Abstract
Background & Aims:
Our aim is to explain the lack of clarity in the ways in which anxiety and depression, which are common in defecatory disorders (DD), may contribute to the disorder. In this study, we evaluate the effects of mental stress and relaxation on anal pressures and the mechanisms thereof.
Methods:
In 38 healthy women and 36 DD patients, rectoanal pressures were assessed at rest and during mental stressors (ie, word-color conflict [Stroop] and mental arithmetic tests) and mental relaxation, before and after randomization to placebo or the adrenergic α1-antagonist alfuzosin.
Results:
During the baseline Stroop test, the anal pressure increased by 6±13 mm Hg (mean±SD, P=.004) in healthy women and 9±10 mm Hg (P=.0001) in constipated women. During mental arithmetic, the anal pressure increased in healthy (4±8 mm Hg, P=.002) and constipated women (5± 9mm Hg, P=.004). After relaxation, anal pressure declined (P=.0004) by 3±4 mm Hg in DD but not in controls. Alfuzosin reduced (P=.0001) anal resting pressure (by 31±19 mm Hg) versus placebo (16±18 mm Hg). However, during the post-drug Stroop test, anal pressure increased (P=.0001) in participants who received alfuzosin but not placebo.
Conclusions:
In healthy controls and DD patients, mental stressors likely increased anal pressure by contracting the internal anal sphincter; relaxation reduced anal pressure in DD patients. Alfuzosin reduced anal resting pressure but did not block the Stroop-mediated contractile response, which suggests that this response is not entirely mediated by adrenergic α1 receptors.
Keywords: alfuzosin, anal pressure, constipation, mental stressors, Stroop
Graphical Abstract
Abbreviated abstract: •Mental stress increases anal pressures in healthy and constipated women. By comparison, mental relaxation has lesser effects, and decreases anal pressures in constipated women only.
Introduction
Defecatory disorders (DD) result from reduced rectal propulsive forces and/or decreased anal relaxation during defecation (1, 2). Among those with DD, nearly 48% have a psychiatric diagnosis (44% with depression and 17% with anxiety), 30% have irritable bowel syndrome (IBS), and 18% have a history of abuse before the DD is diagnosed (3). Whether anxiety and depression predispose to or are a consequence of DD is unknown. While mental stress can induce colonic sensorimotor dysfunctions, the effects of mental stress and relaxation on the anal sphincter have not been evaluated (4, 5).
In dogs, monkeys, and humans, sympathetic motor nerves innervate the internal anal sphincter, which maintains approximately 70% of anal resting tone (6). These effects are primarily exerted via adrenergic alpha-1 (α1) receptors. In vitro studies suggest that these receptors contribute up to 50% of anal resting pressure in humans and dogs (7, 8). In healthy and constipated women, the adrenergic α1-antagonist alfuzosin reduced anal resting pressure by 30% to 40% (9). Adrenergic stimulation doubled contraction in the internal anal sphincter in monkeys (10). Since mental stress increases sympathetic activity (11), the hypothesis of this study was that mental stress would increase anal pressure by adrenergic α1-receptor–mediated contraction of the internal anal sphincter, while mental relaxation would reduce anal resting pressure.
Central adrenergic α1-mediated mechanisms may also contribute to the effects of stress on the internal sphincter. Thus, α1-adrenergic stimulation affects the stress-mediated release of corticotrophin releasing factor (CRF) in the hypothalamus (12) and the startle response, possibly by modulating CRF release from brainstem nuclei (13). The α1-adrenergic antagonist prazosin reversed the excessive startle response induced by α1 agonists (14).
The aims of this study were to 1) evaluate the effects of acute mental stress and relaxation on anal pressure and 2) assess the effects of the adrenergic α1-receptor antagonist alfuzosin on anal pressures at baseline and in response to mental stress and relaxation in healthy controls and patients with DD.
Materials and Methods
Study Design
This was a double-blind, placebo-controlled, randomized, parallel-group study conducted at Mayo Clinic in, Rochester, Minnesota. This study was approved by the Mayo Clinic Institutional Review Board, registered on clinicaltrials.gov (NCT 01834729), and conducted between March 7, 2013 and March 31, 2017. All authors had access to the study data and have reviewed and approved the final manuscript. Anal pressures at rest and the effects of mental stress and relaxation on those pressures were recorded before and after randomization to alfuzosin or placebo on the same day in all participants. Some of the results of this study (ie, the effects of alfuzosin on anal pressures and bowel habits), but not the effects of stress, have been published (9).
Participants
Thirty-eight healthy women (mean±SD age 40±16 y, body mass index [BMI] 26±7 kg/m2) and 36 women with constipation for 1 year or longer (age 40±12 y, BMI 26±6 kg/m2) participated in this study. Healthy women did not have a functional bowel disorder. Patients satisfied Rome III symptom criteria for chronic constipation or constipation-predominant IBS (15). All participants had a digital rectal examination and at least one abnormal anorectal test performed in the clinic before the research study, suggestive of a DD (16, 17). The key exclusion criteria included clinical evidence of considerable systemic disease, rectal inflammation or cancer, pregnancy, symptomatic orthostatic hypotension, QTc interval longer than 500 msec on an electrocardiogram, an estimated glomerular filtration rate less than 60 mL/minute, and a history of pelvic radiation, rectosigmoid surgery, or inflammatory bowel disease.
Anorectal Manometry
After participants were given 2 sodium phosphate enemas (Fleets, C.B. Fleet, Lynchburg, VA), anorectal pressures were measured using established techniques with a high-resolution anorectal manometry catheter (Medtronic Inc, Los Angeles, CA). Pressures were measured at rest, during 2 mental stressors, word color-conflict and mental arithmetic, and during mental relaxation at baseline, and, after study drug (i.e., placebo or alfuzosin).
Alfuzosin
The uroselective α1-adrenergic antagonist alfuzosin is 144-fold more selective for the prostate than vascular tissue and has a low risk of orthostatic hypotension (18, 19). It barely penetrates the blood-brain barrier. We used an immediate-release formulation of alfuzosin (2.5 mg, Xatral, Sanofi-Aventis, Paris, France) that is unavailable in the United States and was imported from the United Kingdom under an investigator-initiated Investigational new drug IND (117098) from the US Food and Drug Administration (FDA). For this formulation, the mean bioavailability was 64%, the tmax was 1.0 to 1.5 hours, and the terminal thalf was 3 to 5 hours (18, 19). The post-drug assessment was performed 90 minutes after the drug was administered.
Mental Stress and Relaxation
Three maneuvers, each lasting 6 minutes, were performed in random order. The mental stressors (word-color conflict or Stroop test) and mental arithmetic tests were administered by a licensed clinical psychologist (20). The mental arithmetic test comprised number subtraction of increasing difficulty, beginning with subtraction of 1 digit from 2 digits and increasing up to subtraction of 3 digits from 3 digits. During the Stroop test, participants were asked to rapidly identify the colors in which words representing colors were printed. For example, the correct response for the word red, presented in the color green, was green. External task pacing, for example by periodically informing patients that their correct answers were incorrect, was provided to accentuate the induced mental stress (21). To evoke mental relaxation, patients listened to an audio recording that encouraged them to close their eyes, be comfortable, monitor their breathing, and relax their entire body.
Two 100-mm visual analogue scales (VAS) anchored by the terms tired–energetic and peaceful–tense evaluated arousal and stress, respectively (22, 23). During the Stroop and mental arithmetic tests, the duration for which anal pressure was greater than 50% of the difference between peak anal pressure sustained for 2 seconds during the maneuver and the resting pressure before the maneuver were measured.
Statistical Analysis
The treatment assignments that were balanced on BMI, age, and hysterectomy with a block size of 4 were generated by computer and shared with the pharmacy. The study personnel were blinded until the study was completed. The effects of mental stress and relaxation on anal pressures in all, healthy, and constipated women were analyzed with paired parametric tests. After treatment, the effects of these stressors were assessed among all, and separately in participants receiving alfuzosin or placebo with paired parametric tests. The effects of stress on healthy and constipated women were compared with the Student t test. Comparisons between parameters were evaluated with Spearman correlation coefficients. Data were analyzed using SAS software (version 9.4, Cary, NC).
Sample Size Assessment
Among healthy women and DD patients, the inter-subject coefficient of variation in anal resting pressure was 20% and 11%, respectively (unpublished data). The study had 80% power to detect an effect size of approximately 15% to 20% in healthy women and in DD patients using a 2-sample paired t test with a 2-sided α level of .05. All data are presented as mean±SD.
Results
Baseline Demographic and Clinical Features and Anorectal Tests
Of the 38 healthy participants and 36 patients, 36 healthy participants (95%) and 35 patients with constipation (97%) completed the study (Table 1). One constipated patient withdrew from the study due to rectal pain. Of the 36 patients, 21 (58%) had functional constipation and 15 (42%) had constipation-predominant IBS; 13 (36%) had considerable anxiety and/or depression. As detailed in Table 1, 34 of 36 (94%) had abnormal tests indicative of a DD.
Table 1.
Comparison of Anorectal Sensorimotor Functions in Health and in Chronic Constipation
| Parametera | Healthy (N=38) | Constipation (N=36) | |||||
|---|---|---|---|---|---|---|---|
| Placebo (n=18) | Alfuzosin (n=20) | P Valueb | Placebo (n=18) | Alfuzosin (n=18) | P Valuec | Overall P Valued | |
| Demographic data | |||||||
| Age (y) | 32 (26–51) | 45 (28–53) | .78 | 36 (26–50) | 40 (35–44) | .73 | .61 |
| Body mass index, kg/m2 | 24 (24–29) | 27 (23–29) | .96 | 25 (21–30) | 25 (19–29) | .83 | .63 |
| Baseline data | |||||||
| Functional constipation, No. (%) | 0 | 0 | 9 (50) | 12 (67) | |||
| IBS-C, No. (%) | 0 | 0 | 9 (50) | 6 (33) | |||
| Depression, No. (%) | 0 | 0 | 3 (17) | 2 (11) | |||
| Anxiety, No. (%) | 0 | 0 | 4 (22) | 4 (22) | |||
| Anorectal pressures, mm Hg | |||||||
| Anal pressure at rest | 85 (57–91) | 91 (83–98) | .12 | 83 (76–100) | 81 (73–90) | .23 | .73 |
| Anal pressure at squeeze | 225 (188–265) | 251 (200–277) | .43 | 224 (191–282) | 194 (148–235) | .05 | .19 |
| Rectal pressure―simulated evacuation | 30 (25–45) | 24 (13–39) | .42 | 32 (28–42) | 21 (8–34) | .04 | .68 |
| Rectal pressure increment―simulated evacuation | 27 (19–41) | 26 (11–34) | .54 | 34 (22–38) | 17 (6–33) | .17 | .70 |
| Anal pressure―simulated evacuation | 74 (59–85) | 82 (65–93) | .19 | 86 (70–103) | 78 (71–91) | .72 | .45 |
| Rectoanal gradient―simulated evacuation | −44 (−53 to −26) | −59 (−67 to −39) | .05 | −51 (−61 to −32) | −65 (−81 to −38) | .27 | .33 |
| Anal relaxation―simulated evacuation,%e | 23 (4–33) | 19 (9–25) | .78 | 2 (−7 to 16) | 9 (−11 to 17) | .79 | .05 |
| Anal relaxation―simulated evacuation after rectal distension,% | 13 (−6 to 30) | 11 (3–26) | .52 | 0 (−17 to 10) | −6 (−30 to 14) | .68 | .02 |
| Two or more abnormal manometry features during evacuation, No. (%) | 4 (22) | 5 (25) | .84 | 6 (33) | 8 (44) | .49 | .1 |
| Balloon expulsion time > 60 s, No. (%) | 1 (6) | 2 (10) | .61 | 7 (39) | 9 (50) | .50 | .0005 |
| Abnormal MR or barium proctogram, No. (%) | 0 | 0 | 7 (39) | 7 (39) | |||
| Abnormal anorectal surface EMG exam, No. (%) | 0 | 0 | 8 (44) | 5 (28) | |||
| Abnormal test(s) indicative of DD, No. (%) | 5 (28) | 7 (35) | .62 | 17 (94) | 17 (94) | >.99 | <.0001 |
Abbreviations: DD, Dyssynergic defecation; EMG, Electromyography; IBS-C, Constipation predominant irritable bowel syndrome; MR, Magnetic resonance.
Values are medians (interquartile range) unless specified otherwise. Statistical comparisons were performed only for parameters that were evaluated in all participants.
Comparison of placebo versus alfuzosin in healthy participants.
Comparison of placebo versus alfuzosin in constipated patients.
Comparison of healthy and constipated patients.
Positive larger values represent greater anal relaxation.
Reproduced with permission from Chakraborty S, Feuerhak K, Muthyala A, Harmsen WS, Bailey KR, Bharucha AE. Effects of alfuzosin, an alpha-1 alphadrenergic antagonist on anal pressures and bowel habits, in women with and without defecatory disorders. Clinical Gastroenterology & Hepatology. 2018;18:18.
At baseline, anal resting and squeeze pressures and rectoanal pressures during evacuation were not markedly different between healthy and constipated participants or between patients with versus without anxiety or depression (data not shown).
Effects of Mental Stress and Relaxation on Psychosensory State and Anal Pressures at Baseline (Pre-drug)
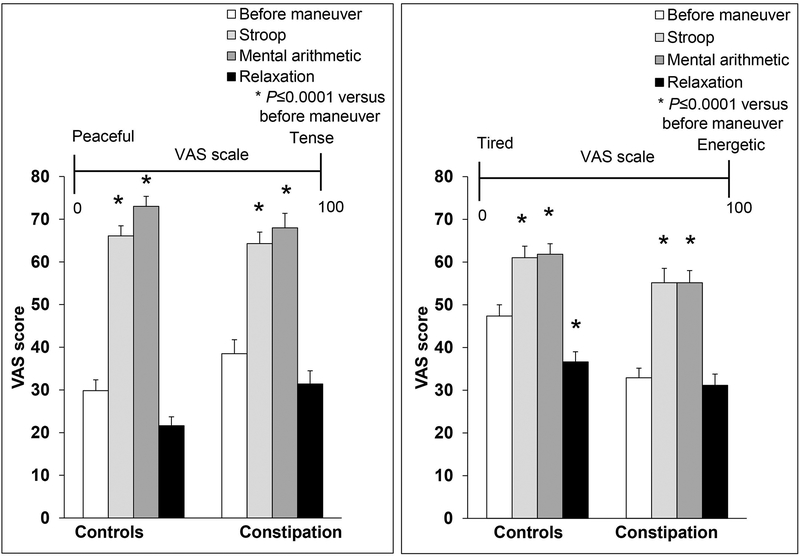
Compared to baseline, the peaceful-tense Visual analog scale (VAS) score was greater (P<.0001) during the Stroop (34±18 before vs 65±15 after) and mental arithmetic (34±18 before vs 71±18 after) tests, indicative of increased stress, in all participants (Figure 1). The tired-energetic VAS score was also significantly higher (P<.0001), indicating greater arousal during the mental arithmetic (40±17 before vs 59±16 after) and Stroop tests (40±17 before vs 58±19 after). By comparison, mental relaxation reduced significantly the VAS tired-energetic score in healthy people only (47±16 before vs 37±15 after, P=0.0001). These VAS scores at baseline and the effects of perturbations were not markedly different between healthy and constipated participants (data not shown).
Figure 1. Effects of mental stressors on VAS scales in healthy women and constipated patients.
Both VAS scores increased significantly in response to the Stroop and mental arithmetic tests. By comparison, mental relaxation had smaller effects on VAS scores. * P≤.0001 versus corresponding score before the maneuver. VAS indicates visual analogue scale.
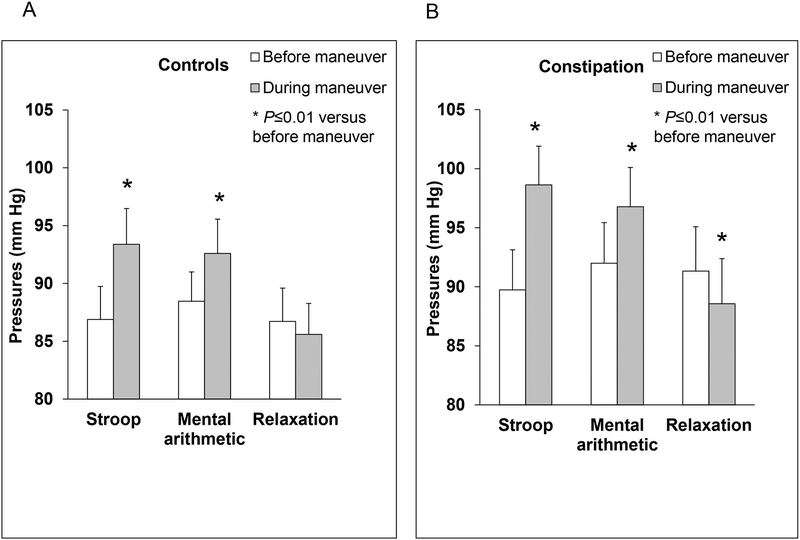
During the Stroop test, the anal pressure increased in healthy (87±18 mm Hg before vs 93±19 mm Hg after, P=.004) and constipated women (90±20 mm Hg before vs 99±20 mm Hg after, P=.0001) (Table 2, Figures 2 and 3). The anal pressure also increased during mental arithmetic in healthy and constipated participants. The increase in anal pressures during the Stroop and mental arithmetic tests were correlated (r=0.51, P<.0001). The pressure increment was sustained for 31 (4–87) seconds [median (interquartile range)] during the Stroop test and for 21 (6–95) seconds during mental arithmetic (Figure 4). By contrast, the anal pressure declined during relaxation in constipated women (91±23 mm Hg before vs 89±23 after, P=.0004) (Table 2, Figure 2). In healthy women, anal pressures were not significantly different (87±18 mm Hg before versus 86±17 mm Hg after, P=0.34).
Table 2.
Effects of Mental Stress and Relaxation on Anal Pressures at Baselinea
| Maneuver | Baseline | During Maneuver | Difference (During – Before) | P Valueb |
|---|---|---|---|---|
| Healthy women (N=38) | ||||
| Stroop test | 87±18 | 93±19 | 6±13 | .004 |
| Mental arithmetic | 89±16 | 93±18 | 4±8 | .002 |
| Relaxation | 87±18 | 86±17 | −1±7 | .34 |
| Constipated women (N=36) | ||||
| Stroop test | 90±20 | 99±20 | 9±10 | .0001 |
| Mental arithmetic | 92±21 | 97±20 | 5±9 | .004 |
| Relaxation | 91±23 | 89±23 | −3±4 | .0004 |
Values are in mm Hg. Data are mean ± SD.
Pressure during maneuver versus baseline.
Figure 2. Effects of mental stressors on anal pressures in healthy women and constipated patients.
Anal pressures increase in response to mental stressors in healthy (panel A) and constipated women (panel B). However during relaxation, anal pressures declined only in constipated women.
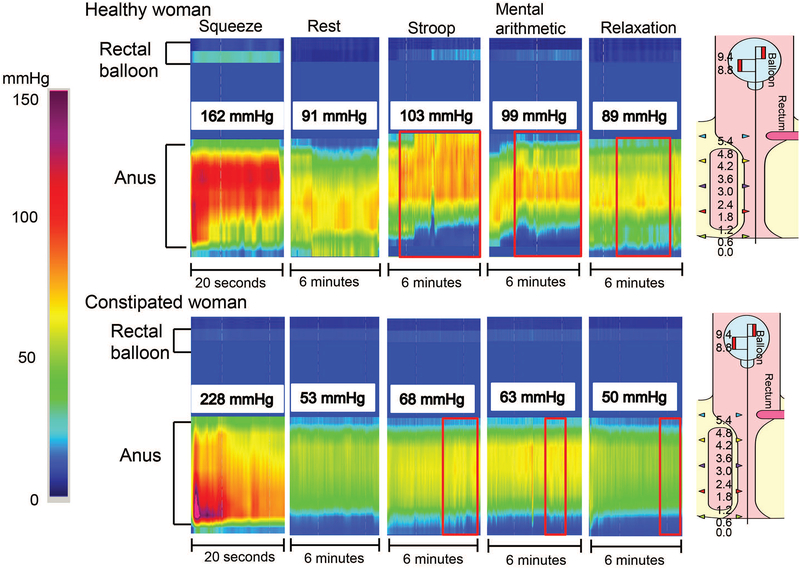
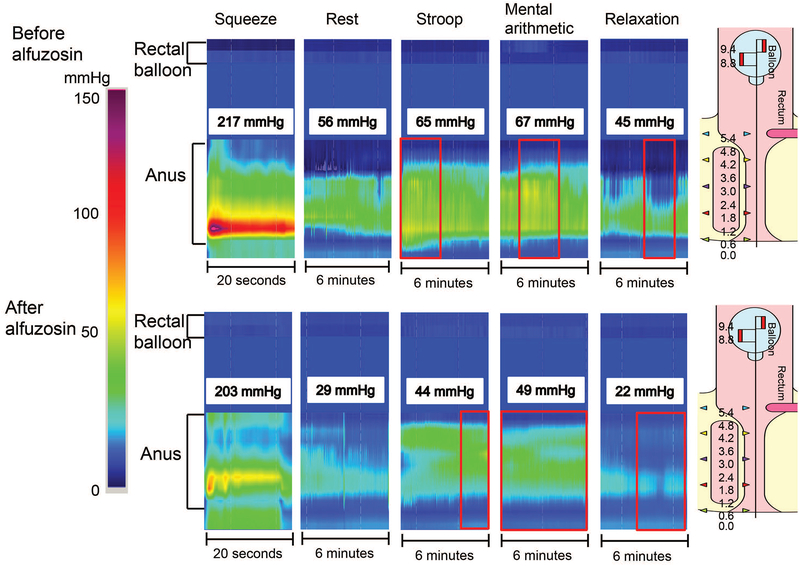
Figure 3. Representative high-resolution manometry tracings showing effects of mental stress and relaxation on rectoanal pressures.
Anal pressures increased considerably during the Stroop and mental arithmetic tests in a healthy woman (upper panel) and a constipated woman (lower panel). During mental stressors, observe that the increased anal pressure was sustained for virtually the entire duration of the maneuver, but was much lower than the pressure during squeeze.
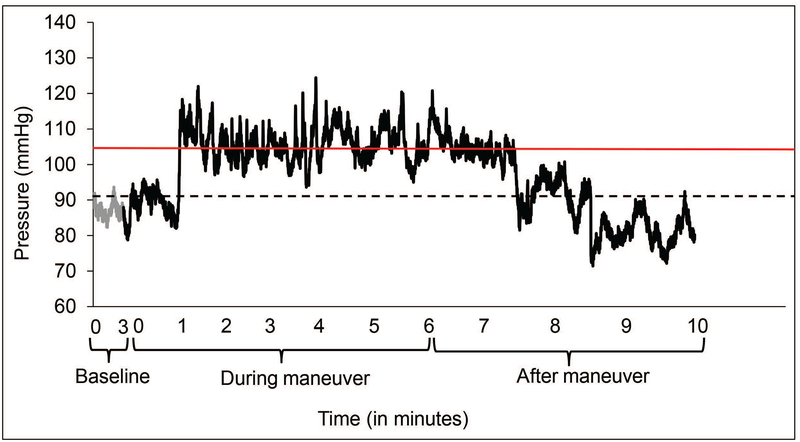
Figure 4. Sustained anal contraction during mental stress.
This example shows anal pressures before and during the Stroop test in the healthy participant shown in Figure 3 (upper panel). The anal pressure increased 1 minute after the test began, was sustained for 5 minutes, and returned to baseline thereafter. As defined in the Materials and Methods section, only the period that was above the threshold (red line) was used to calculate the duration of contraction. Hence, the measured duration often underestimates the actual duration of the contractile response. The black dotted line denotes the average baseline pressure before the maneuver.
Effects of Mental Stress and Relaxation on Psychosensory State and Anal Pressures After Alfuzosin or Placebo
Compared to baseline, the anal pressure was not markedly different during any of the 3 maneuvers among participants randomized to placebo (Table 3).
Table 3.
Effects of Mental Stress and Relaxation on Anal Pressures After Treatmenta
| Maneuver | Before Maneuver | During Maneuver | Difference (During – Before) | P Valueb |
|---|---|---|---|---|
| Placebo (N=36) | ||||
| Stroop test | 81±31 | 83±20 | 2±20 | .55 |
| Mental arithmetic | 79±22 | 80±19 | 1±9 | .47 |
| Relaxation | 77±23 | 77±23 | −0.1±6 | .93 |
| Alfuzosin (N=38) | ||||
| Stroop test | 59±18 | 68±19 | 9±12 | .0001 |
| Mental arithmetic | 62±18 | 66±19 | 4±11 | .06 |
| Relaxation | 59±20 | 58±20 | −1±9 | .49 |
Data are mean ± SD.
Pressure during maneuver versus baseline.
Alfuzosin reduced resting anal pressure (31±19 mm Hg vs 16±18 mm Hg for placebo, P=.0001). The anal pressure increased during the Stroop test (59±18 mm Hg before vs 68±19 after, P=.0001), but not significantly during mental arithmetic (62±18 mm Hg before vs 66±19 mm Hg after, P=.06) or relaxation (59±20 mm Hg before vs 58±20 mm Hg after, P=.49) (Table 3). The duration of sustained contraction was not significantly different (P=.74) before [35 (7–90), median (interquartile range) versus after 34 (8–87) seconds] alfuzosin (Figure 5). Differences between healthy and constipated participants were not significant.
Figure 5. Representative high-resolution manometry tracings showing effects of alfuzosin on rectoanal pressures.
Before alfuzosin (upper panel), anal pressures increased considerably during the Stroop and mental arithmetic tests and declined during relaxation in a healthy woman. In the same patient, alfuzosin considerably reduced anal resting pressure but did not prevent the contractile response to stress (lower panel).
Adverse Effects
Among participants who received alfuzosin, mean blood pressure was not different (85±10 mm Hg before vs 83±13 mm Hg after drug administration, P=.22). Likewise, heart rate was nearly identical (63±11 mm Hg before vs 64±12 mm Hg after drug administration, P=.71).
Adverse effects included orthostatic hypotension in 1 patient treated with alfuzosin.
Discussion
In this study, the Stroop and mental arithmetic tests increased anal pressure to a similar extent in healthy people and patients with DD. The VAS scores for mental stress and arousal during mental stressors were substantially greater than at baseline and compared to a previous study (24). While the effects of these stressors on anal pressures were statistically significant, they were numerically small. Anal pressure increased by approximately 5 mm Hg during mental arithmetic in healthy participants and constipated patients and during the Stroop test in healthy participants, and by approximately 10 mm Hg during the Stroop test in constipated patients.
Both the mental arithmetic and Stroop tests increased sympathetic traffic in the peroneal nerve (25, 26). The temporo-spatial anal pressure profile during mental stress is more suggestive of contraction of the internal rather than the external anal sphincter. On average, this contractile response was sustained for approximately 60 seconds, which exceeds the maximum contractile duration of the external sphincter (6). The pressure during mental stressors was lower than pressure during voluntary contraction of the external anal sphincter. However, alfuzosin did not block the anal contractile response during the Stroop test, which suggests that this response may also be mediated by other mechanisms, such as adrenergic α2 receptors that mediate the anal contractile response to electrical stimulation in dogs (27) and venoconstriction induced by acute stress in animal models (28).
Mental relaxation did not substantially reduce the “peaceful-tense” VAS scores in healthy or constipated participants, perhaps because the baseline VAS score was relatively low (~30–40/100), suggesting a floor effect. However, in healthy people, mental relaxation did considerably reduce the “tired-energetic” VAS score, which was higher (~50/100) at baseline (before mental relaxation). Mental relaxation only reduced anal pressures in constipated patients. After administration of placebo, these mental stressors did not markedly affect anal pressures, suggesting habituation (29).
This study has several strengths and limitations. Both mental stress maneuvers increased anal pressures to a similar extent. However, the effects of acute mental stress on anal pressures were variable among subjects. Similarly, a stress-induced cortisol response was observed in only 40% of normotensive participants (30). This inter-individual variability may be related to the perceived stress induced by the stressor and/or the biological responses to stress. For example, the cortisol response to stress is greater when participants are confronted with social-evaluative challenges and an element of uncontrollability rather than purely psychomotor and problem-solving tasks (31). In this study, the stressors did include some elements of social-evaluative threat (ie, an evaluative audience) and uncontrollability (ie, false feedback).
Are these findings useful for understanding the pathogenesis of DD? This study evaluated the effects of acute, but not longstanding, mental stress. The stress-induced augmentation of anal pressure was relatively modest and comparable in healthy controls and DD patients, which argues against the hypothesis that DD patients have an exaggerated anal contractile response to stress. While the Stroop test and mental arithmetic increased sympathetic nerve traffic in the peroneal nerve, the responses to stress may be region-specific. The peroneal nerve may not represent sympathetic nerve input to the internal anal sphincter (25, 26). In normotensive individuals, the acute cortisol response to stress predicted incident hypertension 3 years later (30). To speculate, perhaps the acute effects of mental stress on anal pressures in asymptomatic people may predict the risk of developing DD in future. In that regard, the blood pressure response to the Stroop test is representative of the response to real-life stressors (32).
In conclusion, mental stressors likely increase anal pressure in healthy controls and DD patients by contracting the internal anal sphincter. Mental relaxation reduces anal pressure in constipated women. The contractile response of the anal sphincter to stress is not entirely mediated by stimulation of adrenergic alpha 1 receptors.
Key points.
While defecatory disorders (DD) are associated with anxiety, there is no evidence that anxiety causes DD. Indeed, the effects of stress on the anal sphincters are unknown.
Mental stress increases anal pressures in healthy and constipated women. By comparison, mental relaxation has lesser effects, and decreases anal pressures in constipated women only.
The effects of mental stress on anal pressures may predispose to or aggravate DD.
Acknowledgments and Disclosures
Funding: This work was supported by Grant R01 DK 78924 from the National Institutes of Health to Dr Bharucha.
Abbreviations.
- α1
alpha-1
- BMI
body mass index
- CRF
corticotrophin releasing factor
- DD
dyssynergic defecation
- FDA
US Food and Drug Administration
- GI
gastrointestinal
- IBS
irritable bowel syndrome
- IND
investigational new drug
- VAS
visual analogue scale
Footnotes
Competing interests: The authors have no competing interests.
References
- 1.Rao S, Bharucha AE, Chiarioni G, Felt-Bersma RJ, Knowles CH, Malcolm A, et al. Functional anorectal disorders. Gastroenterology. 2016;150(6):1430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharucha AE, Wald A. Chronic constipation. Mayo Clin Proc. 2018;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noelting J, Eaton J, Choung RS, Zinsmeister AR, Locke GR 3rd, Bharucha AE. The Incidence Rate and Characteristics of Clinically Diagnosed Defecatory Disorders in the Community. Neurogastroenterol Motil. 2016;28(11):1690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford MJ, Camilleri M, Zinsmeister AR, Hanson RB. Psychosensory modulation of colonic sensation in the human transverse and sigmoid colon. Gastroenterology. 1995;109(6):1772–80. [DOI] [PubMed] [Google Scholar]
- 5.Van Oudenhove L, Levy RL, Crowell MD, Drossman DA, Halpert AD, Keefer L, et al. Biopsychosocial Aspects of Functional Gastrointestinal Disorders: How Central and Environmental Processes Contribute to the Development and Expression of Functional Gastrointestinal Disorders. Gastroenterology. 2016;150(6):1355–67.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil. 2006;18(7):507–19. [DOI] [PubMed] [Google Scholar]
- 7.Frenckner B, Ihre T. Influence of autonomic nerves on the internal and sphincter in man. Gut. 1976;17(4):306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizutani M, Neya T, Ono K, Yamasato T, Tokunaga A. Histochemical study of the lumbar colonic nerve supply to the internal anal sphincter and its physiological role in dogs. Brain Res. 1992;598(1–2):45–50. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborty S, Feuerhak K, Muthyala A, Harmsen WS, Bailey KR, Bharucha AE. Effects of Alfuzosin, an Alpha-1 Alphadrenergic Antagonist on Anal Pressures and Bowel Habits, in Women With and Without Defecatory Disorders. Clinical Gastroenterology & Hepatology. 2018;18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobine CA, Fong M, Hamilton R, Keef KD. Species dependent differences in the actions of sympathetic nerves and noradrenaline in the internal anal sphincter. Neurogastroenterol Motil. 2007;19(11):937–45. [DOI] [PubMed] [Google Scholar]
- 11.Esler M Mental stress and human cardiovascular disease. Neurosci Biobehav Rev. 2017;74(Pt B):269–76. [DOI] [PubMed] [Google Scholar]
- 12.Kiss A, Aguilera G. Role of alpha-1-adrenergic receptors in the regulation of corticotropin-releasing hormone mRNA in the paraventricular nucleus of the hypothalamus during stress. Cell Mol Neurobiol. 2000;20(6):683–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risbrough VB, Hauger RL, Pelleymounter MA, Geyer MA. Role of corticotropin releasing factor (CRF) receptors 1 and 2 in CRF-potentiated acoustic startle in mice. Psychopharmacology (Berl). 2003;170(2):178–87. [DOI] [PubMed] [Google Scholar]
- 14.Carasso BS, Bakshi VP, Geyer MA. Disruption in prepulse inhibition after alpha-1 adrenoceptor stimulation in rats. Neuropharmacology. 1998;37(3):401–4. [DOI] [PubMed] [Google Scholar]
- 15.Bharucha AE, Locke GR, Seide B, Zinsmeister AR. A New Questionnaire for Constipation and Fecal Incontinence. Alimentary Pharmacology & Therapeutics. 2004;20:355–64. [DOI] [PubMed] [Google Scholar]
- 16.Ratuapli S, Bharucha AE, Noelting J, Harvey D, Zinsmeister AR. Phenotypic Identification and Classification of Functional Defecatory Disorders Using High Resolution Anorectal Manometry Gastroenterology. 2013;144:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratuapli S, Bharucha AE, Harvey D, Zinsmeister AR. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterol Motil. 2013;25(12):e813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salva P, Bianchetti G, Morselli P, Garcia-Teresa G, Costa J. Pharmacokinetics of alfuzosin after single oral administration to healthy volunteers, of three different doses. Biopharm Drug Dispos. 1992;13(8):583–90. [DOI] [PubMed] [Google Scholar]
- 19.Roehrborn CG. Alfuzosin: overview of pharmacokinetics, safety, and efficacy of a clinically uroselective alpha-blocker. Urology. 2001;58(6 Suppl 1):55–63; discussion -4. [DOI] [PubMed] [Google Scholar]
- 20.Jensen AR, Rohwer WD Jr. The Stroop color-word test: a review. Acta Psychol (Amst). 1966;25(1):36–93. [DOI] [PubMed] [Google Scholar]
- 21.Renaud P, Blondin J-P. The stress of Stroop performance: physiological and emotional responses to color–word interference, task pacing, and pacing speed. Int J Psychophysiol. 1997;27(2):87–97. [DOI] [PubMed] [Google Scholar]
- 22.Mackay C, Cox T, Burrows G, Lazzerini T. An inventory for the measurement of self-reported stress and arousal. Br J Soc Clin Psychol. 1978;17(3):283–4. [DOI] [PubMed] [Google Scholar]
- 23.Cox T, Mackay C. The measurement of self-reported stress and arousal. Br J Psychol. 1985;76(Pt 2):183–6. [DOI] [PubMed] [Google Scholar]
- 24.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53(8):1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson EA, Wallin BG, Mark AL. Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension. 1987;9(6 Pt 2):Iii114–9. [DOI] [PubMed] [Google Scholar]
- 26.Hjemdahl P, Fagius J, Freyschuss U, Wallin BG, Daleskog M, Bohlin G, et al. Muscle sympathetic activity and norepinephrine release during mental challenge in humans. Am J Physiol. 1989;257(5 Pt 1):E654–64. [DOI] [PubMed] [Google Scholar]
- 27.Nie Y, Chen JD. Effects and mechanisms of anal electrical stimulation on anorectal compliance and tone in dogs. Dis Colon Rectum. 2006;49(9):1414–21. [DOI] [PubMed] [Google Scholar]
- 28.Martin DS, Appelt C, Rodrigo MC, Egland MC. Acute stress increases venomotor tone in conscious rats. Am J Physiol. 1996;271(4 Pt 2):H1375–83. [DOI] [PubMed] [Google Scholar]
- 29.Gerra G, Zaimovic A, Mascetti GG, Gardini S, Zambelli U, Timpano M, et al. Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology. 2001;26(1):91–107. [DOI] [PubMed] [Google Scholar]
- 30.Hamer M, Steptoe A. Cortisol responses to mental stress and incident hypertension in healthy men and women. J Clin Endocrinol Metab. 2012;97(1):E29–34. [DOI] [PubMed] [Google Scholar]
- 31.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–91. [DOI] [PubMed] [Google Scholar]
- 32.Kamarck TW, Schwartz JE, Janicki DL, Shiffman S, Raynor DA. Correspondence between laboratory and ambulatory measures of cardiovascular reactivity: a multilevel modeling approach. Psychophysiology. 2003;40(5):675–83. [DOI] [PubMed] [Google Scholar]