < 0.05 using Paired t-tests.
P < 0.05 using ANCOVA.
BMI = body mass index. FFM = fat-free mass. FPG = fasting plasma glucose. FPI = fasting plasma insulin. PI = plasma insulin. PCpep = plasma C-peptide. PG = plasma glucose. iAUC = incremental area under the curve.
Dosage Information/Dosage Regimen:
A registered dietitian designed and monitored the diets, which were based on individual needs (i.e. resting metabolic rate × 1.3 activity factor) as previously described [16]. The macronutrient composition of the diets was matched and consisted of 50 g per 1000 kcal of whole-grains or refined-grains, respectively. Subjects were provided the whole-grain or refined-grain diets for 8 weeks to consume by mouth with an 8–10 week washout period during which the participants resumed their usual diets. All meals and fluids were provided throughout the study, and recipes were identical between diets, with only frozen ready meals and breakfast cereals differing in the source of carbohydrate (whole grain or refined grain). Any visible and taste differences between WG and RG meals was masked by the use of dark colored sauces. The whole-grain diet included wheat (57%), rice (21%) and oats (16%), while the refined grains were wheat (73%) and rice (27%). A sample daily menu and the composition of the meals are reported in Supplementary Material Table 1. The dose of whole grains was targeted at about 100g per day to provide direct achievability by diet.
Diet Intervention:
The dose of our diet was monitored, as food compliance was estimated by weekly food container weigh backs, and defined as the difference between prescribed and actual caloric intake. Alkylresorcinols, a biomarker of whole-grain wheat and rye intake, were measured using liquid chromatography-tandem mass spectrometry to objectively confirm diet adherence [18, 19]. Diet analysis was performed using ESHA Food Processor Pro v.10.80 (Salem, OR).
Metabolic Control Period:
All metabolic testing was conducted during a 3-day inpatient stay at our Clinical Research Unit. Subjects were provided their study meals and refrained from strenuous physical activity, alcohol, and caffeine for 48-hour prior to metabolic testing.
Anthropometrics:
Weight was assessed on a digital platform with minimal clothing, and height was obtained via a wall-mounted stadiometer (Veeder-Root, Elizabethtown, NC). BMI was calculated as body mass (kg) divided by height (m)2. Dual-energy x-ray absorptiometry (DXA, Lunar Prodigy CORE Scan, Madison, WI) was used to assess total body fat and fat-free mass (FFM).
Insulin Secretion:
After an approximate 10-hour overnight fast, a 75 gram oral glucose tolerance test (OGTT) with a stable isotopic glucose tracer was administered to assess glucose metabolism and insulin sensitivity [17]. Plasma glucose, insulin and C-peptide were determined throughout the 240-minute OGTT. Glucose-stimulated insulin secretion (GSIS) was determined using plasma C-peptide incremental area under the curve (iAUC) divided by glucose (GLC) iAUC during the first 30-minutes (early phase) and 240-minutes (total phase) of the OGTT. iAUC during the OGTT was calculated using the trapezoidal method. The oral disposition index (DI) was used to determine β-cell function as previously described by our group [20, 21] since insulin secretory function varies according to the degree of insulin sensitivity. Early and total phase DI was defined as: GSISearly phase × insulin sensitivity and GSIStotal phase × insulin sensitivity. Insulin sensitivity was assessed from the rate of disappearance of deuterated glucose and insulin concentration as variables during the OGTT. Hepatic extraction was also estimated by dividing insulin AUC by C-peptide AUC during 0–30 and 0–240 minutes of the OGTT [22].
Biochemical Analysis:
Plasma glucose was measured immediately after collection using the glucose oxidase method (YSI 2300 STAT Plus, Yellow Springs, OH). All measurements pre- and post-intervention were analyzed on the same plate to minimize inter-assay variability. Samples for plasma insulin, C-peptide, PYY and ghrelin were collected in vacutainers containing EDTA and the protease inhibitor aprotonin, and samples were analyzed using a radioimmunoassay or ELISA (Millipore, Billerica, MA). GLP-1 and GIP were also collected in vacutainers containing EDTA, aprotonin and DPP-IV and analyzed by ELISA (Millipore, Billerica, MA). All blood samples were centrifuged at 1,000 rpm for 10 min at 4°C to separate plasma.
Statistical Analysis:
Data were analyzed using the statistical program R (Vienna, Austria, 2014). Skewed GSIS and pancreatic function data were log transformed for statistical analysis to meet normality requirements. Paired t-tests were used to compare baseline differences and post-test differences. Analysis of variance (ANOVA) with linear mixed-effects was used to compare differences (i.e. change from pre to post-test) between diets. We also conducted a 2-way ANOVA (i.e., group × test) at each time-point for gut hormone analysis. In the event of significant group differences, analysis of co-variance (ANCOVA) with linear mixed-effects was used to compare differences between diets using co-variates: order effect, period effect, age, sex, baseline levels of the respective outcome, and changes (i.e., delta) in body fat and dietary fiber. Pearson’s correlation was used to test associations between outcomes using delta-delta (i.e., whole-group) values. Significance was accepted as P<0.05 and trends are reported as P≤0.10. Data are reported as mean±SEM.
RESULTS
Diet:
Food compliance was excellent at 91.4±2.2 vs. 90.5±2.2% of target intake for whole-grain and refined-grain diet, respectively (P=0.73). There was no difference between whole-grain and refined-grain diet periods for energy (2034±92 vs. 2016±94 kcal/d, P=0.77), total carbohydrate (54.5±0.7 vs. 53.8±0.7%, P=0.56), fat (28.4±0.3 vs. 28.5±0.4%, P=0.91), or protein (18.2±0.4 vs. 17.5±0.3%, P=0.17) intake. As expected, whole-grain (89.8±4.9 vs. 0±0 g/d, P<0.001) and dietary fiber consumption (27.6 ± 1.5 vs. 22.2±2.1 g/d, P<0.001) were higher during the whole-grain compared with refined-grain intervention. In addition, plasma alkylresorcinols were significantly higher after whole-grain compared with refined-grain intake (185.9±48.7 vs. 9.4±5.4 nM, P<0.002), independently confirming that all subjects were compliant.
Body Composition:
Both whole-grain and refined-grain interventions induced approximately 2 kg weight and fat loss (Table 1). Further, whole-grain intake was associated with approximately 1.1 kg loss of FFM compared with a 0.2 kg loss following refined-grains (between-effect; P=0.05).
Plasma Glucose, Insulin and C-peptide:
Whole-grain intake had no statistically significant effect on fasting plasma glucose, although 2-hr and iAUC for 240 min glucose tended to be lower when compared to the refined grain diet (P=0.08 and P=0.06, respectively). iAUC glucose for 30 min was also lower after treatment (P=0.05; Table 1). Moreover, whole-grain intake reduced 2-hour circulating insulin significantly more than refined-grains (between-effect; P<0.01; Table 1). However, while both diets increased iAUC C-peptide for 240 min compared with pre-test (P<0.05), the refined grain diet tended to have higher concentrations after treatment (P=0.09; Table 1).
Pancreatic Function:
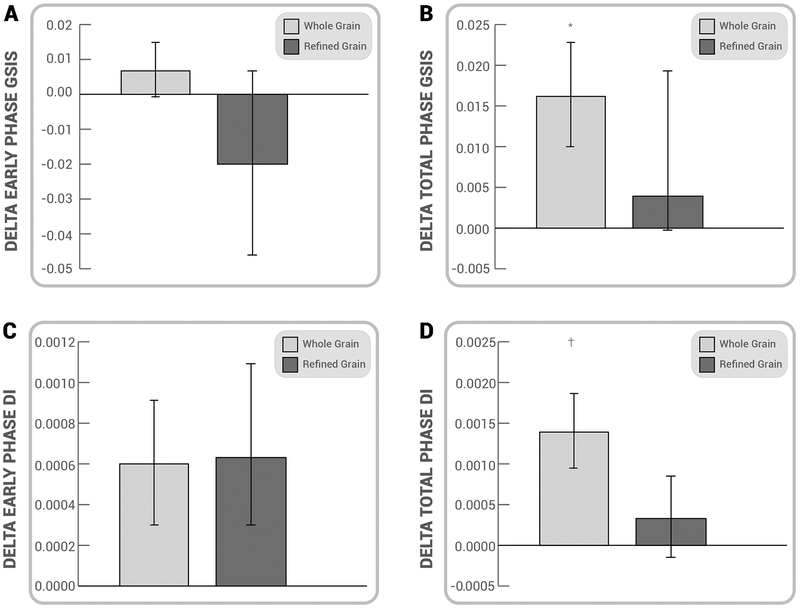
Early and total phase GSIS responses were not statistically different at baseline between whole-grain and refined grain diets (early phase: 0.04 ± 0.01 vs. 0.07 ± 0.03, P= 0.36 and 0.06 ± 0.01 vs. 0.07 ± 0.01, total phase P=0.24, respectively). The total, but not early phase, GSIS was increased after the whole-grain diet relative to baseline, although there was no between group difference (Figure 1). In addition, early and total phase β-cell function calculations were not statistically different at baseline between whole-grain and refined grain diets (early phase: 0.002 ± 0.001 vs. 0.002 ± 0.000, P=0.35 and total phase: 2 × 10−8 ± 1 × 10−8 vs. 3 × 10−8 ± 1 × 10−8 P=0.08, respectively). Whole-grains increased the total β-cell function response when compared with a decrease/maintenance following refined-grains (Figure 1). After the intervention, there was a trending association between improved β-cell function and the plasma glucose response (glucose iAUC for 240 min; r=-0.49, P=0.09). Neither whole-grain, nor refined grain interventions had an effect on hepatic insulin extraction in response to the OGTT after the intervention (early phase: 0.003 ± 0.003 vs. −0.005 ± 0.013 ng/ml/μU/ml-30 minute; between-effect; P=0.57, and total response 0.010 ± 0.005 vs. 0.007 ± 0.004 ng/ml/μU/ml-240 minute; between-effect; P=0.71).
Figure 1.
Effect of Whole-Grain and Refined-Grain Diets on Glucose-Stimulated Insulin Secretion. Data are reported as mean ± SEM. Compared to Pre, *P<0.05 using Paired t-tests. †P<0.05 using ANCOVA. Post-test total phase disposition index (DI) tended to be higher after WG compared with RG P=0.09 using Paired t-tests, although glucose-stimulated insulin secretion (GSIS) early phase did not (P=0.57). Whole-grain (WG); Refined grain (RG).
Gut Hormones:
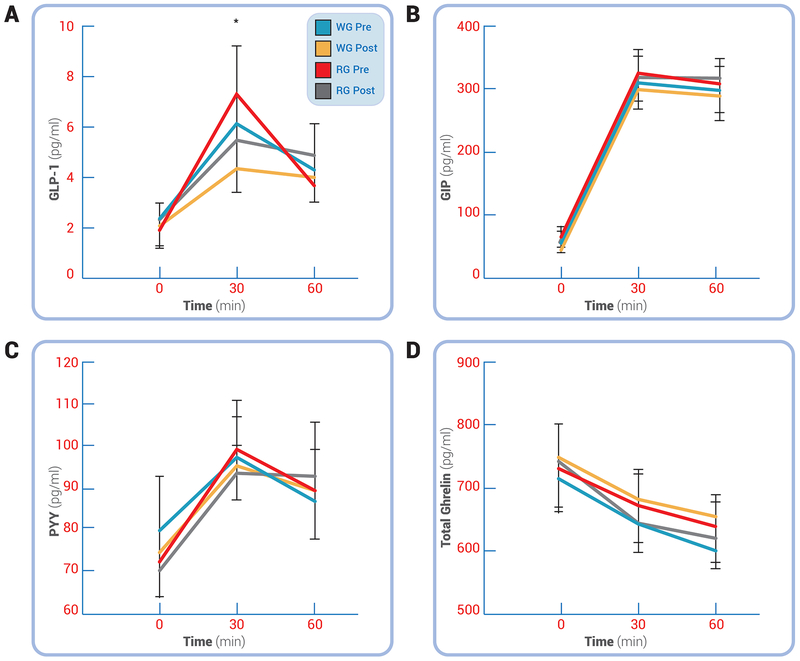
Whole-grain consumption had no significant effect on fasting GLP-1 when compared with refined-grains. However, refined-grains increased circulating GLP-1 at the 30-minute time-point of the OGTT more than was observed after the whole-grain diet (between-effect; P<0.05, Figure 2). Neither interventions affected fasting or post-prandial GIP or PYY (Figure 2), but both interventions resulted in significantly higher post-prandial ghrelin levels following the intervention (Figure 2, time effect; P<0.05).
Figure 2.
Effect of Whole-Grain and Refined-Grain Diets on Gut Hormones. Glucagon-like peptide 1 (GLP-1; A), Glucose-dependent insulinotropic polypeptide (GIP; B), peptide tyrosine-tyrosine (PYY; C) and total ghrelin (D) are shown. No post-test differences existed for any outcome using Paired t-tests, except for GLP-1 being higher after RG vs. WG (P=0.055). Data are reported as mean ± SEM. *P<0.05 for WG vs. RG, #P<0.05 for main effect of test. Whole-grain (WG); Refined grain (RG).
DISCUSSION
β-cell secretion is a biphasic process, whereby the initial, or early phase release is derived from a readily available pool of insulin that is stored in secretory granules, whereas the second phase depends on synthesis of new insulin granules [23]. Loss of early and total β-cell response is widely accepted as a primary defect in the development of type 2 diabetes [24] and appears to occur prior to any changes in insulin resistance. Treatments including exercise and/or caloric restriction that restore insulin secretion are thus highly relevant for the prevention and/or reversal of type 2 diabetes [21]. The primary finding in this report is that a whole-grain diet intervention significantly improved the total GSIS response in middle-aged overweight/obese adults at risk for type 2 diabetes, but refined grains did not. Importantly, this effect was maintained after correction for ambient insulin sensitivity and suggests that diets with a high proportion of whole-grains may prevent or attenuate a decline in pancreatic GSIS. Increasing whole-grain consumption may provide an effective dietary strategy to enhance β-cell function, and thus improve insulin secretion so as to compensate for insulin resistance. Such an approach could serve to prevent the progression to diabetes in an at-risk obese population with prediabetes. Our data extend current knowledge on the effects of whole-grains on pancreatic function, which to date have been limited to studies using whole-grain rye bread and effects on early phase insulin secretion alone using OGTT or intravenous glucose tolerance test [10, 11, 25]. To our knowledge, our data are the first to suggest that eating whole-grain forms of commonly consumed grains such as wheat, rice and oat, from commercially available meals, improves total phase GSIS and prevent declines in pancreatic function across the post-prandial state in a relatively young obese population who are at increased risk of developing type 2 diabetes.
There are several mechanisms by which whole-grains may increase GSIS. Whole-grain intake is reported to promote fat loss [26], which in turn would reduce blood lipids and/or inflammatory mediators that are known to impair β-cell insulin secretion [13, 20]. However, in our study both whole-grain and refined-grain interventions reduced body weight and fat loss, and despite our finding that refined grains induced a ~0.5 kg greater fat loss compared to whole-grains, the effect of whole-grain treatment on improving GSIS remained after co-varying for fat loss. While prior studies have suggested that whole-grains induce loss of fat mass when compared to refined grains [27, 28], other studies do not support such observations [29, 30]. As such, it remains unclear if whole-grains are a clinically relevant strategy for inducing weight loss and longer duration studies using more sensitive analytical techniques (e.g., doubly-labelled water or biopsies) to elucidate energetic mechanisms and changes in body composition are needed. Until these studies are completed, the data reported herein serve to suggest that the improvement in GSIS following whole-grain intake is independent of weight/fat loss and more likely related to the ability to synthesize new insulin during the postprandial state. Interestingly, dietary fiber has been shown to improve early phase insulin secretion in overweight adults [31]. Since fiber consumption was modestly higher during the whole-grain arm, it is reasonable to expect increased pancreatic function and improved glucose tolerance. Indeed, prior single meal or OGTT studies suggest that soluble fiber from whole-grains is important for glycemic control [32, 33]. While these prior studies [32, 33] fed barely-based meals rich in soluble fiber beta-glucan, we used primarily wheat and rice as the main grains in our study, which contain little beta-glucan. Oats were a minor component (16%) of the whole-grains on the whole-grain arm and intake and intake of beta-glucan would unlikely to be substantially different between diets. We noted no improvement in early phase GSIS, which is likely due to the small (8 g/d) difference in fiber intake we observed during the whole-grain arm. This compares to a difference of 30 g/d for earlier studies on dietary fiber [31]. To test the effect of this modest fiber difference on GSIS, we co-varied for changes in dietary fiber in our statistical model and confirmed that fiber did not contribute to the improved total GSIS response after the whole-grain intervention. This observation is supported by literature data showing that the addition of fiber (inulin) to rye porridge in another study did not lead to differences in post-prandial GLP-1 either [34], and fiber and weight-loss did not explain improvement in fasting glucose status in subjects with prediabetes following a controlled WG diet [12]. These studies further support the idea that fiber is unlikely to explain the improved GSIS results following increased whole-grain intake and suggests that other compounds or food structure likely explain the response.
Another possible explanation for the whole-grain induced improvement in GSIS may relate to gut hormones. Prior work suggests that carbohydrate modification impacts GLP-1, PYY, and GIP, as well as ghrelin [34, 35]. In particular, GLP-1 and GIP, commonly referred to as incretins, account for approximately 60% of the post-prandial insulin secretion response to nutrient ingestion. Further, high PYY is related to declines in metabolic health [36]. Since whole-grains increased the GSIS response, it would be reasonable to hypothesize that GLP-1, PYY and/or GIP would be elevated and correlate with this improved pancreatic insulin secretion and glucose tolerance. However, we observed that whole-grain intake had no effect on GLP-1 and PYY or GIP when compared with refined-grains. In contrast, we found an increase in GLP-1 following refined-grain intake. The physiologic and clinical relevance of this observation is unclear, as we found that the refined-grain diet resulted in no improvement in GSIS or glucose tolerance, and pancreatic function declined. Ghrelin, a known orexigenic stimulant, is an additional gut-derived hormone that has been shown to reduce GSIS [37]. We examined the effect of our intervention on ghrelin as a potential modulating factor contributing to the changes in GSIS. Ghrelin appeared to increase during the post-prandial period following both dietary interventions. These findings are consistent with prior weight loss studies demonstrating that ghrelin concentrations rise during periods of negative energy balance [38] and highlight that whole-grains exert effects on pancreatic function that are independent of ghrelin, PYY, GLP-1 and GIP. We note that we did detect a moderate, albeit non-statistically significant correlation between the improvement in post-prandial plasma glucose tolerance and pancreatic function. This association is consistent with the idea that glucotoxicity may be a factor related to changes in insulin secretion [23]. This suggests that improved glucose tolerance mediated by insulin sensitivity may be an important mechanism by which whole-grains improve glucose regulation [17]. However, the observation that GSIS improves, despite enhanced insulin sensitivity suggests a disconnect between the well-described hyperbolic relation and highlights a potential nutrient specific effect on distinct tissues. As a result, it remains possible that some other factors, e.g., the gut microbiota and/or fermentation of whole-grain carbohydrate, modified circulating factors that influenced pancreatic function. Indeed, gut-derived short-chain fatty acids could in theory be related to the oxidation of fat in the pancreas [39] as well as activation of PPARγ receptors in adipose tissue in a similar manner to thiazolidinediones [40]. Further work is needed to identify the mechanism(s) that underlie these effects.
This present study has some limitations. We used the OGTT as opposed to intravenous glucose methods (e.g. hyperglycemic clamp) to assess pancreatic function. Subsequently, it is possible that other factors directly or indirectly impacted insulin secretion. In addition, we measured gut hormones up to 60-minutes post-meal, and this may have underestimated the effect of our intervention on interpreting gut hormone effects. However, changes in gut hormones typically occur within the first 60-minutes after eating [34, 35, 41]. We also studied individuals with impaired glucose tolerance on average (i.e. 2-hr glucoses > 140 mg/dl). As a result, these findings may not translate to individuals with type 2 diabetes. There was also some imbalance in terms of sex, with mainly female subjects (77 %) which limits the generalizability of the findings and similar studies with larger sample sizes and better gender balance are needed to confirm these results. Furthermore, it is not possible from this study to discern whether compounds within whole-grains (e.g. vitamins, phenolic compounds, magnesium, and phytoestrogens), either in isolation or in combination, produced elevations in insulin secretion, and so future work is needed to determine the effects of grain type and food structure on insulin secretion [42]. Of interest is a study showing that purified alkylresorcinols, phenolic lipids present in high amounts in whole-grain wheat and rye reduced insulin resistance and leptin responses in mice fed a high in sucrose diet [43]. Thus, the increase in plasma alkylresorcinols during the whole-grain intervention may have also contributed to the improved insulin secretion. Lastly, we recognize that the probability of a type 1 error is 5% in each case when the null hypothesis is true, and thus these data are likely preliminary in nature. Even though we used a cross-over design, there may be a limitation in terms of sample size, and we may be underpowered to detect statistically significant findings for some outcomes. Herein, we include 0.80 power estimates at the significance level of 0.05 to inform future randomized trials examining whole grains vs. refined grains on GSIS (early phase: delta = 0.03, standard deviation (SD) = 0.09, n = 142; total phase: delta = 0.01, SD = 0.03, n = 142) and disposition index (early phase: delta = 0.001, SD = 0.003, n = 142; total phase: delta = 0.00000002, SD = 0.00000002, n = 17) to expand and confirm the current findings. We also report minimal detectable change scores at 95% for the delta of whole grain vs. refined grains on GSIS (early phase: 0.021; total phase: 0.017) and DI (early phase: 0.0008; total phase: 0.0012). Nonetheless, a strength of the current study is that all subjects served as their own control and ate a mixed-whole-grain diet based on commercial meals, meaning that these dietary changes are readily achievable and strengthen the clinical application of our findings. Moreover, C-peptides were used to assess pancreatic function. This bolsters the idea that whole-grains impact insulin secretion independent of hepatic insulin extraction.
In conclusion, whole-grain intake improved insulin secretion (total GSIS) and maintained pancreatic function in overweight/obese adults at risk for type 2 diabetes while the refined grain diet did not. Further, the improvement in insulin secretion tended to relate to circulating glucose, independent of body fat loss. This suggests that whole-grains may have the potential to exert beneficial effects on pancreatic function in obese adults at risk factor for diabetes. Since our findings were not paralleled by improvements in GLP-1, GIP, PYY or ghrelin, the effect of whole-grains on insulin secretion appears to be due to some other physiologic mechanism. Future work is warranted to address how whole-grains lead to better glucose control in obese adults in order to optimize nutritional therapies for preventing and/or delaying on the onset of type 2 diabetes and cardiovascular disease.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the CRU nursing staff, CORE lab, and participants for their outstanding efforts. In particular, we thank Jacob Haus and Julianne Filion for study coordination, Kay Stelmach for CRU support, Marianne Fischer, RD, for dietetic support, Velma Stephens and Brenda Foley-Murray for excellent organization of food distribution and biospecimen collection, Teresa Markle for outstanding biospecimen organization, Jeff Hammel for statistical advice, Ciarán Fealy for technical support, Isabelle Breton and Anne-France Kapp for biochemical analyses, and the staff of Nestlé PTC Solon and Cereal Partners Worldwide for providing the study meals and foods.
Funding: Investigator-initiated trial from Nestlé (JPK); NIH T32 DK007319; and NIH Research Resources Grant UL1RR024989
Footnotes
Trial Registration: NCT01411540
CONFLICTS OF INTEREST
JPG is employed by Nestlé, while the remaining authors report no conflict of interest.
REFERENCES
- 1.Kahn SE, Hull RL, Utzschneider K. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444(7121):840–6. [DOI] [PubMed] [Google Scholar]
- 2.Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr 2012;142(7):1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Q, Spiegelman D, van Dam RM, Holmes MD, Malik VS, Willett WC, Hu FB. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med 2010;170(11):961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, Nadolsky K, Pessah-Pollack R, Plodkowski R; Reviewers of the AACE/ACE ObesityClinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract 2016;22 Suppl 3:1–203. [DOI] [PubMed] [Google Scholar]
- 5.Standards of medical care in diabetes-2015: summary of revisions. Diabetes Care 2015;38 Suppl:S4. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MK, Koh Banerjee P, Franz M, Sampson L, Gronbaek M, Rimm EB. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation. Am J Clin Nutr 2006;83(2):275–83. [DOI] [PubMed] [Google Scholar]
- 7.Rave K, Roggen K, Dellweg S, Heise T, tom Dieck H. Improvement of insulin resistance after diet with a whole-grain based dietary product: results of a randomized, controlled cross-over study in obese subjects with elevated fasting blood glucose. Br J Nutr 2007;98(5):929–36. [DOI] [PubMed] [Google Scholar]
- 8.Andersson A, Tengblad S, Karlstrom B, Kamal-Eldin A, Landberg R, Basu S, Aman P, Vessby B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr 2007;137(6):1401–7. [DOI] [PubMed] [Google Scholar]
- 9.Pereira MA, Jacobs DR, Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr 2002;75(5):848–55. [DOI] [PubMed] [Google Scholar]
- 10.Laaksonen DE, Toppinen LK, Juntunen KS, Autio K, Liukkonen KH, Poutanen KS, Niskanen L, Mykkänen H. Dietary carbohydrate modification enhances insulin secretion in persons with the metabolic syndrome. Am J Clin Nutr 2005;82(6):1218–27. [DOI] [PubMed] [Google Scholar]
- 11.Juntunen KS, Laaksonen DE, Poutanen KS, Niskanen LK, Mykkänen H. High-fiber rye bread and insulin secretion and sensitivity in healthy postmenopausal women. Am J Clin Nutr 2003;77(2):385–91. [DOI] [PubMed] [Google Scholar]
- 12.Harris Jackson K, West SG, Vanden Heuvel JP, Jonnalagadda SS, Ross AB, Hill AM, Grieger JA, Lemieux SK, Kris-Etherton PM. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled-feeding trial. Am J Clin Nutr 2014;100(2):577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, Leonetti DL, McNeely MJ, Fujimoto WY, Kahn SE. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32(2):335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juntunen SK, Niskanen LK, Liukkonen KH, Poutanen KS, Holst JJ, Mykkänen HM. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am J Clin Nutr 2002;75(2):254–62. [DOI] [PubMed] [Google Scholar]
- 15.Kanat M, Mari A, Norton L, Winnier D, DeFronzo RA, Jenkinson C, Abdul-Ghani MA. Distinct beta-cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes 2012;61(2):447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirwan JP, Malin SK, Scelsi AR, Kullman EL, Navaneethan SD, Pagadala MR, Haus JM, Filion J, Godin JP, Kochhar S, Ross AB. A Whole-Grain Diet Reduces Cardiovascular Risk Factors in Overweight and Obese Adults: A Randomized Controlled Trial. J Nutr 2016;146(11):2244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malin SK, Kullman EL, Scelsi AR, Haus JM, Filion J, Pagadala MR, Godin JP, Kochhar S, Ross AB, Kirwan JP. Whole Grain Diet Reduces Peripheral Insulin Resistance and Improves Glucose Kinetics in Obese Adults: A Randomized Controlled Trial. Metabolism 2018; 82:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross AB, Svelander C, Savolainen OI, Lind MV, Kirwan JP, Breton I, Godin JP, Sandberg AS. A high-throughput method for liquid chromatography-tandem mass spectrometry determination of plasma alkylresorcinols, biomarkers of whole grain wheat and rye intake. Anal Biochem 2016;499:1–7. [DOI] [PubMed] [Google Scholar]
- 19.Ross AB. Present status and perspectives on the use of alkylresorcinols as biomarkers of wholegrain wheat and rye intake. J Nutr Metab 2012;2012:462967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malin SK, Finnegan S, Fealy CE, Filion J, Rocco MB, Kirwan JP. β-Cell Dysfunction Is Associated with Metabolic Syndrome Severity in Adults. Metab Syndr Relat Disorder 2014; 12(2):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malin SK, Solomon TPJ, Blaszczak A, Finnegan S, Filion J, Kirwan JP. Pancreatic beta cell function increases in a linear dose-response manner following exercise training in adults with prediabetes. Am J Physiol Endocrinol Metab 2013; 305(10):E1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry RR, Brechtel G, Griver K. Secretion and hepatic extraction of insulin after weight loss in obese noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1988;66(5):979–86. [DOI] [PubMed] [Google Scholar]
- 23.Cobelli C, Toffolo G, Dalla Man C, Campioni M, Denti P, Caumo A, Butler P, Rizza R. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 2007;293(1):E1–E15. [DOI] [PubMed] [Google Scholar]
- 24.Kanat M, Norton L, Winnier D, Jenkinson C, DeFronzo RA, Abudl-Ghani MA. Impaired early- but not late-phase insulin secretion in subjects with impaired fasting glucose. Acta Diabetol 2011;48(3):209–17. [DOI] [PubMed] [Google Scholar]
- 25.Juntunen KS, Niskanen LK, Liukkonen KH, Poutanen SK, Holst JJ, Mykkänen HM. Postprandial glucose, insulin, and incretin responses to grain products in healthy subjects. Am J Clin Nutr 2002;75(2):254–62. [DOI] [PubMed] [Google Scholar]
- 26.Karl JP, Meydani M, Barnett JB, Vanegas SM, Goldin B, Kane A, Rasmussen H, Saltzman E, Vangay P, Knights D Chen CO, Das SK, Jonnalagadda SS, Meydani SN, Roberts SB. Substituting whole grains for refined grains in a 6-wk randomized trial favorably affects energy-balance metrics in healthy men and postmenopausal women. Am J Clin Nutr 2017;105(3):589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, Kris-Etherton PM. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr 2008;87(1):79–90. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, Bugel S, Tetens I, Astrup A. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr 2012;142(4):710–6. [DOI] [PubMed] [Google Scholar]
- 29.Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr 2013;98(4):872–84. [DOI] [PubMed] [Google Scholar]
- 30.Harris Jackson K, West SG, Vanden Heuvel JP, Jonnalagadda SS, Ross AB, Hill AM, Grieger JA, Lemieux SK, Kris-Etherton PM. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled-feeding trial. Am J Clin Nutr 2014;100(2):577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bodinham CL, Smith L, Wright J, Frost G, Robertson MD. Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS ONE 2012;7(7):e40834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Priebe M, Wang H, Weening D, Schepers M, Preston T, Vonk RJ. Factors related to colonic fermentation of nondigestible carbohydrates of a previous evening meal increase tissue glucose uptake and moderate glucose-associated inflammation. Am J Clin Nutr 2010;91(1):90–7. [DOI] [PubMed] [Google Scholar]
- 33.Thorburn A Muir J, Proietto J. Carbohydrate fermentation decreases hepatic glucose output in healthy subjects. Metabolism 1993;42(6):780–5. [DOI] [PubMed] [Google Scholar]
- 34.Lee I, Shi L, Webb DL, Hellstrom PM, Riserus U, Landberg R. Effects of whole-grain rye porridge with added inulin and wheat gluten on appetite, gut fermentation and postprandial glucose metabolism: a randomised, cross-over, breakfast study. Br J Nutr 2016;116(12):2139–49. [DOI] [PubMed] [Google Scholar]
- 35.Hartvigsen ML, Gregersen S, Laerke HN, Holst JJ, Bach Knudsen KE, Hermansen K. Effects of concentrated arabinoxylan and beta-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: a randomized study. Eur J Clin Nutr 2014;68(1):84–90. [DOI] [PubMed] [Google Scholar]
- 36.Ukkola OH, Puurunen VP, Piira OP, Niva JT, Lepojarvi ES, Tulppo MP, Huikuri HV. High serum fasting peptide YY (3–36) is associated with obesity-associated insulin resistance and type 2 diabetes. Regul Pept 2011;170(1–3):38–42. [DOI] [PubMed] [Google Scholar]
- 37.Tong J, Prigeon R, Davis H, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010;59(9):2145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings D, Weigle D, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002;346(21):1623–30. [DOI] [PubMed] [Google Scholar]
- 39.Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 2010;1801(11):1175–83. [DOI] [PubMed] [Google Scholar]
- 40.Campbell IW. The Clinical Significance of PPAR Gamma Agonism. Curr Mol Med 2005;5(3):349–63. [DOI] [PubMed] [Google Scholar]
- 41.Falkn Y, Hellstrm PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab 2011;96(7):2227–35. [DOI] [PubMed] [Google Scholar]
- 42.Kärkkäinen O, Lankinen MA, Vitale M, Jokkala J, Leppänen J, Koistinen V, Lehtonen M, Giacco R, Rosa-Sibakov N, Micard V, Rivellese AAA, Schwab U, Mykkänen H, Uusitupa M, Kolehmainen M, Riccardi G, Poutanen K, Auriola S, Hanhineva K. Diets rich in whole grains increase betainized compounds associated with glucose metabolism. Am J Clin Nutr. 2018. 1;108(5):971–979. [DOI] [PubMed] [Google Scholar]
- 43.Oishi K, Yamamoto S, Itoh N, Nakao R, Yasumoto Y, Tanaka K, Kikuchi Y, Fukudome S, Okita K, Takano-Ishiawa Y. Wheat alkylresorcinols suppress high-fat, high-sucrose diet-induced obesity and glucose intolerance by increasing insulin sensitivity and cholesterol excretion in male mice. J Nutr 2015;145(2):199–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.