Abstract
Objective:
To assess performance of risk stratification schemes in predicting adverse cardiac outcomes in pregnant women with congenital heart disease and to compare these schemes to clinical factors alone.
Design:
Single-center retrospective study
Setting:
Tertiary care academic hospital
Patients:
Women ≥ 18 years with International Classification of Diseases, Ninth Revision, Clinical Modification codes indicating congenital heart disease who delivered between 1998 and 2014. CARPREG I and ZAHARA risk scores and modified World Health Organization (WHO) criteria were applied to each woman.
Outcome measures:
The primary outcome was defined by ≥1 of the following: arrhythmia, heart failure/pulmonary edema, transient ischemic attack, stroke, dissection, myocardial infarction, cardiac arrest, death during gestation and up to 6 months post-partum.
Results:
Of 178 women, the most common congenital heart disease lesions were congenital aortic stenosis (15.2%), ventricular septal defect (13.5%), atrial septal defect (12.9%), and tetralogy of Fallot (12.9%). Thirty-five women (19.7%) sustained 39 cardiac events. Observed vs. expected event rates were 9.9% vs 5% (p=0.02) for CARPREG I score 0 and 26.1% vs. 7.5% (p<0.001) for ZAHARA scores 0.51 – 1.5. ZAHARA outperformed CARPREG I at predicting adverse cardiovascular outcomes (AUC 0.80 vs. 0.72, p=0.03) but was not significantly better than modified WHO. Clinical predictors of adverse cardiac event were symptoms (p = 0.002), systemic ventricular dysfunction (p < 0.001), and subpulmonary ventricular dysfunction (p = 0.03) with an AUC 0.83 comparable to ZAHARA (p=0.66).
Conclusions:
CARPREG I and ZAHARA scores underestimate cardiac risk for lower risk pregnancies in these women. Of the three risk schemes, CARPREG I performed least well in predictive capacity. Clinical factors specific to the population studied are comparable to stratification schemes.
Keywords: Adult congenital heart disease, outcomes, pregnancy, risk stratification
Introduction
As survival of those born with congenital heart disease shifts towards adulthood, there are a growing number of females with congenital heart disease (CHD) who reach childbearing age. From 1998 to 2007, the proportion of hospitalizations for delivery increased by 34.9% in women with CHD compared to 21.3% in the female general population.1 Pregnancy outcomes for women with CHD can be highly variable and pose special challenges for congenital cardiovascular specialists and high-risk obstetricians who are tasked with counseling these women and managing them throughout pregnancy, delivery and post-partum period. Risk stratification schemes predicting adverse outcomes in pregnant women with CHD have been published2–6 with variable accuracy.7–12 The objectives of this study were 1) to assess the performance of CARPREG I, ZAHARA, and the modified WHO classification in predicting adverse cardiovascular outcomes in women with CHD at a single tertiary care center with an established adult CHD program in an urban North American setting and 2) to compare these classification and scoring systems to clinical factors alone in predicting adverse outcomes.
Methods
This was a retrospective cohort study of women age 18 years or older with CHD who had a pregnancy cared for and delivered at a single tertiary care center with an established adult CHD program in an urban North American setting. The electronic medical records were reviewed for women with an International Classification of Diseases, Ninth Revision (ICD-9) code indicating a CHD diagnosis and an ICD-9 code 648.0–648.9 for the supervision and management of high risk pregnancy. Women who delivered at the Hospital of the University of Pennsylvania between January 1, 1998 and December 31, 2014 were included. Women with patent foramen ovale or mitral valve prolapse as their sole congenital diagnosis were excluded. Only the first pregnancy was counted for women who delivered more than once during the study time period. Twin gestations and pregnancies that ended in a termination or a miscarriage (defined as less than 24 weeks gestation) were excluded. Written informed consent was waived and the study was approved by the institutional review board.
Clinical and demographic data were collected through review of the electronic medical records by cardiac clinicians specialized in the care of adults with CHD. All baseline data were captured up to one year prior to conception including variables necessary to calculate CARPREG I score (0, 1, ≥2) and ZAHARA score (0–13) as described previously.2,4 Echocardiographic variables were collected from baseline echocardiograms up to 1 year pre-conception. Functional capacity was assessed by review of the electronic medical records using the New York Heart Association (NYHA) classification.13
CARPREG I and ZAHARA scores were calculated for each woman2,4 and they were assigned a modified WHO classification (class I – IV). For those who fell under modified WHO classification II-III, the clinician assigned the woman to one category or another based on the presence of any other associated defects and symptoms of functional incapacity or heart failure. A separate adult CHD cardiologist subsequently validated the CARPREG I and ZAHARA risk scores as well as the modified WHO classification for all women. Maternal CHD complexity was categorized as simple, moderate, or complex.14
Adverse cardiovascular outcome was defined as any cardiac event during pregnancy up to 6 months post-partum which included at least one of the following: heart failure or pulmonary edema, arrhythmia requiring treatment, thromboembolic complications including deep vein thrombosis or pulmonary embolism, transient ischemic attack, stroke, endocarditis, myocardial infarction, aortic dissection, cardiac arrest, or cardiac death.4 Heart failure or pulmonary edema was a combined endpoint that was based on CARPREG I (chest X-ray documentation or rales heard more than 1/3 up lung fields) and ZAHARA (heart failure requiring treatment at least including drug therapy) definitions. Study data were collected and managed using REDCap (Research Electronic Data Capture), a secure web-based data capture application hosted at the Hospital of the University of Pennsylvania.15
Statistical Methods
Characteristics of all study variables were reported as mean with standard deviation or counts with percentages. For our first objective (assessing the performance of CARPREG I, ZAHARA, and modified WHO classification in predicting adverse cardiac event), the CARPREG I score, ZAHARA score, and modified WHO classification were compared as predictor variables among women with and without an adverse cardiac event. To test predictive capacity of CARPREG I and ZAHARA risk scores on adverse cardiovascular outcomes, one-sample proportions tests were used to determine if observed cardiovascular event rates were significantly different from the predicted values for each ZAHARA and CARPREG I risk category. As modified WHO is a classification and not a risk score, it was not included in that initial analysis. Next, the predictive capacity of each of CARPREG I, ZAHARA, and the modified WHO for an adverse cardiovascular event was evaluated using Receiver Operating Curves and the area under the curve for each was calculated.
For our second objective (comparing the classification and scoring systems to clinical factors along in predicting outcomes), clinical predictor variables were analyzed to build a multivariable model. Baseline maternal comorbidities that were accounted for as potential confounders included age at conception, obesity, tobacco use, and history of any of the following: arrhythmia, heart failure, endocarditis, hypertension, stroke or transient ischemic attack, mechanical valve, pacemaker or defibrillator, pulmonary hypertension, venous thromboembolism, diabetes mellitus, genetic syndrome, chronic kidney disease, cirrhosis, chronic lung disease, anxiety or depression. Univariate logistic regression models were used to calculate odds ratios to assess the impact of each covariate on the odds of having a cardiac event. A preliminary multivariable model was fit to include the covariates determined to be significant with p < 0.05 in the univariate models. After backward stepwise regression was performed, all factors associated with a cardiac event with p < 0.05 were included in the final multivariable model. A final set of ROC curves were constructed and the AUC values of the final multivariable model, as well as those for ZAHARA and CARPREG I scores and modified WHO classification were compared.
To address the issue of missing data in the multivariable model, we completed a second multivariable regression using multiple imputation. We used imputation by chained equations to impute missing values of the covariates in the regression model with missing values. This multivariate approach uses the conditional distribution of each covariate, given other predictor variables, to cycle between filling the missing values for each covariate. We repeated the imputation process 500 times, to create 500 data sets with complete data. We then ran the multivariable regression model on each imputed data set and combined the results using Proc MI in SAS.
All statistical analyses were performed using SAS version 9.4 (SAS Institute). ROC Curves were constructed using Stata 14 (StataCorp LLC).
Results
There were a total of 268 women identified by the search strategy above, of which 90 women were excluded: 47 women had no personal history of CHD, 21 had patent foramen ovale, 5 had fetuses with CHD but no maternal CHD, 4 had dextrocardia alone, 4 delivered at an affiliated hospital in the health system, 3 did not have records available for review, 3 underwent termination, 2 were not pregnant and 1 had isolated mitral valve prolapse. There were 178 women in the final cohort.
Table 1 describes the baseline demographic and clinical characteristics of the women included. Mean maternal age was 29.0 ± 6.1 years and 32% were black. The most common CHD lesions were congenital aortic stenosis, ventricular septal defect, atrial septal defect, and tetralogy of Fallot. Half had moderate or complex forms of CHD including 40 women (22.5%) who were born with cyanotic CHD (e.g. tetralogy of Fallot, D-transposition of the great arteries, double outlet right ventricle). Five women had Fontan physiology, nine had systemic right ventricles, and one had cyanosis with saturations less than 90%. None of the women included in the study had Eisenmenger syndrome or renal insufficiency. The two most common comorbidities prior to pregnancy was history of arrhythmia (19.1%) and tobacco use (26.4%). One-quarter of these women were on a cardiac medication prior to becoming pregnant. Systemic ventricular dysfunction was found in 12%.
Table 1.
Maternal baseline characteristics, n=178
| Characteristics | n | (%) |
|---|---|---|
| Maternal age at conception (years ± SD) | 29.0 | ± 6.1 |
| Body Mass Index (kg/m2 ± SD) | 25.3 | ± 5.0 |
| Race | ||
| White | 92 | 51.7 |
| Black | 57 | 32.0 |
| Asian | 7 | 3.9 |
| Other | 15 | 8.4 |
| Not reported | 5 | 2.8 |
| Parity | ||
| 0 | 96 | 53.9 |
| 1 | 32 | 18.0 |
| ≥ 2 | 23 | 12.9 |
| Unknown | 27 | 15.2 |
| Congenital heart disease complexity | ||
| Simple | 88 | 49.4 |
| Moderate | 66 | 37.1 |
| Complex | 24 | 13.5 |
| Congenital heart disease diagnosis (congenital heart disease complexity) | ||
| Congenital aortic stenosis/bicuspid aortic valve (simple) | 27 | 15.2 |
| Ventricular septal defect (simple) | 24 | 13.5 |
| Atrial septal defect (simple) | 23 | 12.9 |
| Tetralogy of Fallot (TOF) including TOF with pulmonary atresia (moderate or complex) | 231 | 12.9 |
| Pulmonary stenosis or infundibular obstruction (moderate) | 5 | 8.4 |
| Coarctation of the aorta (moderate) | 11 | 6.2 |
| D-transposition of the great arteries (complex) | 7 | 3.9 |
| Double outlet right ventricle (complex) | 6 | 3.4 |
| Atrioventricular canal defect (moderate) | 5 | 2.8 |
| Sinus venosus defect (moderate) | 5 | 2.8 |
| Coronary artery anomaly (N/A) | 4 | 2.2 |
| Partial or total anomalous pulmonary venous connection (moderate) | 4 | 2.2 |
| Patent ductus arteriosus (simple) | 4 | 2.2 |
| Subvalvar or supravalvar aortic stenosis (moderate) | 4 | 2.2 |
| Marfan syndrome (N/A) | 4 | 2.2 |
| L-transposition of the great arteries (complex) | 3 | 1.7 |
| Ebstein anomaly (moderate) | 7 | 1.7 |
| Othera | 3.9 | |
| Past medical history | ||
| Tobacco use | 44 | 26.4 |
| Arrhythmia | 34 | 19.1 |
| Obesity (Body Mass Index > 30 kg/m2) | 25 | 14.0 |
| Chronic lung disease | 19 | 10.7 |
| Pacemaker and/or defibrillator | 15 | 8.4 |
| Depression | 14 | 7.9 |
| Hypertension | 13 | 7.3 |
| Anxiety | 11 | 6.2 |
| Heart failure | 7 | 3.9 |
| Diabetes | 7 | 3.9 |
| Endocarditis | 6 | 3.4 |
| Genetic syndrome | 5 | 2.8 |
| Pulmonary hypertension (PASP > 50 mmHg) | 4 | 2.3 |
| Stroke or transient ischemic attack | 3 | 1.7 |
| Myocardial infarction | 3 | 1.7 |
| Venous thromboembolism | 3 | 1.7 |
| Cirrhosis or chronic liver disease | 3 | 1.7 |
| Mechanical valve | 2 | 1.1 |
| Otherb | 31 | 17.4 |
| New York Heart Association Functional Class | ||
| I | 143 | 80.3 |
| II | 27 | 15.2 |
| III | 2 | 1.1 |
| IV | 0 | 0.0 |
| Missing | 6 | 3.4 |
| Cardiac medication use prior to pregnancy (up to 1 year preconception) | ||
| None | 132 | 74.2 |
| Beta-blocker | 16 | 9.0 |
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 12 | 6.7 |
| Aspirin | 11 | 6.2 |
| Diuretic | 10 | 5.6 |
| Digoxin | 6 | 3.4 |
| Anti-arrhythmic medication | 4 | 2.3 |
| Calcium channel blocker | 3 | 1.7 |
| Warfarin | 2 | 1.1 |
| Heparin or low molecular weight heparin | 1 | 0.6 |
| Echocardiographic parameters | ||
| Systemic ventricular dysfunction (n=163) | ||
| None | 144 | 88.3 |
| Mild | 16 | 9.8 |
| Moderate | 2 | 1.2 |
| Severe | 1 | 0.6 |
| Left heart obstruction (peak gradient >30 mmHg, AVA <1.5 cm2, MVA <2 cm2); n=160 | 17 | 10.6 |
| Left heart obstruction (peak gradient >50 mmHg, AVA <1.0 cm2); n=162 | 14 | 8.6 |
| Systemic atrioventricular valve regurgitation (at least moderate); n=161 | 11 | 6.8 |
| Aortic valve regurgitation (at least moderate); n=161 | 12 | 7.5 |
| Subpulmonary ventricular dysfunction (at least moderate); n=155 | 3 | 1.9 |
| Right heart obstruction (peak gradient >50 mmHg); n=160 | 6 | 3.8 |
| Pulmonary atrioventricular valve regurgitation (at least moderate); n=156 | 14 | 8.9 |
| Pulmonary valve regurgitation (at least moderate); n=152 | 24 | 15.8 |
AVA, aortic valve area; MVA, mitral valve area; PASP, pulmonary arterial systolic pressure; SD, standard deviation
Diagnoses included in “other” include mitral valve disorder (2), Hypoplastic Left Heart Syndrome (1), double inlet left ventricle (1), truncus arteriosus (1), branch pulmonary artery stenosis (1), and mid-aortic syndrome (1).
Other comorbidities include thyroid disorder (9), hematologic disorder (5), seizure disorder, hypoparathyroidism (2), hyperlipidemia (2), bioprosthetic valve (2), gestational diabetes (2), substance abuse (2), history of breast cancer (1), polycystic kidney disease (1), multiple sclerosis (1), and human immune deficiency virus (1).
Figure 1 shows the distribution of CARPREG I scores, ZAHARA scores, and modified WHO class for our cohort. Approximately one-third of the women had scores conferring elevated risk: 31% had CARPREG I score of 1 or greater and 30% had ZAHARA score >1.5. One-quarter were modified WHO classification III or IV.
Figure 1.
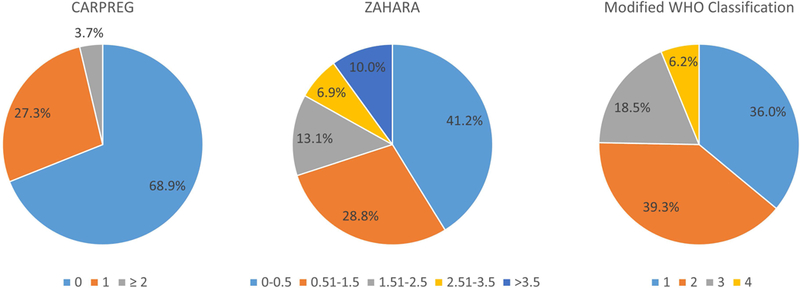
Distribution of pregnancies according to CARPREG I score (n = 161), ZAHARA score (n = 160), and modified WHO classification (n = 178).
There were 35 women (19.7%) who sustained 39 cardiac events during pregnancy or within 6 months post-partum. Pulmonary edema or heart failure were the most common adverse events and occurred in 24 (68.6%) of these pregnancies, symptomatic arrhythmia occurred in 14 (40%), and aortic dissection in 1. Four women had both pulmonary edema/heart failure and arrhythmia. There were no deaths.
For our first objective, we performed two analyses. First we compared the frequency of expected adverse cardiovascular outcomes based on the CARPREG I and ZAHARA scores to the observed frequency of outcomes in our cohort (Figure 2). CARPREG I and ZAHARA underestimated the risk of events for women in the lowest risk groups. For women with a CARPREG I score of 0, the frequency of observed adverse cardiovascular outcomes was significantly higher than the expected frequency (9.9% vs. 5%, p=0.02). For women with a CARPREG I score of 1, while not statistically significant, our cohort again had a higher observed frequency than expected (38.6 vs. 27%, p=0.08). There was no difference in the frequency of observed and expected cardiac outcomes among women with a CARPREG I score of ≥2. When using the ZAHARA score, observed and expected frequencies of events were not different except for women with a ZAHARA risk score of 0.51–1.5. These women had a significantly higher frequency of observed outcomes compared to expected (26.1% vs. 7.5%, p<0.001). Despite sustaining higher than predicted events, women with lower risk (CARPREG I score = 0 and ZAHARA score 0–1.5) had baseline demographics that were similar to the overall cohort.
Figure 2.
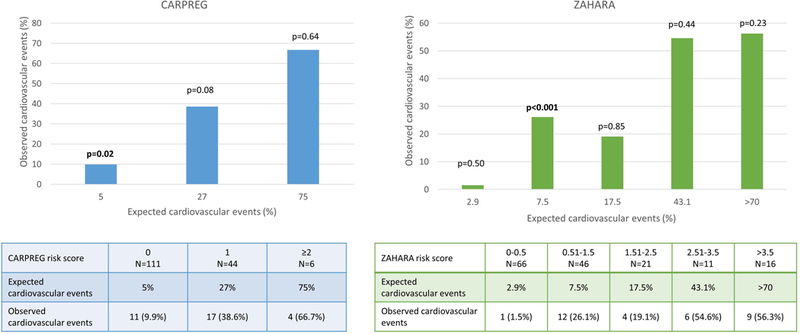
Comparison of observed cardiac events during pregnancy to predicted event rate by CARPREG I and ZAHARA scores.
The second analysis for objective 1 utilized univariate logistic regression to examine the relationship between CARPREG I, ZAHARA, and modified WHO and adverse cardiac outcomes. When evaluating the area under the curve (Figure 3), ZAHARA performed better than CARPREG I at predicting adverse cardiovascular outcomes (AUC 0.80 vs. 0.72, p=0.03). There was no other significant difference when comparing the different risk scores and stratification schemes to each other Figure 3.
Figure 3.
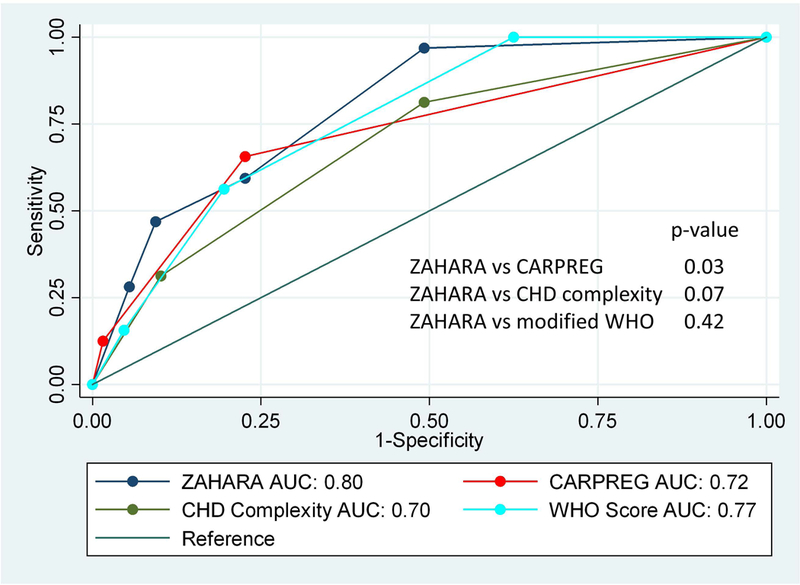
Area under the receiver operating curve of logistic regression analyses examining association between CARPREG I, ZAHARA, modified WHO, and congenital heart disease (CHD) complexity and adverse maternal cardiac event.
For our second objective, clinical characteristics were examined by univariate analyses. As noted in Table 2, NYHA class, Fontan physiology, systemic right ventricle, cyanotic heart disease, history of arrhythmia or heart failure, history of pacemaker, cardiac medication use, systemic and subpulmonary ventricular dysfunction were found to be associated with a cardiac event. In our multivariable model, women with NYHA class II-IV had a 5-fold higher odds of a cardiac event; women with systemic ventricular dysfunction had a 22-fold higher odds of cardiac event, and women with subpulmonary ventricular dysfunction had a 4-fold higher odds of cardiac event (Table 3). The AUC for the final multivariable model with these clinical characteristics performed similarly to that of ZAHARA (AUC 0.83 vs. 0.80, p = 0.66).
Table 2.
Univariate logistic regression analyses of clinical factors associated with adverse cardiac events
| Univariate Analysis | OR (95% CI) | p-value |
|---|---|---|
| Age* | 1.05 (0.98 – 1.11) | 0.16 |
| Race | ||
| White | -- | 1.00 |
| Black | 1.10 (0.48 – 2.49) | 0.83 |
| Asian | 1.64 (0.30 – 9.17) | 0.57 |
| Other | 0.65 (0.17 – 2.44) | 0.52 |
| Body Mass Index* | 1.05 (0.97 – 1.15) | 0.24 |
| Tobacco use | 0.74 (0.30 – 1.87) | 0.60 |
| NYHA class | ||
| I | 1.0 | -- |
| II - IV | 4.61 (2.0 – 10.47) | <0.001 |
| Fontan physiology | 6.61 (1.06 – 41.2) | 0.04 |
| Systemic right ventricle | 5.79 (1.47 – 22.85) | 0.01 |
| Cyanotic heart disease (corrected or uncorrected) | 3.54 (1.6 – 7.85) | 0.002 |
| History of arrhythmia | 4.9 (2.14 – 11.17) | <0.001 |
| History of heart failure | 29.38 (3.41 – 253.32) | 0.002 |
| History of pacemaker or defibrillator | 4.22 (1.41 – 12.59) | 0.01 |
| Cardiac medication use prior to pregnancy | 3.17 (1.46 – 6.9) | 0.004 |
| Systemic outflow tract obstruction (peak gradient >30 mmHg or AVA <1.5 cm2 or MVA <2 cm2) | 0.88 (0.24 – 3.27) | 0.85 |
| Systemic ventricular dysfunction, at least mild | 14.25 (4.84 – 42.02) | <0.001 |
| Systemic AV valve regurgitation, at least mild | 1.19 (0.54 – 2.63) | 0.66 |
| Subpulmonary ventricular dysfunction | 3.69 (1.27 – 10.74) | 0.02 |
| Subpulmonary AV valve regurgitation, at least mild | 1.42 (0.63 – 3.16) | 0.40 |
| Pulmonary regurgitation, at least mild | 0.99 (0.44 – 2.24) | 0.99 |
AV, atrioventricular; AVA, aortic valve area; CI, confidence interval; MVA, mitral valve area; NYHA, New York Heart Association; OR, odds ratio
For every year/unit increase
Table 3.
Multivariable logistic regression analyses of clinical factors associated with adverse cardiac events
| Multivariable analysis | OR (95% CI) | p-value |
|---|---|---|
| NYHA class | ||
| I | 1.0 | -- |
| II - IV | 5.3 (1.8 – 15.2) | 0.002 |
| Systemic ventricular dysfunction, at least mild | 22.3 (5.9 – 84.4) | <0.001 |
| Subpulmonary ventricular dysfunction, at least mild | 4.1 (1.2 – 14.2) | 0.03 |
CI, confidence interval; NYHA, New York Heart Association; OR, odds ratio
A total of 17 women were excluded from analyses of CARPREG I scores and 18 women from analyses of ZAHARA scores because of incomplete data required to calculate the scores. A sensitivity analysis was performed with imputed datasets for missing data and the results were unchanged.
Discussion
In this cohort of women with CHD from an urban, North American tertiary care referral center, 19.7% had an adverse cardiac outcome characterized by heart failure or arrhythmia except for one dissection in the context of Marfan syndrome. We found that the CARPREG I and ZAHARA scores significantly underestimated risk of event in the lowest risk groups. Of CARPREG I, ZAHARA, and modified WHO, we found ZAHARA was superior to CARPREG I in predictive capacity. The modified WHO classification, which is not a risk score, was equivalent to ZAHARA in predicting adverse cardiac outcomes in this cohort.
Interestingly, however, we found a combination of clinical factors (including NYHA class, systemic ventricular dysfunction, and subpulmonary ventricular dysfunction) yielded a similar performance in predicting adverse cardiac events compared to ZAHARA. Prior research has identified risk factors for untoward maternal cardiovascular outcomes in women with CHD and stratification schemes have been proposed.2,4,5 The performance and validation of the CARPREG I and ZAHARA risk scores have been variable depending on population studied and may not be accurate for higher risk pregnancies.7–10 CARPREG II was recently published and built on prior findings from the original CARPREG I study with other lesion-specific risk factors found to be predictive of outcome but has yet to be independently validated.16 Recent studies suggest the modified WHO classification may be superior to the CARPREG I and ZAHARA scores in predicting poor outcomes, highlighting the challenges inherent in applying and validating these risk schemes.11,12
Our findings are similar to other studies that have been unable to validate the predictive capacity of CARPREG I and ZAHARA scores across the spectrum of risk.11 In contrast to Balci et al11 who found both CARPREG I and ZAHARA overestimated risk in the higher risk categories, CARPREG I and ZAHARA underestimated risk for lower risk categories in our population. It is notable that the adverse cardiac event rate in our cohort (19.7%) was higher than in CARPREG I (13%) and ZAHARA (7.6%) studies.
The significant variability in performance of risk scoring systems such as CARPREG I and ZAHARA is likely population-specific. For example, we only included women with congenital heart defects, not acquired heart disease, and had an underrepresentation of women with connective tissue disorders compared to women in CARPREG I and ZAHARA. Therefore, it is plausible that CARPREG I did not perform well in our cohort given the fact that CARPREG I was derived and validated in a population that included 25% non-CHD diagnoses whereas our population was comprised entirely of women with CHD. Our patients are derived from a specialized, high-volume adult CHD center with nearly one in five women (18%) having a prior history of arrhythmia. This, along with the fact that more than 50% of our population had moderate to highly complex forms of CHD, potentially reflects a more at-risk population. The hospital to which this center belongs serves an urban community that is significantly more racially diverse than prior studies with close to 50% of our population being non-Caucasian. It is possible that these patient characteristics account for variable accuracy in applying traditional risk scores derived from other populations to our cohort.10
While the modified WHO classification has been identified as superior to other prediction methods,11,12,17,18 we found it to be equivalent to ZAHARA in our study. However, there were two women who sustained clinical events that did not meet the definition of an adverse cardiac event that are noteworthy, and highlight the advantage of the modified WHO risk classification scheme over a risk score. One woman with critical aortic stenosis in the setting of a unicuspid aortic valve developed dyspnea, chest discomfort and dizziness shortly after delivery at 35 weeks gestation by elective cesarean section. She underwent mechanical aortic valve replacement with aortic annulus enlargement 3 months post-partum. Her CARPREG I score was 1 and ZAHARA score 2.5. Similarly, a second woman with severe LVOT obstruction with baseline peak and mean gradients 85 mmHg and 50 mmHg, respectively, was hospitalized at 32 weeks for worsening dyspnea, orthopnea and tachycardia. She was placed on bed rest in the hospital and delivered four weeks later by elective cesarean section that was uncomplicated. Her CARPREG I score was 1 and ZAHARA score 3.25. Notably, both women were categorized as modified WHO class 4. Though neither pregnancies met the definition of an adverse cardiovascular outcome, it is undeniable that both had serious cardiovascular complications and speaks to the caveats in defining the outcome of interest when using risk scores.
There were a number of strengths and limitations of the study. A strength of this study was the fact that two independent clinicians performed chart review with all risk stratifications validated by a cardiologist trained in CHD. This limits misclassification bias that can otherwise occur when using ICD codes alone to compare exposures and outcomes. This study excluded pregnancies that ended in termination or miscarriage which could have resulted in a bias towards women with less complex disease and fewer medical comorbidities who completed a pregnancy; however our population still included a high percentage of women with moderate and complex disease. The study spanned a 16 year period during which time research on pregnancy outcomes in CHD grew significantly. Outcomes as a result of changes in counseling and practice could have been impacted by newer data which is not captured in our analysis. Although there were missing data for a subset of patients limiting the ability to calculate a ZAHARA or CARPREG I score for all women, sensitivity analyses demonstrated no impact of the missing data on our overall results. While this was a single center study and our results may not be generalizable to other clinical settings, it was a unique opportunity to study a high-risk urban population within the United States, a previously understudied group.
Risk scores such as CARPREG I and ZAHARA are derived from studied populations and therefore are subject to inaccuracy when applied to an individual or group of individuals, as shown in this study. Given the heterogeneity of CHD, clinical behavior under pregnancy conditions can vary widely. Pre-existing medical comorbidities also play a significant role in the assessment of pregnancy risk, the presence of which may increase risk for a woman with simple CHD beyond that of a woman with more complex disease alone. In translating these findings to practice, there may be no incremental value of risk scores for an individual beyond sound clinical judgment and “common sense”. Current guidelines suggest as much with emphasis on individualized counseling to each woman keeping in mind anatomic, physiologic, and other clinical factors that are specific to her alone.19
Acknowledgements
We would like to thank Lacey Gleason for assistance with this project. Lacey Gleason is currently affiliated with the Emory University Rollins School of Public Health. This project was supported by the generosity of Big Hearts to Little Hearts.
Dr. Levine is supported by a grant from the National Institute of Child Health and Human Development, Bethesda, Maryland (K12-HD001265–15).
Footnotes
The authors report no conflict of interest.
References
- 1.Opotowsky AR, Siddiqi OK, D’Souza B, Webb GD, Fernandes SM, Landzberg MJ. Maternal cardiovascular events during childbirth among women with congenital heart disease. Heart. 2012;98(2):145–151. [DOI] [PubMed] [Google Scholar]
- 2.Siu SC, Sermer M, Colman JM, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104(5):515–521. [DOI] [PubMed] [Google Scholar]
- 3.Khairy P, Ouyang DW, Fernandes SM, Lee-Parritz A, Economy KE, Landzberg MJ. Pregnancy outcomes in women with congenital heart disease. Circulation. 2006;113(4):517–524. [DOI] [PubMed] [Google Scholar]
- 4.Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31(17):2124–2132. [DOI] [PubMed] [Google Scholar]
- 5.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(24):3147–3197. [DOI] [PubMed] [Google Scholar]
- 6.Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart. 2006;92(10):1520–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jastrow N, Meyer P, Khairy P, et al. Prediction of complications in pregnant women with cardiac diseases referred to a tertiary center. Int J Cardiol. 2011;151(2):209–213. [DOI] [PubMed] [Google Scholar]
- 8.Curtis SL, Marsden-Williams J, Sullivan C, et al. Current trends in the management of heart disease in pregnancy. Int J Cardiol. 2009;133(1):62–69. [DOI] [PubMed] [Google Scholar]
- 9.Ford AA, Wylie BJ, Waksmonski CA, Simpson LL. Maternal congenital cardiac disease: outcomes of pregnancy in a single tertiary care center. Obstet Gynecol. 2008;112(4):828–833. [DOI] [PubMed] [Google Scholar]
- 10.Diller GP, Uebing A. Predicting the risks of pregnancy in congenital heart disease: the importance of external validation. Heart. 2014;100(17):1311–1312. [DOI] [PubMed] [Google Scholar]
- 11.Balci A, Sollie-Szarynska KM, van der Bijl AG, et al. Prospective validation and assessment of cardiovascular and offspring risk models for pregnant women with congenital heart disease. Heart. 2014;100(17):1373–1381. [DOI] [PubMed] [Google Scholar]
- 12.Lu CW, Shih JC, Chen SY, et al. Comparison of 3 Risk Estimation Methods for Predicting Cardiac Outcomes in Pregnant Women With Congenital Heart Disease. Circ J. 2015;79(7):1609–1617. [DOI] [PubMed] [Google Scholar]
- 13.The Criteria Committee of the New York Heart Association: Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed Boston: Little, Brown & Co; 1994. [Google Scholar]
- 14.Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation. 2008;118(23):e714–833. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silversides CK, Grewal J, Mason J, et al. Pregnancy Outcomes in Women with Heart Disease: The CARPREG II Study. J Am Coll Cardiol. 2018;71(20):2419–2430. [DOI] [PubMed] [Google Scholar]
- 17.Ruys TP, Roos-Hesselink JW, Hall R, et al. Heart failure in pregnant women with cardiac disease: data from the ROPAC. Heart. 2014;100(3):231–238. [DOI] [PubMed] [Google Scholar]
- 18.Pijuan-Domenech A, Galian L, Goya M, et al. Cardiac complications during pregnancy are better predicted with the modified WHO risk score. Int J Cardiol. 2015;195:149–154. [DOI] [PubMed] [Google Scholar]
- 19.Canobbio MM, Warnes CA, Aboulhosn J, et al. Management of Pregnancy in Patients With Complex Congenital Heart Disease: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2017;135(8):e50–e87. [DOI] [PubMed] [Google Scholar]


