Abstract
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder and is characterized by the loss of neurons in the substantia nigra that project to the striatum and release dopamine (DA), which is required for normal movement. Common non-motor symptoms likely involve abnormalities with other neurotransmitters, such as serotonin, norepinephrine, acetylcholine, glycine, glutamate and gamma-aminobutyric acid (GABA). As part of a broad effort to provide better PD research tools, the Michael J. Fox Foundation for Parkinson’s Research funded the generation and characterization of knockout (KO) rats for genes with PD-linked mutations, including PINK1, Parkin, DJ-1 and LRRK2. Here we extend the phenotypic characterization of these lines of KO rats to include in vivo microdialysis to measure both basal and potassium-induced release of the above neurotransmitters and their metabolites in the striatum of awake and freely moving rats at ages 4, 8 and 12 months compared to wild-type (WT) rats. We found age-dependent abnormalities in basal DA, glutamate and acetylcholine in PINK1 KO rats and age-dependent abnormalities in basal DA metabolites in Parkin and LRRK2 KO rats. Parkin KO rats had increased glycine release while DJ-1 KO rats had decreased glutamate release and increased acetylcholine release compared to WT rats. All lines except DJ-1 KO rats showed age-dependent changes in release of one or more neurotransmitters. Our data suggest these rats may be useful for studies of PD-related synaptic dysfunction and neurotransmitter dynamics as well as studies of the normal and pathogenic functions of these genes with PD-linked mutations.
Keywords: Parkinson’s disease, microdialysis, animal model
Introduction
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder. Clinically, PD is defined by slowness of movement, rigidity, postural instability, gait abnormalities, and tremor. The motor symptoms are predominantly due to the loss of neurons in the substantia nigra pars compacta that project to the striatum and release dopamine (DA), which is required for normal movement. Symptoms typically emerge gradually and, in all cases, increase in severity over the course of many years. In addition to prominent motor symptoms, many non-motor PD symptoms are common, including gastrointestinal or genito-urinary autonomic dysfunction, sleep disorders, reduced (hyposmia) or complete loss of smell (anosmia), anxiety, depression, and cognitive deficits, especially at advanced stages (Zis et al., 2015;Sauerbier et al., 2016;Schapira et al., 2017). Motor symptoms are most widely treated pharmacologically with DA replacement therapy in the form of the DA precursor L-DOPA, which can be administered orally with a non-brain penetrant aromatic amino acid decarboxylase inhibitor to allow brain-restricted conversion of L-DOPA to DA and to minimize adverse effects caused by peripheral DA. Most non-motor symptoms are not effectively treated by L-DOPA and deficits in other neurotransmitters in the striatum and other parts of the brain have been implicated, including serotonin, acetylcholine, and norepinephrine (Schapira et al., 2017). These neurotransmitters are most abundant in brain regions other than the basal ganglia that are also affected in PD, including the dorsal raphe (the largest serotonergic nucleus), the locus coeruleus (the principle noradrenergic nucleus) and the nucleus basalis (rich in acetylcholine). The striatum receives some projections from these areas in addition to the major glutamatergic projections from the cortex and thalamus as well as dopaminergic projections from the substantia nigra.
There are currently no treatments that are proven to slow down PD progression, which is attributed to the continuing loss of neurons, synapses and neurotransmitters. For most cases, the cause of PD remains unknown. The primary risk factor is age, with peak incidence at age 70-79 and greater incidence for males compared to females (Hirsch et al., 2016). Although historically PD was not thought to have a strong genetic component, numerous genes have been identified in the past two decades with mutations causally linked to PD or with polymorphisms associated with risk of developing PD (Deng et al., 2018). The identification of human mutations causally linked to inherited forms of PD provided the opportunity to generate genetic PD animal models with the potential to reproduce the spontaneous adult-onset and progressive neurodegeneration characteristics of PD. Loss-of-function mutations in PINK1, Parkin and DJ-1 have been linked to recessively inherited PD and gain-of-function mutations in LRRK2 and α-synuclein have been linked to dominantly inherited PD (Polymeropoulos et al., 1997;Kitada et al., 1998;Bonifati et al., 2003;Paisan-Ruiz et al., 2004;Valente et al., 2004;Zimprich et al., 2004). Although the expression of these genes is not restricted to the nigrostriatal pathway, many studies have suggested they could be important for regulating neurotransmitter release, reuptake or turnover (Goldberg et al., 2003;Goldberg et al., 2005;Kitada et al., 2007;Melrose et al., 2010).
Preclinical research on Parkinson’s disease molecular mechanisms, pathophysiology and therapeutics relies heavily on rodent models of PD. In an effort to obtain better animal models of PD and to accelerate preclinical PD research, the Michael J. Fox Foundation for Parkinson’s research has generated over 30 lines of mice and rats with targeted disruption, commonly referred to as gene knockout (KO), or transgenic expression of genes with mutations linked to PD (Baptista et al., 2013). As part of this effort, rats with targeted disruption of the PD-linked genes PINK1, Parkin, DJ-1 and LRRK2 were generated and the loss-of-function genetic models were systematically characterized at ages 4, 6 and 8 months for locomotor behavior, neurochemistry and neuropathology (Dave et al., 2014). PINK1 and DJ-1, but not Parkin, KO rats showed locomotor deficits and progressive neurodegeneration with ~50% loss of dopaminergic neurons in the substantia nigra at age 8 months along with a significant increase in total striatal tissue content of DA and serotonin (5-HT) at age 8 months compared to WT rats (Dave et al., 2014). Here we describe the results of extending the phenotypic characterization of these lines of knockout rats to include in vivo microdialysis to sample neurotransmitters in the striatum of awake and freely moving rats at ages 4, 8 and 12 months. Microdialysis provides a means to measure both basal and stimulated release of neurotransmitters and metabolites with ~10 minute temporal resolution. This widely-used method has been employed for decades to study numerous animal models of Parkinson’s disease and other neurological disorders (Di Giovanni et al., 2009).
In addition to providing a more rigorous and thorough characterization of these rat PD models, this study was motivated by the need to better understand the earliest stages of PD-related neurodegeneration, where efforts to develop neuroprotective therapies to slow disease progression may be most fruitful. It is widely accepted that substantial neurodegeneration occurs prior to the onset of motor symptoms and, for many patients, the initial symptoms appear months or years before the clinical diagnosis of PD. Multiple lines of evidence indicate that degeneration of axon terminals is one of the earliest stages of PD and that abnormal nerve terminal function precedes the loss of dopaminergic neuronal cell bodies in the substantia nigra (Burke and O’Malley, 2013). Extrapolation from longitudinal PET imaging studies of PD cases and controls using multiple radioligands to measure nigrostriatal axon terminal integrity suggests that compensatory synaptic adaptations occur at early PD stages (de la Fuente-Fernandez et al., 2011). Similar studies indicate abnormalities in DA turnover (de la Fuente-Fernandez et al., 2001). There is also evidence that compensatory synaptic adaptations other than altered DA release or metabolism likely occur in PD (Bezard et al., 2003). This prompted us to investigate the extent to which any of these KO rats could serve as animal models for studying these phenomena, even in the absence of significant neuronal loss.
Furthermore, postmortem analyses show significantly reduced levels of 5-HT and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the striatum of PD cases compared to controls, which may partly underlie motor and non-motor symptoms (Kish et al., 2008). Loss of noradrenergic axon terminals has been associated with anxiety and depression symptoms in PD (Remy et al., 2005). Depression and cognitive deficits in PD have also been linked to degeneration of cholinergic terminals (Bohnen et al., 2007). Therefore, we sought to determine the extent to which Parkin, PINK1, DJ-1 or LRRK2 KO rats at ages 4, 8 and 12 months have altered basal neurotransmitter levels or potassium-evoked neurotransmitter release as well as alterations in turnover, measured by levels of neurotransmitter metabolites. We used in vivo microdialysis and mass spectrometry to measure striatal levels of DA, the DA metabolites 3,4-dihydroxyphenylacetic (DOPAC) and homovanillic acid (HVA), 5-HT and its metabolite 5-HIAA, as well as other neurotransmitters including acetylcholine (Ach), norepinephrine (NE), glutamate (Glu) glycine (Gly), and gamma-aminobutyric acid (GABA), which are important for synaptic plasticity at striatal terminals and may also relate to the non-motor symptoms of PD.
Experimental Procedures
Animals
Rats with targeted disruption of Parkin, DJ-1, PINK1 and LRRK2 genes were generated as previously described (Dave et al., 2014). Rats were maintained on a Long Evans Hooded genetic background from Charles River Laboratories (Crl: LE). Prior to surgery, male wild-type (WT) and knockout (KO) rats were housed 2–3 animals per cage with ad libitum access to food and water and maintained on a 12/12 hour light/dark cycle with lights on at 7 AM. All experiments were standardized for each age and genotype and were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of Brains On-Line, LLC and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Sample sizes
Separate cohorts of male WT and KO rats were studied at ages 4, 8 and 12 months. The sample sizes used for analysis were as follows: at age 4 months, n=10 WT, n=10 Parkin KO, n=10 LRRK2 KO, n=10 PINK1 KO, n=9 DJ-1 KO; at age 8 months, n=9 WT, n=10 Parkin KO, n=8 LRRK2 KO, n=10 PINK1 KO, n=9 DJ-1 KO; at age 12 months, n=14 WT, n=10 Parkin KO, n=7 LRRK2 KO, n=11 PINK1 KO, n=11 DJ-1 KO.
Surgeries
Rats were anesthetized using isoflurane (2%, 800 mL/min O2). Bupivacaine/epinephrine was used for local analgesia and carprofen was used for peri-/post-operative analgesia. Anesthetized animals were placed in a stereotaxic frame (Kopf instruments, USA). I-shaped microdialysis probes (polyacrylonitrile membrane; BrainLink, the Netherlands) were inserted vertically into the striatum (STR; 3 mm exposed surface) with the following coordinates for the tips of the probes: posterior (AP) = +0.9 mm to bregma, lateral (L) = −3.0 mm to midline and ventral (V) = −6.0 mm to dura with the toothbar set at −3.3 mm (Paxinos and Watson, 2007). After surgery, animals were housed individually with food and water ad libitum and maintained on a 12/12 hour light/dark cycle with lights turned on at 7 AM. Dialysates were collected between 10 AM and 4 PM within individual home cages, as described below.
Microdialysis
One day after surgery, microdialysis probes were connected with flexible PEEK tubing to a microperfusion pump (Harvard PHD 2000 Syringe pump, Holliston, MA or similar). Microdialysis probes were perfused with artificial cerebrospinal fluid (aCSF) containing 147 mM NaCl, 3.0 mM KCl, 1.2 mM CaCl2 and 1.2 mM MgCl2, at a flow rate of 1.5 μL/min. Microdialysis samples were collected in 20 minute periods by an automated fraction collector (820 Microsampler, Univentor, Malta) into 300uL polystyrene mini-vials already containing 10 μL 0.02 M formic acid (FA) and 0.04% ascorbic acid in ultrapurified H2O. Four basal samples were collected before the perfusion solution was switched to aCSF containing 60 mM KCl (90 mM NaCl, 60 mM KCl, 1.2 mM CaCl2, and 1.2 mM MgCl2). Infusion of high potassium aCSF was continued for 60 min. After 60 min, the perfusion solution was switched back to aCSF and samples were collected for an additional 120 minutes. All the dialysis samples were stored at −80 °C until analysis by mass spectrometry (MS) using established methods essentially as previously published and described in detail below (Giorgetti et al., 2010;DeKorver et al., 2017). Following experimentation, rats were sacrificed, and brains were harvested and analyzed for probe placement verification. Tail biopsies were collected and analyzed for genotype verification.
Analysis of neurotransmitters and metabolites
Acetylcholine:
Samples (10 μl) were mixed with 4 μl internal standard (acetylcholine-d9) and 5 μl of the mixture was injected into the HPLC-MS/MS system by an automated sample injector (SIL-20ACHT, Shimadzu, Japan). Chromatographic separation was performed on an ion exchange (150 × 2.1 mm, 5 μm) analytical column (Thermo Scientific BioBasic SCX, keystone, USA) held at a temperature of 30° C. Components were separated using a linear gradient of 15 mM ammonium acetate and 10 mM ammonium formate to 2 mM ammonium formate in acetonitrile (ACN)/ultrapurified H2O (80/20 %v/v) (pH = 4.0; flow rate 0.3 mL/min). The flow of the LC was directed to the MS from 2.5 to 5.2 minutes of the run for detection of acetylcholine (post column make up flow 50 μL/min (acetonitrile/0.1% FA)). MS analyses were performed using an API 4000 MS/MS system consisting of an API 4000 MS/MS detector and a Turbo Ion Spray interface (both from AB Sciex, USA). The acquisitions were performed in positive ionization mode, with ion spray voltage set at 4.5 kV with a probe temperature of 450° C. The instrument was operated in multiple-reaction-monitoring (MRM) mode. Data were calibrated and quantified using the Analyst data system (Applied Biosystem, version 1.4.2).
Glutamate, glycine, GABA, norepinephrine, dopamine and serotonin:
Samples (20 μl) were mixed with 4 μl internal standard (deuterated version of the respective analytes) and were derivatized with SymDaq automatically in the autosampler (SIL-10ADvp, Shimadzu, Japan), by addition of 30 μL of reagent into the sample vial. After a reaction time of 2 minutes 45 μl of the mixture was injected into the LC system by an automated sample injector (SIL-10ADvp, Shimadzu, Japan). Chromatographic separation was performed on a reversed phase Phenomenex Synergi max-RP 3.0 * 100 mm, particle size: 2.5 μm (Phenomenex, USA) held at a temperature of 35° C. Components were separated using a linear gradient of mobile phase B consisting of 70 % ACN, 30 % ultrapurified H2O and 0.1%FA and mobile phase A which consisted of 99.7 % ultrapurified H2O, 0.2 % ACN and 0.1% FA at a flow rate 0.35 ml/min. The flow of the LC was diverted to the waste for 3.9 minutes, after which it was switched to the MS for detection of the neurotransmitters. MS analyses were performed using an API 4000 MS/MS system consisting of an API 4000 MS/MS detector and a Turbo Ion Spray interface (both from Applied Biosystems, USA). The acquisitions were performed in positive ionization mode, with ion spray voltage set at 3 kV with a probe temperature of 200°C. The instrument was operated in multiple-reaction-monitoring (MRM). The collision gas (nitrogen) pressure was held at 2 psig. Data were calibrated and quantified using the Analyst data system (Applied Biosystem, version 1.4.2).
DOPAC, HVA and 5-HIAA:
5 μl of dialysate was mixed with 19 μl internal standard and injected into the LC system (Agilent infinity binary pump 1290, USA) by an automated sample injector (CTC, PAL Switzerland). The analytical column used for separation was a Luna C18(2)-HST 100 × 3.0 mm, particle size: 2.5 micron (Phenomenex, USA). Components were separated using a linear gradient of ACN/0.1% FA in ultrapurified H2O/ACN 99.8/0.2 + 0.1% FA 5 mM ammonium formate (flow rate 0.3 ml/min). MS analyses were performed using an API 5500 MS/MS QTrap system consisting of an API 5500 MS/MS detector and a Turbo Ion Spray interface (both from Applied Biosystems, USA). The acquisitions were performed in negative ionization mode, with ion spray voltage set at −4.5 kV with a probe temperature of 500°C. The instrument was operated in multiple-reaction-monitoring (MRM). The collision gas (nitrogen) pressure was held at the medium setting. Data were calibrated and quantified using the Analyst data system (Applied Biosystems, version 1.5.2).
Data analysis
Neurotransmitter/metabolite concentrations were calculated based on deuterated internal standards that were added to each sample, as described above. For each animal, baseline neurotransmitter levels were calculated as the mean of the first 4 samples collected (designated as time points −60, −40, −20 and 0 minutes prior to switching the aCSF in the perfusate from 3 mM to 60 mM KCl). Potassium-evoked neurotransmitter levels were calculated as the sum of the baseline-normalized area under the curve (AUC) for the 3 samples collected 20, 40 and 60 minutes after switching the perfusate from 3 mM to 60 mM KCl.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 7.0). All data were normally distributed according to both the Shapiro Wilk normality test and the D’Agostino and Pearson’s normality test, except for basal glutamate, which was log transformed to yield a normal distribution. All data were determined to have equal variances using the Brown-Forsythe test, except for post-potassium GABA prior to log transformation. Means were compared using 2-way ANOVA, with age and genotype as factors. Neurotransmitter levels of each genotype were compared to WT at each age and Dunnett’s multiple comparisons test was used to determine significance. For comparisons between each age within genotypes, the arithmetic mean of each age group was compared to every other age group and Tukey’s multiple comparisons test was used to determine significance. Prism adjusted p values (taking into account multiple comparisons of genotypes and ages) less than 0.05 were considered statistically significant.
Results
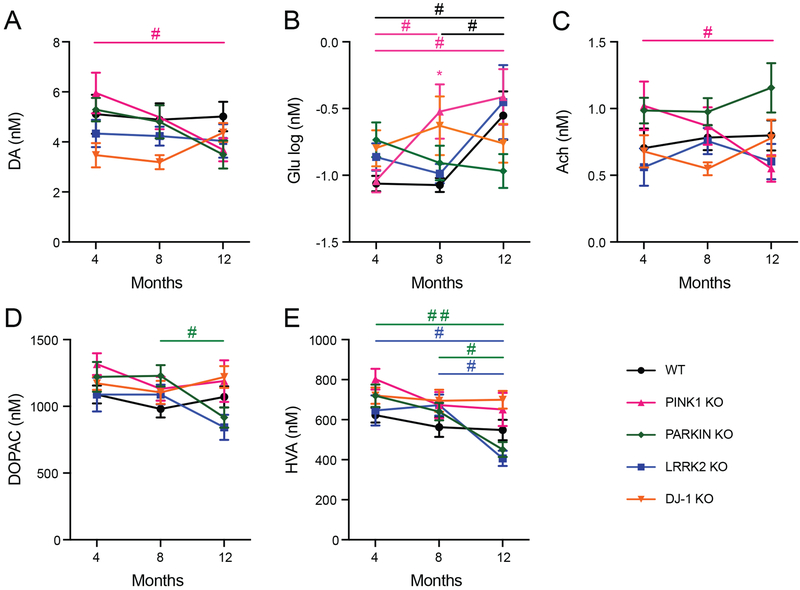
In vivo microdialysis was used to sample extracellular levels of neurotransmitters in the dorsal striatum of awake and freely moving WT and KO rats at ages 4, 8 and 12 months. We first examined basal levels of neurotransmitters and metabolites in each line of KO rats relative to WT control rats at each age. The day after the microdialysis probe was surgically implanted, aCSF was perfused through the probe at a flow rate of 1.5 μL/minute and perfusate samples were continuously collected over 20-minute periods. Frozen perfusate samples were subsequently analyzed by mass spectrometry and compared to internal standards to determine the concentration of each analyte. The values from four consecutive samples were averaged to obtain the basal level for each rat, then the means for each genotype were compared to WT controls. 2-way ANOVA with age and genotype as factors showed a main effect of genotype for DA [F(4, 132) = 2.71, p ≤ 0.05], HVA [F(4, 134) = 4.43, p ≤ 0.01], and Ach [F(4, 132) = 4.36, p ≤ 0.01], and a main effect of age for HVA [F(2, 134) = 9.77, p ≤ 0.001], Glu [F(2, 134) = 4.08, p ≤ 0.05], Gly [F(2, 133) = 4.66, p ≤ 0.05], and GABA [F(2, 132) = 3.18, p ≤ 0.05], as well as a significant interaction between age and genotype for Glu [F(8, 134) = 2.14, p ≤ 0.05].
Tukey’s multiple comparisons test was used for pairwise comparisons of basal levels among the different age groups of each line. As shown by the pound signs in Figure 1, age-dependent changes were found in the levels of DA, Glu, Ach, DOPAC and HVA (Figure 1 A–E respectively). PINK1 KO rats had decreased DA and Ach at age 12 months compared to 4 months, as well as decreased Glu at 4 months compared to 8 and 12 months (Figure 1 A–C). The levels of DA and Ach in PINK1 KO rats at age 8 months were intermediate and not significantly different compared to 4 months or 12 months. Parkin KO rats showed a decrease in DOPAC and HVA at age 12 months compared to 8 months (Figure 1D, E), as well as a decrease in HVA at 12 months compared to 8 months of age. LRRK2 KO rats showed a similar decrease in HVA at age 12 months compared to 4 months (Figure 1E). The only significant age-dependent changes observed for WT rats was Glu, which was significantly higher at age 12 months compared to 4 and 8 months. All other neurotransmitters tested showed no changes with respect to age in any of the animal models in this study.
Figure 1. Basal levels of neurotransmitters with significant differences.
Mean +/−SEM basal levels of DA (A), Glu (B), Ach (C), DOPAC (D) and HVA (E) in striatal microdialysates of rats at ages 4, 8 and 12 months. Asterisk shows significant difference between WT and KO line indicated by color legend (* p ≤ 0.05, Dunnett’s Multiple Comparisons test). Pound signs show significant differences between ages within each line indicated by color legend (# p ≤ 0.05, ## p ≤ 0.01, Tukey’s Multiple Comparisons test).
Pairwise comparisons between WT and mutant rats using Dunnett’s multiple comparisons test showed a significant increase in basal Glu in PINK1 KO rats compared to WT rats at age 8 months (Figure 1B; asterisk p ≤ 0.05). Notable trends were also observed with PINK1 KO rats for an increase in HVA at 4 months (p= 0.073) and an increase in Gly at 8 months (p= 0.057) compared to WT rats of the same age. Appendix A lists the mean +/−SEM basal levels of Gly, 5-HT, 5HIAA, NE and GABA, which showed no significant differences between ages or between WT and KO rats.
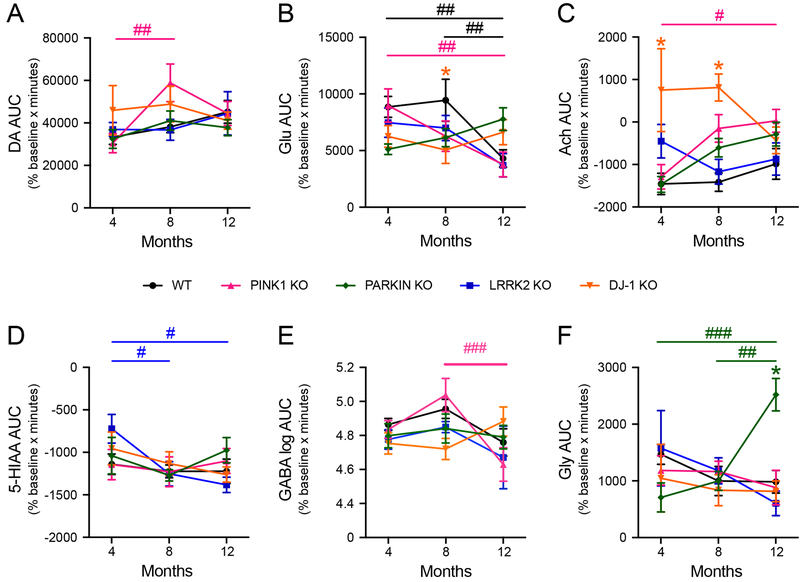
After collecting samples for measuring basal levels, neurotransmitter release was assessed by switching the perfusate to aCSF containing 60 mM KCl for 60 minutes to induce release of synaptic vesicles, then the perfusion solution was switched back to aCSF containing 3 mM KCl and samples were collected for an additional 120 minutes. For all rats, the infusion of high potassium induced substantial increases in extracellular neurotransmitter levels compared to baseline and decreases in metabolites, as expected from previous studies (Stanford et al., 2000). Neurotransmitter release was quantified as the area under the curve (AUC) of the percent basal levels for the first 3 microdialysate samples collected 0–20, 20–40 and 40–60 minutes after switching to high potassium. 2-Way ANOVA with genotype and age as factors showed a main effect of genotype for Ach [F(4, 128) = 8.05, p ≤ 0.001] and NE [F(4, 118) = 2.90, p ≤ 0.05], a main effect of age for Glu [F(2, 133) = 4.71, p ≤ 0.01], GABA [F(2, 132) = 3.20, p ≤ 0.05], and 5-HIAA [F(2, 131) = 3.21, p ≤ 0.05], and a significant genotype-age interaction for Glu [F(8, 133) = 3.13, p ≤ 0.01], Ach [F(8, 128) = 2.64, p ≤ 0.01], and Gly [F(8, 134) = 3.04, p ≤ 0.01].
Age-dependent changes in neurotransmitter release were evaluated using Tukey’s multiple comparisons test. PINK1 KO rats showed significant perturbations in release of multiple neurotransmitters following potassium infusion. PINK1 KO rats showed a significant increase in DA release at 8 months compared to 4 months (Figure 2A), a significant decrease in Glu release at 12 months compared to 4 months (Figure 2B), and a significant increase in Ach release at 12 months compared 4 months (Figure 2C). PINK1 KO rats also showed a significant decrease in GABA release at 12 months compared to 8 months (Figure 2E). We observed significant decreases in extracellular 5-HIAA in LRRK2 KO rats at 8 months and 12 months compared to age 4 months (Figure 2D). We found a significant increase in Gly release in Parkin KO rats at 12 months compared 4 and 8 months (Figure 2F). It should be noted that while WT rats showed no age-dependent changes in potassium evoked release in all other neurotransmitters, there was a decrease in Glu release at 12 months compared to both 4 and 8 months (Figure 2B).
Figure 2. Potassium-evoked levels of neurotransmitters with significant differences.
Mean +/−SEM basal levels of DA (A), Glu (B), Ach (C), 5-HIAA (D), GABA (E) and Gly (F) in striatal microdialysates of rats at ages 4, 8 and 12 months. Asterisks show significant differences between WT and KO lines indicated by color legend (* p ≤ 0.05, Dunnett’s Multiple Comparisons test). Pound signs show significant differences between ages within each line indicated by color legend (# p ≤ 0.05, ## p ≤ 0.01, ### p ≤ 0.001, Tukey’s Multiple Comparisons test).
Pairwise comparisons using Dunnett’s multiple comparison test showed that DJ-1 KO rats had decreased Glu release at 8 months (Figure 2B) and increased Ach release at both 4 and 8 months compared to age matched WT rats (Figure 2C). Parkin KO rats at age 12 months showed an increase in potassium-evoked Gly compared to WT rats (Figure 2F). Although 2-way ANOVA showed a main effect of genotype for NE, there was not a significant main effect of age or interaction between age and genotype and Dunnett’s multiple comparisons test showed no significant differences between WT and any of the KO rats at any age. Appendix B lists the mean +/−SEM potassium evoked levels of NE, 5-HT, DOPAC and HVA, which showed no significant differences between ages or between WT and KO rats.
Discussion
Much of our understanding of the time course of neurodegeneration in PD is inferred from imaging studies and from postmortem examination of PD brains. Based on these studies and substantial research on PD animal models, it is presumed that loss of dopaminergic projections to the striatum precedes the loss of neurons in the substantia nigra that is observed at the end stage of disease (Burke and O’Malley, 2013;O’Keeffe and Sullivan, 2018). Even prior to frank loss or degeneration of nerve terminals, abnormal synaptic function is likely. Therefore, we sought to determine if rats with disruption of these PD-linked genes develop defects in basal or potassium-evoked striatal neurotransmitter release even prior to significant nigral cell loss, which has previously been shown to occur in PINK1 and DJ-1 KO rats at age 8–9 months (Dave et al., 2014;Villeneuve et al., 2016).
PINK1 KO rats showed more age or genotype-dependent neurotransmitter differences than Parkin, DJ-1 or LRRK2 KO rats. Unlike Parkin, DJ-1 or LRRK2 KO rats, PINK1 KO rats showed significant age-dependent differences in both basal DA levels and potassium-evoked DA release. The significant decrease in basal striatal DA in 12-month-old PINK1 KO rats (Figure 1A) is consistent with the significant age-dependent decrease in dopaminergic neurons in the substantia nigra previously reported for this line (Dave et al., 2014;Villeneuve et al., 2016). The significant increase in potassium-evoked striatal DA release we observed for PINK1 KO rats at age 8 months (Figure 2A) aligns with the increase in striatal DA tissue content previously reported for PINK1 KO rats at age 8 months (Dave et al., 2014). This surprising increase in evoked striatal DA release could be an indication of compensatory adaptations at nigrostriatal synapses in response to neurodegeneration, as has been proposed to explain the appearance of clinical PD symptoms only after a large proportion of nigrostriatal synaptic terminals are lost (Zigmond et al., 1990). Failure of such compensatory mechanisms with advancing neurodegeneration could explain why this was observed at age 8 but not 12 months (Figure 2A).
In addition to dysregulation of striatal DA, PINK1 KO rats also showed age-dependent abnormalities in basal glutamate (Figure 1B) and acetylcholine (Figure 1C). Contrary to the simple model in which DA release in the striatum is controlled solely by firing of action potentials originating at the soma of nigral dopaminergic neurons, DA release at presynaptic terminals in the striatum is also regulated by acetylcholine and glutamate, which are proposed to exert more local control or “fine-tuning” of DA release (Cachope and Cheer, 2014). Moreover, optogenetic studies demonstrate that glutamatergic input to the dorsal striatum from the cortex and thalamus drives striatal DA release via ionotropic glutamate receptors on striatal cholinergic interneurons, which activate nicotinic acetylcholine receptors on DA axons (Kosillo et al., 2016). Further studies would be needed to determine the extent to which the alterations in glutamate and acetylcholine are a cause of DA dysfunction or an adaptation to the basal or evoked DA abnormalities in PINK1 KO rats. It is important to note that WT rats unexpectedly showed increased basal glutamate at age 12 months (Figure 1B), as well as decreased potassium-evoked release at age 12 months (Figure 2B) to levels comparable to 12-month old PINK1 KO rats, yet WT rats showed no changes in basal DA (Figures 1A, 2A). This would argue against the increased basal glutamate being a cause of the decrease in basal DA in 12-month old PINK1 KO rats. In either case, it has been proposed that altered coupling between glutamatergic and dopaminergic firing at nigrostriatal synapses could play a role in PD-related neurodegeneration via over-activation of glutamate receptors or metabolic stress (Calabresi et al., 1997). A recent imaging and biochemical analysis of PINK1 KO rats revealed altered glutathione and ATP in various brain regions including striatum, suggesting that our observation of multiple neurotransmitter alterations in PINK1 KO rats at baseline (Figure 1A–C) and upon high potassium stimulation (Figure 2A–C, E) are part of a broader set of neurobiological abnormalities resulting from PINK1 deficiency (Ferris et al., 2018).
Parkin KO rats had significantly decreased baseline levels of the DA metabolites DOPAC and HVA at age 12 months (Figure 1D–E) and what appears to be a similar decrease in DA at age 12 months (Figure 1A), but the decrease in DA was not statistically significant. The decreases in DOPAC and HVA could be due to decreases in both DA synthesis and metabolism, resulting in almost normal steady-state DA levels but decreased DA metabolites. By contrast, DA turnover is thought to be increased in early PD as a compensatory mechanism within the striatum (Zigmond, 1997). The baseline level of glycine was normal in Parkin KO rats; however, 12-month-old Parkin KO rats showed significantly greater potassium-evoked release of glycine compared to WT controls at age 12 months and compared to Parkin KO rats at ages 4 months and 8 months (Figure 2F). Glycinergic neurons are predominantly located in the spinal cord, cerebellum and brainstem and project widely to many areas including the striatum, but more to the thalamus and basal forebrain (Zeilhofer et al., 2005). Glycine is an NMDA receptor co-agonist and binding at the glycine site is required, together with glutamate, for full activation of NMDA receptors. In addition to the above-mentioned coupling of glutamatergic and dopaminergic firing at nigrostriatal synapses, NMDA receptors in particular are regulated by a multitude of highly complex mechanisms that are important for basal ganglia function, which has fueled efforts targeting NMDA receptors for PD therapeutic development (Hallett and Standaert, 2004). Because excessive activation of NMDA receptors could cause excitotoxicity, most studies have focused on NMDA receptor antagonists, which have shown some promise in PD animal models (Hallett and Standaert, 2004). This suggests that Parkin KO rats could be more vulnerable to excitotoxicity due to the increased potassium-evoked release of the co-agonist glycine at age 12 months. Alternatively, the increased glycine release could help Parkin KO rats maintain normal basal ganglia function by compensating for other synaptic defects. In support of this, Parkin KO rats at age 2 months were shown to have decreased striatal trace amine-associated receptor 1 and D2 dopamine receptor levels, which were interpreted as potential compensatory changes to maintain normal striatal DA, DOPAC and HVA (Gemechu et al., 2018).
LRRK2 KO rats showed decreased basal HVA at age 12 months compared to ages 4 months and 8 months (Figure 1E) and decreased potassium-evoked 5-HIAA at ages 12 months and 8 months, compared to age 4 months (Figure 2D). This suggests age-dependent decreases in DA and serotonin turnover. DJ-1 KO rats showed no age-dependent changes and no basal neurotransmitter defects, however, DJ-1 KO rats had significantly less potassium-induced glutamate release at age 8 months (Figure 2B) and significantly greater acetylcholine release at ages 4 months and 8 months (Figure 2C) compared to WT control rats at the same ages. It is possible that the apparent differences in stimulated neurotransmitter release in DJ-1 KO rats compared to WT rats is due to altered reuptake rather than release, as has been shown for DJ-1 KO mice (Goldberg et al., 2005).
Overall, we observed significant differences between KO and WT or between ages for each of the ten neurochemicals except NE and 5-HT. The lack of any changes in 5-HT is surprising given the significant increase in total striatal tissue content of 5-HT previously observed in 8-month, but not 4 or 6-month old PINK1 KO rats compared to WT (Dave et al., 2014). Nevertheless, the lack of any differences in NE and 5-HT is consistent with the much lower levels of NE and 5-HT in the striatum compared to the other eight neurochemicals measured. Moreover, our study was limited to the striatum and it remains possible that we would have observed significantly altered NE or 5-HT in other brain regions. This might be expected based on PET imaging studies that show much greater reductions of 5-HT transporter radioligand binding in more rostral brain regions, such as cingulate cortex, relative to the striatum in early PD patients compared to normal controls (Albin et al., 2008). Likewise, ELISA analysis showed NE was significantly decreased in the locus coeruleus and increased in the substantia nigra of 8 month old PINK1 KO rats compared to WT, while levels of NE in the striatum were below the limit of detection (Kelm-Nelson et al., 2018).
The significant differences observed here should be interpreted with some caution because, although the p-values were adjusted for multiple comparisons for both the Dunnett’s test (for comparisons of each genotype to WT) and the Tukey’s test (for comparisons among all the ages within each genotype), no correction was made for testing ten different neurotransmitters/metabolites from each sample. Correcting for this is complicated by the lack of complete independence of the values measured for neurotransmitters and their metabolites, as well as some shared enzymatic pathways for biosynthesis and degradation (for example DA and NE share three biosynthetic enzymes). If one makes the extreme assumption that the level of each neurotransmitter/metabolite is completely independent, thus providing an independent test of the null hypothesis, then there are 120 independent comparisons to WT rats (10 neurotransmitters × 3 ages × 4 genotypes compared to WT) and 150 independent comparisons for age-dependence (10 neurotransmitters × 5 genotypes with 3 comparisons between ages). If the threshold for statistical significance is set at p < 0.05, the number of significant differences expected by chance would be 6 (comparisons with WT) and 7.5 (comparisons between ages). This is close to the observed numbers of 1 (Figure 1) and 4 (Figure 2) significant differences compared to WT and 11 (Figure 1) and 10 (Figure 2) significant differences between ages. Nevertheless, PINK1 KO rats had 9 significant neurotransmitter abnormalities as well as trends for abnormalities in basal glycine (multiple comparison adjusted p = 0.0570) and HVA (adjusted p = 0.0730) compared to age-matched WT rats, which suggests PINK1 KO rats may have more unequivocal synaptic defects compared to the other lines, which had 6, 4 and 3 neurotransmitter abnormalities for Parkin, LRRK2, and DJ-1 KO rats, respectively. It should also be noted that human mutations in Parkin, DJ-1 and PINK1 that are causally linked to PD are loss-of-function mutations, however, PD-linked mutations in LRRK2 are more consistent with gain-of-function mutations and LRRK2 KO rats may not reproduce the effects of these mutations. In line with our analysis of LRRK2 KO rats, a similar microdialysis study of LRRK2 KO mice found no abnormalities in basal or potassium-evoked DA release (Hinkle et al., 2012).
Because the genetic evidence linking mutations in these genes to PD is unequivocal, greater abnormalities in basal or evoked neurotransmitter levels or turnover might be expected from this study, particularly from the KO rats that model loss-of-function mutations linked to PD. However, the time resolution of microdialysis is limited to the order of minutes and our results do not exclude the possibility that more robust abnormalities occur at sub-second time scales detectable by methods with greater temporal resolution, such as fast scan cyclic voltammetry or electrophysiology. These methods have been used to identify significant abnormalities in DA axon terminal function in PINK1, Parkin and DJ-1 KO mice even in the absence of nigral cell loss (Goldberg et al., 2003;Goldberg et al., 2005;Kitada et al., 2007;Kitada et al., 2009a;Kitada et al., 2009b;Oyama et al., 2010;Beccano-Kelly et al., 2015;Maas et al., 2017). It is also possible that subtle neurotransmitter release abnormalities could go undetected using microdialysis with high potassium, which depolarizes all cells and stimulates the release of synaptic vesicles non-specifically.
The precise mechanisms by which mutations in Parkin, PINK1, DJ-1 and LRRK2 cause human PD remain uncertain. Many studies indicate that Parkin and PINK1 function in the same biochemical pathway to target dysfunctional mitochondria for degradation and thereby protect against oxidative stress and inflammation (Narendra et al., 2012;McLelland et al., 2014;Sliter et al., 2018). DJ-1 is also neuroprotective against oxidative stress (Biosa et al., 2017). PD-linked point mutations in LRRK2 are associated with increased kinase activity (West, 2017). Proteomic studies have identified Rab GTPases as substrates for LRRK2 kinase activity as well as substrates for PINK1 kinase activity, which suggests another common pathway related to PD (Lai et al., 2015;Steger et al., 2016). Multiple Rab GTPases have been identified as substrates for PINK1, LRRK2 or both, and several are known to regulate synaptic vesicle exocytosis, endocytosis or recycling, as well as neurotransmitter receptor endocytosis or recycling (Ng and Tang, 2008;Lai et al., 2015;Clague and Rochin, 2016;Steger et al., 2016). It is possible that altered phosphorylation of one or more Rab GTPases could underlie the neurotransmitter abnormalities identified in the KO rats studied here by microdialysis. The results of this study support the utility of these KO rats for testing this and other mechanisms related to PD pathogenesis, progression or therapeutic development.
Acknowledgments
Funding
This work was supported by grant 15058 from the Michael J. Fox Foundation for Parkinson’s Research. Rose B. Creed is supported by NIH NINDS Award F99NS108458.
Appendix A. Neurotransmitters and metabolites with no significant differences between WT and KO or between ages in basal levels
Table A.1.
Mean +/−SEM basal Glycine (μM)
| 4 month | 8 month | 12 month | |
|---|---|---|---|
| WT | 0.768 +/− 0.042 | 0.842 +/− 0.056 | 1.219 +/− 0.153 |
| PINK1 KO | 0.873 +/− 0.074 | 1.379 +/− 0.230 | 1.300 +/− 0.143 |
| Parkin KO | 0.985 +/− 0.096 | 0.976 +/− 0.102 | 1.156 +/− 0.185 |
| LRRK2 KO | 0.991 +/− 0.091 | 0.961 +/− 0.044 | 1.146 +/− 0.158 |
| DJ-1 KO | 0.979 +/− 0.150 | 1.246 +/− 0.282 | 1.254 +/− 0.212 |
Table A.2.
Mean +/−SEM basal 5-HT (nM)
| 4 month | 8 month | 12 month | |
|---|---|---|---|
| WT | 0.122 +/− 0.010 | 0.105 +/− 0.006 | 0.124 +/− 0.015 |
| PINK1 KO | 0.104 +/− 0.009 | 0.102 +/− 0.009 | 0.117 +/− 0.025 |
| Parkin KO | 0.110 +/− 0.009 | 0.121 +/− 0.013 | 0.094 +/− 0.006 |
| LRRK2 KO | 0.108 +/− 0.010 | 0.143 +/− 0.038 | 0.116 +/− 0.013 |
| DJ-1 KO | 0.133 +/− 0.034 | 0.090 +/− 0.006 | 0.101 +/− 0.006 |
Table A.3.
Mean +/−SEM basal 5-HIAA (nM)
| 4 month | 8 month | 12 month | |
|---|---|---|---|
| WT | 179.0 +/− 11.07 | 184.3 +/− 10.04 | 213.9 +/− 14.95 |
| PINK1 KO | 197.5 +/− 17.77 | 176.4 +/− 17.14 | 218.8 +/− 20.10 |
| Parkin KO | 177.7 +/− 10.57 | 191.9 +/− 14.89 | 189.7 +/− 10.98 |
| LRRK2 KO | 179.8 +/− 24.05 | 218.3 +/− 11.96 | 164.8 +/− 19.45 |
| DJ-1 KO | 162.3 +/− 10.63 | 162.7 +/− 12.05 | 202.1 +/− 14.78 |
Table A.4.
Mean +/−SEM basal NE (nM)
| 4 month | 8 month | 12 month | |
|---|---|---|---|
| WT | 0.097 +/− 0.010 | 0.106 +/− 0.006 | 0.105 +/− 0.006 |
| PINK1 KO | 0.104 +/− 0.009 | 0.121 +/− 0.006 | 0.151 +/− 0.053 |
| Parkin KO | 0.117 +/− 0.013 | 0.099 +/− 0.009 | 0.098 +/− 0.008 |
| LRRK2 KO | 0.103 +/− 0.014 | 0.106 +/− 0.012 | 0.116 +/− 0.007 |
| DJ-1 KO | 0.095 +/− 0.009 | 0.111 +/− 0.012 | 0.104 +/− 0.013 |
Table A.5.
Mean +/−SEM basal GABA (nM)
| 4 month | 8 month | 12 month | |
|---|---|---|---|
| WT | 11.91 +/− 0.760 | 12.34 +/− 0.602 | 24.25 +/− 5.394 |
| PINK1 KO | 12.60 +/− 1.187 | 24.69 +/− 6.253 | 26.89 +/− 10.21 |
| Parkin KO | 19.71 +/− 4.271 | 20.59 +/− 5.847 | 12.35 +/− 0.844 |
| LRRK2 KO | 14.71 +/− 1.425 | 12.64 +/− 0.612 | 31.80 +/− 12.96 |
| DJ-1 KO | 14.09 +/− 1.029 | 21.47 +/− 4.870 | 18.49 +/− 4.928 |
Appendix B. Neurotransmitters and metabolites with no significant differences between WT and KO or between ages in potassium evoked levels
Table B.1.
Mean +/−SEM potassium evoked NE (area under the curve of % baseline × minutes)
| 4 month | 8 month | 12 month | |
|---|---|---|---|
| WT | 50078 +/− 5679 | 46200 +/− 6595 | 56568 +/− 7072 |
| PINK1 KO | 40075 +/− 4859 | 53144 +/− 4755 | 40586 +/− 6080 |
| Parkin KO | 47619 +/− 3286 | 53270 +/− 8300 | 37976 +/− 4841 |
| LRRK2 KO | 45989 +/− 3886 | 49248 +/− 8297 | 51305 +/− 8580 |
| DJ-1 KO | 33645 +/− 5345 | 28667 +/− 2642 | 44334 +/− 5919 |
Table B.2.
Mean +/−SEM potassium evoked 5-HT (area under the curve of % baseline × minutes)
| 4 month | 8 month | 12 month | |
|---|---|---|---|
| WT | 11395 +/− 1641 | 13273 +/− 961 | 16131 +/− 1822 |
| PINK1 KO | 15271 +/− 1828 | 19778 +/− 2149 | 14101 +/− 2544 |
| Parkin KO | 14070 +/− 1279 | 15564 +/− 2489 | 12355 +/− 1565 |
| LRRK2 KO | 13790 +/− 1488 | 14420 +/− 2696 | 11458 +/− 2442 |
| DJ-1 KO | 10674 +/− 1810 | 12210 +/− 1028 | 15940 +/− 2993 |
Table B.3.
Mean +/−SEM potassium evoked DOPAC (area under the curve of % baseline × minutes)
| 4 month | 8 month | 12 month | |
|---|---|---|---|
| WT | −1211 +/− 107 | −1489 +/− 69 | −1437 +/− 133 |
| PINK1 KO | −1424 +/− 125 | −1458 +/− 148 | −1421 +/− 113 |
| Parkin KO | −1405 +/− 191 | −1534 +/− 72 | −1335 +/− 121 |
| LRRK2 KO | −1128 +/− 129 | −1465 +/− 66 | −1310 +/− 85 |
| DJ-1 KO | −1242 +/− 157 | −1253 +/− 111 | −1586 +/− 87 |
Table B.4.
Mean +/−SEM potassium evoked HVA (area under the curve of % baseline × minutes)
| 4 month | 8 month | 12 month | |
|---|---|---|---|
| WT | −1505 +/− 101 | −1732 +/− 65 | −1672 +/− 126 |
| PINK1 KO | −1506 +/− 106 | −1401 +/− 158 | −1466 +/− 116 |
| Parkin KO | −1563 +/− 209 | −1666 +/− 71 | −1492 +/− 111 |
| LRRK2 KO | −1385 +/− 126 | −1609 +/− 109 | −1634 +/− 59 |
| DJ-1 KO | −1323 +/− 179 | −1394 +/− 107 | −1681 +/− 64 |
Footnotes
Conflict of interest statement
None declared
References
- Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, and Frey KA (2008). Spared caudal brainstem SERT binding in early Parkinson’s disease. J Cereb Blood Flow Metab 28, 441–444. [DOI] [PubMed] [Google Scholar]
- Baptista MA, Dave KD, Sheth NP, De Silva SN, Carlson KM, Aziz YN, Fiske BK, Sherer TB, and Frasier MA (2013). A strategy for the generation, characterization and distribution of animal models by The Michael J. Fox Foundation for Parkinson’s Research. Dis Model Mech 6, 1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccano-Kelly DA, Volta M, Munsie LN, Paschall SA, Tatarnikov I, Co K, Chou P, Cao LP, Bergeron S, Mitchell E, Han H, Melrose HL, Tapia L, Raymond LA, Farrer MJ, and Milnerwood AJ (2015). LRRK2 overexpression alters glutamatergic presynaptic plasticity, striatal dopamine tone, postsynaptic signal transduction, motor activity and memory. Hum Mol Genet 24, 1336–1349. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, and Brotchie JM (2003). Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci 26, 215–221. [DOI] [PubMed] [Google Scholar]
- Biosa A, Sandrelli F, Beltramini M, Greggio E, Bubacco L, and Bisaglia M (2017). Recent findings on the physiological function of DJ-1: Beyond Parkinson’s disease. Neurobiol Dis 108, 65–72. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Constantine GM, Mathis CA, and Moore RY (2007). Cortical cholinergic denervation is associated with depressive symptoms in Parkinson’s disease and parkinsonian dementia. J Neurol Neurosurg Psychiatry 78, 641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, Van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, Van Dongen JW, Vanacore N, Van Swieten JC, Brice A, Meco G, Van Duijn CM, Oostra BA, and Heutink P (2003). Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259. [DOI] [PubMed] [Google Scholar]
- Burke RE, and O’malley K (2013). Axon degeneration in Parkinson’s disease. Exp Neurol 246, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, and Cheer JF (2014). Local control of striatal dopamine release. Front Behav Neurosci 8, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Centonze D, and Bernardi G (1997). Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev 21, 519–523. [DOI] [PubMed] [Google Scholar]
- Clague MJ, and Rochin L (2016). Parkinson’s Disease: A Traffic Jam? Curr Biol 26, R332–334. [DOI] [PubMed] [Google Scholar]
- Dave KD, De Silva S, Sheth NP, Ramboz S, Beck MJ, Quang C, Switzer RC 3rd, Ahmad SO, Sunkin SM, Walker D, Cui X, Fisher DA, Mccoy AM, Gamber K, Ding X, Goldberg MS, Benkovic SA, Haupt M, Baptista MA, Fiske BK, Sherer TB, and Frasier MA (2014). Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol Dis 70, 190–203. [DOI] [PubMed] [Google Scholar]
- De La Fuente-Fernandez R, Lu JQ, Sossi V, Jivan S, Schulzer M, Holden JE, Lee CS, Ruth TJ, Calne DB, and Stoessl AJ (2001). Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neurol 49, 298–303. [DOI] [PubMed] [Google Scholar]
- De La Fuente-Fernandez R, Schulzer M, Kuramoto L, Cragg J, Ramachandiran N, Au WL, Mak E, Mckenzie J, Mccormick S, Sossi V, Ruth TJ, Lee CS, Calne DB, and Stoessl AJ (2011). Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol 69, 803–810. [DOI] [PubMed] [Google Scholar]
- Dekorver NW, Lichty D, Van Der Hart M, Rassoulpour A, and Bonasera SJ (2017). Increased whole cerebellar serotonin in aged C57BL/6 mice. Matters (Zur) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Wang P, and Jankovic J (2018). The genetics of Parkinson disease. Ageing Res Rev 42, 72–85. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Esposito E, and Di Matteo V (2009). In vivo microdialysis in Parkinson’s research. J Neural Transm Suppl, 223–243. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Morrison TR, Iriah S, Malmberg S, Kulkarni P, Hartner JC, and Trivedi M (2018). Evidence of Neurobiological Changes in the Presymptomatic PINK1 Knockout Rat. J Parkinsons Dis 8, 281–301. [DOI] [PubMed] [Google Scholar]
- Gemechu JM, Sharma A, Yu D, Xie Y, Merkel OM, and Moszczynska A (2018). Characterization of Dopaminergic System in the Striatum of Young Adult Park2(−/−) Knockout Rats. Sci Rep 8, 1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Gibbons JA, Bernales S, Alfaro IE, Drieu La Rochelle C, Cremers T, Altar CA, Wronski R, Hutter-Paier B, and Protter AA (2010). Cognition-enhancing properties of Dimebon in a rat novel object recognition task are unlikely to be associated with acetylcholinesterase inhibition or N-methyl-D-aspartate receptor antagonism. J Pharmacol Exp Ther 333, 748–757. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Fleming SM, Palacino JJ, Cepeda C, Lam HA, Bhatnagar A, Meloni EG, Wu N, Ackerson LC, Klapstein GJ, Gajendiran M, Roth BL, Chesselet MF, Maidment NT, Levine MS, and Shen J (2003). Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem 278, 43628–43635. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, and Shen J (2005). Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron 45, 489–496. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, and Standaert DG (2004). Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol Ther 102, 155–174. [DOI] [PubMed] [Google Scholar]
- Hinkle KM, Yue M, Behrouz B, Dachsel JC, Lincoln SJ, Bowles EE, Beevers JE, Dugger B, Winner B, Prots I, Kent CB, Nishioka K, Lin WL, Dickson DW, Janus CJ, Farrer MJ, and Melrose HL (2012). LRRK2 knockout mice have an intact dopaminergic system but display alterations in exploratory and motor co-ordination behaviors. Mol Neurodegener 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch L, Jette N, Frolkis A, Steeves T, and Pringsheim T (2016). The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology 46, 292–300. [DOI] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Trevino MA, and Ciucci MR (2018). Quantitative Analysis of Catecholamines in the Pink1 −/− Rat Model of Early-onset Parkinson’s Disease. Neuroscience 379, 126–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, and Furukawa Y (2008). Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain 131, 120–131. [DOI] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, and Shimizu N (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Karouani M, Haburcak M, Martella G, Tscherter A, Platania P, Wu B, Pothos EN, and Shen J (2009a). Impaired dopamine release and synaptic plasticity in the striatum of parkin−/− mice. J Neurochem 110, 613–621. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, and Shen J (2007). Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A 104, 11441–11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Tong Y, Gautier CA, and Shen J (2009b). Absence of nigral degeneration in aged parkin/DJ-1/PINK1 triple knockout mice. J Neurochem 111, 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosillo P, Zhang YF, Threlfell S, and Cragg SJ (2016). Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb Cortex 26, 4160–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YC, Kondapalli C, Lehneck R, Procter JB, Dill BD, Woodroof HI, Gourlay R, Peggie M, Macartney TJ, Corti O, Corvol JC, Campbell DG, Itzen A, Trost M, and Muqit MM (2015). Phosphoproteomic screening identifies Rab GTPases as novel downstream targets of PINK1. EMBO J 34, 2840–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas JW, Yang J, and Edwards RH (2017). Endogenous Leucine-Rich Repeat Kinase 2 Slows Synaptic Vesicle Recycling in Striatal Neurons. Front Synaptic Neurosci 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclelland GL, Soubannier V, Chen CX, Mcbride HM, and Fon EA (2014). Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J 33, 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melrose HL, Dachsel JC, Behrouz B, Lincoln SJ, Yue M, Hinkle KM, Kent CB, Korvatska E, Taylor JP, Witten L, Liang YQ, Beevers JE, Boules M, Dugger BN, Serna VA, Gaukhman A, Yu X, Castanedes-Casey M, Braithwaite AT, Ogholikhan S, Yu N, Bass D, Tyndall G, Schellenberg GD, Dickson DW, Janus C, and Farrer MJ (2010). Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol Dis 40, 503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Walker JE, and Youle R (2012). Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng EL, and Tang BL (2008). Rab GTPases and their roles in brain neurons and glia. Brain Res Rev 58, 236–246. [DOI] [PubMed] [Google Scholar]
- O’keeffe GW, and Sullivan AM (2018). Evidence for dopaminergic axonal degeneration as an early pathological process in Parkinson’s disease. Parkinsonism Relat Disord 56, 9–15. [DOI] [PubMed] [Google Scholar]
- Oyama G, Yoshimi K, Natori S, Chikaoka Y, Ren YR, Funayama M, Shimo Y, Takahashi R, Nakazato T, Kitazawa S, and Hattori N (2010). Impaired in vivo dopamine release in parkin knockout mice. Brain Res 1352, 214–222. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, Van Der Brug M, Lopez De Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, De Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, and Singleton AB (2004). Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44, 595–600. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates (6th ed.), Academic Press Inc., San Diego. [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, and Nussbaum RL (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276, 2045–2047. [DOI] [PubMed] [Google Scholar]
- Remy P, Doder M, Lees A, Turjanski N, and Brooks D (2005). Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128, 1314–1322. [DOI] [PubMed] [Google Scholar]
- Sauerbier A, Jenner P, Todorova A, and Chaudhuri KR (2016). Non motor subtypes and Parkinson’s disease. Parkinsonism Relat Disord 22 Suppl 1, S41–46. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Chaudhuri KR, and Jenner P (2017). Non-motor features of Parkinson disease. Nat Rev Neurosci 18, 509. [DOI] [PubMed] [Google Scholar]
- Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, Cai H, Borsche M, Klein C, and Youle RJ (2018). Parkin and PINK1 mitigate STING-induced inflammation. Nature 561, 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JA, Giardina K, and Gerhardt GA (2000). In vivo microdialysis studies of age-related alterations in potassium-evoked overflow of dopamine in the dorsal striatum of Fischer 344 rats. Int J Dev Neurosci 18, 411–416. [DOI] [PubMed] [Google Scholar]
- Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, Fiske BK, Fell MJ, Morrow JA, Reith AD, Alessi DR, and Mann M (2016). Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, and Wood NW (2004). Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 304, 1158–1160. [DOI] [PubMed] [Google Scholar]
- Villeneuve LM, Purnell PR, Boska MD, and Fox HS (2016). Early Expression of Parkinson’s Disease-Related Mitochondrial Abnormalities in PINK1 Knockout Rats. Mol Neurobiol 53, 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB (2017). Achieving neuroprotection with LRRK2 kinase inhibitors in Parkinson disease. Exp Neurol 298, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer HU, Studler B, Arabadzisz D, Schweizer C, Ahmadi S, Layh B, Bosl MR, and Fritschy JM (2005). Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J Comp Neurol 482, 123–141. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ (1997). Do compensatory processes underlie the preclinical phase of neurodegenerative disease? Insights from an animal model of parkinsonism. Neurobiol Dis 4, 247–253. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, and Stricker EM (1990). Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci 13, 290–296. [DOI] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, and Gasser T (2004). Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607. [DOI] [PubMed] [Google Scholar]
- Zis P, Erro R, Walton CC, Sauerbier A, and Chaudhuri KR (2015). The range and nature of non-motor symptoms in drug-naive Parkinson’s disease patients: a state-of-the-art systematic review. NPJ Parkinsons Dis 1, 15013. [DOI] [PMC free article] [PubMed] [Google Scholar]