Abstract
Objective:
Patients undergoing surgical repair of aortic coarctation have a 50% risk of pathologic left ventricular remodeling (increased left ventricular mass or relative wall thickness). Endothelin 1, ST2, galectin 3, norepinephrine, and B-natriuretic peptide are biomarkers that have been associated with pathologic LV change in adult populations but their predictive value following pediatric coarctation repair are not known.
Hypothesis:
Biomarker levels at coarctation repair will predict persistent left ventricular remodeling at 1-year follow up.
Design:
Prospective, cohort study of 27 patients age 2 days-12 years with coarctation of the aorta undergoing surgical repair. Echocardiograms were performed pre-operation, post-operation, and at 1 year follow-up. Plasma biomarker levels were measured at the peri-operative time points. Association between biomarker concentrations and echocardiographic parameters was assessed.
Results:
Neither left ventricular mass index nor relative wall thickness varied from pre-op to post-op. At pre-op, relative wall thickness was elevated in 52% and left ventricular mass index was elevated in 22%; at follow-up, relative wall thickness was elevated in 13% and left ventricular mass index was elevated in 8%. Presence of residual coarctation did not predict left ventricular remodeling (AUC 0.59; p>0.05). Multivariable receiver operating characteristic curve combining pre-op ST2 and endothelin 1 demonstrated significant predictive ability for late pathologic left ventricular remodeling (AUC 0.85; p=0.02).
Conclusions:
Persistent left ventricular hypertrophy and abnormal relative wall thickness at intermediate term follow-up was rare compared to previous studies. A model combining pre-op endothelin 1 and ST2 level demonstrated reasonable accuracy at predicting persistent abnormalities in this cohort. Larger studies will be needed to validate this finding and further explore the mechanism of persistent left ventricular remodeling in this population.
Keywords: Endothelin-1 (ET-1), B-type natriuretic peptide (BNP), norepinephrine (NE), ST-2, Galectin-3 (Gal-3), Relative wall thickness (RWT)
Introduction:
Isolated coarctation of the aorta affects 800 of every million live births, approximately 8% of all congenital heart disease.1 Open surgical repair, via thoracotomy or sternotomy, is required for most patients presenting with severe obstruction to relieve the pressure load on the left ventricle (LV). Although perioperative mortality is low in the modern era, the post-operative course is frequently complicated by either low cardiac output syndrome or recalcitrant hypertension.2,3 Additionally, affected children are at risk for long-term complications including persistent hypertension, a hypertensive response to exercise, altered cardiac mechanics, and LV hypertrophy in as many as 50% of cases.4,5
For many patients, evidence of cardiac remodeling is already present at the time of initial surgery; pre-operatively, approximately 65% of all children with coarctation of the aorta will have LV hypertrophy.6 Echocardiographic evidence of LV remodeling, present at diagnosis, suggests that there may be early activation of critical pathologic pathways. Persistence of LV abnormalities at late follow-up in 50% of patients despite adequate anatomic repair suggests that reversal of this process is often incomplete.7 The mechanisms underlying early LV remodeling and variable reverse remodeling in this population have not been described. Better understanding of such pathways and their patterns of activation offers significant promise to understand the mechanisms of disease progression, improve prognostic accuracy, and guide future therapy.
Blood levels of certain protein biomarkers may correlate with activation of the pathologic response to a high LV pressure load. Multiple protein biomarkers, including ET-1, ST-2, Gal-3, BNP, and NE have demonstrated promise in pre-clinical animal studies of coarctation of the aorta or in adult pressure overload lesions. However, none have been assessed in the peri-operative period surrounding pediatric coarctation of the aorta repair. A preliminary report from a subset of this cohort demonstrated early ET-1 activation in neonates undergoing coarctation repair and a modest correlation between ET-1 concentration and early LV thickening.8 No prior study has evaluated the peri-operative concentration of other protein biomarkers or their utility as predictors of persistent LV abnormalities in this population.
Here we present a prospective, cohort study of protein biomarkers and pathologic LV remodeling in pediatric patients undergoing surgical relief of LV obstructive lesions. We hypothesized that peripheral blood concentration of a panel of protein biomarkers would be associated with echocardiographic evidence of persistent LV abnormalities at intermediate-term follow-up.
Methods:
The Colorado Multiple Institution Review Board approved this study. Written informed consent was obtained from the study subjects’ parents in all cases. Written assent was obtained from all subjects aged between seven years and eighteen years. A manuscript reporting early peri-operative ET-1 and echocardiography results from a subset of this cohort has been previously published.8
Subjects
We prospectively enrolled consecutive subjects (aged 0 to 18 years) undergoing surgical relief of an isolated coarctation of the aorta with or without associated aortic arch hypoplasia at Children’s Hospital Colorado from September 2015 through March 2017. Exclusion criteria included patients with significant co-morbid heart disease, those with a prior intervention (surgical or trans-catheter) on their left ventricular outflow tract, and those weighing less than 2 kg, due to limitations in acceptable sample blood volumes for research. Subjects <=30 days of age on the day of surgery were included in the sub-cohort of “neonates”.
Clinical Data
Clinical information was extracted from the electronic medical record (Epic Systems, Verona, WI). Demographic variables, peri-operative details, key clinical variables, and follow up data were recorded. Study data were collected and managed using REDCap electronic data capture tools hosted at University of Colorado.
Laboratory Data
Blood samples were obtained within 24 hours prior to surgery and between 12–48 hours post-operatively. Extracted plasma aliquots were stored at −80 degrees C for batch analysis. ET-1, Gal-3, ST-2, NE, and BNP analysis were performed by enzyme-linked immunosorbent assay (ELISA) per manufacturer’s recommendations (ET-1, Gal-3, and ST-2: R&D Systems, Inc. Minneapolis, MN. BNP: Beckman Coulter, Inc., Brea, CA. NE: Biorad Laboratories, Hercules, Ca) in the core laboratory at Children’s Hospital Colorado, a College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA) accredited facility. Normal ranges are not well described in pediatric patients for ET-1, Gal 3, ST-2, and norepinephrine levels. At our institution, 99 pg/mL is taken as the upper limit of normal for BNP concentration.
Echocardiographic Data
Echocardiograms were obtained immediately prior to surgical repair, between 24 and 72 hours post-operatively, and at 1-year follow-up. All images were obtained with a GE Vivid E9 or E95 machine (General Electric, Chicago, Ill). Relative wall thickness (RWT) was measured at end-diastole from the parasternal short axis view at the mid-papillary level as the ratio of the sum of the posterior and septal mural thickness to the left ventricular internal end-diastolic diameter (Fig 1); a value of 0.41 is conventionally taken as the upper limit of normal for RWT.4 LV mass was calculated by the area-length (AL) method, indexed to height2.7 (LV mass index, LVMI), and compared to previously published normal values with LVMI > 95%ile for age and gender taken as abnormal.9,10
Figure 1:
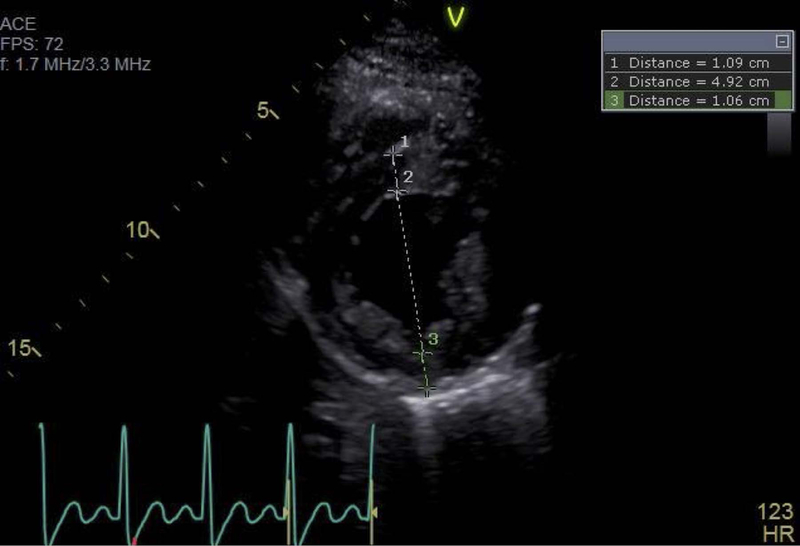
Representative echocardiographic image, obtained at end diastole in the parasternal short axis view at the level of the mitral valve papillary muscles, demonstrating a typical relative wall thickness measurement. 1 = inter-ventricular septal thickness, 2 = left ventricular end diastolic internal diameter, 3 = left ventricular posterior wall thickness. In this example, RWT = (1.09 + 1.06)/4.92 = 0.44.
Statistical Analysis
Demographics were summarized using descriptive statistics as indicated by the distribution of the data. Changes in echocardiographic indices were compared using the Signed-Rank test. Pearson’s correlation test, two-sample T-Test, and general linear modeling compared biomarker levels among groups and correlation with echocardiographic indices. All the statistical analyses were performed with SAS V9.4 or JMP V14 (SAS, Cary, NC).
Results:
Twenty-seven patients consented and enrolled in the study; twenty-four patients had complete data through follow-up and were included in the final analysis. Three patients were lost to follow-up between the post-op and follow-up time points due to the family moving to a different city (1) or due to the patient being clinically lost to follow-up (2). Their demographics are presented in Table 1. Five patients, all in the neonatal cohort, underwent aortic arch reconstruction on cardio-pulmonary bypass, while the other twenty-two underwent coarctectomy by lateral thoracotomy without bypass. Eight of the neonatal subjects had evidence of a patent ductus arteriosus on echocardiogram and were on prostaglandin infusion at the time of repair. In each of those patients, ductal flow was right-to-left in systole, indicating that pressure in the pulmonary artery was equal to or greater than the pressure in the aorta. Two patients were on continuous milrinone prior to repair. One patient was on a nitroprusside infusion prior to surgery. No patients were receiving any other vasoactive medications at the time of pre-operative sampling. At the time of post-operative sampling, five patients were receiving continuous milrinone, five patients were receiving continuous nicardipine, seven patients were receiving continuous nitroprusside, and four patients were receiving continuous esmolol. No patient received an endothelin receptor antagonist during the study period. No patient had a diagnosed significant systemic illness or genetic diagnosis.
Table 1:
Demographics
| Subjects | Entire Cohort (n=27) | Neonates (n=17) | Older Children (n=10) | P-value |
|---|---|---|---|---|
| Age at repair [yr] | 0.70 (0.01, 11.06) | 0.05 (0.01, 2.34) | 5.46 (2.54, 11.06) | < .0001 |
| Weight at repair [kg] | 5.90 (3.10, 81.8) | 3.82 (3.10, 13.10) | 20.55 (12.4, 81.8) | <.0001 |
| Male Gender [n] | 20 (74%) | 12 (71%) | 8 (80%) | 0.6784 |
| Cardio-Pulmonary Bypass [n] | 5 (18.5%) | 5 (29.4%) | 0 (0%) | 0.1240 |
| Time to follow up [d] | 260 (108, 544) | 271 (108, 431) | 252 (191, 544) | 0.7756 |
| Anti-hypertensive therapy pre-op [n] | 5 (15.5%) | 1 (5.9%) | 4 (40.0%) | 0.0473 |
| Anti-hypertensive therapy at discharge [n] | 19 (70.4%) | 9 (52.9%) | 10 (100%) | 0.0119 |
| Anti-hypertensive therapy at follow-up [n] | 8 (33.3%) | 4 (25.0%) | 4 (50.0%) | 0.3625 |
| Residual outflow obstruction [n] | 5 (21.7%) | 4(26.7%) | 1 (12.5%) | 0.6214 |
Data presented as n (%) or median (range). P-values refer to comparison between neonatal and older children groups.
Clinical presentation varied by age at diagnosis. Eight of the neonates were diagnosed prenatally, started on prostaglandin infusion within the first hours of life, and remained stable until repair. Two neonates presented within the first week of life with clinical evidence of systemic hypoperfusion and were medically stabilized prior to operative repair. The patients between one month and one year of life had the greatest variability in clinical presentation, ranging from asymptomatic murmur to symptomatic left ventricular failure with decreased tissue oxygen delivery. Children older than one year were all clinically stable at presentation, referred for right upper extremity hypertension, decreased femoral pulses, or an asymptomatic murmur.
At follow-up, four patients had evidence of mild residual coarctation by echocardiography (gradient range 30–40 mmHg by echocardiography) and one had moderate coarctation (gradient 55 mmHg). Eight subjects required anti-hypertensive therapy at follow-up with five receiving angiotensin-converting enzyme inhibitor (ACE) therapy, two receiving beta-blocker, and one receiving combination ACE and beta-blocker therapy. No patients had clinical evidence of heart failure, were receiving a continuous infusion, or required hospitalization at the follow-up time point.
Biomarker concentration
At the pre-op time point, plasma concentrations of ET-1 (2.2 v. 0.8 pg/mL, p=0.001), NE (668 v. 236 pg/mL, p=0.001), Gal-3 (5.8 v. 3.8 pg/mL, p=0.02), and BNP (889 v. 32 pg/mL, p=0.001) were higher in the neonatal population than in the older children. Within the neonatal cohort, pre-op ET-1 concentration was higher among subjects who ultimately required repair on cardio-pulmonary bypass compared to those who required thoracotomy. (3.8 v. 1.8 pg/mL, p=0.01) There was no association between pre-operative concentration of the other biomarkers and type of surgical repair. There was no significant association between any pre-op biomarker concentration and exposure to pre-op prostaglandin infusion, pre-op milrinone infusion, or presence of prenatal coarctation diagnosis. There were no differences in ST-2 concentration at pre-op by age within the cohort. At the post-op time point, concentrations of ET-1, and BNP were decreased from pre-op in the neonatal cohort and increased from pre-op in the older children. NE was unchanged from pre-op in the neonates and increased from pre-op in the older children. ST-2 was increased at post-op in all patients, and Gal-3 was unchanged from pre-op to post-op in all patients. There were no differences in post-op biomarker concentrations between subjects requiring cardio-pulmonary bypass compared to those requiring a thoracotomy. Biomarker concentrations at the pre-operative and post-operative time points are shown in Fig 2.
Figure 2:
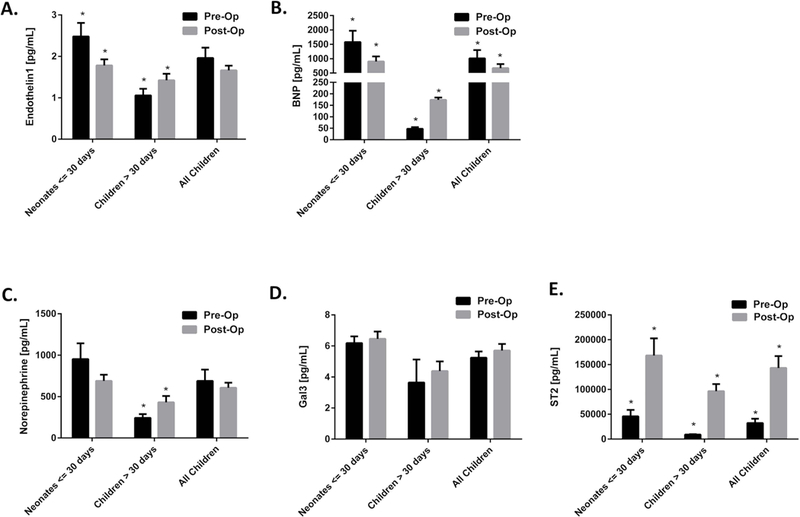
Biomarker plasma concentrations at the pre-operative and post-operative time points in the different age groups demonstrate distinct patterns of activation for most biomarkers in subjects of different ages: A) Endothelin-1, B) B-type natriuretic peptide (BNP), C) Norepinephrine, D) Galectin-3 (Gal3), E) ST-2. * Signifies p < 0.05 between the time points for a given age group.
Echocardiography
Mean values for RWT and LVMI at the various time points are shown in Table 2. Neither LVMI nor RWT varied statistically from pre-op to post-op. At pre-op, RWT was elevated in 48% and LVMI was elevated for age in 22% of subjects (Fig 3). Both average LVMI (42.1 v. 33.1, p<0.001) and average RWT (0.41 v. 0.28, p<0.0001) were higher at pre-op than at follow-up. At follow-up, RWT was elevated in 13% and LVMI was elevated in 8% of subjects. LVMI was higher for neonates than older children at all time points. RWT did not vary significantly by age. Neither RWT nor LVMI at any of the time points varied between subjects requiring cardio-pulmonary bypass and those repaired via thoracotomy.
Table 2:
Echocardiography Results
| Pre-Op | Post-Op | Follow-Up | P value | ||
|---|---|---|---|---|---|
| LVMI | Neonates | 53.0 (29.0, 133.3) | 66.5 (35.2, 98.6) | 37.2 (30.8, 82.6) | <.0001 |
| Older Children | 36.2 (20.6, 44.0) | 39.4 (26.7, 49.3) | 28.6 (25.1, 31.8) | 0.0004 | |
| Entire Cohort | 42.1 (20.6, 133.3) | 45.5 (26.7, 98.6) | 33.1 (25.1, 82.6) | <.0001 | |
| RWT | Neonates | 0.43 (0.22, 0.58) | 0.45 (0.21, 0.60) | 0.30 (0.21, 0.53) | 0.0002 |
| Older Children | 0.38 (0.27, 0.54) | 0.37 (0.28, 0.51) | 0.26 (0.18, 0.30) | <.0001 | |
| Entire Cohort | 0.41 (0.22, 0.58) | 0.40 (0.21 0.60) | 0.28 (0.18, 0.53) | <.0001 |
Data presented as median (range). P values refer to comparison between pre-op and follow-up time points.
Figure 3:
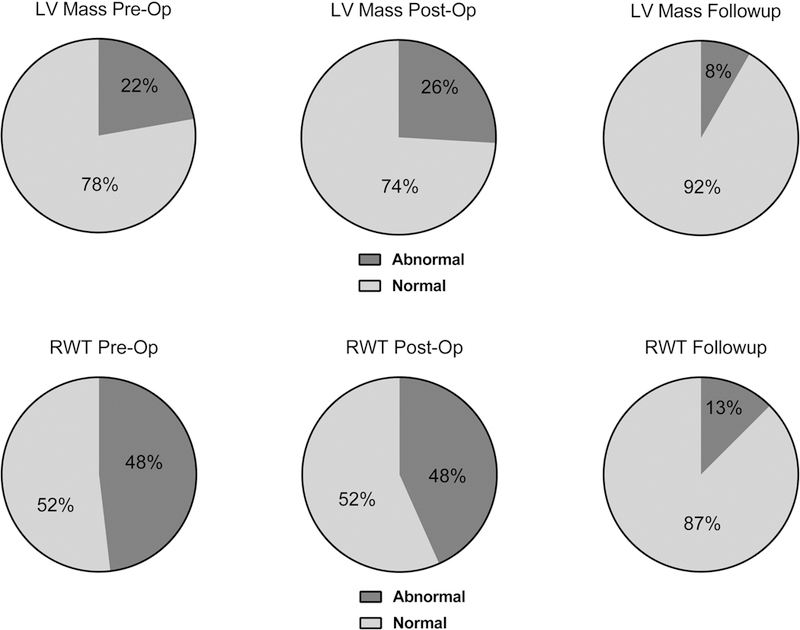
Serial echocardiograms demonstrate decreased incidence of abnormal left ventricular geometry at intermediate term follow-up compared to the peri-operative period.
Biomarkers as Predictors of LV remodeling
Pre-op ET-1 demonstrated a moderate positive linear correlation with post-op RWT (r = 0.43, p=0.01) and LVMI (r = 0.44, p=0.01). Pre-op ST-2 had a modest negative correlation with post-op RWT (r = 0.39, p=0.03) and LVMI (r=0.38, p=0.04). Pre-op BNP, NE, and Gal3 showed no linear relationship with post-op LVMI or RWT. No single pre-operative biomarker demonstrated a significant linear correlation with LVMI or RWT at follow-up.
We created receiver operator characteristic curves using previously defined normal ranges (fixed at 0.41 for RWT, age specific for LVMI) to evaluate the utility of pre-op biomarkers as predictors of abnormal echocardiographic parameters. Age on date of surgery (AUC 0.61) and presence of residual coarctation (AUC 0.59) did not predict persistence of abnormal LVMI or RWT. Pre-op ET-1 alone provided only modest predictive value for persistent, pathologic LV remodeling (abnormal LVMI or RWT, AUC 0.66) while none of the other pre-operative biomarkers were predictors of abnormal LV geometry at follow-up on univariate analysis. We then evaluated whether any of the remaining biomarkers acted as precision variables for ET-1 and found that addition of ST-2 improved the predictive accuracy of the model. A multivariable receiver operator characteristic curve combining ET-1 and ST-2 demonstrated this improved predictive ability (AUC 0.85, p=0.02; R2=0.35). For each unit increase in pre-operative ET-1, the odds of abnormal remodeling at intermediate follow-up increased 790% (p<0.05). Conversely, for each 1000 unit decrease in ST2 the odds of abnormal remodeling increased by 8.7%.
Discussion:
This is the first study to evaluate the association between protein biomarkers from the pre-operative period and abnormal LV geometry and at intermediate term follow-up in pediatric coarctation of the aorta. This study demonstrated that a multivariable model combining pre-op ET-1 and ST-2 levels was independently predictive for persistent myocardial abnormalities at 1-year post-surgery despite a lower incidence of LV abnormalities than has been previously reported for this population. Additional key findings included distinct patterns of activation between older children and neonates for ET-1, BNP, NE, and Gal-3 and a marked post-operative rise in ST-2 in all ages.
Persistently abnormal LV geometry remains a significant source of morbidity in pediatric patients status post coarctectomy. Our finding that the combination of elevated pre-operative ET-1 and depressed ST-2 plasma levels was associated with echocardiographic abnormalities at 1 year post-operation is significant for multiple reasons. First, this finding provides preliminary evidence for the potential utility of an ET-1/ST-2 biomarker panel as a clinical test to risk stratify patients prior to surgery. Such a panel, if validated in larger studies, could be useful to clinicians both as a means of guiding the frequency of echocardiographic surveillance and as a counseling tool for families. Second, linking the incompletely reversible myocardial changes seen prior to repair with chronic exposure to increased blood levels of ET-1 raises the possibility that ET-1 could be a driver of the pathologic changes seen.
Animal and in vitro studies have demonstrated a number of potential mechanisms by which ET-1 leads to myocardial hypertrophy in many coarctation patients. Using a mouse model of transverse aortic coarctation, Tsai, et al demonstrated increased myocardial tissue ET-1 concentration in mice with coarctation-induced myocardial hypertrophy.11 That study implicated Rho-kinase signaling and resultant increased superoxide production in activating the oxidative stress response leading to the myocardial hypertrophy. Other in vitro and animal work demonstrated that myocardial ET-1 exposure increases inositol triphosphate–diacylglycerol second messenger signaling thereby promoting genesis of new sarcomeres.12,13 Prior studies have also implicated VEGF overexpression, heightened TGF-β1 signaling, and ERK 1/2 activation as mechanisms for ET-1 induced myocardial hypertrophy.14–16 Further studies are needed to determine whether ET-1 exposure has a causal role for myocardial hypertrophy in our population and to clarify the specific mechanisms of action of ET-1 in pediatric pressure overload lesions.
Our findings are aligned with previous data linking higher blood levels of ET-1 to cardiac pathology. In coarctation of the aorta, Tavli, et al demonstrated an association between ET-1 concentration and persistent LV hypertrophy at a mean of 58 months after coarctectomy.17 Previous studies in adult subjects with heart failure due to reduced ejection fraction, ST-elevation myocardial infarction, and hypertension have each also shown an association between blood ET-1 concentration and clinical worsening.18–20 Higher ET-1 concentration was additionally linked to larger shunt volume in pre-operative pediatric subjects with left-to-right shunts.21 Our observation that ET-1 concentration was higher pre-operatively in those neonates requiring aortic arch reconstruction rather than simple coarctectomy could additionally reflect ET-1 activation in cases of more significant physiologic derangement. Taken with our findings, these studies support continued interest in plasma ET-1 concentration as a potentially useful clinical biomarker of a pathologic cardiac stress response.
Alterations in ST-2 biology have also been linked to changes in cardiac physiology. Under normal physiologic conditions, ST-2 exists in two predominant isoforms: membrane-bound ST-2 ligand is the true receptor for IL-33 on myocardial cells and exerts anti-hypertrophic effects, while soluble ST-2 is a decoy IL-33 receptor thereby promoting cardiac fibrosis and hypertrophy.22 In adult patients with both acute and chronic heart failure, plasma ST-2 concentration is known to be prognostic of clinical worsening.23,24 Previous studies have also demonstrated a significant increase in plasma ST-2 concentration after cardio-pulmonary bypass in adult patients.25 This response may be in part due to an increase in the soluble, pro-fibrotic ST-2 isoform driven by increased IL-1 and TNF-alpha activity.25 The pattern of peri-operative ST-2 concentration in our study is aligned with the findings of Szerafin et al, as all subjects showed a significant increase in ST-2 level post-operatively. However, the direction of the relationship between pre-operative ST-2 concentration and late myocardial change was unexpected, with lower pre-operative ST-2 concentration associating with myocardial pathology in strengthening the predictive capacity of our multivariate model. One possible explanation for this is that the lower pre-operative ST-2 concentration could in part reflect decreased expression of the membrane-bound, anti-hypertrophic ST-2 ligand isoform, which our assay cannot distinguish from soluble ST-2.
The rate of persistent LV abnormalities in our population was lower than has previously been reported.4–7,26 There are several potential reasons to explain this finding. Prior studies have typically assessed LV performance and geometry either prior to or several years after repair, while our study followed subjects to one year post-op. This raises the possibility that the prevalence of LV echocardiographic abnormalities would increase if our population were followed longer-term. We also used LV mass index normal values based on age to most appropriately dichotomize subjects.27 Given the higher upper limit of normal of LV mass index for infants and neonates compared to older children, our methodology may be more conservative in assigning a subject as abnormal than what was used in the prior studies.
Although we did not find evidence for an association between changes in LV geometry and BNP, NE, or Gal-3 concentration in the pediatric coarctation population, we did observe interesting patterns of activation for each. BNP levels were significantly elevated in the neonatal population at pre-op with a significant post-operative decline. As several patients in this group had pre-operative left ventricular dysfunction with post-operative improvement, this pattern likely reflects the hemodynamic effect of improved ventricular performance. Among older children, all of whom had normal markers of peri-operative ventricular function, BNP levels were normal at baseline with only a modest post-operative increase. NE levels were higher in neonates than in older children throughout, likely reflecting the greater degree of critical illness among the younger subjects. Older children demonstrated higher NE levels post-op compared to pre-op, consistent with the previously demonstrated catecholamine surge thought to be causative of early post-coarctectomy hypertension.2 Gal-3 concentration, a known marker of myocardial fibrosis in adult heart failure, did not vary significantly by age or time point within our cohort.28 Further studies will be needed to clarify the role of these molecules in other pediatric populations.
This study has several potential limitations. Due to being a single-center study and the low incidence of coarctation of the aorta in the population, the sample size is small. The moderate sample size does not allow us to pursue multivariate modeling to fully evaluate all potential confounding variables, such as whether there is an interaction between specific vasoactive medications and biomarker levels at the post-operative time point. The heterogeneity of clinical presentation and age in our cohort additionally introduces potential factors that could affect biomarker concentrations independent of or in association with LV pressure loading. As previously noted, the prevalence of persistent LV abnormalities in our population was also lower than in previous reports, further limiting statistical power. Additionally, while prospective and longitudinal, this study is observational in nature so we cannot make any conclusions about a causal relationship between biomarkers and myocardial changes. Validation of these findings at other centers and in larger cohorts will be of great importance.
In summary, we conclude that ET-1, ST-2, BNP, NE, and Gal-3 levels change significantly in response to surgical repair of coarctation of the aorta. We find evidence for an association between pre-operative ET-1 and ST-2 concentration and intermediate-term persistence of elevated LVMI or RWT regardless of age at time of repair. Further studies will be needed to evaluate whether increased ET-1 and ST-2 concentration are causative of LV abnormalities in this population and to validate their role as markers of the pathologic change.
Acknowledgments
Funding Sources Pertaining to this Research: Supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082–04 and NIH/NHLBI K23HL123634. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. The study sponsor had no input into the study design, collection, analysis, and interpretation of data, the writing of the report, and the decision to submit the paper for publication.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose. Dr. Davidson is supported by NIH/NHLBI K23HL123634.
Contributor Information
Benjamin S Frank, University of Colorado Denver, Department of Pediatrics, Division of Cardiology.
Tracy T Urban, Children’s Hospital Colorado Research Institute.
Karlise Lewis, Children’s Hospital Colorado Research Institute.
Suhong Tong, University of Colorado Denver, Department of Biostatistics.
Courtney Cassidy, Children’s Hospital Colorado, Department of Pediatric Cardiology.
Max B. Mitchell, University of Colorado Denver, Department of Surgery.
Christopher S. Nichols, University of Colorado Denver, Department of Anesthesiology.
Jesse A. Davidson, University of Colorado Denver, Department of Pediatrics, Division of Cardiology.
References:
- 1.Hoffman JIE, Kaplan S. The Incidence of Congenital Heart Disease. J Am Coll Cardiol 2002;39(12):1890–1900. [DOI] [PubMed] [Google Scholar]
- 2.Roccini AP, Rosenthal A, Barger AC, et al. Pathogenesis of Paradoxical Hypertension after Coarctation Resection. Circulation 1978;54(3):382–387. [DOI] [PubMed] [Google Scholar]
- 3.Lim C Aortic arch reconstruction using regional perfusion without circulatory arrest. European Journal of Cardio-Thoracic Surgery 2003;23(2):149–155. [DOI] [PubMed] [Google Scholar]
- 4.Bocelli A, Favilli S, Pollini I, et al. Prevalence and long-term predictors of left ventricular hypertrophy, late hypertension, and hypertensive response to exercise after successful aortic coarctation repair. Pediatr Cardiol 2013;34(3):620–629. [DOI] [PubMed] [Google Scholar]
- 5.Krieger EV, Clair M, Opotowsky AR, et al. Correlation of exercise response in repaired coarctation of the aorta to left ventricular mass and geometry. Am J Cardiol 2013;111(3):406–411. [DOI] [PubMed] [Google Scholar]
- 6.Klitsie LM, Roest AA, Kuipers IM, et al. Enhanced characterization of ventricular performance after coarctation repair in neonates and young children. Ann Thorac Surg 2013;96(2):629–636. [DOI] [PubMed] [Google Scholar]
- 7.Crepaz R, Cemin R, Romeo C, et al. Factors Affecting Left Ventricular Remodeling and Mechanics in the Long-Term Follow-Up after Successful Repair of Aortic Coarctation. Cardiol Young 2005;15:160–167. [DOI] [PubMed] [Google Scholar]
- 8.Frank BSUT, Tong S, Cassidy C, Mitchell MB, Nichols CS, Davidson JA. Endothelin-1 Activation in Pediatric Patients Undergoing Surgical Coarctation of the Aorta Repair. World Journal of Cardiology 2018;9(12):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Simone G, Daniels SR, Deverux RB, et al. Left Ventricular Mass and Body Size in Normotensive Children and Adults: Assessment of Allometric Relations and Impact of Overweight. J Am Coll Cardiol 1992;20:1251–1260. [DOI] [PubMed] [Google Scholar]
- 10.Daniels SR, Kimball TR, Morrison JA et al. Indexing Left Ventricular Mass to Account for Differences in Body Size in Children and Adolescents without Cardiovascular Disease. Am J Cardiol 1995;76:699–701. [DOI] [PubMed] [Google Scholar]
- 11.Tsai SH, Lu G, Xu X, Ren Y, Hein TW, Kuo L. Enhanced endothelin-1/Rho-kinase signalling and coronary microvascular dysfunction in hypertensive myocardial hypertrophy. Cardiovasc Res 2017;113(11):1329–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shubeita HE, McDonough PM, Harris AN, et al. Endothelin Induction of Inositol Phospholipid Hydrolysis, Sarcomere Assembly, and Cardiac Gene Expression in Ventricular Myocytes. Journal of Biological Chemistry 1990;265(33):20555–20562. [PubMed] [Google Scholar]
- 13.Drawnel FM; Archer CR; Roderick HL The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. British Journal of Pharmacology 2013;168(1):296–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimojo N, Jesmin S, Zaedi S, et al. Eicosapentaenoic acid prevents endothelin-1-induced cardiomyocyte hypertrophy in vitro through the suppression of TGF-beta 1 and phosphorylated JNK. Am J Physiol Heart Circ Physiol 2006;291(2):H835–845. [DOI] [PubMed] [Google Scholar]
- 15.Shimojo N, Jesmin S, Zaedi S, et al. Contributory role of VEGF overexpression in endothelin-1-induced cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol 2007;293(1):H474–481. [DOI] [PubMed] [Google Scholar]
- 16.Marshall AK, Barrett OP, Cullingford TE, Shanmugasundram A, Sugden PH, Clerk A. ERK1/2 signaling dominates over RhoA signaling in regulating early changes in RNA expression induced by endothelin-1 in neonatal rat cardiomyocytes. PLoS One 2010;5(4):e10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavli V, Saritas T, Guven B, et al. Myocardial performance after successful intervention for native aortic coarctation. Cardiol Young 2010;20(1):33–38. [DOI] [PubMed] [Google Scholar]
- 18.Gottlieb SS, Harris K, Todd J, et al. Prognostic significance of active and modified forms of endothelin 1 in patients with heart failure with reduced ejection fraction. Clin Biochem 2015;48(4–5):292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eitel I, Nowak M, Stehl C, et al. Endothelin-1 release in acute myocardial infarction as a predictor of long-term prognosis and no-reflow assessed by contrast-enhanced magnetic resonance imaging. Am Heart J 2010;159(5):882–890. [DOI] [PubMed] [Google Scholar]
- 20.Ergul A, Jupin D, Johnson MH, Prisant LM. Elevated Endothelin-1 Levels are Associated with Decreased Arterial Elasticity in Hypertensive Patients. J Clin Hypertension 2006;8:549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent JA, Ross RD, Kassab J, et al. Relation of Elevated Plasma Endothelin in Congenital Heart Disease to Increased Pulmonary Blood Flow. Am J Cardiol 1993;71:1204–1207. [DOI] [PubMed] [Google Scholar]
- 22.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest 2007;117(6):1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frioes F, Lourenco P, Laszczynska O, et al. Prognostic value of sST2 added to BNP in acute heart failure with preserved or reduced ejection fraction. Clin Res Cardiol 2015;104(6):491–499. [DOI] [PubMed] [Google Scholar]
- 24.Piper SE, Sherwood RA, Amin-Youssef GF, Shah AM, McDonagh TA. Serial soluble ST2 for the monitoring of pharmacologically optimised chronic stable heart failure. Int J Cardiol 2015;178:284–291. [DOI] [PubMed] [Google Scholar]
- 25.Szerafin T, Niederpold T, Mangold A, et al. Secretion of soluble ST2 - possible explanation for systemic immunosuppression after heart surgery. Thorac Cardiovasc Surg 2009;57(1):25–29. [DOI] [PubMed] [Google Scholar]
- 26.Pacileo G, Pisacane C, Russo MG et al. Left Ventricular Remodeling and Mechanics After Successful Repair of Aortic Coarctation. Am J Cardiol 2001;87:748–752. [DOI] [PubMed] [Google Scholar]
- 27.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR. Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 2009;22(6):709–714. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Zhang R, An T, et al. The utility of galectin-3 for predicting cause-specific death in hospitalized patients with heart failure. J Card Fail 2015;21(1):51–59. [DOI] [PubMed] [Google Scholar]


