Abstract
Objective
To examine sex differences and trends in comorbid disease and health care utilization in individuals with newly diagnosed Parkinson disease (PD).
Design
Retrospective cohort study.
Participants
Over 133,000 Medicare beneficiaries with a new PD diagnosis in 2002 followed through 2008.
Methods
We compared the prevalence and cumulative incidence of common medical conditions, trends in survival and health care utilization between men and women with PD.
Results
Female PD patients had higher adjusted incidence rate ratio (IRR) of depression (IRR: 1.28, 1.25–1.31), hip fracture (IRR: 1.51, 1.45–1.56), osteoporosis (3.01, 2.92–3.1), and rheumatoid/osteoarthritis (IRR: 1.47, 1.43–1.51) than men. In spite of greater survival, women with PD used home health and skilled nursing facility care more often, and had less outpatient physician contact than men throughout the study period.
Conclusions
Women experience a unique health trajectory after PD diagnosis as suggested by differing comorbid disease burden and health care utilization compared to men. Future studies of sex differences in care needs, care quality, comorbidity related disability, PD progression, and non-clinical factors associated with disability are needed to inform research agendas and clinical guidelines that may improve quality survival for women with PD.
Keywords: Parkinson disease, Comorbidity, Gender, Epidemiology, Health services, Disparities
INTRODUCTION
Parkinson disease (PD) is a common, neurodegenerative condition that primarily affects older adults. While the incidence of PD is consistently found to be higher in men, more recent research has found sex differences in motor and non-motor features as well [1–4]. Women have a slightly older age of onset, are more likely to present with tremor and have a shorter time to and higher likelihood of developing motor fluctuations (particularly levodopa induced dyskinesias) [5, 6]. Despite a higher propensity to develop motor fluctuations, women are less likely to receive the most efficacious treatment for fluctuations, deep brain stimulation surgery [7–9]. Non-motor features also differ between the sexes, with women more often reporting or displaying depression, fatigue, nervousness, constipation, pain, and restless legs, as well as reporting higher disability and lower quality of life [1, 2, 10, 11]. Conversely, excessive daytime sleepiness, drooling and sexual problems were found to be more common and severe in men with PD [12, 13].
PD epidemiology and outcomes research studies have generally been limited to academic center populations and focus on classic PD motor and non-motor symptoms, ignoring other determinants of health and health care use. Unfortunately, limited to no data are available on populations not adequately represented in clinical trials or academic centers, particularly women. Administrative data not only allow for the study of these populations, but also afford the opportunity to study a decline in health in ways a clinic based study cannot. Increased use of health care services may suggest barriers to preventative and therapeutic disease management, increased susceptibility to adverse outcomes, or lack of social support. Improving quality survivorship for PD requires a better understanding of how health and health care use changes after PD diagnosis. To address this gap in knowledge, we identified 131,950 Medicare beneficiaries with a new diagnosis of PD and examined sex differences in the incidence of comorbid disease, health care service use and survival.
METHODS
This study was approved by human studies research office of University of Pennsylvania Perelman School of Medicine.
Design
This was a retrospective cohort study of Medicare beneficiaries diagnosed with PD in the year 2002 and followed through December 31, 2008. Medicare is a government-mandated insurance program used by 98% of adults aged 65 years and older and a portion of the disabled population below the age of 65.
Participants
Medicare beneficiaries with an incident PD diagnosis in the year 2002 were identified using the Centers for Medicare & Medicaid Services (CMS) Carrier file, which contains diagnosis and treatment claims for provider services. We required that a beneficiary have at least two years of Medicare eligibility prior to a new claim for Parkinson disease (ICD-9 code “332.0”). Our case identification methods are published elsewhere [14], but briefly, the Carrier files were searched to identify beneficiaries with claims containing ICD-9 codes for “Parkinson disease” (332) or “Paralysis agitans” (332.0) [15]. Beneficiaries that had a diagnostic claim for “Secondary/Drug induced Parkinsonism” (332.1) or “Atypical Parkinson Syndromes” (333.0) were excluded. We also excluded those with PD who were younger than the age of standard Medicare eligibility (65 years) because these individuals likely have different clinical courses and health care needs.
Claims data from incident PD cases were then linked to the CMS Beneficiary Annual Summary File (BASF) from 2002–2008 using the beneficiary identification number. The BASF contains demographic variables (race, date of birth and sex), annual data on health service use, chronic/comorbid conditions and date of death.
The primary study outcomes were comorbid disease diagnosis and health service use. We examined survival through 2008 as a secondary outcome. We determined the frequency of a diagnosis of atrial fibrillation, acute myocardial infarction, depression, dementia, cataract, chronic obstructive pulmonary disease, congestive heart failure, diabetes, glaucoma, hip fracture, ischemic heart disease, osteoporosis, rheumatoid arthritis/osteoarthritis, stroke, breast cancer, uterine cancer, lung cancer, and prostate cancer. Diagnosis dates were used to examine the timing of comorbid disease relative to PD diagnosis.
Health care utilization analyses were performed on the subpopulation of newly diagnosed PD patients that were still alive at the end of the observation period (December 31, 2008) to minimize survival and perceived futility bias. We extracted BASF data on annual use of home health care, skilled nursing facility care, and hospice services by beneficiaries with PD. Home health care services are covered by Medicare when the beneficiary is determined by a provider to be homebound. Skilled nursing facility care in our dataset includes both services provided in an acute rehabilitation facility as well as initial services in a chronic nursing facility.
Statistical Methods
The primary focus of this study was to examine sex differences in health and health care service use; all analyses were either stratified by sex or compared women to men. Baseline patient characteristics and comorbid conditions were determined using data from the year 2002. Follow-up time for each patient was calculated as time to event, time to death or time to end of study, whichever came first. We calculated the cumulative incidence (per 100 PD patient years) of each comorbid diagnosis from 2003 through the end of 2008. Incident rate ratios (IRR) were calculated to compare selected comorbid diseases by sex. Models were adjusted for age at PD diagnosis and race.
Health service use was calculated annually and reported as the proportion (crude prevalence) of the PD population in receipt of a given service. Trends in health service utilization from 2002–2008 were stratified by sex. Generalized estimating equations (GEE) and logistic regression using binary or poisson distribution were used to estimate health service utilization while adjusting for year, sex, age at diagnosis, race and comorbid conditions. Cox proportional hazards models were used to estimate the risk of death associated with sex, adjusting for race, age and comorbid disease. The time to event variable was measured in months from January 1, 2002 to the date of death. Surviving cases were censored on December 31, 2008. All statistical analyses were generated using SAS software, Version 9.4.
RESULTS
Patient Characteristics
A total of 133,133 Medicare beneficiaries with a diagnosis of PD recorded in 2002 met the inclusion criteria. Of these, 70,458 (52.9%) were women (Table 1). Over 90% of both men and women diagnosed with PD were white. A slightly greater proportion (6.7% n=4,691) of women with PD were Black compared to 5.5% of men. Hispanic, Asian and Native Americans were equally represented between the sexes. The women with PD were older: 55.6% of women were over 80 years old at the time of diagnosis, compared to 48.0% of the men.
Table 1.
Demographic Characteristics of 133,133 Medicare Beneficiaries with Incident Diagnosis of Parkinson Disease
| Characteristic | Women | Men | |
|---|---|---|---|
| n (%) 70,458 (52.9) |
n (%) 62,675 (47.1) |
Chi Square, p-value | |
| Race/Ethnicity | <0.0001 | ||
| White | 63,466 (90.1) | 57,153 (91.2) | |
| Black | 4,691 (6.7) | 3,422 (5.5) | |
| Asian | 741 (1.1) | 718 (1.2) | |
| Hispanic | 1,532 (2.2) | 1,362 (2.2) | |
| Native American | 28 (0.0) | 22 (0.0) | |
|
| |||
| Age group | <0.0001 | ||
| 67–69 | 4,347 (6.2) | 4,830 (7.7) | |
| 70–74 | 10,829 (15.4) | 11,617 (18.5) | |
| 75–79 | 16,281 (23.1) | 16,194 (25.8) | |
| 80–85 | 20,939 (29.7) | 18,424 (29.4) | |
| 85+ | 18,062 (25.6) | 11,610 (18.5) | |
Data shown are number (percent).
Comorbid Disease Burden
Women and men with PD differed in their comorbid disease burden throughout the observation period. At baseline, women with PD had a lower crude prevalence of atrial fibrillation, acute myocardial infarction, chronic obstructive pulmonary disease, diabetes, colorectal cancer, ischemic heart disease, chronic kidney disease, stroke/transient ischemic attack, and lung cancer (Table 2). Conversely, women had higher baseline prevalence of cataracts, depression, dementia, glaucoma, hip fracture, osteoporosis, rheumatoid arthritis/osteoarthritis, and congestive heart failure, consistent with the known sex predilection of these conditions.[16]
Table 2.
Sex Differences in Comorbid Disease Burden in Incident Parkinson Disease Cohort
| Cumulative Incidence of CCW condition (2002–2008) (per 100 PD patient years) adjusted for race (95%CI) | IRR (95%) (women vs men) adjusted for race | p-value | ||||
|---|---|---|---|---|---|---|
| Comorbid Disease | ||||||
| N | Men | N | Women | |||
| Atrial Fibrillation | 7,472 | 3.46 (3.27–3.65) | 7,597 | 2.78 (2.63–2.93) | 0.8 (0.78–0.83) | <.0001 |
| Dementia | 18,612 | 16.34 (15.8–16.89) | 19,058 | 15.68 (15.17–16.2) | 0.96 (0.94–0.98) | <.0001 |
| Myocardial Infarction | 3,908 | 1.76 (1.63–1.89) | 3,650 | 1.33 (1.23–1.43) | 0.75 (0.72–0.79) | <.0001 |
| Cataract | 8,059 | 11.77 (11.17–12.41) | 7,506 | 10.91 (10.35–11.5) | 0.93 (0.9–0.96) | <.0001 |
| Congestive Heart Failure | 14,956 | 12.75 (12.3–13.22) | 15,694 | 11.7 (11.28–12.13) | 0.92 (0.9–0.94) | <.0001 |
| Colorectal Cancer | 992 | 0.46 (0.4–0.53) | 945 | 0.36 (0.31–0.42) | 0.78 (0.71–0.85) | <.0001 |
| COPD | 10,018 | 6.73 (6.43–7.03) | 9,944 | 5.23 (5–5.46) | 0.78 (0.76–0.8) | <.0001 |
| Depression | 12,122 | 7.36 (7.06–7.68) | 13,448 | 9.43 (9.04–9.83) | 1.28 (1.25–1.31) | <.0001 |
| Diabetes | 7,025 | 7.17 (6.85–7.51) | 8,264 | 6.92 (6.61–7.24) | 0.97 (0.93–1) | 0.0283 |
| Glaucoma | 3,340 | 2.44 (2.29–2.61) | 3,855 | 2.37 (2.22–2.52) | 0.97 (0.93–1.02) | 0.1929 |
| Hip Fracture | 4,422 | 1.28 (1.19–1.38) | 7,390 | 1.92 (1.79–2.07) | 1.51 (1.45–1.56) | <.0001 |
| Ischemic Heart Disease | 9,641 | 14.76 (14.12–15.43) | 11,358 | 12.37 (11.84–12.93) | 0.84 (0.82–0.86) | <.0001 |
| Chronic Kidney Disease | 14,640 | 9.23 (8.91–9.57) | 13,640 | 6.75 (6.51–7) | 0.73 (0.71–0.75) | <.0001 |
| Lung Cancer | 1,291 | 0.53 (0.47–0.61) | 928 | 0.32 (0.27–0.36) | 0.59 (0.54–0.64) | <.0001 |
| Osteoporosis | 6,347 | 3.33 (3.18–3.5) | 11,548 | 10.03 (9.59–10.48) | 3.01 (2.92–3.1) | <.0001 |
| Arthritis | 9,431 | 7.64 (7.31–7.99) | 11,373 | 11.23 (10.75–11.73) | 1.47 (1.43–1.51) | <.0001 |
| Stroke/TIA | 10,893 | 8.32 (7.99–8.66) | 12,355 | 7.89 (7.59–8.21) | 0.95 (0.92–0.97) | <.0001 |
Over the next six years of observation, women displayed a higher cumulative incidence of hip fracture, osteoporosis, and rheumatoid arthritis/osteoarthritis (Figure 1). After adjusting for age and race, the incidence ratios for depression (IRR: 1.28, 1.25–1.31), hip fracture (IRR: 1.51, 1.45–1.56), osteoporosis (3.01, 2.92–3.1), and rheumatoid/osteoarthritis (IRR: 1.47, 1.43–1.51) remained elevated for women compared to men. In contrast, almost all other conditions studied were more common in men, including atrial fibrillation (IR=0.8, 0.78–0.83), acute myocardial infarction (IR=0.75, 0.72–0.79), colorectal cancer (IR= 0.78, 0.71–0.85), and chronic obstructive pulmonary disease (IR=0.78, 0.) were more common in men. The incidence ratios for dementia, diabetes, and glaucoma were very close to 1, suggesting a nearly equal age and race adjusted incidence of these diseases between men and women after PD diagnosis.
Figure 1. Sex Differences in Comorbid Disease in PD.
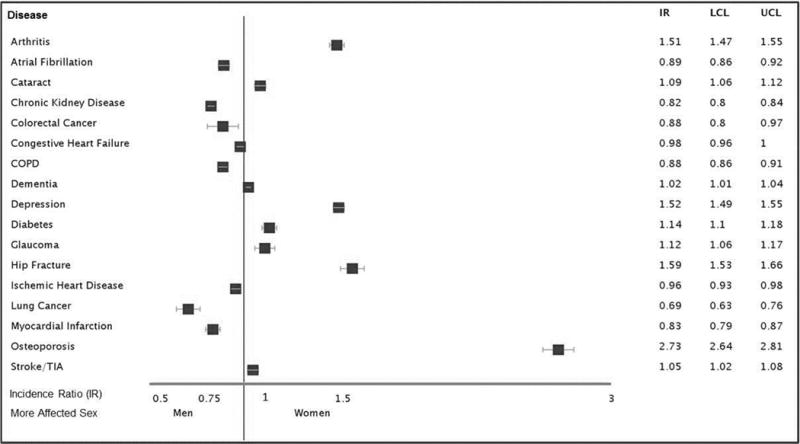
Data shown are the incidence ratios of comorbid disease in Parkinson’s disease between men and women, adjusted for age and race. An incidence ratio below 1 indicates the incidence of disease is higher in men, while an incidence ratio above 1 indicates the incidence of disease is higher in women.
Abbreviations: IR = Incidence Ratio; LCL = Lower Confidence Limit; UCL = Upper Confidence Limit
Trends in Health Care Use
In general, we found that both women and men were frequent users of Medicare services, including physician office visits (POVs), skilled nursing facility care (SNF), home health care (HH), and hospice care (HS). Figure 2 displays the trends in use of these services by sex in the subpopulation of incident PD cases that were still living at the end of 2008 (n=42,611). Both men and women showed an initial increase in use of POV, SNF and HH care in the year of being diagnosed with PD. In the years that followed the diagnosis, the annual average number of outpatient physician office visits decreased, which may reflect consolidation of care or difficulty accessing health care as the disease progresses. Women had fewer physician office visits than men at the time of diagnosis and at every point of observation thereafter. Furthermore, the decline in physician care was greater in women compared to men (−37.4% versus −22.3%, chi square p<0.01). Our previous data on gender disparities in specialty care for PD demonstrated that women were less likely than men to have neurologists involved in their care [17], which may explain a portion of these observed differences.
Figure 2. Use of Health Care Services by Sex.
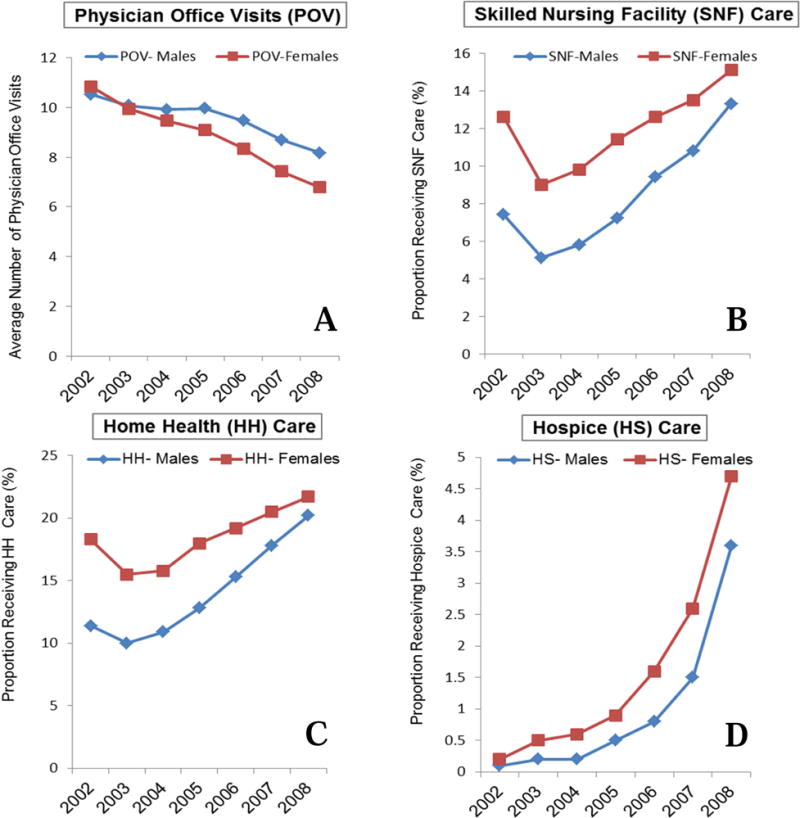
A: Average number of physician office visits by year and sex
B: Proportion of Medicare beneficiaries with PD receiving skilled nursing facility (SNF) care by sex.
C: Proportion of Medicare beneficiaries with PD receiving home health (HH) care by sex.
D: Proportion of Medicare beneficiaries with PD receiving hospice (HS) care by sex.
The receipt of skilled nursing facility care in our dataset may reflect acute rehabilitation services (such as after a stroke or elective knee replacement) or services provided along the path to long term nursing facility care. We found higher SNF service use in the year of PD diagnosis for both sexes, however, the proportion of women that utilized SNF care (12.6%) was 41.3% greater than men (7.4%, chi-square p<0.01). After an initial decline in the first year after diagnosis, a steady increase in the proportion of PD patients receiving SNF care was observed for both men and women. However, women remained the greater users of SNF care throughout the study period. This relationship remained after adjusting for year, sex, age at diagnosis, race and comorbid conditions, as well as including the full cohort instead of the survivor only cohort.
To be eligible for home health care, an individual must demonstrate cognitive or physical disability that prevents the use of outpatient services, and be referred for home health care by a medical care provider. Similar to SNF care, annual utilization of home health care increased over time for men and women, and women used home health services more than men at every measured time point. The sex associated difference in home health care use lessened over time, becoming nearly identical between men and women at year seven after PD diagnosis (20.2% and 21.7% respectively) (Figure 2). Again, this relationship remained after adjusting for year, sex, age at diagnosis, race and comorbid conditions, as well as including the full cohort instead of the survivor only cohort. In this incident PD cohort, annual hospice use rates were uniformly low at less than 5%. Although women used hospice care less than men, the absolute difference was negligible (−1.1%). This relationship remained after adjusting for year, sex, age at diagnosis, race and comorbid conditions. However, when the whole cohort was included, men had a slightly higher use of hospice than women.
Sex Related Differences in Survival
In spite of greater use of health care services reserved for individuals with high levels of disability, women with PD appear to have a survival advantage over men. After adjusting for age at diagnosis, race, and comorbid diseases which affect both sexes, women were almost 25% less likely to die during the observation period (Adjusted Hazard Ratio, AHR 0.76, 95% CI: 0.75–0.77). For both sexes, having a diagnosis of dementia at baseline was associated with the greatest risk of death (AHR 1.76, 95% CI 1.73–1.80). Other common illnesses associated with increased death when present prior to PD diagnosis included chronic obstructive pulmonary disease (AHR 1.20, 95% CI 1.18–1.22), hip fracture (AHR 1.16, 95% CI 1.12–1.19), ischemic heart disease (AHR 1.40, 95% CI 1.37–1.42), chronic kidney disease (AHR 1.26, 95% CI 1.25–1.30), and lung cancer (AHR 1.48, 95% CI 1.38–1.58).
DISCUSSION
In this study, we examined data on 133,133 Medicare beneficiaries with a new PD diagnosis. We found sex-specific patterns of comorbid disease, and found that women experience greater use of advanced nursing care (SNF, home health, hospice), and lower use of direct physician contact. Our data demonstrate demographic differences in PD diagnosis, congruent with previous data and the current theory that there are race and sex differences in PD risk [3, 14]. The age- and race-adjusted incidence of PD in our cohort was higher in men than in women, and the majority of PD patients were white. As anticipated, PD incidence increased with age for both men and women. However, women in our cohort on average were older than men at the time of diagnosis, a finding which may be explained by a later disease onset, delay in diagnosis, or longer life expectancy [5]. In this cohort, the percentage of males and females with PD were similar, which at first may seem to be at odds with the known association of PD with male sex [3]. However, the sex differences in PD relate to incidence and not the sex distribution within an all-PD cohort.
The cumulative incidence of depression, hip fracture, osteoporosis and rheumatoid arthritis were more common in women than in men in our PD cohort. The greater incidence of hip fracture in women is not surprising, as women in general are more susceptible to hip fractures due to higher prevalence of osteoporosis [18, 19]. However, more data are needed to determine whether women with PD are more likely to fall, or more likely to suffer from a hip fracture when they fall. Increased falling among women may reflect medication misadventures, such as use of sedatives, hypnotics, benzodiazepines, all of which should be avoided, or, undertreatment of PD motor symptoms. Hip fracture is associated with significant mortality and is a strong predictor of long-term care facility placement among PD patients [19], underscoring the need for a sex-specific approach to preventable contributors to disability in PD.
Our health care utilization data shows that women, although more likely to survive, have greater need for advanced care services after being diagnosed with PD. More studies are needed to define contributing factors, but our data presents initial evidence that PD is more ‘disabling’ for women, according to several models of disability. The medical model of disability is perhaps the most familiar, and guides reimbursement policy for advanced care, like home health or skilled nursing facility [20]. This model regards disability as the consequences of an illness or disease which cannot be cured. Whether PD in women is actually more severe is unclear. Women are less likely to see a neurologist [17], and misdiagnosis is higher in GPs (47% in one study)[21] than neurologists – perhaps women are underdiagnosed until their symptoms are more severe [22]. Women have a greater reported burden of non-motor symptoms [12, 13, 23], but objective evidence (e.g. biomarker or pathological) data on sex differences in PD pathology is sparse. The evidence that does exist suggests men with PD have greater physiological disturbance. For example, men have been found to have greater burden of mitochondrial dysfunction on spectroscopy [24]. In a study of 253 consecutive PD patients, women had persistently greater striatal [123I] FP-CIT binding than men throughout the disease course [5]. Our previous data, which demonstrate that women are less likely to have specialist care [17] and less likely to receive DBS [7] could not ascertain need for these services, which would be affected by disease course. Regardless of whether neurodegeneration differs by sex, women may be at greater risk of lower quality care (PD related or general medical care), which could eventually manifest as greater need for HH and SNF care. If so, a portion of the observed overutilization of SNF and HH services in women is avoidable. The extent to which medical disability in women with PD is preventable will be the focus of future studies.
The rehabilitation model of disability defines disability as a loss of function that requires a rehabilitation professional. Our data supports the hypothesis that this aspect of disability may factor prominently in the health care experiences of the female PD population, particularly in the absence of data that supports worse neurodegeneration. Home health and SNF provide substantial rehabilitative and supportive services to users, with the goal of returning the person to outpatient utilization, if at all possible. Referral for SNF or HH may be preferred for patients with borderline criteria for these services if social support is lacking to obtain required services on an outpatient basis. Women in general are less supported through chronic illness, particularly in older age, because they are more likely to live alone [25, 26]. Thus, our data may be systemic evidence of compensatory strategies that have evolved to provide needed services to women who lack adequate or effective caregiver support. Future studies that examine the use of rehabilitative services by women with PD on a more granular level are necessary to examine the comparative- and cost-effectiveness of such approaches. Limitations of this study include the possibility of selection bias and misclassification of outcome or exposure variables, as it is retrospective in design. Other limitations are associated with Medicare and administrative datasets such as coding errors, inability to currently adjust for disease severity, and case mix bias. Unknown and unobservable factors may contribute to health care utilization and diagnoses. Additionally, this study includes patients 65 years or older, so the results may not be generalizable to younger PD patients.
In conclusion, this study highlights the importance of sex-specific data on comorbidities and healthcare experiences of individuals with PD. Understanding the underlying contributors to disability in PD will help develop targeted interventions to improve not just survival in PD, but high quality survivorship. These data generate multiple hypotheses for future studies of the nature and pervasiveness of sex variability in comorbid disease and healthcare utilization, which have the potential to improve overall understanding of PD.
HIGHLIGHTS.
We examined data on 133,133 Medicare beneficiaries with a new diagnosis of PD.
We found sex-specific patterns of comorbid disease in PD.
Women experienced greater use of advanced nursing care after PD diagnosis.
Men had greater use of direct physician contact than women.
Acknowledgments
This work was supported by NIH K23NS081087.
Dr. Drew Kern has served as an advisor for Michael J. Fox Foundation and AbbVie Pharmaceutics; he has received honorarium from Merz Pharma, AbbVie Pharmaceutics and SAI-Med Partners, LLC and has received grants from the Parkinson’s Society of Canada and University of Colorado Skin Disease Research Center.
Dr. Schwalb is funded by the NIH for research and clinical trials.
Dr. Urrea-Mendoza received honorarium from Great Lakes NeuroTechnologies.
Dr. Shulman is funded by the NIH for research and clinical trials, as well as the Fox foundation, The Veteran’s Administration Medical Center, PCORI, The Rosalyn Newman foundation, The Brin Family and Biotie Therapies.
Dr. Dahodwala receives grant funding from the NIH, National Parkinson Foundation, Parkinson Counsil, Biotie and Abbvie.
Dr. Willis received research support from the NIH, the Patient Centered Outcomes Research Institute (PCORI), the Parkinson Foundation, the St. Louis Chapter of the American Parkinson Disease Association, the University of Pennsylvania, the John Middleton Fund, Walter and Connie Donius and The Robert Renschen Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosures:
Dr. Fullard receives support from NIH training grant #T32NS061779-07 and joint support from the AAN, the American Brain Foundation and the Parkinson’s Foundation.
Mr. Thibault reports no disclosures.
Ms. Torado reports no disclosures.
Ms. Foster reports no disclosures.
Ms. Katz reports no disclosures.
Ms. Morgan reports no disclosures.
Author Roles:
1. Research project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
M.F: 2C, 3A, 3B
D.T: 1C, 2A, 2B
R.T.: 2C, 3B
S.F.: 2C, 3B
L.K.: 2C, 3B
R.M.: 2C, 3B
D.K.: 2A, 3B
J.S.: 2C, 3B
E.M.: 2C, 3B
L.S.: 2C, 3B
N.D.: 2C, 3B
A.W.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
References
- 1.Miller IN, Cronin-Golomb A. Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov Disord. 2010;25(16):2695–703. doi: 10.1002/mds.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavon JM, Whitson HE, Okun MS. Parkinson’s disease in women: a call for improved clinical studies and for comparative effectiveness research. Maturitas. 2010;65(4):352–8. doi: 10.1016/j.maturitas.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. American journal of epidemiology. 2003;157(11):1015–22. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 4.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? Journal of neurology, neurosurgery, and psychiatry. 2004;75(4):637–9. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. Gender differences in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 2007;78(8):819–24. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassin-Baer S, Molchadski I, Cohen OS, Nitzan Z, Efrati L, Tunkel O, Kozlova E, Korczyn AD. Gender effect on time to levodopa-induced dyskinesias. Journal of neurology. 2011;258(11):2048–53. doi: 10.1007/s00415-011-6067-0. [DOI] [PubMed] [Google Scholar]
- 7.Willis AW, Schootman M, Kung N, Wang XY, Perlmutter JS, Racette BA. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology. 2014;82(2):163–71. doi: 10.1212/WNL.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan AK, McGovern RA, Brown LT, Sheehy JP, Zacharia BE, Mikell CB, Bruce SS, Ford B, McKhann GM., 2nd Disparities in access to deep brain stimulation surgery for Parkinson disease: interaction between African American race and Medicaid use. JAMA neurology. 2014;71(3):291–9. doi: 10.1001/jamaneurol.2013.5798. [DOI] [PubMed] [Google Scholar]
- 9.Hariz GM, Nakajima T, Limousin P, Foltynie T, Zrinzo L, Jahanshahi M, Hamberg K. Gender distribution of patients with Parkinson’s disease treated with subthalamic deep brain stimulation; a review of the 2000–2009 literature. Parkinsonism & related disorders. 2011;17(3):146–9. doi: 10.1016/j.parkreldis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Dahodwala N, Pei Q, Schmidt P. Sex Differences in the Clinical Progression of Parkinson’s Disease. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN. 2016;45(5):749–56. doi: 10.1016/j.jogn.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shulman LM, Bhat V. Gender disparities in Parkinson’s disease. Expert review of neurotherapeutics. 2006;6(3):407–16. doi: 10.1586/14737175.6.3.407. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Martin P, Pecurariu C Falup, Odin P, Hilten JJ van, Antonini A, Rojo-Abuin JM, Borges V, Trenkwalder C, Aarsland D, Brooks DJ, Chaudhuri K Ray. Gender-related differences in the burden of non-motor symptoms in Parkinson’s disease. Journal of neurology. 2012;259(8):1639–47. doi: 10.1007/s00415-011-6392-3. [DOI] [PubMed] [Google Scholar]
- 13.Solla P, Cannas A, Ibba FC, Loi F, Corona M, Orofino G, Marrosu MG, Marrosu F. Gender differences in motor and non-motor symptoms among Sardinian patients with Parkinson’s disease. Journal of the neurological sciences. 2012;323(1-2):33–9. doi: 10.1016/j.jns.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Willis A Wright, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34(3):143–51. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noyes K, Liu H, Holloway R, Dick AW. Accuracy of Medicare claims data in identifying Parkinsonism cases: comparison with the Medicare current beneficiary survey. Mov Disord. 2007;22(4):509–14. doi: 10.1002/mds.21299. [DOI] [PubMed] [Google Scholar]
- 16.Tan A, Kuo YF, Goodwin JS. Predicting life expectancy for community-dwelling older adults from Medicare claims data. American journal of epidemiology. 2013;178(6):974–83. doi: 10.1093/aje/kwt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology. 2011;77(9):851–7. doi: 10.1212/WNL.0b013e31822c9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang KP, Center JR, Nguyen TV, Eisman JA. Incidence of hip and other osteoporotic fractures in elderly men and women: Dubbo Osteoporosis Epidemiology Study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19(4):532–6. doi: 10.1359/JBMR.040109. [DOI] [PubMed] [Google Scholar]
- 19.Safarpour D, Thibault DP, DeSanto CL, Boyd CM, Dorsey ER, Racette BA, Willis AW. Nursing home and end-of-life care in Parkinson disease. Neurology. 2015;85(5):413–9. doi: 10.1212/WNL.0000000000001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.International Classification of Functioning, Disability and Health, WHO.
- 21.Meara J, Bhowmick BK, Hobson P. Accuracy of diagnosis in patients with presumed Parkinson’s disease. Age and ageing. 1999;28(2):99–102. doi: 10.1093/ageing/28.2.99. [DOI] [PubMed] [Google Scholar]
- 22.Pagan FL. Improving outcomes through early diagnosis of Parkinson’s disease. The American journal of managed care. 2012;18(7 Suppl):S176–82. [PubMed] [Google Scholar]
- 23.Picillo M, Erro R, Amboni M, Longo K, Vitale C, Moccia M, Pierro A, Scannapieco S, Santangelo G, Spina E, Orefice G, Barone P, Pellecchia MT. Gender differences in non-motor symptoms in early Parkinson’s disease: a 2-years follow-up study on previously untreated patients. Parkinsonism & related disorders. 2014;20(8):850–4. doi: 10.1016/j.parkreldis.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 24.Weiduschat N, Mao X, Beal MF, Nirenberg MJ, Shungu DC, Henchcliffe C. Sex differences in cerebral energy metabolism in Parkinson’s disease: a phosphorus magnetic resonance spectroscopic imaging study. Parkinsonism & related disorders. 2014;20(5):545–8. doi: 10.1016/j.parkreldis.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 25.DiGiacomo M, Lewis J, Nolan MT, Phillips J, Davidson PM. Transitioning from caregiving to widowhood. Journal of pain and symptom management. 2013;46(6):817–25. doi: 10.1016/j.jpainsymman.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Donelan K, Falik M, DesRoches CM. Caregiving: challenges and implications for women’s health. Women’s health issues : official publication of the Jacobs Institute of Women’s Health. 2001;11(3):185–200. doi: 10.1016/s1049-3867(01)00080-9. [DOI] [PubMed] [Google Scholar]


