Abstract
The curative potential of autologous hematopoietic cell transplantation (autoHCT) for male germ cell tumors (GCTs) is well established. The optimal timing and number (single transplant [ST] versus tandem transplants [TT] versus triple transplants) of autoHCT are controversial, with wide practice variations. We examined survival trends among 2395 recipients of autoHCT for male GCTs between 1990 and 2015 reported to the Center for International Blood and Marrow Transplant Research. Trends and outcomes were analyzed by year of transplantation for intervals 1990 to 1994 (N = 288), 1995 to 1999 (N = 351), 2000 to 2004 (N = 376), 2005 to 2009 (N = 509), and 2010 to 2015 (N = 871). Multivariate analysis was restricted to the subset from 2000 to 2015 with research-level data (n = 267). The median duration of follow-up was 51 months. The median age at autoHCT was 31 years; 633 patients (26%) had primary extragonadal GCT, and 1167 (49%) underwent TT. The 3-year progression-free (PFS) and overall survival (OS) improved from 24% (95% confidence interval [CI], 18% to 31%) and 35% (95% CI, 29% to 40%), respectively, in 1990 to 1994 to 47% (95% CI, 43% to 50%) and 54% (95% CI, 50% to 57%), respectively, in 2010 to 2015 (P < .0001). TT recipients were more likely than ST recipients to undergo autoHCT as first salvage treatment. The proportion of TTs increased from 38% of all autoHCTs in 2000 to 2004 to 77% in 2010 to 2015. Nonseminoma histology, residual disease at autoHCT, >1 line of pretransplantation chemotherapy, and ST versus TT were associated with inferior PFS and OS. Post-transplantation survival has improved significantly over time for relapsed/refractory male GCT and is associated with the increased use of TTs (compared with STs) and performance of autoHCT earlier in the disease course.
Keywords: Autologous, Transplantation, Germ cell cancers
INTRODUCTION
Germ cell tumors (GCTs) represent one of the most common curable malignancies affecting adolescent and young adult men in both Europe and North America [1,2]. Despite high rates of platinum sensitivity, up to 40% of men with intermediate-or poor-risk advanced GCT will require salvage treatment [3]. The prognosis for relapsed/refractory male GCTs remains poor, and death from disease accounts for the greatest average number of years of life lost due to any adolescent or adult solid tumor [4]. The current standard of care for salvage of relapsed/refractory GCT includes either conventional-dose chemotherapy (CDCT) consisting of cisplatin, ifosfamide, and vinblastine, paclitaxel, or etoposide, or high-dose chemotherapy (HDCT) with autologous hematopoietic cell transplantation (autoHCT) [5–8]. Currently, there are no randomized data to inform the optimal sequencing of these approaches and the number of transplantations (single transplant [ST] versus tandem transplant [TT] versus triple transplant) in the salvage setting.
The era of autoHCT for GCT began with the introduction of high-dose carboplatin in 1986 [9]. In a phase 2 study led by the Eastern Cooperative Oncology Group (ECOG) for patients with relapsed/refractory GCT, a regimen of HDCT with carboplatin and etoposide with autoHCT led to cure in 23% of cases[10]. Several other single-arm prospective and retrospective studies demonstrated a benefit with HDCT [7,11,12]. In an observational multicenter study of patients with advanced GCT who received either HDCT or CDCT as first-line salvage treatment, HDCT demonstrated better progression-free survival (PFS) in all prognostic groups [13]. However, a randomized study failed to demonstrate a benefit with HDCT [14]. These results could be attributed to a high dropout rate in the transplantation arm secondary to the use of multiple lines of CDCT before autoHCT and the use of HDCT considered suboptimal. In a prospective study comparing 1 cycle of CDCT followed by 3 cycles of HDCT to 3 cycles of CDCT followed by 1 cycle of HDCT in patients with relapsed/refractory GCT, there was no statistical differences in outcomes between the 2 arms, likely because the trial was stopped prematurely after excessive nonrelapse mortality (NRM) was detected in the ST arm [15].
These conflicting data have led to the lack of a standard of care in the salvage setting, resulting in wide variation in clinical practice worldwide, underscoring the need for a randomized prospective study, which is currently ongoing (TIGER; Clinical Trials.gov identifier NCT02375204). Given that the current out-come data are mainly from highly specialized centers, we analyzed the outcomes of patients with relapsed/refractory male GCT who underwent autoHCT and were reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). The CIBMTR reporting mechanism captures the majority of autoHCTs performed in North America [16].
METHODS
Data Source
The CIBMTR is a prospectively maintained registry that collects HCT data from more than 500 centers worldwide [16]. Data are collected prospectively at 2 levels: registration and research. The registration data include disease type, age, sex, date of diagnosis, conditioning regimen, post-transplantation disease progression, survival, and cause of death for all transplantations reported to the CIBMTR. More detailed data are collected from a subgroup of registered patients selected for research data using a weighted randomization scheme. Data are collected pretransplantation, at 100 days and 6 months post-transplantation, and annually thereafter until death or last follow-up. Protected health information used in the performance of such research is collected and maintained in the CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule. Data accuracy and completeness are maintained by rigorous onsite audits and quality visits as part of a comprehensive data quality program.
Patient Selection
Patients who underwent autoHCT for relapsed/refractory GCT in the United States and Canada between January 1, 1990, and December 31, 2015, and registered with CIBMTR were included. These included 2395 patients on the registration track, that is, all GCT patients reported to the CIBMTR. Data were analyzed in arbitrarily grouped 5-year cohorts based on the year of autoHCT: 1990 to 1994, 1995 to 1999, 2000 to 2004, 2005 to 2009, and 2010 to 2015. This dataset was used to assess survival trends. Research-level data were available for a subgroup of 267 patients who underwent autoHCT between 2000 and 2015 and were analyzed in multivariable analyses. Key characteristics and survival for the patients who underwent autoHCT between 2000 and 2015 were compared between the registration set (N = 1489) and the research subset (N = 267) to confirm that the subgroup was representative of the overall dataset (Supplementary Table 1). Data accuracy of the CIBMTR database is ensured by mandatory audits of centers and a robust quality system.[17,18]. For this study, it was further enhanced by the investigator’s review of discrepancies and queries to centers whenever necessary.
Definitions of Outcomes
Overall survival (OS) was defined as death from any cause with censoring of surviving patients at last follow-up. PFS was defined as survival without disease or relapse from complete response. Patients alive and without relapse/progression were censored at last follow-up. Relapse/ progression was defined as the time to first evidence of recurrence or progression of GCT and summarized by the cumulative incidence estimate with NRM as the competing risk. NRM was defined as death from any cause in the first 28 days after transplantation or death beyond 28 days after transplantation in the absence of persistent disease, recurrence, or tumor progression. Cisplatin resistance was defined as disease progression (tumor marker increase with/without radiographic progression) within 6 months after cisplatin-based chemotherapy. Cisplatin refractoriness was defined as disease progression (tumor marker increase with/without radiographic progression) during treatment or within 4 weeks after cisplatin-based chemotherapy.
Statistical Analysis
Descriptive statistics of patient-, disease-, and transplantation-related factors were recorded as median and range for continuous variables and a percentage of the total for categorical variables. Estimates of outcomes were reported as probabilities with 95% confidence intervals (CI). The probabilities of PFS and OS were estimated using the Kaplan-Meier method, and comparisons were performed using the log-rank test. The probabilities of relapse/progression and NRM were estimated using the cumulative incidence function. Multivariate analysis was performed using Cox proportional hazards models to identify risk factors for the outcomes of interest. The variables tested in the multivariate analysis were year of transplantation group (main variable), age, race, Karnofsky Performance Status score, hematopoietic cell transplantation-comorbidity index (HCT-CI), disease site, histology, presence of yolk sac tumor, International Germ Cell Consensus Classification Group (IGCCCG) stage, Beyer score at transplantation (calculated based on the presence/absence of mediastinal nonseminoma primary tumor, refractory or absolute refractory disease to conventional-dose cisplatin, and human chorionic gonadotropin [HCG] level >1000 U/L before HDCT), lines of chemotherapy before HDCT, best response to initial chemotherapy (provided by transplantation centers using response evaluation criteria in solid tumors [RECIST] criteria and captured on CIBMTR forms), platinum sensitivity, disease status before transplantation, intent for TT, time from diagnosis to transplantation, and conditioning regimen. HCT-CI was analyzed as a categorical variable (0 versus 1 versus 2 versus ≥3), but this information was not captured before 2007 as the HCT-CI was not introduced until 2007.
The proportional hazards assumption was assessed for each factor. If the proportional hazards assumption was violated, the covariates with nonproportional hazards were included in the final model as time-dependent covariates. Stepwise variable selection was used to identify variables that were significant at the .05 level.
RESULTS
Patient-, Disease-, and Transplantation-Related Variables
Patient characteristics of the registration cohort (N = 2395) and the research subset (N = 267) are summarized in Tables 1 and 2, respectively. For the research subset, the median age at transplantation was 32 years, 17% of patients had primary extragonadal GCT (5% primary mediastinal GCT) at diagnosis, and seminoma accounted for only 19% of the GCTs for which autoHCT was performed. Almost one-half (49%) of the patients had intermediate/poor-risk disease based on the IGCCCG classification, and 55% had intermediate/high-risk disease based on Beyer scoring, although the Beyer risk was not calculable in 36% of the population (due to missing variables). In addition, 29% had platinum-resistant/refractory disease.
Table 1.
Characteristics of North American Male Patients Who Underwent AutoHCT for GCT Registered with the CIBMTR between 1990 and 2015
| Variable | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2015 |
|---|---|---|---|---|---|
| Number of patients | 288 | 351 | 376 | 509 | 871 |
| Number of centers | 73 | 108 | 129 | 126 | 137 |
| Age at transplantation, yr, median (range) | 31 (17–63) | 32 (12–59) | 31 (12–61) | 31 (11–76) | 30 (11–70) |
| Karnofsky Performance Status score, n (%) | |||||
| 80–100 | 83 (29) | 202 (58) | 241 (64) | 353 (69) | 745 (86) |
| <80 | 9 (3) | 21 (6) | 28 (7) | 44 (9) | 91 (10) |
| Missing | 196 (68) | 128 (36) | 107 (28) | 112 (22) | 35 (4) |
| Subdisease, n (%) | |||||
| Testicular | 269 (93) | 305 (87) | 251 (67) | 323 (63) | 614 (70) |
| GCT, extragonadal | 19 (7) | 46 (13) | 125 (33) | 186 (37) | 257 (30) |
| Time from diagnosis to autoHCT, mo, n (%) | |||||
| 0–6 | 18 (6) | 59 (17) | 65 (17) | 57 (11) | 104 (12) |
| 6–12 | 81 (28) | 109 (31) | 126 (34) | 185 (36) | 351 (40) |
| 12–18 | 46 (16) | 66 (19) | 60 (16) | 93 (18) | 151 (17) |
| 18–24 | 24 (8) | 28 (8) | 36 (10) | 55 (11) | 86 (10) |
| >24 | 60 (21) | 82 (23) | 78 (21) | 101 (20) | 177 (20) |
| Missing | 59 (20) | 7 (2) | 11 (3) | 18 (4) | 2 (<1) |
| Number of transplantations, n (%) | |||||
| Single autoHCT | 212 (74) | 233 (66) | 214 (57) | 179 (35) | 240 (28) |
| Double autoHCT | 73 (25) | 116 (33) | 158 (42) | 313 (61) | 507 (58) |
| Triple autoHCT | 3 (1) | 2 (<1) | 4 (1) | 17 (3) | 124 (14) |
| Follow-up of survivors, mo, median (range) | 96 (3–313) | 86 (4–245) | 80 (3–191) | 72 (3–129) | 27 (3–79) |
Table 2.
Characteristics of North American Male Patients with Detailed Research Data Who Underwent First AutoHCT for GCTs in 2000–2015 and Reported to the CIBMTR
| Variable | 2000–2004 | 2005–2009 | 2010–2015 |
|---|---|---|---|
| Number of patients | 81 | 143 | 43 |
| Number of centers | 43 | 60 | 26 |
| Age at autoHCT, yr, median (range) | 35 (15–58) | 31 (16–76) | 32 (13–57) |
| Karnofsky Performance Status score, n (%) | |||
| 80–100 | 72 (89) | 115 (80) | 36 (84) |
| < 80 | 7 (9) | 17 (12) | 7 (16) |
| Missing | 2 (2) | 11 (8) | 0 |
| Disease site at diagnosis, n (%) | |||
| Testicular | 68 (84) | 119 (83) | 34 (79) |
| Extragonadal | |||
| Retroperitoneal | 5 (6) | 15 (10) | 2 (5) |
| Mediastinum | 6 (7) | 4 (3) | 4 (9) |
| Other | 2 (2) | 5 (3) | 3 (7) |
| Histology at diagnosis, n (%) | |||
| Seminoma | 18 (22) | 27 (19) | 6 (14) |
| Nonseminoma | |||
| Mixed nonseminoma | 44 (54) | 75 (52) | 25 (58) |
| Other pure histology* | 17 (20) | 41 (28) | 10 (24) |
| Missing | 4 (5) | 12 (8) | 2 (5) |
| Beyer score, n (%) | |||
| Low risk | 10 (12) | 11 (8) | 4 (9) |
| Intermediate risk | 38 (47) | 73 (51) | 21 (49) |
| High risk | 4 (5) | 8 (6) | 3 (7) |
| Not evaluable | 29 (36) | 51 (36) | 15 (35) |
| Prognosis at diagnosis (IGCCCG stage), n (%) | |||
| Seminoma | 18 (22) | 27 (19) | 6 (14) |
| Nonseminoma good | 10 (12) | 14 (10) | 7 (16) |
| Nonseminoma intermediate | 8 (10) | 27 (19) | 3 (7) |
| Nonseminoma poor | 29 (36) | 49 (34) | 15 (35) |
| Nonseminoma not specified | 14 (17) | 26 (18) | 10 (23) |
| Missing | 2 (2) | 0 | 2 (5) |
| Lines of chemotherapy, n (%) | |||
| 1 | 10 (12) | 30 (21) | 17 (40) |
| ≥2 | 71 (87) | 113 (79) | 26 (61) |
| Planned vs performed autoHCTs, n (%) | |||
| Single autoHCT | 50 (62) | 52 (36) | 10 (23) |
| Double autoHCT | 31 (38) | 91 (64) | 33 (77) |
| Disease status before transplantation, n (%) | |||
| PRm+ | 31 (38) | 66 (46) | 23 (53) |
| PRm- | 34 (42) | 53 (37) | 15 (35) |
| Elevated markers only | 10 (12) | 20 (14) | 4 (9) |
| Missing | 6 (7) | 4 (3) | 1 (2) |
| Conditioning regimen† | |||
| Carboplatin + vp16 (no cyclophosphamide) | 46 (57) | 114 (80) | 43 (100) |
| Nonstandard regimens | 35 (43) | 29 (20) | 0 |
| Time from diagnosis to transplantation, mo, n (%) | |||
| ≥12 | 42 (52) | 65 (45) | 18 (42) |
| 12–24 | 24 (30) | 51 (36) | 14 (33) |
| >24 | 15 (19) | 27 (19) | 11 (26) |
| Follow-up of survivors, mo, median (range) | 70 (4–167) | 72 (6–124) | 16 (5–54) |
PRm+ indicates residual tumor with elevated markers; PRm-, residual tumor with normal markers.
Pure histology included patients with pure embryonal cancer, yolk sac tumor, or choriocarcinoma.
Nonstandard regimens included cyclophosphamide, thiotepa, busulfan, and various combinations not involving carboplatin and etoposide together
Nearly one-half (47%) of patients underwent autoHCT within 12 months of initial diagnosis, and 80% did so within 24 months. Transplantation was performed earlier in the disease course in later years; the proportion receiving transplantation as first salvage therapy in 2010 to 2015 was higher compared with earlier years (40% versus 12% for 2000 to 2004 and 21% for 2005 to 2009). The proportion of TTs increased over time, from 38% in 2000 to 2004 to 64% in 2005 to 2009 to 77% in 2010 to 2015. Almost one-half (45%) of patients had residual disease with elevated tumor markers (PRm+) at the time of HDCT, and 38% underwent HDCT with residual disease and normal tumor markers (PRm-). A carboplatin/etoposide-based regimen as the conditioning regimen was increasingly preferred over time, at 57% in 2000 to 2004, 80% in 2005 to 2009, and 100% in 2010 to 2015, respectively. The median follow-up of survivors from transplantation was 51 months (range, 3 to 313 months).
Trend in Outcomes in 1990 to 2015 (N=2395)
Univariate outcomes for the overall cohort showed no statistically significant change in NRM over time. However, the 3-year relapse/progression rates decreased, and both PFS and OS serially improved over the 5 time intervals (Table 3). Day +100 NRM was 8% (95% CI, 4% to 12%) in 1990 to 1994 versus 4% (95% CI, 3% to 5%) in 2010 to 2015 (P = .20). The 3-year probability of relapse/progression decreased from 68% (95% CI, 61% to 75%) in 1990 to 1994 to 42% (95% CI, 38% to 45%) in 2010 to 2015 (P < .001). Similarly, the 3-year PFS improved from 24% (95% CI, 18% to 31%) in 1990 to 1994 to 47% (95% CI, 43% to 50%) in 2010 to 2015 (P < .0001) and the 3-year OS improved from 35% (95% CI, 29% to 40%) in 1990 to 1994 to 54% (95% CI, 50% to 57%) in 2010 to 2015 (P < .0001) (Figure 1).
Table 3.
Univariate Analysis of Outcomes by Year of Transplantation
| 1990–1994(N=288) | 1995–1999 (N=351) | 2000–2004 (N=376) | 2005–2009 (N=509) | 2010–2015 (N=871) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Eval. n | Prob. % (95% Cl) | Eval. n | Prob. %(95% Cl) | Eval. n | Prob. %(95% Cl) | Eval. n | Prob. % (95% Cl) | Eval, n | Prob. % (95% Cl) | P Value |
| TRM | 165 | 299 | 349 | 476 | 863 | .19 | |||||
| 30 d | 7(3–11) | 4(2–7) | 3(2–5) | 2(1–4) | 3(2–4) | .16 | |||||
| 100 d | 8(4–12) | 6(4–9) | 5(3–7) | 4(2–6) | 4(3–5) | .20 | |||||
| Relapse | 165 | 299 | 349 | 476 | 863 | <.001 | |||||
| 1 yr | 53(45–60) | 44(39–50) | 44(38–49) | 47(42–51) | 37(34–41) | <.001 | |||||
| 2 yr | 64(57–71) | 50(45–56) | 49(44–54) | 52 (48–57) | 40(37–44) | <.001 | |||||
| 3 yr | 68(61–75) | 53 ( 47–58) | 51(45–56) | 53(49–58) | 42(38–45) | <.001 | |||||
| PFS | 165 | 299 | 349 | 476 | 863 | <.001 | |||||
| 1 yr | 41(34–49) | 48 (43–54) | 51(46–56) | 47(43–52) | 54(50–57) | .02 | |||||
| 2 yr | 29(22–36) | 39(34–45) | 42(37–48) | 40(36–45) | 48(44–51) | <.001 | |||||
| 3yr | 24(18–31) | 36(31–42) | 40(35–45) | 39(34–43) | 47 (43–50) | <.001 | |||||
| OS | 288 | 351 | 376 | 509 | 871 | <.001 | |||||
| 1 yr | 52 (46–58) | 62(57–67) | 71(66–75) | 68(63–72) | 69(66–72) | <.001 | |||||
| 2 yr | 40(34–45) | 50(44–55) | 55(49–60) | 55(51–60) | 56(53–60) | <.001 | |||||
| 3yr | 35 (29–40) | 44(39–50) | 48(43–53) | 52(47–56) | 54(50–57) | <.001 | |||||
Eval indicates evaluable; Prob, probability.
Figure 1.
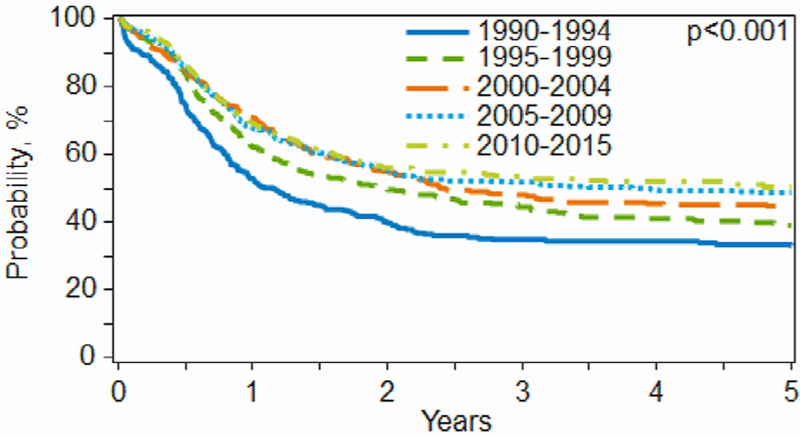
OS by year of transplantation.
Figure 2.
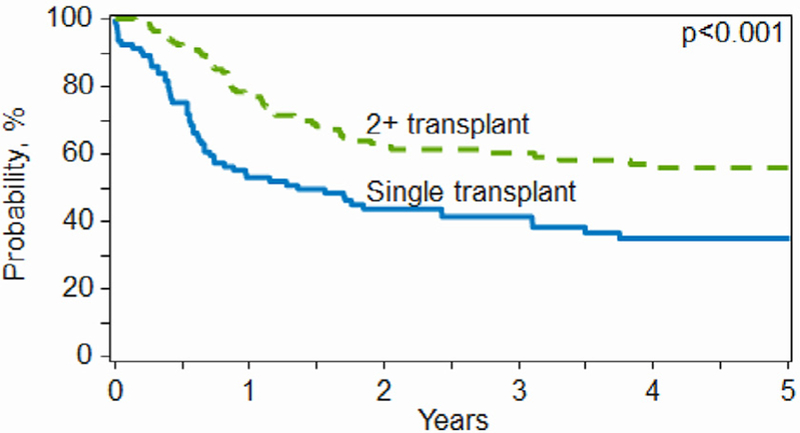
OS by single versus tandem autoHCT.
Multivariate Analysis of Outcomes (N=267)
Table 4 presents the results of the multivariate analysis of outcomes, including relapse/progression, PFS, and OS. Residual disease (PRm-/PRm+) at the time of transplantation, >1 line of chemotherapy before HDCT, and nonseminoma histology were associated with a higher incidence of relapse/progression, inferior PFS, and inferior OS. Furthermore, TT was associated with superior PFS and OS compared with ST. Year of transplantation was not significant for OS when adjusted for all these factors.
Table 4.
Multivariate Analysis of Outcomes
| Relapse/Progression | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | N | HR | P Value | HR | P Value | HR | P Value | |
| Year of transplantation | Overall | .27 | .04 | .10 | ||||
| 2000–2004 | 62 | 1.00 | 1.00 | 1.00 | ||||
| 2005–2009 | 108 | 1.45 | .11 | 1.76 | .01 | 1.69 | .03 | |
| 2010–2015 | 33 | 1.24 | .50 | 1.47 | .22 | 1.36 | .43 | |
| Histology | ||||||||
| Seminoma | 41 | 1.00 | 1.00 | 1.00 | ||||
| Nonseminoma | 162 | 2.71 | <.01 | 1.97 | .02 | 1.93 | .05 | |
| Disease staging before HCT | Overall | <.01 | <.01 | <.01 | ||||
| Marker+ | 28 | 1.00 | 1.00 | 1.00 | ||||
| PRm+ | 79 | 2.92 | <.01 | 3.23 | <.01 | 3.62 | <.01 | |
| PRm+ | 96 | 3.45 | <.01 | 3.7 | <.01 | 4.89 | <.01 | |
| Lines of chemotherapy | Overall | <.01 | <.01 | .01 | ||||
| 1 | 42 | 1.00 | 1.00 | 1.00 | ||||
| 2 | 101 | 2.21 | <.01 | 2.35 | <.01 | 1.76 | .08 | |
| 3+ | 60 | 3.25 | <.01 | 3.11 | <.01 | 2.72 | <.01 | |
| Number of autoHCTs | ||||||||
| 2+ | 122 | - | - | 1.00 | ||||
| 1 | 81 | - | - | 1.52 | .03 | 2.13 | <.01 | |
| Karnofsky Performance Status score | ||||||||
| 80–100 | 180 | 1.00 | - | - | - | - | ||
| <80 | 23 | 2.10 | <.01 | - | - | - | - | |
HR indicates hazard ratio; Marker+, elevated tumor markers only.
Characteristics of TT Recipients
Compared with ST, TT recipients were younger (31 years [95% CI, 16–62 years] versus 34 years [95% CI, 13 to 76 years]), had a lower HCT-CI, and were more likely to have intermediate/poor-risk disease at primary diagnosis based on IGCCCG stage. TT recipients were also more likely to be platinum-resistant/ refractory and to receive HDCT after 1 line of chemotherapy (28% versus 9%) within 1 year of diagnosis (51% versus 38%). There was increasing use of TT over ST in later cohorts (38% of transplantations were TT in 2000 to 2004 versus 77% in 2010 to 2015), and this was also associated with a higher proportion of TT receiving a carboplatin/etoposide-based conditioning regimen (85% with TT versus 51% with ST).
An interval of c28 days between day 0 of the 2 transplantations for TT recipients was associated with the best univariate outcomes, with lower relapse rates at 1 year (31%; 95% CI, 26% to 37%) and 3 years (37%; 95% CI, 31% to 43%) compared with those associated with an interval of >28 days between transplantations (41% [95% CI, 37% to 44%] and 48% [95% CI, 44% to 51%], respectively) (P = .003). Similarly, PFS was superior at a 28-day interval, with a 3-year PFS of 55% (95% CI, 49% to 61%) versus 46% (95% CI, 42% to 49%) for a >28-day interval (P = .009). No significant difference in NRM or OS was associated with the interval between transplantations in TT recipients.
DISCUSSION
Cisplatin-based combination chemotherapy has transformed advanced GCT from a uniformly lethal disease into one of the most curable neoplasms [19]. In the late 1980s, the role of HDCT followed by autoHCT and salvage chemotherapy was established [5,9,20]. Currently, either of these approaches is considered appropriate in a second-line salvage setting. Using a longitudinal dataset, we examined outcomes after autoHCT in a large cohort of patients with GCTs. The practice patterns identified are reflective of the real-world clinical setting. To our knowledge, this is the largest study evaluating the role of autoHCT for GCT in North America.
We report several clinically important observations. First, there is a wide variation in clinical practice in the use of autoHCT for GCT in North America. Second, the outcomes are superior with TT compared with ST. Third, autoHCT is associated with better outcomes when used in an earlier line (first salvage) at relapse rather than in later lines. Fourth, the out-comes with autoHCT have improved over time, presumably associated with the increasing use of TT and incorporation of HDCT in the second line (as opposed to later in the disease course). Finally, we explored outcomes for GCT based on the timing between the 2 transplantations and found that c28 days provided the best outcomes with lower relapse and PFS, without an increase in TRM. We attempted to perform additional analyses, such as a difference in transplantation outcomes based on early versus late relapse, but identified only 13 patients in the CRF cohort that had a transplantation for late relapse (>24 months from diagnosis) and thus were unable to perform this analysis.
Although autoHCT has been established as an effective salvage strategy for GCT, the lack of an established consensus on the timing and the number of transplantations is evident from the variation in practice noted in our study. Over the past 3 decades, practice has become more uniform, and an increasing number of centers are performing autoHCT for GCT, which likely explains the improvement in outcomes over time. In the most recent cohort (2010 to 2015), we noted the near-uniform use of carboplatin/etoposide conditioning regimen and use of TT. In our overall cohort, however, the intent for a TT was only 62%, and 9% of these patients did not undergo TT owing to progressive disease or toxicity after the first transplantation of the planned TT. These data suggest that ST is still considered an effective salvage strategy by many physicians. The use of HDCT for patients with extragonadal GCT also has increased over time. AutoHCT is predominantly used in a second salvage or later setting (in 79%), indicating that CDCT remains a preferred approach in the first-line salvage setting in many centers. Similar to previous studies, autoHCT when used in patients age c40 years was curative [21]. Despite indirect evidence that adding a third drug to carboplatin plus etoposide might not be beneficial [22], wide discrepancy was noted in the conditioning regimens used for autoHCT until the most recent cohort. Non-carboplatin-etoposide-based regimens were administered to a significant proportion of patients (43%), although a difference in outcomes based on conditioning regimen was not observed in multivariate analysis. Given the heterogeneity in practice patterns and curative role of transplantation, these patients would benefit from expertise at tertiary care centers of testicular cancer excellence.
Importantly, TT was associated with superior PFS and OS, and it appears that over time, TT is being increasingly adopted into practice. Historically, treatment-related toxicity was high in the era of marrow grafts when HDCT for GCT was developed at Indiana University [9]. In the late 1990s, the use of growth factors, blood-derived hematopoietic cell grafts, and rapid engraftment led to the development of TT. A similar study using the CIBMTR database conducted in 1989 to 2002 analyzed 300 autoHCT recipients with relapsed/refractory GCT and found no benefit with TT over ST [23]. The improved outcomes in the most recent cohort in our study corroborates reported single-institution experience and could be due to the numerous improvements in peritransplantation care [24,25]. However, there are no randomized prospective comparisons of ST versus TT. In the modern era, investigators from Indiana University reported 2-year PFS and OS of 60% and 66%, respectively, in a cohort of 364 consecutive patients uniformly treated with TT [26]. In our study, in a much more real-world setting, the 1-year PFS and 2-year PFS in the latest cohort are 54% and 48%, respectively, a substantial improvement over previous years.
Multivariate analysis suggests that the increasing use of TT and performance of autoHCT earlier as first salvage treatment are key practice changes that have likely contributed to the improved outcomes seen in the latest cohort. Many of the currently identified prognostic models could not be validated in our dataset, owing mainly to a lack of uniform and consistent reporting of disease-related data [27,28]. Investigators at Memorial Sloan Kettering Cancer Center (MSKCC) recommend 3 courses of HDCT as salvage therapy [12,29]. In our study, only 6% underwent 3 courses of HDCT, suggesting that this approach is not as widely used in North America. Notably, although there were 48 patients from MSKCC and 306 from Indiana University in the registration dataset, there were no patients from MSKCC and Indiana University in the research dataset used for multivariate models. Owing to the small number of patients with the triple transplantation approach, we did not formally compare outcomes for this approach.
The optimal timing of autoHCT in GCT has also been debated. Several retrospective studies have reported better outcomes with transplantation as a first salvage treatment [7,13]. In the present analysis, only 21% overall underwent autoHCT as first salvage treatment, and this was associated with a lower risk of relapse and superior PFS and OS. The use of transplantation as a first salvage treatment has increased over time, with 40% in the latest cohort, compared with 12% in the early cohort. Although our results provide further corroboration that early autoHCT may be superior to CDCT in first-line salvage treatment, the results of the prospective study are awaited.
Encouragingly, PFS and OS with transplantation for GCT has clearly improved over time, indicating that substantial numbers of patients are being cured across many centers. As in other recent series, nonseminoma histology, evidence of residual tumor at autoHCT, later line transplantation as well as ST (versus TT), were associated with worse PFS and OS [7,26]. In our multivariate model, cisplatin sensitivity did not predict outcomes, very likely due to the extremely small number of patients (n = 10) who had true cisplatin-refractory disease reported to the CIBMTR database within the research cohort. The rate of secondary malignancy with autoHCT in our series (<1%) is similar to that reported in the recent literature [30].
We acknowledge several limitations of this study, including the retrospective nature of our dataset and accrual of patients over a 25-year period at multiple institutions, during which time supportive measures and peritransplantation care evolved. However, examining the changing outcomes of transplantation for GCT in the real world setting over time was our intention. The following caveats should be borne in mind. Prognostic data on several risk factors were missing for the earlier cohorts (due to the long accrual period), limiting our ability to study their impact across the whole population. Patient selection bias may explain the improved survival seen with early TT over ST. Finally, the CIBMTR only captures data on patients who underwent a transplantation, and thus we are unable to make comparisons with patients who did not undergo a transplantation. Conversely, the strength of the dataset lies in the large multicenter cohort with excellent longitudinal follow-up data on disease outcomes, as well as the fact that the vast majority of transplantations performed across the region are in this dataset. Thus, we are able to provide a general view of the use of transplantations for GCT in North America. In conclusion, these data validate previous knowledge from large institutional series at specialized centers in a real-world setting and suggest a benefit with the adoption of early TT in relapsed/refractory GCT in the absence of prospective randomized trial results.
Supplementary Material
ACKNOWLEDGMENTS
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases; Grant/Cooperative Agreement 4U10HL069294 from the NHLBI and NCI; Contract HHSH25020170006C with the Health Resources and Services Administration; Grants N00014–17-1–2388 and N0014–17-1–2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, *Amgen, *Amneal Biosciences; *Angiocrine Bioscience, anonymous donation to the Medical College of Wisconsin, Astellas Pharma US, Atara Biotherapeutics, Be the Match Foundation, *bluebird bio, *Bristol Myers Squibb Oncology, *Celgene, Cerus, *Chimerix, Fred Hutchinson Cancer Research Center, Gamida Cell, Gilead Sciences, HistoGenetics, Immucor, *Incyte, Janssen Scientific Affairs, *Jazz Pharmaceuticals, Juno Therapeutics, Karyopharm Therapeutics, Kite Pharma, Medac, MedImmune, The Medical College of Wisconsin, *Mediware, *Merck & Co, *Mesoblast, MesoScale Diagnostics, Millennium, the Takeda Oncology Co, *Miltenyi Biotec, National Marrow Donor Program, *Neovii Biotech NA, Novartis Pharmaceuticals, Otsuka Pharmaceutical, PCORI, *Pfizer, *Pharmacyclics, PIRCHE, *Sanofi Genzyme, *Seattle Genetics, Shire, Spectrum Pharmaceuticals, St. Baldrick’s Foundation, *Sunesis Pharmaceuticals, Swedish Orphan Biovitrum, Takeda Oncology, Telomere Diagnostics, and the University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, the Health Resources and Services Administration, or any other agency of the US Government.
*Corporate member.
Footnotes
Financial disclosure: See Acknowledgments on page 7.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.bbmt.2019.02.015.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Le Cornet C, Lortet-Tieulent J, Forman D, et al. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur J Cancer 2014;50:831–839. [DOI] [PubMed] [Google Scholar]
- 3.de Wit R, Stoter G, Sleijfer DT, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer 1998;78:828–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mead GM, Cullen MH, Huddart R, et al. A phase II trial of TIP (paclitaxel, ifosfamide and cisplatin) given as second-line (post-BEP) salvage chemotherapy for patients with metastatic germ cell cancer: a medical research council trial. Br J Cancer 2005;93:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol 2005;23:6549–6555. [DOI] [PubMed] [Google Scholar]
- 6.Loehrer PJ Sr, Lauer R, Roth BJ, Williams SD, Kalasinski LA, Einhorn LH. Salvage therapy in recurrent germ cell cancer: ifosfamide and cisplatin plus either vinblastine or etoposide. Ann Intern Med 1988;109:540–546. [DOI] [PubMed] [Google Scholar]
- 7.Einhorn LH, Williams SD, Chamness A, Brames MJ, Perkins SM, Abonour R. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med 2007;357:340–348. [DOI] [PubMed] [Google Scholar]
- 8.Necchi A, Nicolai N, Mariani L, et al. Modified cisplatin, etoposide, and ifosfamide (PEI) salvage therapy for male germ cell tumors: long-term efficacy and safety outcomes. Ann Oncol 2013;24:2887–2892. [DOI] [PubMed] [Google Scholar]
- 9.Nichols CR, Tricot G, Williams SD, et al. Dose-intensive chemotherapy in refractory germ cell cancer: a phase I/II trial of high-dose carboplatin and etoposide with autologous bone marrow transplantation. J Clin Oncol 1989;7:932–939. [DOI] [PubMed] [Google Scholar]
- 10.Nichols CR, Andersen J, Lazarus HM, et al. High-dose carboplatin and etoposide with autologous bone marrow transplantation in refractory germ cell cancer: an Eastern Cooperative Oncology Group protocol. J Clin Oncol 1992;10:558–563. [DOI] [PubMed] [Google Scholar]
- 11.Siegert W, Beyer J, Strohscheer I, et al. High-dose treatment with carboplatin, etoposide, and ifosfamide followed by autologous stem-cell transplantation in relapsed or refractory germ cell cancer: a phase I/II study. The German Testicular Cancer Cooperative Study Group. J Clin Oncol 1994;12:1223–1231. [DOI] [PubMed] [Google Scholar]
- 12.Feldman DR, Sheinfeld J, Bajorin DF, et al. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: results and prognostic factor analysis. J Clin Oncol 2010;28:1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorch A, Bascoul-Mollevi C, Kramar A, et al. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: evidence from a large international database. J Clin Oncol 2011;29:2178–2184. [DOI] [PubMed] [Google Scholar]
- 14.Pico JL, Rosti G, Kramar A, et al. A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann Oncol 2005;16: 1152–1159. [DOI] [PubMed] [Google Scholar]
- 15.Lorch A, Kleinhans A, Kramar A, et al. Sequential versus single high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: long-term results of a prospective randomized trial. J Clin Oncol 2012;30:800–805. [DOI] [PubMed] [Google Scholar]
- 16.D’Souza A, Fretham C Current uses and outcomes of hematopoietic cell transplantation (HCT) Available at: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx. Accessed XXX.
- 17.Center for International Blood and Marrow Transplant Research. Data use and processing policy Available at: http://www.cibmtr.org/About/Documents/CIBMTR%20Data%20Use%20and%20Processing%20Policy.pdf. Accessed XXX.
- 18.Center for International Blood and Marrow Transplant Research. CIBMTR Audit program Available at: http://www.cibmtr.org/DataManagement/AuditProgram/Pages/index.aspx. Accessed XXX.
- 19.Einhorn LH, Donohue J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med 1977;87:293–298. [DOI] [PubMed] [Google Scholar]
- 20.Loehrer PJ Sr, Gonin R, Nichols CR, Weathers T, Einhorn LH. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol 1998;16:2500–2504. [DOI] [PubMed] [Google Scholar]
- 21.Necchi A, Lo Vullo S, Rosti G, et al. Administration of high-dose chemotherapy with stem cell support in patients 40 years of age or older with advanced germ cell tumours: a retrospective study from the European Society for Blood and Marrow Transplantation database. Bone Marrow Transplant 2017;52:1218–1220. [DOI] [PubMed] [Google Scholar]
- 22.Broun ER, Nichols CR, Tricot G, Loehrer PJ, Williams SD, Einhorn LH. High-dose carboplatin/VP-16 plus ifosfamide with autologous bone marrow support in the treatment of refractory germ cell tumors. Bone Marrow Transplant 1991;7:53–56. [PubMed] [Google Scholar]
- 23.Lazarus HM, Stiff PJ, Carreras J, et al. Utility of single versus tandem auto-transplants for advanced testes/germ cell cancer: a Center For International Blood and Marrow Transplant Research (CIBMTR) analysis. Biol Blood Marrow Transplant 2007;13:778–789. [DOI] [PubMed] [Google Scholar]
- 24.Ku K, Ibrahim S, Adra N, et al. A retrospective analysis of patients with metastatic germ cell tumor (GCT) treated at Indiana University (IU) from 2000 to 2012. J Clin Oncol 2015;33(15 suppl):4539. [Google Scholar]
- 25.Albany C, Adra N, Snavely AC, et al. Multidisciplinary clinic approach improves overall survival outcomes of patients with metastatic germ-cell tumors. Ann Oncol 2018;29:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: the Indiana University experience. J Clin Oncol 2017;35:1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.International Prognostic Factors Study Group. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol 2010;28:4906–4911. [DOI] [PubMed] [Google Scholar]
- 28.Beyer J, Kramar A, Mandanas R, et al. High-dose chemotherapy as salvage treatment in germ cell tumors: a multivariate analysis of prognostic variables. J Clin Oncol 1996;14:2638–2645. [DOI] [PubMed] [Google Scholar]
- 29.Selle F, Wittnebel S, Biron P, et al. A phase II trial of high-dose chemotherapy (HDCT) supported by hematopoietic stem-cell transplantation (HSCT) in germ-cell tumors (GCTs) patients failing cisplatin-based chemotherapy: the Multicentric TAXIF II study. Ann Oncol 2014;25:1775–1782. [DOI] [PubMed] [Google Scholar]
- 30.Necchi A, Lo Vullo S, Secondino S, et al. Secondary malignancies after highdose chemotherapy in germ cell tumor patients: a 34-year retrospective study of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 2018;53:722–728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


