Abstract
Organic anion transporter 3 (OAT3) plays a vital role in removing a broad array of anionic drugs from kidney, thereby avoiding their possibly toxic side effects in the body. We earlier demonstrated that OAT3 is subjected to a specific type of post-translational modification called SUMOylation. SUMOylation is a dynamic event, where de-SUMOylation is catalyzed by a class of SUMO-specific proteases. In the present investigation, we assessed the role of SUMO-specific protease Senp2 in OAT3 SUMOylation, expression and function. We report here that overexpression of Senp2 in COS-7 cells led to a reduced OAT3 SUMOylation, which correlated well with a decreased OAT3 expression and transport activity. Such phenomenon was not observed in cells overexpressing an inactive mutant of Senp2. Furthermore, transfection of cells with Senp2-specific siRNA to knockdown the endogenous Senp2 resulted in an increased OAT3 SUMOylation, which correlated well with an enhanced OAT3 expression and transport activity. Coimmunoprecipitation experiments showed that Senp2 directly interacted with OAT3 in the kidneys of rats. Together these results provided first demonstration that Senp2 is a significant regulator for OAT3-mediated organic anion/drug transport.
Keywords: Organic Anion Transporter, Drug Transport, Regulation, SUMO-specific protease, deSUMOylation
1. Introduction
Organic anion transporter 3 (OAT3) belongs to a family of organic anion transporters, playing vital parts in the removal of numerous drugs from the kidney, including anti-viral, anticancer, anti-hypertension, and anti-inflammation drugs, and thereby avoiding their possibly toxic side effects in the body [1-6].
The activity of OAT3 is critically reliant on their level of expression at the cell surface. Our lab has established that OATs regularly endocytoses/internalize from and recycle back to cell surface [7]. A crucial event that precedes OAT endocytosis is the conjugation of ubiquitin to the lysine residues of the transporter. The attachment of ubiquitin to cell surface OAT triggers OAT to endocytose from cell surface to intracellular early endosomes, where OAT either becomes deubiquitinated and recycles back to cell surface or target to proteolytic system for degradation. We further established that activation of protein kinase C (PKC) suppresses OAT transport activity by enhancing OAT ubiquitination, which results in an acceleration of OAT internalization from cell surface to intracellular early endosomes and subsequent degradation without affecting OAT recycling. Thus, the quantity of OAT at the cell surface is significantly reduced, resulting in a significant decrease in OAT transport activity [7-10].
Another important ubiquitin-like modifier is the small ubiquitin-related modifier SUMO [11, 12]. SUMO family consists of three functional isoforms SUMO1-3. All three are polypeptides of ~12 kDa, and are broadly detected in many tissues, including brain, liver, and kidney. SUMO2 and SUMO3 are regularly mentioned as SUMO2/3 as they have 97% identity. In contrast, SUMO2/3 is only ~50% identical in the sequence with SUMO-1. Consistent with such sequence differences, SUMO1 and SUMO2/3 modify different substrates in vivo. Recently, it has become obvious that ubiquitin and SUMO may target the identical site in a competitive manner of the shared substrate proteins [13, 14]. We recently revealed that, contrary to the ubiquitination-dependent inhibitory effect of PKC on OAT3, activation of PKA stimulated OAT3 expression and transport activity, which correlated with an enhanced SUMOylation of OAT3 and a decreased ubiquitination, suggesting that PKC and PKA regulate OAT3 activity, possibly through the cross-talk between ubiquitination and SUMOylation [15].
SUMOylation is a dynamic and reversible event, and SUMO-specific proteases including members of Ulp (in yeast) and SENP family (in mammals) remove the SUMO moiety (deSUMOylation) from their substrates [16]. Till now, six human Senp proteins have been cloned and identified with the ability to de-conjugate SUMO. Among these six members [17], Senp2 is known to shuttle between the nucleus and the cytoplasm, regulating the function of several integral membrane proteins such as potassium channels and TGFβ receptor by deSUMOylation [18-21]. In the present study, we evaluated the role of Senp2 in OAT3 SUMOylation, expression and function.
2. Materials and Methods
2.1. Materials
COS-7 cells were purchased from American Type Culture Collection (Manassas, VA). [3H]-labeled estrone sulfate (ES) was purchased from PerkinElmer (Waltham, MA). Membrane-impermeable biotinylation reagent NHS-SS-biotin (Catalog number: 21331), streptavidin-agarose (Catalog number: 20349) and protein G-agarose (Catalog number: 20399) were purchased from Thermo Fisher Scientific (Waltham, MA). cDNAs for SUMO-2 and Ubc9 were generously provided by Dr. Jorge A Iñiguez-Lluhí from University of Michigan Medical School. cDNAs for wild type Senp2 (#18047) and mutant Senp2 (R577L, K578M) (#18713) were purchased from Addgene (Watertown, MA). Mouse anti-Myc antibody (9E10) was purchased from Roche (Indianapolis, IN). Rabbit anti-HA antibody (ab9110), rabbit anti-SUMO 2/3 antibody (ab3742), rabbit anti-Senp2 (ab58418) and mouse anti-E-cadherin (ab76055) were purchased from Abcam (Cambridge, MA). Mouse anti - β - actin (sc-47778), mouse anti-OAT3 (sc-293264) and normal mouse IgG (sc-2025) were purchased from Santa Cruz (Santa Cruz, CA). Senp2-specific siRNA oligonucleotides (Silencer® Select, catalog number AM16708, Assay ID 105222) and negative control siRNA oligonucleotides (Silencer® Select, catalog number AM4611) were purchased from Thermo Fisher Scientific (Waltham, MA). Senp2-specific siRNA was designed to target an identical sequence between human Senp2 and green monkey Senp2 (predicted), and therefore can knock down both human and monkey Senp2. All other reagents were from Sigma-Aldrich (St. Louis, MO).
2.2. Cell culture and Transfection
Parental COS-7 cells were newly purchased from ATCC and cultured in Dulbecco's modified Eagle's medium (DMEM) (Product Number 17-204-CI, Corning Life Science, Tewksbury, MA) supplemented with 10% fetal bovine serum (Catalog number: 16000044, Thermo Fisher Scientific, Waltham, MA) at 37 °C in 5% CO2. Parental COS-7 cells were used at passage 5-15. We followed the manufacturer’s instruction to carry out the plasmids transfection by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA).
2.3. Transport Measurements
The drug transport assay was adapted from previously published research article[9]. Briefly, cells were seeded in 48-well plates. Uptake solution (phosphate-buffered saline (PBS)/Ca2+/Mg2+with 0.3 μM [3H] ES) was added into each well. At the times indicated, uptake process was stopped by removing the uptake solution and immediately washing the cells twice with ice-cold PBS. The cells were then lysed and the amount of [3H] ES transported into cells measured by liquid scintillation counting (Beckman LSC LS6500).
2.4. Cell Surface Biotinylation
We followed the biotinylation methods published previously by our lab to detect the surface expression level of hOAT3 [8]. Cells were cultured in 6-well plates. Each well of cells was washed with cold PBS twice followed by incubating with 1 ml of freshly made NHS-SS-biotin (0.5 mg/ml in cold PBS/CM) in two successive 20 min incubations on ice. After biotinylation, each well was briefly rinsed with 2 ml of PBS/CM containing 100 mM glycine then quenched with the same solution for 20 min on ice. The cells were then lysed on ice for 30 min in 400 ml of regular lysis buffer. The cell lysates were cleared by centrifugation at 16,000g at 4 °C. The supernatant was added to 40 μl of streptavidin-agarose beads to isolate cell membrane proteins. hOAT3 was detected in the pool of surface proteins by SDS-PAGE and immunoblotting using an anti-Myc antibody 9E10.
2.5. Preparation of rat kidney slices
Male Sprague-Dawley rats (250–300 g) were euthanized by CO2 inhalation, and the kidneys were immediately placed into freshly oxygenated ice-cold saline. Tissue slices (<0.5 mm, 5–10 mg wet weight) were cut with a Stadie-Riggs microtome, homogenized and lysed for immunoprecipitation assay.
All animal experiments were conducted following guidelines described in the guide for the care and use of laboratory animals (Association for Assessment and Accreditation of Laboratory Animal Care) as well as requirements established by the animal protocol approved by the Rutgers Institutional Animal Care and Use Committee.
2.6. Immunoprecipitation
We followed the immunoprecipitation method developed previously by our lab to detect the hOAT3 SUMOylation and the hOAT3 associated Senp2 [8]. Transfected cells were solubilized with lysis buffer I, containing 150mM NaCl with 1% proteinase inhibitor cocktail and 2% N-ethylmaleimide (NEM). The total cell lysate was precleared with protein G-agarose for 2 hours at 4°C, followed by immunoprecipitation with anti-Myc antibody which was conjugated to protein G beads at 4°C overnight. After an extensive wash with lysis buffer I, containing 500 mM NaCl for three times, the immunoprecipitated proteins were eluted with Urea buffer containing β-mecaptoethanol and analyzed by immunoblotting with indicated antibodies.
2.7. Electrophoresis and Immunoblotting
Protein samples were separated by 7.5% SDS-PAGE minigels (Bio-Rad, Hercules, CA) and transferred to polyvinylidene difluoride membranes (Thermo Fisher Scientific, Waltham, MA). The blots were blocked for 1 hour at room temperature with 5% nonfat dry milk in PBS-0.05% Tween 20 and incubated overnight at 4 °C with appropriate primary antibodies, followed by horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 hour. Chemiluminescence was determined by SuperSignal West Dura Extended Duration Substrate kit (Thermo Fisher Scientific, Waltham, MA). Nonsaturating, immunoreactive protein bands were quantified by scanning densitometry with the FluorChem 8000 imaging system (Alpha Innotech Corp., San Leandro, CA).
2.8. Data Analysis
Each experiment was repeated a minimum of three times. The statistical analysis was from multiple experiments (biological replicates: each experiment, we used COS-7 cells at different passage numbers). Statistical analysis was performed using Student’s paired t-tests between two groups. Among multiple treatments, one-way ANOVA, Tukey’s test was applied by using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). A p-value of <0.05 was considered significant for all analysis.
3. Results
3.1. Effect of Senp2 on hOAT3 SUMOylation
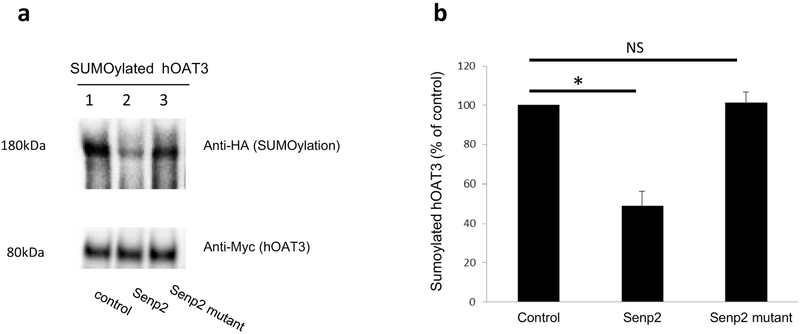
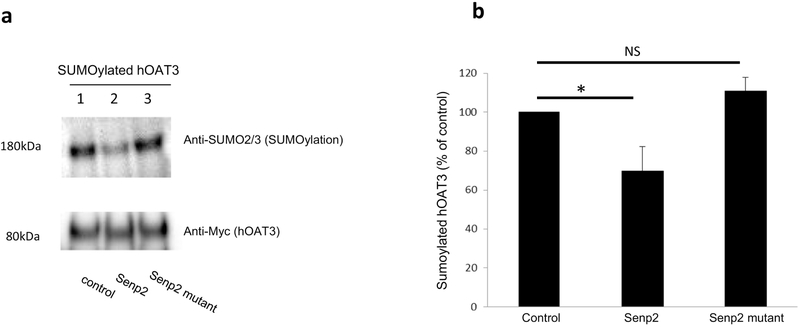
Senp2 is a deSUMOylation enzyme. To examine whether Senp2 specifically deSUMOylates hOAT3, cells were co-transfected with hOAT3, HA-tagged SUMO2, Ubc9 (a SUMO-conjugating enzyme) together with Senp2 or an inactive mutant of Senp2 (R577L, K578M). hOAT3 was pulled down from the transfected cells by anti-Myc antibody (hOAT3 was tagged with epitope Myc), followed by immunoblotting using anti-HA antibody to detect the amount of SUMO2 conjugated to hOAT3. As shown in Fig. 1a. top panel, lane 1, SUMOylated hOAT3 was detected, in control cells, at ~ 180 kDa, 80 kDa larger than unSUMOylated hOAT3 (~ 80 kDa). Given that SUMO is a 12 kDa polypeptide, this suggests that hOAT3 is likely multi- or poly-SUMOylated by SUMO2. However, in cells transfected with Senp2 (lane 2), the level of SUMOylated hOAT3 was much reduced in comparison to that in control cells. In cells transfected with the inactive mutant of Senp2 (lane 3), SUMOylated hOAT3 remained the same as that in control cells. The difference in the hOAT3 SUMOylation was not owing to the variance in the amount of hOAT3 immunoprecipitated because comparable quantity of hOAT3 was pulled down in every sample. Similar results were obtained when experiment was conducted to examine hOAT3 SUMOylation by endogenous SUMO2/3 conjugation (Fig. 2), indicating that Senp2 deSUMOylates not only the exogenous SUMO2 conjugation on hOAT3 but also the endogenous SUMO2/3 conjugation on hOAT3.
Fig. 1. Effect of Senp2 on hOAT3 SUMOylation.
(a). Top panel: COS-7 cells were cotransfected with hOAT3, HA-SUMO2, Ubc9 together with control vector, Senp2 or inactive mutant of Senp2 for 48h. The cells were then lysed. hOAT3 was immunoprecipitated by anti-Myc antibody (epitope Myc was tagged to hOAT3), followed by immunoblotting with anti-HA antibody to detect SUMOylated hOAT3. Bottom panel: The same immunoblot from Fig. 1a, Top panel was reprobed by anti-Myc antibody. (b). Densitometry plot of results from Fig. 1a as well as from other experiments. The values are mean ± SD (n = 3). *P<0.05. NS: statistically not significant.
Fig. 2. Effect of Senp2 on hOAT3 SUMOylation by endogenous SUMO2/3.
(a). Top panel: hOAT3 together with control vector, Senp2 or the inactive mutant of Senp2 were co-transfected into COS-7 cells for 48h. The cells were then lysed. hOAT3 was immunoprecipitated by anti-Myc antibody, followed by immunoblotting with anti-SUMO2/3 antibody. Bottom panel: The same immunoblot from Fig. 2a, Top panel was reprobed by anti-Myc antibody. (b). Densitometry plot of results from Fig. 2a as well as from other experiments. The values are mean ± SD (n = 3). *P<0.05. NS: statistically not significant.
3.2. Effect of Senp2 on hOAT3 transport activity
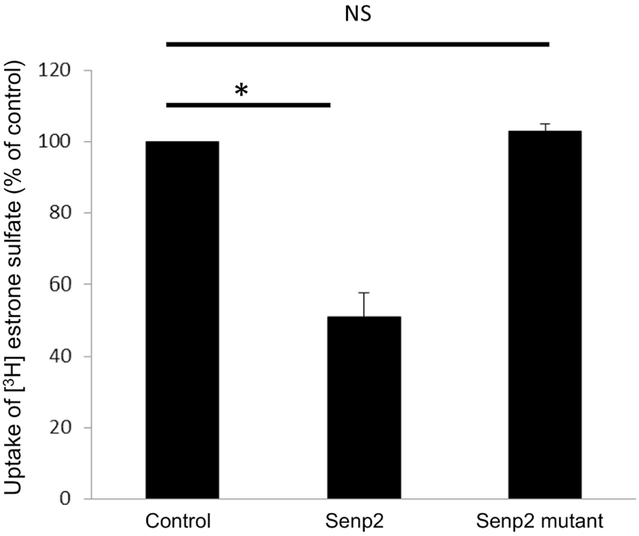
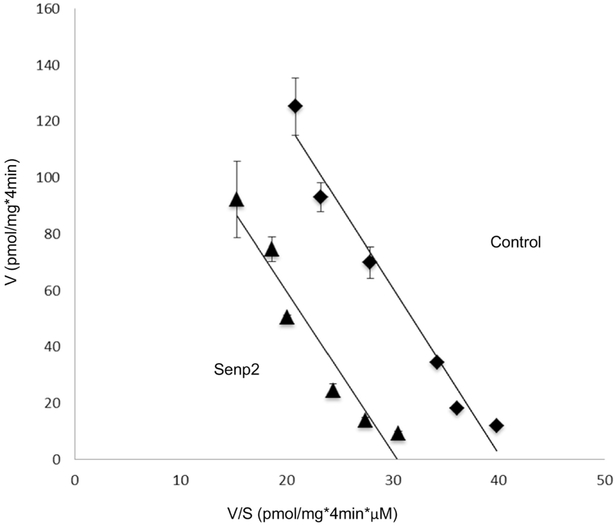
To investigate the role of Senp2 in hOAT3 transport activity, we co-transfected cells with hOAT3 and Senp2 (wild type or mutant), followed by measuring hOAT3-mediated uptake of [3H] estrone sulfate (ES), a prototypical substrate for hOAT3. As shown in Fig. 3, Senp2 significantly inhibited the hOAT3-mediated uptake by ~ 50% in comparison to that in control cells, while the catalytically inactive mutant of Senp2 was lacking any effect. To dissect the mechanism of Senp2-induced suppression of hOAT3 activity, we measured hOAT3-mediated uptake of [3H] ES at various substrate concentrations. An Eadie-Hofstee examination (Fig. 4) showed that Senp2 reduced the maximal transport velocity Vmax of hOAT3 (170.14 ± 14.87 pmol·mg−1·4min−1 with control cells and 235.96 ± 21.73 pmol·mg−·4min−1 with cells transfected with Senp2), without causing notable change in the substratebinding affinity Km of the transporter (5.83 ± 0.28 μM with control cells and 5.54 ± 0.32 μM with cells transfected with Senp2).
Fig. 3. Effect of Senp2 on hOAT3 transport activity.
hOAT3 together with control vector, Senp2 or inactive mutant of Senp2 were co-transfected into COS-7 cells for 48h, followed by the measurement of the uptake of [3H] estrone sulfate (4min, 0.3 μM). Uptake activity was expressed as a percentage of the uptake measured in control cells. The data represent uptake into hOAT3-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± SD. (n = 3). *P<0.05. NS: statistically not significant.
Fig. 4. Effect of Senp2 on the kinetics of hOAT3-mediated transport of estrone sulfate.
hOAT3 together with control vector or Senp2 were co-transfected into COS-7 cells for 48h, and initial uptake (4 min) of [3H] estrone sulfate was measured at the concentration of 0.1–10 μM. The data represent uptake into hOAT3-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are means ± SD (n = 3). V, velocity; S, substrate concentration.
3.3. Effect of Senp2 on hOAT3 Expression
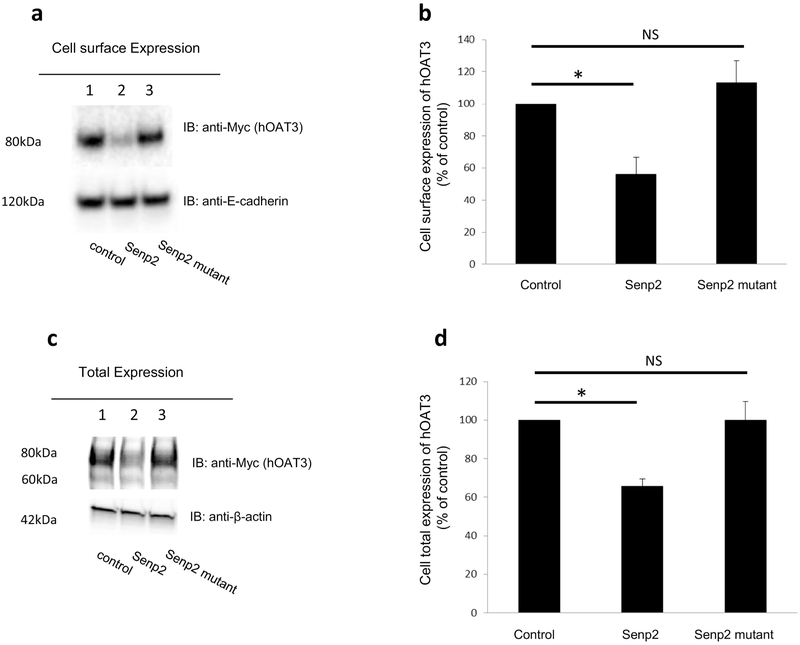
Two possibilities could explain a decreased maximal transport velocity Vmax of hOAT3 shown above: either a reduced quantity of the transporter at the plasma membrane or a reduced turnover rate of the transporter. We performed tests that delineate these possibilities by examining transporter expression both at the plasma membrane and in the total cell extracts. We showed that co-transfection of cells with Senp2 and hOAT3 led to a decrease of hOAT3 expression at the cell surface (Fig. 5a, top panel), and in total cell extracts (Fig. 5c, top panel). Such a change in hOAT3 expression was not owing to the general disruption of membrane and cellular proteins as the expressions of cell surface membrane protein marker E-cadherin and cellular protein marker β-actin were unaffected (Fig. 5a, bottom panel, and Fig. 5c, bottom panel). hOAT3 at the cell surface displayed a single band at 80 kDa (Fig. 5a, top panel), whereas hOAT3 showed two bands at 60 kDa and 80 kDa in total cell extracts (Fig.5c, top panel). Our lab previously illustrated [22, 23] that OAT undergoes glycosylation as a maturation process in the endoplasmic reticulum-Golgi complex. The immature form is a non-glycosylated form of 60 kDa, which matures in ER-Golgi complex to a glycosylated form of 80 kDa. Only the mature form (80 kDa) can target to cell surface.
Fig. 5. Effect of Senp2 on hOAT3 expression.
(a). Cell surface expression of hOAT3. Top panel: hOAT3 together with control vector, Senp2 or the inactive mutant of Senp2 were cotransfected into COS-7 cells for 48h. Transfected cells were labeled with biotin. Biotinylated/cell surface proteins were separated with streptavidin beads, followed by immunoblotting (IB) with an anti-Myc antibody (epitope Myc was tagged to hOAT3 to facilitate the immunodetection). Bottom panel: The same blot as the top panel was re-probed with anti-E-cadherin antibody. E-cadherin is a cell membrane marker protein. (b). Densitometry plot of results from Fig. 5a, Top panel, as well as from other experiments. The values are mean ± SD. (n = 3). *P<0.05. NS: statistically not significant. (c). Total expression of hOAT3. hOAT3 together with control vector, Senp2 or the inactive mutant of Senp2 were co-transfected into COS-7 cells for 48h. Cells were lysed, followed by immunoblotting (IB) with an anti-Myc antibody. (d). Densitometry plot of results from Fig. 5c, top panel as well as from other experiments. The values are mean ± SD (n = 3). *P<0.05. NS: statistically not significant.
3.4. Effectiveness of Senp2-specific siRNA on knocking down the endogenous Senp2
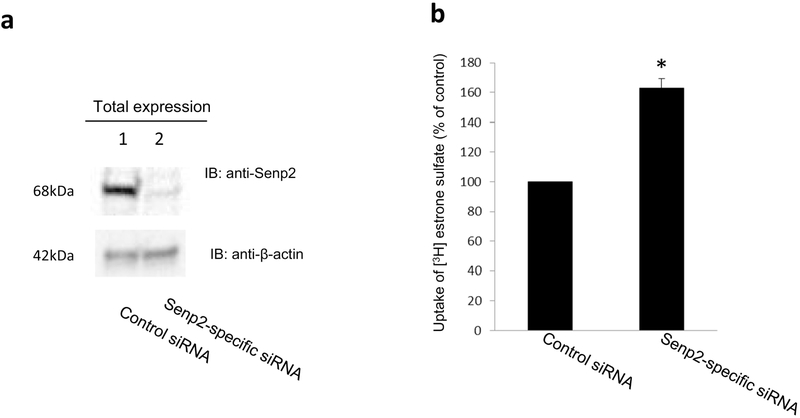
The experiments above (Figs. 1-5) examined the effect of exogenously transfected Senp2 on hOAT3 SUMOylation, expression and transport activity. In the following experiments, we investigated effects of endogenous Senp2 on hOAT3 SUMOylation and transport activity. For such purpose, we first examined the effectiveness of Senp2-specific siRNA on knocking down the endogenous Senp2. As shown in Fig. 6a, Top panel, the expression of endogenous Senp2 was much lower in cells transfected with Senp2-specific siRNA in comparison to that in control cells, whereas the expression of a cellular protein marker actin was unaffected (Fig. 6a, Bottom panel), demonstrating the specificity and effectiveness of the siRNA used for our studies.
Fig. 6. (a) Effect of Senp2-specific siRNA on the expression of endogenous Senp2.
Top panel: COS-7 cells were co-transfected with hOAT3 and scrambled control siRNA or with hOAT3 and Senp2-specific siRNA. The effectiveness of Senp2-specific siRNA was tested by probing the lysis sample with anti-Senp2 antibody. Bottom panel: The same blot from the top panel was re-probed with anti-actin antibody. β-Actin is a cellular marker protein. (b) Effect of Senp2-specific siRNA on hOAT3 transport activity. COS-7 cells were co-transfected with hOAT3 and scrambled control siRNA or with hOAT3 and Senp2-specific siRNA. Transfected cells were then measured for the uptake of [3H]-estrone sulfate (4-min uptake and 0.3 μM estrone sulfate). The data represent uptake into hOAT3-transfected cells minus uptake into mock cells (parental COS-7 cells). Values are mean ± SD (n = 3). *P < 0.05.
3.5. Effect of Senp2-specific siRNA on hOAT3 transport activity
We co-transfected cells with hOAT3 and control siRNA or with hOAT3 and Senp2-specific siRNA. The transfected cells were assessed for hOAT3-mediated uptake of [3H] estrone sulfate (ES). As shown in Fig. 6b, hOAT3 transport activity was significantly enhanced in cells transfected with Senp2-specific siRNA in comparison to that in control cells.
3.6. Effect of Senp2-specific siRNA on hOAT3 Expression
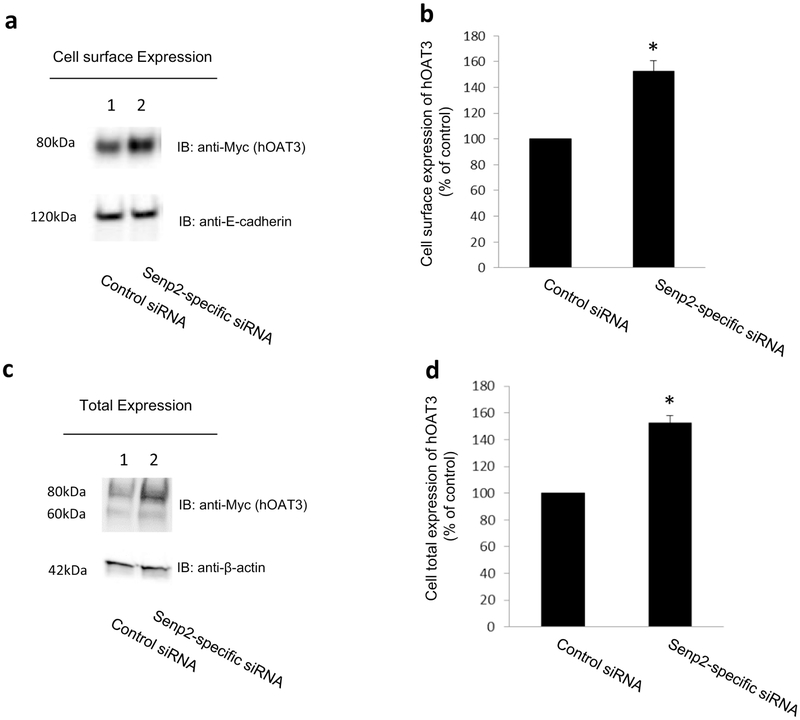
We co-transfected cells with hOAT3 and control siRNA or with hOAT3 and Senp2-specific siRNA. hOAT3 expression in these cells was then examined. Our results showed that knocking down the endogenous Senp2 by Senp2-specific siRNA significantly enhanced the expression of hOAT3 at the cell surface (Fig. 7a, top panel) and in total cell lysates (Fig. 7c, top panel). The expression of the cell surface protein marker E-cadherin (Fig. 7a, bottom panel) and total cell protein marker β-actin (Fig. 7c, bottom panel) was not changed under such condition.
Fig. 7. Effect of Senp2-specific siRNA on hOAT3 expression.
Cell surface expression of hOAT3. Top panel: COS-7 cells were co-transfected with hOAT3 and scrambled control siRNA or with hOAT3 and Senp2-specific siRNA for 48h. Transfected cells were labeled with biotin. Biotinylated/cell surface proteins were separated with streptavidin beads, followed by immunoblotting (IB) with an anti-Myc antibody (epitope Myc was tagged to hOAT3 to facilitate the immunodetection). Bottom panel: The same blot as the top panel was re-probed with anti-E-cadherin antibody. E-cadherin is a cell membrane marker protein. (b). Densitometry plot of results from Fig. 7a, Top panel, as well as from other experiments. The values are mean ± SD. (n = 3). *P<0.05. (c). Total expression of hOAT3. COS-7 cells were co-transfected with hOAT3 and scrambled control siRNA or with hOAT3 and Senp2-specific siRNA for 48h. Cells were lysed, followed by immunoblotting (IB) with an anti-Myc antibody. (d). Densitometry plot of results from Fig. 7c, top panel as well as from other experiments. The values are mean ± SD (n = 3). *P<0.05.
3.7. Effect of Senp2-specific siRNA on hOAT3 SUMOylation
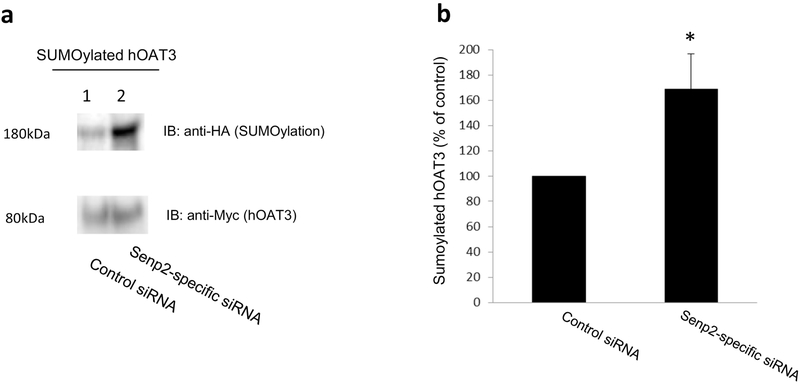
We transfected hOAT3-expressing cells with HA-SUMO2, Ubc9 (a SUMO-conjugating enzyme) and with scramble siRNA or Senp2-specific siRNA. hOAT3 was then pulled down from transfected cells by anti-Myc antibody (hOAT3 was tagged with epitope Myc), followed by immunoblotting with anti-HA antibody. Our results (Fig. 8a, top panel) showed that the level of SUMOylated hOAT3 was much higher in cells transfected with Senp2-specific siRNA in comparison to that in control cells. The difference in hOAT3 SUMOylation was not owing to the different amount of hOAT3 pulled down as hOAT3 was immunoprecipitated with similar quantity in each sample (Fig. 8a, bottom panel).
Fig. 8. Effect of Senp2-specific siRNA on hOAT3 SUMOylation.
(a). Top panel: COS-7 cells were co-transfected with hOAT3, HA-tagged SUMO2, UBC9 and scrambled control siRNA or with hOAT3, HA-SUMO2, UBC9 and Senp2-specific siRNA for 48h. The cells were then lysed. hOAT3 was immunoprecipitated by anti-Myc antibody, followed by immunoblotting (IB) with anti-HA antibody. Bottom panel: The same immunoblot from Fig. 8a, Top panel was reprobed by anti-Myc antibody. (b). Densitometry plot of results from Fig. 8a, as well as from other experiments. The values are mean ± SD (n = 3). *P<0.05.
3.8. The interaction between Senp2 and hOAT3
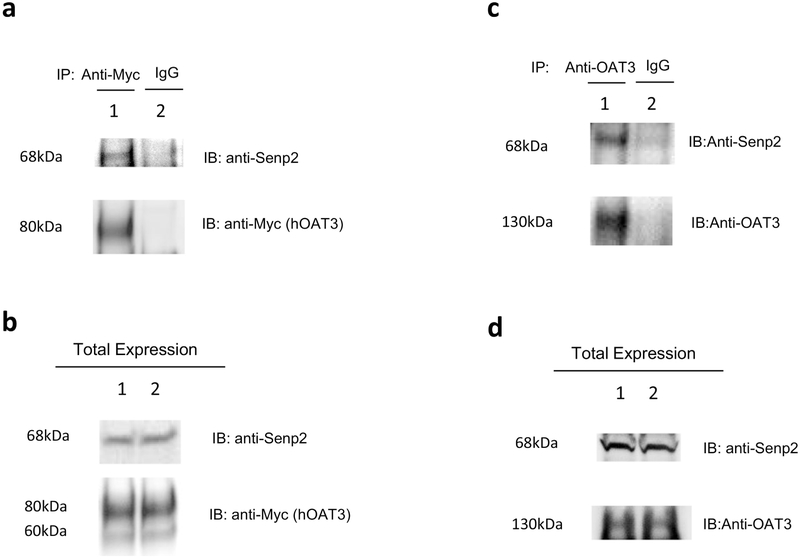
We next assessed whether the effect of Senp2 on hOAT3 occurred through a direct contact between these two proteins. hOAT3-expressing cells were transfected with Senp2. hOAT3 was then pulled down by anti-Myc antibody or normal mouse IgG (as control), followed by immunoblotting with anti-Senp2 antibody. As shown in Fig. 9a, top panel, substantial amount of Senp2 was detected in hOAT3 immunoprecipitates (line 1) in comparison to that in control group (line 2), suggesting a direct association between Senp2 and hOAT3. A similar test was subsequently conducted with rat kidney slice, where both Oat3 and Senp2 were endogenously expressed. As shown in Fig. 9c, top panel, Senp2 was detected in OAT3 immunoprecipitates from the rat kidney slice. These results further confirmed that there was a direct interaction between Oat3 and Senp2 not only in vitro but also in vivo.
Fig. 9. Interaction between Senp2 and hOAT3.
(a) The Interaction of Senp2 with hOAT3 in COS-7 cells. Top panel: COS7 cells were co-transfected with hOAT3 and Senp2. Transfected cells were then lysed, and hOAT3 was immunoprecipitated with anti-Myc antibody or with normal mouse IgG (as negative control), followed by immunoblotting (IB) with anti-Senp2 antibody. Bottom panel: The same immunoblot from Fig. 9a, Top panel was reprobed by anti-Myc antibody. (b) Total expression of hOAT3 and Senp2. COS-7 cells were co-transfected with hOAT3 and Senp2 for 48h. Top panel: Cells were lysed, followed by immunoblotting (IB) with an anti-Senp2 antibody. Bottom panel: The same immunoblot from Fig. 9b, Top panel was reprobed by anti-Myc antibody. Line 1 and line 2 represent 2 samples. (c) The interaction of Senp2 with rOAT3 in rat kidney slice. Top panel: The kidney slices from rat were lysed, and rOAT3 was then immunoprecipitated with anti-OAT3 antibody or with normal mouse IgG (as negative control), followed by immunoblotting (IB) with anti-Senp2 antibody. Bottom panel: The same immunoblot as Fig. 9c, top panel was reprobed by anti-OAT3 antibody. (d) Total expression of rOAT3 and Senp2. Top panel: The kidney slices from rat were lysed, followed by immunoblotting (IB) with an anti-Senp2 antibody. Bottom panel: The same immunoblot from Fig. 9d, Top panel was reprobed by anti-OAT3 antibody. Line 1 and line 2 represent 2 samples.
4. Discussion
Active drug transport mediated by OATs is a major determining factor of the outcomes of therapeutical reagents and toxic chemicals [1-6]. Hence, mechanistic understanding of OAT regulation is clinically and pharmacologically significant. Our current study explored the role of SUMO-specific protease Senp2 in the SUMOylation, expression and transport activity of OAT3 and revealed a function of Senp2 in the regulation of this transporter. We chose to conduct our work in kidney COS-7 cells and in rat kidney slices. COS-7 cells have been widely used as a model system for characterizing OATs because the properties of OATs in these cells mimic those in vivo [7, 8, 24]. The consistent results between COS-7 cells and rat kidney slices, where OAT3 and Senp2 are endogenously expressed, suggest that our studies are physiologically relevant.
Our lab previously established that OATs constitutively internalize from and recycle back to cell surface. We further demonstrated that activation of protein kinase A enhances OAT3 SUMOylation and increases OAT3 expression at the plasma membrane and OAT3 transport activity [25]. Post-translational modification of target protein by SUMOylation is a dynamic and reversible event [16]. The deSUMOylation is catalyzed by a class of SUMO-specific proteases [17]. SUMO-specific protease Senp2 has been shown to regulate several integral membrane proteins [19-21]. However, it is the first study of this protease on any of the drug transporters.
Here we found that overexpression of Senp2 in OAT3-expressing cells resulted in a significant deSUMOylation of the transporter (Fig. 1-2), which correlated well with a decrease of OAT3 expression at the cell surface and a decrease in OAT3-mediated drug transport (Fig. 3-5). The role of Senp2 in hOAT3 was further reinforced when we knocked down the endogenous Senp2 by Senp2-specific siRNA. Knocking down the endogenous Senp2 resulted in a significant increase in OAT3 SUMOylation, which correlated well with an increase in OAT3 expression and OAT3-mediated drug transport (Fig. 6-8).
Senp2 expression has been reported in the major human tissues such as brain, liver, lung and kidney [26, 27]. Our co-immunoprecipitation experiment showed that Senp2 is physically associated with OAT3 both in cultured cells and in rat kidney suggesting the Senp2 may regulate OAT3 through a direct interaction (Fig. 9). The senp2-specific SUMOylation site(s) will be identified by mass spectroscopy in conjunction with site-directed mutagenesis.
Senps are crucial in maintaining a balance between SUMOylated and un-SUMOylated proteins which are essential for normal cellular physiology. A number of studies have reported the changes of Senps expression level under pathophysiological conditions and Senps were linked to the progress of various diseases, particular cancer. For instance, Senp2 was down-regulated in bladder cancer and hepatocellular carcinoma (HCC) tissues, and the overexpression of Senp2 contributed to the suppressions on both bladder cancer metastasis and HCC development [21, 29]. In addition, Senp2 regulated the deSUMOylation of p53, which was an important protein to suppress the development of various tumors. Beside the cancers, the overexpression of Senp2 contributed to congenital heart defects and cardiac dysfunction through stimulating deSUMOylation. Therefore, Senps have become attractive targets for drug discovery. 1,2,5-Oxadiazoles have been developed as a novel class of Senp2 inhibitors, which could have therapeutic potential of various diseases [30]. Further studies investigating the effect of Senp2 inhibitors on hOAT3 transport activity, surface expression and SUMOylation would be very interesting.
5. Conclusions
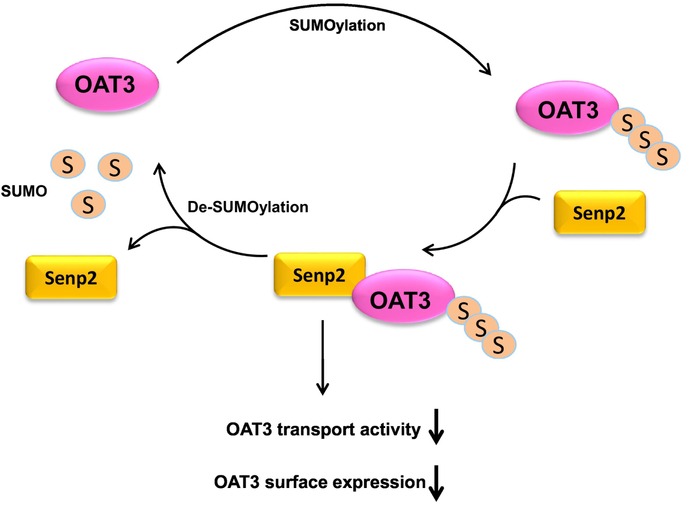
In summary, our investigation identifies a functional role of Senp2 in regulating OAT3 (Fig. 10) and suggests its candidacy as a new target for modulating OAT3-mediated drug transport. In vivo study about the role of Senp2 in OAT3 activity will be our future effort.
Fig. 10. Senp2 Regulates SUMOylation, Expression and Function of hOAT3.
Highlights.
Overexpression of Senp2 in renal cells led to a reduced OAT3 SUMOylation, which correlated well with a decreased OAT3 expression and transport activity.
Knocking-down the endogenous Senp2 in renal cells resulted in an increased OAT3 SUMOylation, which correlated well with an enhanced OAT3 expression and transport activity.
Senp2 regulated OAT3 SUMOylation, expression and transport activity by directly interacting with OAT3 both in cultured cells and in rat kidneys.
Acknowledgement
This work was supported by grants (to Dr. Guofeng You) from National Institute of General Medical Sciences (R01-GM079123 and R01-GM097000).
We would like to thank Dr. Jorge A Iñiguez-Lluhí for his generous gifts of cDNAs for SUMO-2 and Ubc9.
Abbreviation:
- OAT
Organic Anion Transporter
- ES
Estrone Sulfate
- PKC
Protein Kinase C
- PKA
Protein Kinase A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- [1].You G, Structure, function, and regulation of renal organic anion transporters, Med Res Rev, 22 (2002) 602–616. [DOI] [PubMed] [Google Scholar]
- [2].Srimaroeng C, Perry JL, Pritchard JB, Physiology, structure, and regulation of the cloned organic anion transporters, Xenobiotica, 38 (2008) 889–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dantzler WH, Wright SH, The molecular and cellular physiology of basolateral organic anion transport in mammalian renal tubules, Biochim Biophys Acta, 1618 (2003) 185–193. [DOI] [PubMed] [Google Scholar]
- [4].VanWert AL, Gionfriddo MR, Sweet DH, Organic anion transporters: discovery, pharmacology, regulation and roles in pathophysiology, Biopharm Drug Dispos, 31 (2010) 1–71. [DOI] [PubMed] [Google Scholar]
- [5].Ahn SY, Nigam SK, Toward a systems level understanding of organic anion and other multispecific drug transporters: a remote sensing and signaling hypothesis, Mol Pharmacol, 76 (2009) 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Terada T, Inui K, Gene expression and regulation of drug transporters in the intestine and kidney, Biochem Pharmacol, 73 (2007) 440–449. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Q, Hong M, Duan P, Pan Z, Ma J, You G, Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway, J Biol Chem, 283 (2008) 32570–32579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang Q, Li S, Patterson C, You G, Lysine 48-linked polyubiquitination of organic anion transporter-1 is essential for its protein kinase C-regulated endocytosis, Mol Pharmacol, 83 (2013) 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang Q, Suh W, Pan Z, You G, Short-term and long-term effects of protein kinase C on the trafficking and stability of human organic anion transporter 3, Int J Biochem Mol Biol, 3 (2012) 242–249. [PMC free article] [PubMed] [Google Scholar]
- [10].Xu D, Zhang J, Zhang Q, Fan Y, Liu C, You G, PKC/Nedd4-2 Signaling Pathway Regulates the Cell Surface Expression of Drug Transporter hOAT1, Drug Metab Dispos, 45 (2017) 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gareau JR, Lima CD, The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition, Nat Rev Mol Cell Biol, 11 (2010) 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ulrich HD, The fast-growing business of SUMO chains, Mol Cell, 32 (2008) 301–305. [DOI] [PubMed] [Google Scholar]
- [13].Desterro JM, Rodriguez MS, Hay RT, SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation, Mol Cell, 2 (1998) 233–239. [DOI] [PubMed] [Google Scholar]
- [14].Ulrich HD, Mutual interactions between the SUMO and ubiquitin systems: a plea of no contest, Trends Cell Biol, 15 (2005) 525–532. [DOI] [PubMed] [Google Scholar]
- [15].Wang H, You G. Regulation of Human Organic Anion Transporters 3 by Protein Kinases A: Crosstalk between Sumoylation and Ubiqitination (abstract), American Association of Pharmaceutical Scientists (AAPS) Annual Meeting; Nov 4-7 2018; Washington, DC Abstract No. W1030-05-036, (2018). [Google Scholar]
- [16].Yeh ET, Gong L, Kamitani T, Ubiquitin-like proteins: new wines in new bottles, Gene, 248 (2000) 1–14. [DOI] [PubMed] [Google Scholar]
- [17].Yeh ET, SUMOylation and De-SUMOylation: wrestling with life's processes, J Biol Chem, 284 (2009) 8223–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Itahana Y, Yeh ET, Zhang Y, Nucleocytoplasmic shuttling modulates activity and ubiquitination-dependent turnover of SUMO-specific protease 2, Mol Cell Biol, 26 (2006) 4675–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Benson MD, Li QJ, Kieckhafer K, Dudek D, Whorton MR, Sunahara RK, Iniguez-Lluhi JA, Martens JR, SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5, Proc Natl Acad Sci U S A, 104 (2007) 1805–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qi Y, Wang J, Bomben VC, Li DP, Chen SR, Sun H, Xi Y, Reed JG, Cheng J, Pan HL, Noebels JL, Yeh ET, Hyper-SUMOylation of the Kv7 potassium channel diminishes the M-current leading to seizures and sudden death, Neuron, 83 (2014) 1159–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tan M, Zhang D, Zhang E, Xu D, Liu Z, Qiu J, Fan Y, Shen B, SENP2 suppresses epithelial-mesenchymal transition of bladder cancer cells through deSUMOylation of TGF-betaRI, Mol Carcinog, 56 (2017) 2332–2341. [DOI] [PubMed] [Google Scholar]
- [22].Zhou F, Xu W, Hong M, Pan Z, Sinko PJ, Ma J, You G, The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4, Mol Pharmacol, 67 (2005) 868–876. [DOI] [PubMed] [Google Scholar]
- [23].Tanaka K, Xu W, Zhou F, You G, Role of glycosylation in the organic anion transporter OAT1, J Biol Chem, 279 (2004) 14961–14966. [DOI] [PubMed] [Google Scholar]
- [24].Duan P, Li S, You G, Angiotensin II inhibits activity of human organic anion transporter 3 through activation of protein kinase Calpha: accelerating endocytosis of the transporter, Eur J Pharmacol, 627 (2010) 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang H, Zhang J, You G, Activation of Protein Kinase A Stimulates SUMOylation, Expression, and Transport Activity of Organic Anion Transporter 3, AAPS J, 21 (2019) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F, Proteomics. Tissue-based map of the human proteome, Science, 347 (2015) 1260419. [DOI] [PubMed] [Google Scholar]
- [27].Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Bjorling L, Ponten F, Towards a knowledge-based Human Protein Atlas, Nat Biotechnol, 28 (2010) 1248–1250. [DOI] [PubMed] [Google Scholar]
- [28].Li S, Zhang Q, You G, Three ubiquitination sites of organic anion transporter-1 synergistically mediate protein kinase C-dependent endocytosis of the transporter, Mol Pharmacol, 84 (2013) 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shen HJ, Zhu HY, Yang C, Ji F, SENP2 regulates hepatocellular carcinoma cell growth by modulating the stability of beta-catenin, Asian Pac J Cancer Prev, 13 (2012) 3583–3587. [DOI] [PubMed] [Google Scholar]
- [30].Kumar A, Ito A, Takemoto M, Yoshida M, Zhang KY, Identification of 1,2,5-oxadiazoles as a new class of SENP2 inhibitors using structure based virtual screening, J Chem Inf Model, 54 (2014) 870–880. [DOI] [PubMed] [Google Scholar]