Abstract
Pentalogy of Cantrell is a constellation of five congenital defects that pose a unique challenge for surgeons. Defects of the heart, pericardium, diaphragm, sternum, and anterior abdominal wall are pathognomonic. Although the incidence of this defect is small, it is critical to identify it in a timely fashion in order to adequately address all aspects. Early diagnosis, supportive care, and strategic surgical planning with a multidisciplinary team are all key components in managing patients with Pentalogy of Cantrell. In this text we sought to explore the evolution of both the understanding and treatment for this complex entity and provide current recommendations to today’s pediatric caregivers.
Keywords: Pentalogy of Cantrell, Ectopia cordis, Omphalocoele, Congenital Diaphragmatic Hernia, Ventral wall defect, Sternal defect
History of the Disease
When James R. Cantrell defined this pentalogy in 1958 there had been 21 case reports, including five of his own, of patients with a specific constellation of congenital abnormalities without a unifying disease process or title (1). Although case reports of patients suspected of having this pattern date back to the early 1700’s, Cantrell formally grouped all five defects for the first time in his article “A Syndrome of Congenital Defects Involving the Abdominal Wall, Sternum, Diaphragm, Pericardium, and Heart” (1). In that article, Cantrell described the components of the pentalogy, postulated its embryologic development, and proposed recommendations for patient management. Obviously, advanced technologies were not available to him, and as a result prenatal diagnosis, management, and delivery considerations were not included in the original description. He did make a point of noting that in his series, none of the patients’ mothers reported a known family history of congenital disease or experienced complications or exposures during pregnancy, raising questions as to the etiology (1).
While much has changed since 1958 in terms of advancements in surgical and neonatal care, some of Dr. Cantrell’s principles for surgical repair hold true. He stressed the importance of repairing an omphalocoele that was not covered. He also was a proponent of early surgical intervention to repair as many of the five components as possible, with the exception of the intracardiac defects which were too difficult to diagnose or treat in neonates at that time. He endorsed a primary repair in all diaphragmatic hernias and used relaxing incisions in the anterior rectus fascia in order to accomplish primary abdominal wall closures.
Since this landmark paper half a century ago, major advancements in diagnostics, neonatology, and surgical techniques have been made allowing better preparation and skills to treat patients with Pentalogy of Cantrell. Unfortunately, the diagnosis still carries with it significant morbidity and mortality, and each new case poses a unique challenge for neonatologist and surgeon.
Defining Pentalogy of Cantrell
Formally, the Pentalogy of Cantrell is defined as a collection of defects to the midline abdominal wall, lower sternum, anterior diaphragm, diaphragmatic pericardium, and some form of intra-cardiac defect (1). The type of cardiac defect may vary with the most commonly reported being a ventricular septal defect, followed by atrial septal defect, Tetralogy of Fallot, and pulmonary stenosis (2). Very mild sternal cleft to complete thoracoabdominal ectopia cordis is also often present as a result of the ventral midline defects. Ectopia cordis, which is defined as extra-thoracic location of the heart, is one of the most severe Pentalogy of Cantrell cardiac malformations but is not required for diagnosis. Since the pentalogy was established in 1958, varying degrees of severity or penetrance of the disease have been documented in dozens of case reports. Several years later, Toyama devised a classification system in order to better define the disease that remains relevant today (3). He divided patients into three groups: certain, probable, or incomplete pentalogy. Patients were considered certain if they possessed all five defects and probable if they had four defects including an intra-cardiac abnormality and ventral wall defect. The third group of patients classified as the incomplete group, often lacked the intra-cardiac defect or one or more of the remaining defects that prevented classification as certain or probable (3). More recent patient series have demonstrated a similar stratification in severity or penetrance (4).
Recent studies have stressed that strict classification is not as important as a thorough description and understanding of the congenital abnormalities in utero. For example, Coleman et al looked at patients with Pentalogy of Cantrell, OEIS (omphalocoele, exstrophy, imperforate anus, spina bifida), and LBWC (limb-body wall complex) as a spectrum of diseases resulting from improper closure of lateral and craniocaudal folds (5). There are many overlapping features between these three syndromes, and in their cases series, they concluded that the degree of pulmonary hypoplasia was far more indicative of prognosis than any specific classification system would have been (5). In counseling families and planning for delivery and early management, a descriptive diagnosis is more valuable than trying to line characteristics up with a specific syndrome.
Embryology
Based on what was known about embryology at the time, Cantrell suspected that the cause of the resulting defects was two-fold (1). First, he suspected a defect in development of the septum transversum and its immediately adjacent somatic and splanchnic mesoderm, which are ultimately responsible for forming the anterior diaphragm, inferior pericardium, and cardiac structures, respectively. Second, he believed that the primordial sternum failed to migrate and fuse fully, resulting in improper attachment of the anterior abdominal musculature leading to the abdominal wall defects. He hypothesized that the offending event in development most likely occurred between 14 and 18 days of embryonic life (1). In spite of the significant advances in embryology over the last 60 years, these concepts have remained the predominant theory to explain the development of Pentalogy of Cantrell (6). More recently, researchers have found that the defect in development may originate slightly earlier than Cantrell postulated, but the main principles remain the same (6). Additionally, it has been proposed that the wide spectrum of this disease may be a reflection of the time point that the defect in development took place.
Genetics
While there is no discreet genetic mutation that has been deemed responsible for Pentalogy of Cantrell, many associations with genetic abnormalities have been identified. Multiple cases of Pentalogy of Cantrell have been associated with chromosomal abnormalities such as Trisomy 21, Trisomy 18, or Turner’s Syndrome (6). While there have been case reports of siblings with the disease, suggesting a familial inheritance, the majority of cases remain sporadic. There have been three cases in which concurrence of Pentalogy of Cantrell and Goltz-Gorlin syndrome occurred (7). Goltz-Gorlin syndrome has been linked to an X-linked PORCN mutation in almost all identified cases, suggesting that Pentalogy of Cantrell may also be a result of this mutation. PORCN or Porcupine is responsible for mediating Wnt ligand acetylation. Further investigation of this association by Snowball et al found that mesenchymal midline migration required during development was heavily dependent on the Wnt/β-Catenin pathway. Specifically, they found that knockout of Wntlss, which mediates the release of Wnt ligands from cells, resulted in a failure of mesenchymal cell migration in vitro, and midline defects of the sternum in vivo. (8). An additional genetic mutation that has been identified in a patient with Pentalogy of Cantrell is the duplication of ALDH1A2, which encodes an enzyme required for Vitamin A metabolism into trans-retinoic acid. Retinoic acid has been demonstrated to play a critical role in both normal cardiac development and diaphragm development, both key components in the pentalogy (9). While specific genetic aberrations have been identified that could be responsible for the full constellation of defects, a common genetic variant shared by all patients remains to be found.
Epidemiology
Pentalogy of Cantrell remains a rare congenital anomaly with approximately 250 reported cases to date (10). Most documented cases occurred in the United States and Europe (72%) (10). The incidence is about 1 in 65,000 to 200,000 live births with a male predominance of 1.35:1 (10, 11).
Pre-Natal Diagnosis
Prenatal diagnosis of Pentalogy of Cantrell may be made using prenatal 2D ultrasound as early as the first trimester, particularly when a large omphalocoele or an ectopia cordis are present, but is more commonly diagnosed during the second trimester. In cases where large defects are not obviously present, transient pericardial effusion may aid in the diagnosis (11). If a developmental defect is suspected, 3D ultrasound provides a more detailed anatomic survey. Fetal MRI is often obtained to confirm the diagnosis, and if pentalogy is suspected, fetal echocardiography is extremely important to determine the presence of intra-cardiac abnormalities (11). Because Pentalogy of Cantrell has been associated with aneuploidy in several cases, chromosomal analysis is also important at this stage in order to better inform and counsel families. Prenatal diagnosis provides families the opportunity to make informed decisions regarding continuation versus termination of a pregnancy. In addition, in select cases it may allow for prenatal intervention.
Post Natal Evaluation
The presence of an omphalocoele is immediately apparent after birth in most cases of Pentalogy of Cantrell. The combination of the heart defect, cleft sternum, and anterior abdominal wall defect gives the appearance of a visible and palpable pulsatile mass below the skin of the lower chest and upper abdomen on physical exam. The detection of a murmur on auscultation is dependent on the presence of an intra-cardiac lesion. Patients frequently present with dyspnea and cyanosis as well as lung infections.
Plain chest X-ray may demonstrate cardiac dextrorotation or displacement in cases of ectopia cordis. Postnatal echocardiography is essential for the diagnosis of intra-cardiac anomalies and will confirm the prenatal diagnosis. Common cardiac findings as originally described by Cantrell include: ventricular septal defect (100%), atrial septal defect (53%), left ventricular diverticulum (20%), pulmonary stenosis or atresia (33%), and tetralogy of Fallot (20%) (1). Since that original report, it has been determined that although ventricular septal defect is the most common cardiac finding, it is not universally present (72%) (12). Other cardiac malformations that may be found include: dextrocardia, patent ductus arteriosus, hypoplastic left heart syndrome, transposition of the great arteries, and left-sided superior vena cava (13). Echocardiography has certain limitations in complex heart malformations and accurate anatomic and physiologic evaluation of cardiac and respiratory function pre-operatively usually requires cardiac catheterization (14). In some cases, the precise diagnosis is not made until during the surgical intervention.
Thoracoabdominal computed tomography (CT) or CT-angiography are helpful tools and may provide additional information regarding the intra-cardiac defects (15). Multidetector computerized tomography (MCDT) scanning is also useful for detecting intra-cardiac defects and has the advantages of being faster than magnetic resonance imaging (MRI) as well as avoiding the need for anesthesia and ECG-gating acquisition (16). The use of cardiac MRI is advocated especially in cases of a left ventricular diverticulum, defined as partial ectopia cordis and characterized by a muscular or fibrous fingerlike appendix of the ventricular wall beyond the myocardial margin, where early diagnosis is important to avoid impending complications (17, 18).
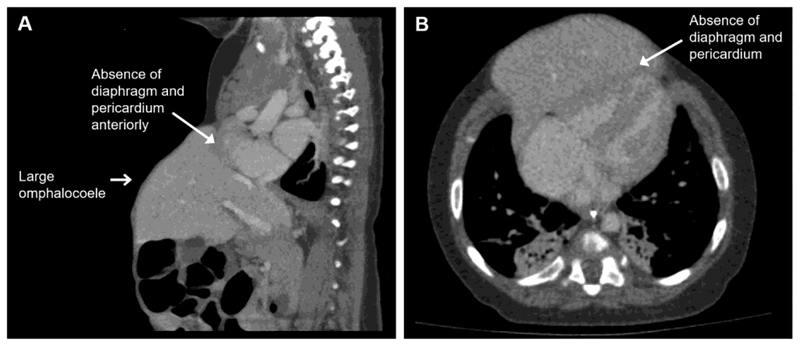
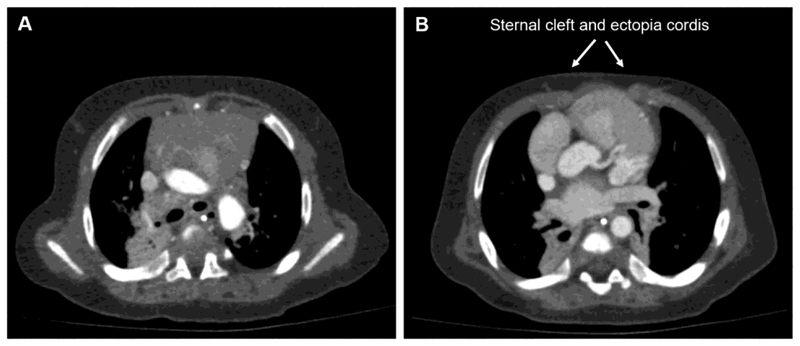
In addition to evaluation of the heart, CT provides anatomic definition of the pericardium, diaphragm and abdominal viscera. Absence of the diaphragm and pericardium may be evident on CT scan (figure 1). CT may also reveal a sternal cleft and ectopia cordis (figure 2). MRI may be useful in cases with small defects of the diaphragm and pericardium that can be otherwise difficult to diagnose (19).
Fig. 1.
(A) Sagittal CT scan of a patient with Pentalogy of Cantrell demonstrates a large omphalocoele (open arrow) accompanied by the loss of diaphragm and diaphragmatic pericardium anteriorly (closed arrow). These are three key features of the disease. (B) An axial view showing the loss of diaphragm and pericardium between the heart and liver.
Fig. 2.
Axial CT angiography of the thorax showing (A) the sternum is present at the level of the aortic arch but (B) is absent inferiorly allowing cardiac protrusion and mild ectopia cordis.
Initial Management and Surgical Repair
The management of patients with Pentalogy of Cantrell necessitates the coordination of a number of subspecialties who should be involved from the prenatal diagnosis until completion of the surgical repair, including neonatal resuscitation, temporary coverage of the defects, palliative or corrective intra-cardiac repair along with thoraco-abdominal reconstruction.
Early surgical intervention may be a risk factor for mortality, and stable neonatal patients may benefit from initial conservative management including prophylactic antibiotics and daily dressing changes to allow epithelialization of the omphalocoele sac (12). Critically ill neonates require full life support and timely surgical intervention and should be managed by a multi- disciplinary team in the neonatal intensive care unit.
Corrective operations for Pentalogy of Cantrell remain a challenge due to the wide spectrum and complexity of the anomalies and the associated high mortality. Surgical repair aims at (1) correcting cardiac malformations, (2) restoring cardiac position and anatomy, and (3) repairing the thoraco-abdominal wall and diaphragmatic defects. The procedures and surgical approaches depend upon the severity of cardiovascular malformation, the cardiopulmonary function, the presence and severity of ectopia cordis, the size of the abdominal wall defect, and the patient’s ability to tolerate the operation. The decision of operative repair should be made after a comprehensive evaluation and the best treatment strategy is well-planned and uniquely individualized to achieve both structural integrity and hemodynamic stability.
Both multi-stage as well as single stage surgical repairs have been described and adopted in complete and incomplete syndromes of Pentalogy of Cantrell (20–25). The basic principle in unstable patients is to focus on saving the child’s life and thus a multi-stage operation may be preferable. According to Saxena et al. staged repair may significantly reduce postoperative respiratory insufficiency, ventilator dependency, and even decrease mortality (26). Although different opinions exist regarding which anomaly should be repaired first in Pentalogy of Cantrell, intra-cardiac anomalies are given priority and typically corrected first to prevent cardiac trauma and since the fragile heart function may be further affected by the thoraco-abdominal reconstruction (27). Subsequently, thoraco-abdominal wall defects are repaired. On the other hand, a single stage operation is possible and advisable in patients with noncomplex intra-cardiac anomalies and if the heart position can be successfully restored without hemodynamic changes (23).
Cardiac and pericardial defects vary and are repaired accordingly. In the event of a ventricular diverticulum, surgical repair is indicated early in life because of the risks of thrombogenic events, spontaneous or traumatic rupture, and sudden death by tachyarrhythmias (18, 28). Some stable intra-cardiac defects, such as an atrial septal defect or a ventricular septal defect may be repaired initially as well. In cases of hemodynamically significant defects with failure to thrive, or tetralogy with severe pulmonary stenosis, palliative procedures are discussed with complete repair planned for later in life. Reduction of the heart into the small thoracic cavity is attempted and care should be taken to avoid compression and kinking of the great vessels resulting in a global reduction in cardiac output and a possible fatal compromise (29). Orthotopic heart transplantation has not been suggested as a viable option for the initial surgical management of ectopia cordis because of the need of immunosuppression in patients with associated chest and abdominal wall defects necessitating several staged repairs (30).
Many options for repair of the sternal cleft are available. Closure of the sternum with direct approximation of the sternal halves was initially described by Maier and Bortone and would be best accomplished in the neonatal period if the defect is very small and repair would not result in cardiac compromise (31). Other techniques that have been described include the use of rotational myocutaneous flaps, which provide only soft tissue coverage of the heart, as well as synthetic materials (artificial struts, and titanium plates) which may limit the growth potential of the chest wall and create a nidus for infection (32–34). The use of autologous tissue such as free rib grafts, costal cartilage grafts, costochondral cartilage rotation, and biocompatible material is thus preferable for chest wall reconstruction (23, 29, 35). Harvesting, placing, and suturing free rib grafts can be avoided by rotating the tissue as a split-thickness cartilage graft instead, as described by Grudziak (36). Regardless of the technique, an optimal sternal repair provides rigid cardiac coverage without compression, avoids the use of synthetic material, and allows for subsequent chest wall expansion and growth.
Abdominal wall defects are repaired by mobilization and gradually stretching of the abdominal muscles with the priority being to preserve intestinal blood flow and achieve a low- tension midline closure. Residual defects that cannot be closed primarily may be covered by the placement of a biocompatible or polytetrafluoroethylene (Gore-Tex) patch (37). The application of a sclerosing solution to promote formation of an amniotic sac eschar has been described in the conservative management of omphalocoeles, with plans to repair the resulting abdominal wall hernia at a later date (14).
Avoiding high postoperative intra-abdominal and intrathoracic pressures after repair of the abdominal wall and sternal defects is crucial. Elevated pressures may lead to cardiac decompensation. Although not routinely measured, the Pediatric Sub-Committee of the World Society of the Abdominal Compartment Syndrome recommended the use of intra-abdominal pressure (IAP) monitoring in the critically-ill patient (38). Divkovic et al suggested that complication of increased IAP such as ventilator dependency, reherniation, and gut ischemia may be prevented by IAP monitoring in the neonatal intensive care unit (37). Clinical studies investigating a critical IAP level in neonates need to be undertaken and may guide further recommendations.
Short and Long-Term Outcomes
Survival rate for Pentalogy of Cantrell remains as low as 37%, with few patients surviving through their early days of life (12). The prognosis depends primarily on the type and severity of associated malformations and intra-cardiac anomalies, but also on the location of the ectopic heart. Complete Cantrell syndrome (as classified by Toyama) and significant ectopia cordis are associated with a more dismal prognosis and carry a higher mortality as opposed to probable and incomplete syndromes without heart defects (15, 39, 40). Very few patients with Pentalogy of Cantrell and significant ectopia cordis survive surgical repair, and the main causes of death include tachyarrhythmias, bradycardia, hypotension, rupture of the diverticulum, and heart failure (29, 30). Outcomes are relatively more favorable with partial ectopia cordis and incomplete presentation (14, 23). In a review of 22 cases by Balderrábano-Saucedo, 13 had total ectopia cordis, 11 of whom died despite various forms of treatment. In contrast, nearly half of those without ectopia cordis survived the operation (14). Analysis of 153 patients with Cantrell’s syndrome reported a surgical mortality of 52% (12). However, the survival rate has improved to 61% with considerable advances in pediatric surgery and neonatal intensive care. Mean age at which patients underwent surgical operation was shown to be a predictor of survival, with older age associated with better prognosis; all non-survivors had surgery on the first postnatal day, whereas mean age of operation of survivors was 9 months (12, 28).
Postoperative care of children with Pentalogy of Cantrell is often complicated by respiratory insufficiency and the prolonged need for ventilator support (41). Other cardiac postoperative complications include residual shunt and low cardiac output syndrome/cardiac insufficiency. Late mortality is usually due to a complication of cardiac dysfunction, infections, or adhesive small bowel obstruction (37).
Current Trends and Implications for Practice
Patients with the diagnosis of Pentalogy of Cantrell should receive antenatal counseling relative to mortality and morbidity risks (40). There are no studies assessing the best mode of delivery in Pentalogy of Cantrell and elective termination of pregnancy may be offered in severe cases following prenatal diagnosis or when amniocentesis shows an abnormal karyotype. In cases of ectopia cordis amenable to surgical palliation or repair, plans for cesarean delivery are made to avoid prolonged cardiac compression and herniated viscera damage or rupture that may occur with vaginal delivery (28). Planning delivery at a tertiary care center, as opposed to transporting a critically ill infant, provides an optimal outcome for the newborn.
Advances in fetal noninvasive and invasive procedures will likely affect the therapeutic options available for patients with Pentalogy of Cantrell. Recently in Spain, in a case of monochorionic twin pregnancy complicated by Pentalogy of Cantrell, ultrasound guided bipolar coagulation of the cord allowed for selective termination of the affected fetus with preservation of the remaining fetus (42). The in utero pericardio-amniotic shunting described by Engels et al for a patient with a large pericardial effusion was the first procedure of its kind for a patient with this disease. The therapeutic draining of the effusion allowed for a suspected improvement in lung growth and development prior to delivery (43). However, aggressive prenatal interventions must be balanced with associated risks as well as informed post-natal expectations for prognosis. A recent study by Antiel et al suggested that pediatric surgeons, neonatologists and maternal- fetal-medicine specialists are divided on the indications for fetal surgery, and parent counseling can often be influenced by the physician’s demographic characteristics or personal beliefs (44).
Advances in therapy for patients with Pentalogy of Cantrell are dependent on a more thorough understanding of the disease process. Aldeiri et al recently developed a murine model in which genetic ablation of TGFβRII in Transgelin (Tagln) expressing cells resulted in midline defects such as diaphragmatic hernia, ventral hernia, and intra-cardiac defects, mimicking the abnormalities seen in the pentalogy. Development of an animal model provides a mechanistic understanding and is critically important as the rarity of the disease makes large-scale human studies impossible (45).
Summary
In summary, Pentalogy of Cantrell is a collection of defects with extreme variation in complexity and severity. This diversity necessitates a comprehensive, multi-disciplinary approach to diagnosis and treatment both in the prenatal and post-natal periods. Although the etiology of the disease is not fully understood, recent discoveries in animal models have begun to shed light on the developmental failures that lead to the pentalogy. Ultimately, the approach to treating these patients requires an individual and personalized approach for each patient based on their severity of disease and specific defects, which in combination with the rarity of the disease, poses a challenge in providing generalizable principles of care for patients with Pentalogy of Cantrell. By addressing the patient’s most life-threatening defect first, surgeons and neonatologists have gradually improved the prognosis for these patients over the last several years, but long-term outcomes reveal that the morbidity of Pentalogy of Cantrell remains substantial.
Acknowledgments
This work was funded, in part, by National Institutes of Health T32 CA183926 (APW) and T32 CA229102 (RM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All work was performed at the University of Alabama at Birmingham
References:
- 1.CANTRELL JR, HALLER JA, RAVITCH MM. A syndrome of congenital defects involving the abdominal wall, sternum, diaphragm, pericardium, and heart. Surg Gynecol Obstet. 1958;107(5):602–14. [PubMed] [Google Scholar]
- 2.Kaouthar H, Jihen A, Faten J, et al. Cardiac anomalies in Cantrell’s pentalogy: From ventricular diverticulum to complete thoracic ectopia cordis. Cardiol Tunis. 2013;9(1):94–7 [PMC free article] [PubMed] [Google Scholar]
- 3.Toyama WM. Combined congenital defects of the anterior abdominal wall, sternum, diaphragm, pericardium, and heart: a case report and review of the syndrome. Pediatrics. 1972;50(5):778–92 [PubMed] [Google Scholar]
- 4.Kaul B, Sheikh F, Zamora IJ, et al. 5, 4, 3, 2, 1: embryologic variants of pentalogy of Cantrell. J Surg Res. 2015;199(1):141–8 [DOI] [PubMed] [Google Scholar]
- 5.Coleman PW, Marine MB, Weida JN, et al. Fetal MRI in the Identification of a Fetal Ventral Wall Defect Spectrum. AJP Rep. 2018;8(4):e264–e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandran S, Ari D. Pentalogy of cantrell: an extremely rare congenital anomaly. J Clin Neonatol. 2013;2(2):95–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smigiel R, Jakubiak A, Lombardi MP, et al. Co-occurrence of severe Goltz-Gorlin syndrome and pentalogy of Cantrell - Case report and review of the literature. Am J Med Genet A. 2011;155A(5):1102–5 [DOI] [PubMed] [Google Scholar]
- 8.Snowball J, Ambalavanan M, Cornett B, et al. Mesenchymal Wnt signaling promotes formation of sternum and thoracic body wall. Dev Biol. 2015;401(2):264–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steiner MB, Vengoechea J, Collins RT. Duplication of the ALDH1A2 gene in association with pentalogy of Cantrell: a case report. J Med Case Rep. 2013;7:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jnah AJ, Newberry DM, England A. Pentalogy of Cantrell: Case Report With Review of the Literature. Adv Neonatal Care. 2015;15(4):261–8 [DOI] [PubMed] [Google Scholar]
- 11.Ergenoğlu MA, Yeniel A, Peker N, et al. Prenatal diagnosis of Cantrell pentalogy in first trimester screening: case report and review of literature. J Turk Ger Gynecol Assoc. 2012;13(2):145–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vazquez-Jimenez JF, Muehler EG, Daebritz S, et al. Cantrell’s syndrome: a challenge to the surgeon. Ann Thorac Surg. 1998;65(4):1178–85 [DOI] [PubMed] [Google Scholar]
- 13.Wheeler DS, St Louis JD. Pentalogy of Cantrell associated with hypoplastic left heart syndrome. Pediatr Cardiol. 2007;28(4):311–3 [DOI] [PubMed] [Google Scholar]
- 14.Balderrábano-Saucedo N, Vizcaíno-Alarcón A, Sandoval-Serrano E, et al. Pentalogy of Cantrell: Forty-two Years of Experience in the Hospital Infantil de Mexico Federico Gomez. World J Pediatr Congenit Heart Surg. 2011;2(2):211–8 [DOI] [PubMed] [Google Scholar]
- 15.Singh N, Bera ML, Sachdev MS, et al. Pentalogy of Cantrell with left ventricular diverticulum: a case report and review of literature. Congenit Heart Dis. 2010;5(5):454–7 [DOI] [PubMed] [Google Scholar]
- 16.Santiago-Herrera R, Ramirez-Carmona R, Criales-Vera S, et al. Ectopia cordis with tetralogy of Fallot in an infant with pentalogy of Cantrell: high-pitch MDCT exam. Pediatr Radiol. 2011;41(7):925–9 [DOI] [PubMed] [Google Scholar]
- 17.Romagnoli A, Ricci A, Morosetti D, et al. Congenital left ventricular diverticulum: Multimodality imaging evaluation and literature review. J Saudi Heart Assoc. 2015;27(1):61–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zani-Ruttenstock E, Zani A, Honjo O, et al. Pentalogy of Cantrell: Is Echocardiography Sufficient in the Neonatal Period? European J Pediatr Surg Rep. 2017;5(1):e9–e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oka T, Shiraishi I, Iwasaki N, et al. Usefulness of helical CT angiography and MRI in the diagnosis and treatment of pentalogy of Cantrell. J Pediatr. 2003;142(1):84. [DOI] [PubMed] [Google Scholar]
- 20.Sakasai Y, Thang BQ, Kanemoto S, et al. Staged repair of pentalogy of Cantrell with ectopia cordis and ventricular septal defect. J Card Surg. 2012;27(3):390–2 [DOI] [PubMed] [Google Scholar]
- 21.Yuan SM, Shinfeld A, Mishaly D. An incomplete pentalogy of Cantrell. Chang Gung Med J. 2008;31(3):309–13 [PubMed] [Google Scholar]
- 22.Abdallah HI, Marks LA, Balsara RK, et al. Staged repair of pentalogy of Cantrell with tetralogy of Fallot. Ann Thorac Surg. 1993;56(4):979–80 [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Xing Q, Sun J, et al. Surgical treatment and outcomes of pentalogy of Cantrell in eight patients. J Pediatr Surg. 2014;49(8):1335–40 [DOI] [PubMed] [Google Scholar]
- 24.Usha MK, Mahimaiha J, Shivanna DN. Incomplete pentalogy of Cantrell: single stage repair. Heart. 2012;98(15):1183. [DOI] [PubMed] [Google Scholar]
- 25.Korver AM, Haas F, Freund MW, et al. Pentalogy of Cantrell : successful early correction. Pediatr Cardiol. 2008;29(1):146–9 [DOI] [PubMed] [Google Scholar]
- 26.Saxena AK, van Tuil C. Delayed three-stage closure of giant omphalocele using pericard patch. Hernia. 2008;12(2):201–3 [DOI] [PubMed] [Google Scholar]
- 27.Harrison MR, Filly RA, Stanger P, et al. Prenatal diagnosis and management of omphalocele and ectopia cordis. J Pediatr Surg. 1982;17(1):64–6 [DOI] [PubMed] [Google Scholar]
- 28.Engum SA. Embryology, sternal clefts, ectopia cordis, and Cantrell’s pentalogy. Semin Pediatr Surg. 2008;17(3):154–60 [DOI] [PubMed] [Google Scholar]
- 29.Morales JM, Patel SG, Duff JA, et al. Ectopia cordis and other midline defects. Ann Thorac Surg. 2000;70(1):111–4 [DOI] [PubMed] [Google Scholar]
- 30.Diaz JH. Perioperative management of neonatal ectopia cordis: report of three cases. Anesth Analg. 1992;75(5):833–7 [DOI] [PubMed] [Google Scholar]
- 31.Maier HC, Bortone F. Complete failure of sternal fusion with herniation of pericardium; report of a case corrected surgically in infancy. J Thorac Surg. 1949;18(6):851–9 [PubMed] [Google Scholar]
- 32.Stephenson JT, Song K, Avansino JR, et al. Novel titanium constructs for chest wall reconstruction in children. J Pediatr Surg. 2011;46(5):1005–10 [DOI] [PubMed] [Google Scholar]
- 33.Hazari A, Mercer NS, Pawade A, et al. Superior sternal cleft: construction with a titanium plate. Plast Reconstr Surg. 1998;101(1):167–70 [DOI] [PubMed] [Google Scholar]
- 34.Lampert JA, Harmaty M, Thompson EC, et al. Chest wall reconstruction in thoracoabdominal ectopia cordis: using the pedicled osteomuscular latissimus dorsi composite flap. Ann Plast Surg. 2010;65(5):485–9 [DOI] [PubMed] [Google Scholar]
- 35.Kim CW, Cho HM, Son BS, et al. Neo-sternum reconstruction using costal cartilage approximation and small Permacol patch repair in the treatment of Cantrell pentalogy: a case report. J Cardiothorac Surg. 2015;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grudziak J, Kogon B. Split-thickness cartilage grafts for chest wall reconstruction in pentalogy of Cantrell. Congenit Heart Dis. 2013;8(1):62–5 [DOI] [PubMed] [Google Scholar]
- 37.Divkovic D, Kvolik S, Sipl M, et al. A successful early gore-tex reconstruction of an abdominal wall defect in a neonate with Cantrell pentalogy: a case report and literature review. Clin Case Rep. 2015;3(1):19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39(7):1190–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Hoorn JH, Moonen RM, Huysentruyt CJ, et al. Pentalogy of Cantrell: two patients and a review to determine prognostic factors for optimal approach. Eur J Pediatr. 2008;167(1):29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mallula KK, Sosnowski C, Awad S. Spectrum of Cantrell’s pentalogy: case series from a single tertiary care center and review of the literature. Pediatr Cardiol. 2013;34(7):1703–10 [DOI] [PubMed] [Google Scholar]
- 41.O’Gorman CS, Tortoriello TA, McMahon CJ. Outcome of children with Pentalogy of Cantrell following cardiac surgery. Pediatr Cardiol. 2009;30(4):426–30 [DOI] [PubMed] [Google Scholar]
- 42.Abehsera D, de la Calle M, Rodríguez R, Revello R, et al. Bipolar cord coagulation for selective feticide in a monochorionic twin pregnancy complicated by pentalogy of Cantrell. Taiwan J Obstet Gynecol. 2016;55(1):135–7 [DOI] [PubMed] [Google Scholar]
- 43.Engels AC, Debeer A, Russo FM, et al. Pericardio-Amniotic Shunting for Incomplete Pentalogy of Cantrell. Fetal Diagn Ther. 2017;41(2):152–6 [DOI] [PubMed] [Google Scholar]
- 44.Antiel RM, Curlin FA, Lantos JD, et al. Attitudes of paediatric and obstetric specialists towards prenatal surgery for lethal and non-lethal conditions. J Med Ethics. 2018;44(4):234–8 [DOI] [PubMed] [Google Scholar]
- 45.Aldeiri B, Roostalu U, Albertini A, et al. Abrogation of TGF-beta signalling in TAGLN expressing cells recapitulates Pentalogy of Cantrell in the mouse. Sci Rep. 2018;8(1):3658. [DOI] [PMC free article] [PubMed] [Google Scholar]