Abstract
The age-associated increase in cardiac and central arterial stiffness is attenuated with lifelong (>25 years) endurance exercise in a dose-dependent manner. Remodelling of the extracellular matrix of cardiovascular structures may underpin these lifelong exercise adaptations in structural stiffness. The primary aim was to examine whether matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMPs) levels are associated with aging and lifelong exercise-related changes in cardiac and central arterial stiffness. Plasma MMPs and TIMPs, left ventricular (LV) (LV stiffness constant) and central arterial stiffness (pulse wave velocity) were examined in healthy adults stratified into five groups based on age and lifelong weekly exercise frequency: (1) young sedentary adults (28-50 years), and older adults (>60 years) who had performed either: (a) sedentary (0-1 sessions/week), (b) casual (2-3 sessions/week), (c) committed (4-5 sessions/week) or (d) athletic (≥ 6 sessions/week) frequency of exercise. MMP-1 was significantly lower in young compared to older sedentary (p =0.049). Except for TIMP-2 (p =0.018 versus committed) and the ratio of MMP-2/TIMP-4 (p =0.047 versus committed), MMP and TIMP expression was not significantly different in lifelong exercise groups (≥ casual) compared to the older sedentary group. MMP-1, −3 had a weak positive relationship with central PWV (r = 0.17 – 0.25, p ≤ 0.050) but there were no significant relationships between MMPs or TIMPs and LV stiffness constant (p ≥ 0.148). In conclusion, there was not a clear or consistent difference in plasma MMPs and TIMPs with lifelong exercise dose despite exhibiting lower cardiovascular stiffness at the highest exercise levels.
Keywords: stiffness, aging, exercise, matrix metalloproteinases, tissue inhibitors of matrix metalloproteinases
The biological mechanisms that underpin the lower cardiac and arterial structural stiffness with vigorous lifelong (>25 years) endurance exercise are poorly understood (Arbab-Zadeh and others 2004; Bhella and others 2014; Shibata and others 2018; Shibata and Levine 2011; Shibata and Levine 2012).
Structural remodelling of the extracellular matrix (ECM) including collagen deposition, non-enzymatic glycation and subsequent collagen cross-linking, which are believed to contribute to stiffening of cardiovascular (CV) structures with aging, are diminished in animals that have performed regular endurance exercise (Aronson 2003; Choi and others 2009; Kwak and others 2010; Woodiwiss and others 1998; Wright and others 2014). These findings suggest that ECM mechanisms may be important pathways for the improved cardiac and arterial stiffness in humans who have performed vigorous and prolonged endurance exercise. However, to date, there is limited information on the effect of lifelong endurance exercise on biomarkers that reflect changes in ECM homeostasis and remodelling in humans to support or refute this contention (Vianello and others 2009).
A family of enzymes, matrix metalloproteinases (MMPs) and their endogenous inhibitors, tissue inhibitors of MMP (TIMPs) regulate ECM structural protein deposition (accumulation) and degradation (turnover). MMP and TIMP expression and balance is reported to be altered with human aging and CV disease (Ahmed and others 2006; Bonnema and others 2007; Li and others 1998; Liu and others 2017; McNulty and others 2006). In these previous investigations and other large epidemiological studies (Hansson and others 2009; Sundstrom and others 2004a; Sundstrom and others 2004b), individual MMPs and TIMPs measured at a single point in time were correlated with cardiac and vascular structure and function suggesting a relationship between ECM remodelling and CV structure and function. However, to date, no studies have examined the relationships between the MMPs/TIMPs profile and cardiac and central arterial stiffness in highly trained older adults who exhibit marked changes in these parameters compared to their untrained peers to determine whether ECM mechanisms are important (Arbab-Zadeh and others 2004; Bhella and others 2014; Shibata and others 2018; Shibata and Levine 2011; Shibata and Levine 2012). We have previously reported a dose-response relationship between the frequency of lifelong exercise and lower cardiac and central arterial stiffness (Bhella and others 2014; Shibata and others 2018). If a similar dose-response relationship in the MMPs/TIMPs profile is demonstrated, it would provide support for an association between ECM mechanisms and CV stiffness with lifelong exercise in humans.
Accordingly, the purpose of this study was three-fold: first, to examine the effect of lifelong endurance exercise on the MMP and TIMP profile compared to sedentary aging; second, to establish whether there is a dose-response relationship between MMPs and TIMPs in older (>60 years) adults who have performed different ‘doses’ of lifelong endurance exercise, and third, to establish whether there is a relationship between plasma MMPs and TIMPs and cardiac and central arterial stiffness in a cohort of young and older sedentary adults and endurance-trained older adults. Based on previous human and animal studies (Bonnema and others 2007; Kwak and others 2010), we hypothesized that a profile of higher MMP and lower TIMP levels (a profile that would favor ECM proteolysis) would be observed in young adults and those with a high lifelong exercise dose and these results would be positively associated with a lower cardiac and central arterial stiffness.
METHODS
Participants and initial screening
This current cohort was recruited as part of a larger investigation examining the effect of sedentary but otherwise healthy aging and lifelong exercise dose on LV and arterial structure and function and exercise performance (Bhella and others 2014; Carrick-Ranson and others 2014; Fujimoto and others 2012; Shibata and others 2018). Twenty-six (17f/9m) young sedentary and early middle-aged (28–50 years) adults were primary recruited from the Dallas Heart Study (Victor and others 2004) and enriched by a random sample of employees at Texas Health Resources, the third largest employer in the Dallas-Fort Worth metroplex and a diverse health care company (Fujimoto and others 2012). One hundred and three (35f/68m) older adults (>60 years) were primarily recruited from the Cooper Center Longitudinal Study (CCLS), a cohort of over 80,000 individuals in whom physical activity and CV disease risk factors have been quantified and followed for greater than 40 years (Wei and others 1999). The exercise-trained cohort was enriched through the recruitment of top performers at regional and national endurance events. Older adults were allocated into four groups based on weekly exercise frequency, with each exercise session defined as being at least 30 minutes in duration: sedentary (0-1 sessions/week, n=28, 13f/15m); casual (2-3 sessions/week, n=25, 9f/16m), committed (4-5 sessions/week, n=25, 5f/20m), and athletic (6-7 sessions per week plus competition, n=25, 8f/17m) as previously described (Bhella and others 2014).
Exercise capacity (maximal oxygen uptake) and invasive and non-invasive measures of CV structure and function (LV stiffness, arterial stiffness, and MRI assessment) were collected during four separate testing sessions. Blood measures were collected after the participant had remained supine for at least 30 minutes during the catheterization session to assess LV stiffness. No strenuous exercise was performed 24-hour prior to the collection of blood measures. All participants were non-smokers, were not taking any CV disease medications, had a 24-h ambulatory blood pressure <140/90 mmHg, body mass index ≤30 kg/m2, and a normal ECG and exercise stress echocardiogram. All participants were informed of the purpose and procedures used in the study and gave their written informed consent to a protocol approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas.
Measurement of maximal oxygen uptake
Maximal oxygen uptake V̇O2max was determined during a treadmill test to maximal exertion as previously described (Arbab-Zadeh and others 2004). A small number of participants (n=5) were tested on an upright cycle because of orthopaedic concerns or participant request. Maximal oxygen uptake was defined as the highest oxygen uptake measured from at least a 30 seconds Douglas bag. Gas fractions were analysed by mass spectrometry and ventilatory volumes by a Tissot spirometer as previously reported (Arbab-Zadeh and others 2004).
Assessment of left ventricular mass
Cardiac MRI was performed on a 1.5-T Philips NT MRI scanner. Short-axis, gradient-echo, cine MRI sequences were obtained to calculate left ventricular (LV) mass, which was computed as the difference between epicardial and endocardial areas multiplied by the density of heart muscle, 1.05 g/mL (Arbab-Zadeh and others 2004).
Left ventricular stiffness
LV stiffness constant assessed by invasive pressure-volume curves was used to describe LV chamber stiffness (or its reciprocal compliance) as previously reported (Arbab-Zadeh and others 2004; Bhella and others 2014; Fujimoto and others 2012). A 6Fr balloon-tipped fluid-filled catheter (Edwards Lifesciences, Irvine, California) was placed using fluoroscopic guidance through an antecubital vein into the pulmonary artery. The catheter was connected to a pressure transducer with the zero-reference point set at 5.0 cm below the sternal angle. Measurements of pulmonary capillary wedge pressure and LV end-diastolic volume were assessed after at least 20 minutes of supine rest and 5 minutes each of −15 and −30 mmHg of lower body negative pressure. After at least a 20-minute break, repeat measurements were used to confirm a return to a steady hemodynamic state. LV filling was then increased by rapid infusion (100-200 ml per min) of warm isotonic saline. Measurements were repeated after 15 and 30 ml per kg of saline infusion. Echocardiographic images were digitally acquired using an iE33 (Philips, Netherlands) and were measured offline in Xcelera cardiovascular image management system (Philips, Netherlands). The modified Simpson’s method was used to determine LV volumes (Arbab-Zadeh and others 2004). LV stiffness constants of these cohorts have been previously reported (Bhella and others 2014; Fujimoto and others 2012).
Central arterial stiffness
Central pulse wave velocity (PWV) was used to characterize central arterial stiffness. Central PWV was measured as previously described (Shibata and others 2018; Shibata and Levine 2011). Briefly, PWV was assessed with Doppler ultrasound (iE33, Phillips, Netherlands), and calculated as the distance between measurement sites divided by the time delay between the two waveforms. Pulse transit time was calculated by subtracting the time between the peak of the R-wave and the foot of the carotid flow profile from the time between the peak of the R-wave and the foot of the femoral flow profile. The distance between arterial measurement sites was calculated by subtracting the distance between the carotid site and the sternal notch from the distance between the sternal notch and the femoral site.
Measurement of plasma MMPs and TIMPs
Circulating MMPs and TIMPs were assessed from a 10-ml blood sample collected in the morning after a light breakfast and the participant had remained supine for at least 30 minutes. Samples were immediately centrifuged, and the plasma layer removed, and the plasma was frozen at −80°C until the assay was run. For measurement, the plasma samples were thawed on ice, with all measurements performed in duplicate. A multiplex suspension array approach (BioRad) was used, which has been described elsewhere (Zile and others 2011). Optimized high-sensitivity, high-throughput plasma biomarker profiles for human MMP (LMPM000, R and D Systems) and human TIMP (LKTM003, R and D Systems) were utilized. All measurements were performed using an internally validated and calibrated instrument that detects a fluorescent signal whereby the sensitivity for all of the assays was in the pg/ml range, with intra-assay coefficient of variations less than 10%. All samples were de-identified and analysed without knowledge of age or lifelong exercise training status.
For this current study, MMP-2, −7, −9 and TIMP-1, −2, −4 and the ratios of MMP-2/TIMP-1, −4 and MMP-9/TIMP-1, −4 were examined as they have been shown to be associated with cardiac and arterial structure and function with aging and regular endurance exercise in humans and animals (Bonnema and others 2007; Kwak and others 2010; Vianello and others 2009). Moreover, animal models have indicated that certain MMPs and TIMPs are important in modulating ECM proteins of CV structures with exercise training (Kwak 2013; Kwak and others 2010; Wright and others 2014). Similar to previous works (Bonnema and others 2007), the ratios of MMPs/TIMPs were used as an indirect index of overall ECM homeostasis (ECM protein accumulation or degradation) such that a higher MMP/TIMP ratio would imply a greater potential for prolonged and greater MMP activity, whereas a lower ratio would imply a reduced MMP proteolysis.
Statistical analysis
A 1-way analysis of variance (ANOVA) was used to determine group differences in participant characteristics, MMPs and TIMPs, with a Tukey’s post hoc test used when a significant main effect was found. Non-parametric data were analyzed via Kruskal-Wallis ANOVA on ranks and a Dunn’s post hoc test used when a significant main effect was found. Unpaired t-tests were used to determine sex-related differences in MMPs and TIMPs in the sedentary adults. Linear regression was used to examine the effect of sedentary aging and V̇O2max on MMPs and TIMPs, and Spearman’s rank-order correlation coefficient were used to determine the relationship between the natural log transformation of MMPs and TIMPs and indices of LV and arterial structure and stiffness. Statistical analyses were performed using SigmaStat (Systat Software, CA, USA) and p <0.05 was considered statistically significant for all analyses.
RESULTS
Participant characteristics
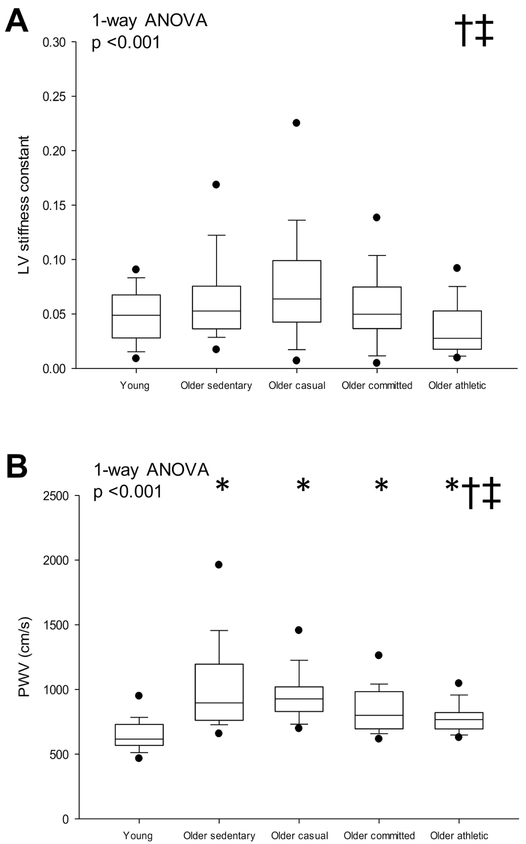
Participant characteristics are shown in Table 1. Total body mass, body surface area and blood pressures were not different among groups (ANOVA p values all p ≥ 0.058). V̇O2max was lower in sedentary older compared to young adults (p =0.030) and was higher in the older athletes compared to the lower frequencies of lifelong exercise (all p <0.001). LV mass index was not significantly different in young versus older sedentary (p =0.867) but was significantly larger with either a committed or athletic frequency of lifelong exercise (all p ≤ 0.040 versus other groups). LV mass-to-volume ratio was not significantly different among groups (p =0.394). LV stiffness constant was lowest in the senior athletes (p ≤ 0.030 versus older sedentary and casual, Fig. 1A), while central PWV was higher in all older groups compared to young sedentary subjects (all p ≤ 0.024) (Fig. 1B).
Table 1.
Participant characteristics
| Young | Older sedentary | Older casual | Older committed | Older athletic | ANOVA p value |
|
|---|---|---|---|---|---|---|
| Participant number, %F | 26 (65) | 28 (46) | 25 (36) | 25 (20) | 25 (32) | |
| Age, years | 40.5 (33.4 - 46.1) | 66.8 (63.2 - 71.0)* | 69.4 (67.4 - 73.8)* | 68.4 (65.2 - 70.7)* | 67.8 (65.7 - 69.2)* | <0.001# |
| Weight, kg | 69.5 ± 13.6 | 69.7 ± 13.3 | 75.8 ± 14.1 | 73.5 ± 11.1 | 65.6 ± 12.1 | 0.058 |
| BSA, m2 | 1.80 ± 0.21 | 1.89 ± 0.19 | 1.90 ± 0.23 | 1.88 ± 0.17 | 1.76 ± 0.21† | 0.060 |
| 24-h systolic BP, mmHg | 121.5 ± 9.0 | 125.0 ± 8.6 | 124.0 ± 6.9 | 124.8 ± 7.9 | 121.5 ± 11.1 (13) | 0.470 |
| 24-h diastolic BP, mmHg | 71.5 ± 5.4 | 72.0 ± 6.5 | 71.1 ± 5.5 | 73.5 ± 6.7 | 73.8 ± 5.6 (13) | 0.525 |
| V̇O2max, ml/kg/min | 28.3 ± 6.1 | 24.0 ± 5.1* | 25.6 ± 4.7 | 32.0 ± 5.9†‡ | 39.5 ± 5.3*†‡ | <0.001 |
| LVMI, g/m2 | 53.1 ± 11.6 (24) | 50.3 ± 8.3 (27) | 51.6 ± 8.2 (22) | 61.7 ± 11.1*†‡ (23) | 67.2 ± 11.8*†‡ (25) | <0.001 |
| LV mass-to-volume ratio, g/ml | 0.82 (0.73 - 0.93) | 0.83 (0.79 - 0.91) | 0.87 (0.77 - 1.05) | 0.88 (0.77 - 1.04) | 0.89 (0.83 - 1.01) | 0.394# |
Values are expressed as mean ± SD except age and LV mass-to-volume ratio expressed as (median, 25 - 75%). Number in parenthesis represents number of observations if different from the total group. BP, blood pressure; V̇O2max, maximal oxygen uptake; LVMI, left ventricular mass index.
Kruskal-Wallis 1-way ANOVA on ranks
p <0.05 versus young
p <0.05 versus older sedentary
p <0.05 versus older casual. P-values are derived from post-hoc tests (Tukey’s method for BSA, V̇O2and LVMI and Dunn’s method for age).
Figure 1.
LV stiffness constant (A) and central PWV (B). Box values are median and 25th and 75th percentile with error bars representing the 10th and 90th percentile. Filled black dots represent 5th and 95th percentile. *p <0.05 versus young sedentary, †p <0.05 versus older sedentary; ‡p <0.05 versus older casual. P-values derived from Kruskal-Wallis on ranks (1-way ANOVA) with Dunn’s post hoc test for between group comparisons.
Plasma MMPs & TIMPs
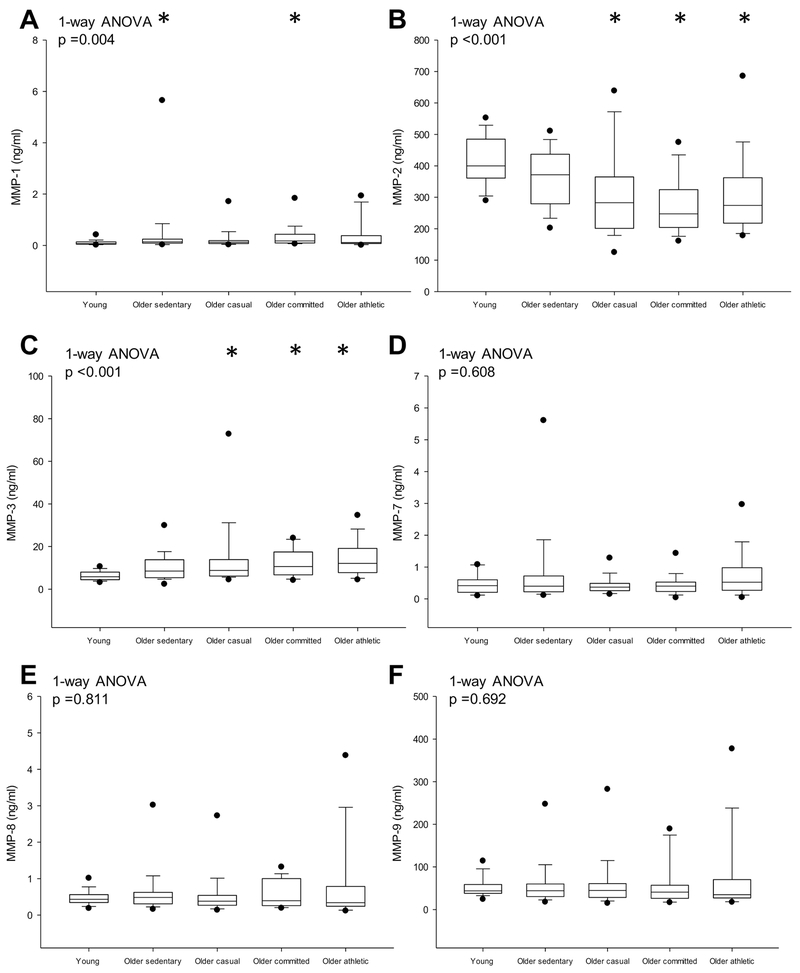
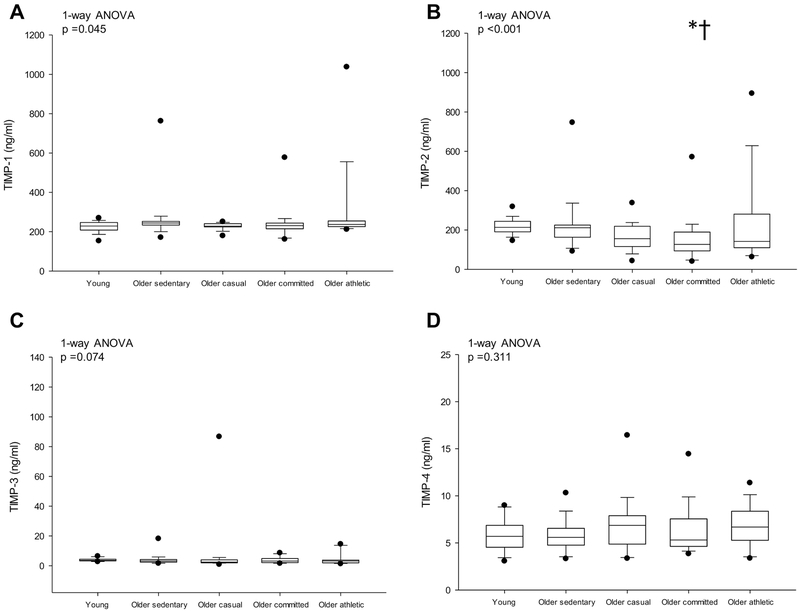
Individual MMPs and TIMPs are presented in Figs. 2 & 3. MMP-1 was higher in the sedentary older and committed groups compared to the young group (p =0.049), but MMP-1 was not different among the older groups (p ≥ 0.835). MMP-2 was lower while MMP-3 was higher in older lifelong exercise groups (≥ casual) compared to young sedentary (p ≤ 0.014) but these were not significantly different among the older groups (all p ≥ 0.059) (Figs. 2B & C). MMP-7, −8, −9 (Figs. 2D-F) and TIMP-1, −3 −4 (Figs. 3A, C & D) were not significantly different among groups. TIMP-2 was lower in older committed compared to the young and older sedentary groups (p ≤ 0.018) (Fig. 3B).
Figure 2.
Detected protein levels of MMP-1 (A), MMP-2 (B), MMP-3 (C), MMP-7 (D), MMP-8 (E) and MMP-9 (F). Box values are median and 25th and 75th percentile with error bars representing the 10th and 90th percentile. Filled black dots represent 5th and 95th percentile. *p <0.05 versus young sedentary; †p <0.05 versus older sedentary; ‡p <0.05 versus older casual. P-values derived from Kruskal-Wallis on ranks (1-way ANOVA) with Dunn’s post hoc test for between group comparisons.
Figure 3.
Detected protein levels of TIMP-1 (A), TIMP-2 (B), TIMP-3 (C) and TIMP-4 (D).Box values are 25th and 75th percentile with error bars representing the 10th and 90th percentile. Filled black dots represent 5th and 95th percentile. *p <0.05 versus young sedentary; †p <0.05 versus older sedentary; ‡p <0.05 versus older casual. P-values derived from Kruskal-Wallis on ranks (1-way ANOVA) with Dunn’s post hoc test for between group comparisons.
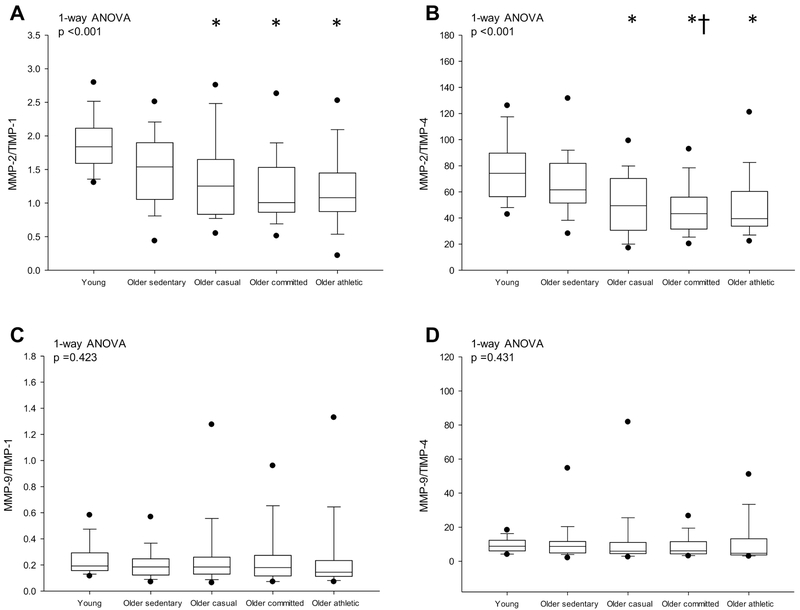
Ratios of MMPs/TIMPs are presented in Fig. 4. MMP-2/TIMP-1 was significantly lower in older lifelong exercise groups compared to young sedentary adults (p ≤ 0.002) but not compared to older sedentary adults (p ≥ 0.436, Fig. 4A). MMP-2/TIMP-4 was lower in older committed compared to young and older sedentary groups (both p ≤ 0.047) (Fig. 4B). MMP-9/TIMP-1, −4 were not significantly different among groups (ANOVA p values ≥ 0.423) (Figs. 4C-D).
Figure 4.
Ratio of MMP-2/TIMP-1 (A), MMP-2/TIMP-4 (B), MMP-9/TIMP-1 (C) and MMP-9/TIMP-4 (D). Box values are median and 25th and 75th percentile with error bars representing the 10th and 90th percentile. Filled black dots represent 5th and 95th percentile. *p <0.05 versus young sedentary. †p <0.05 versus older sedentary. P-values derived from Kruskal-Wallis on ranks (1-way ANOVA) with Dunn’s post hoc test for between group comparisons.
The relationship between plasma MMPs and TIMPs and age, sex and maximal exercise capacity
MMP-3 was higher (p <0.001) in sedentary men versus women (n=54); however, all other MMPs (p ≥ 0.088) and TIMPs (p ≥ 0.056) were not significantly different between the sexes. When examined in all participants (n=129), MMP-2, −7 was higher (p <0.001) and MMP-3 was lower (p =0.006) in women compared to men. In young and older sedentary adults, MMP-2 decreased (r2= 0.119, p =0.011) and MMP-3 increased (r2= 0.159, p =0.003) as a function of age. TIMPs were not significant associated with age (p >0.079). The MMP-2/TIMP-1 ratio decreased with aging (r2 = 0.207, p <0.001). Neither MMPs nor TIMPs were significantly associated with VO2max (p ≥ 0.065).
The relationship between plasma MMPs, TIMPs, LV structure, and LV and arterial stiffness
The relationship between circulating MMPs and TIMPs and LV and arterial structural and functional parameters are presented in Tables 2 & 3. MMP-1, −3 were weakly and positively associated with central PWV (r = 0.17 – 0.26, p ≤ 0.05), while there a weak negative relationship for MMP-2 (r = −0.20, p =0.027). Likewise, there was only a weak-to-moderate positive relationship between MMP-3 and LV mass index and LV mass-to-volume ratio (r = 0.26 – 0.38, p ≤ 0.004). MMP-2/TIMP-1 was weakly and negatively associated with LVMI (r = −0.22, p = 0.017) and PWV (r = −0.19, p =0.031), while a similar relationship was exhibited between MMP-2/TIMP-4 and LVMI (r = −0.19, p =0.038). LV stiffness constant was not significantly associated with MMPs or TIMPs (r = −0.06 – 0.13, p ≥ 0.148).
Table 2.
Correlations of the natural log of plasma MMPs and LV and arterial parameters
| LV stiffness constant (r, p value) n=129 |
LV volume-to-mass ratio (r, p value) n=121 |
LVMI (r, p value) n=121 |
Central PWV (r, p value) n=128 |
|
|---|---|---|---|---|
| Log MMP-1 | 0.02, 0.864 | 0.15, 0.101 | 0.08, 0.376 | 0.25, 0.005 |
| Log MMP-2 | 0.11, 0.222 | −0.15, 0.092 | −0.21, 0.019 | −0.20, 0.027 |
| Log MMP-3 | −0.06, 0.496 | 0.26, 0.004 | 0.38, <0.001 | 0.17, 0.050 |
| Log MMP-7 | 0.12, 0.186 | 0.02, 0.856 | −0.11, 0.226 | 0.01, 0.877 |
| Log MMP-8 | −0.01, 0.917 | 0.11, 0.224 | 0.05, 0.557 | 0.10, 0.250 |
| Log MMP-9 | 0.00, 0.995 | −0.11, 0.229 | −0.06, 0.515 | 0.02, 0.836 |
n, number or observations.
Table 3.
Correlations of the natural log of plasma TIMPs, MMPs/TIMPs ratios and LV and arterial parameters
| LV stiffness constant (r, p value) n=129 |
LV volume-to-mass ratio (r, p value) n=121 |
LVMI (r, p value) n=121 |
Central PWV (r, p value) n=128 |
|
|---|---|---|---|---|
| Log TIMP-1 | −0.05, 0.589 | 0.09, 0.303 | 0.11, 0.246 | 0.01, 0.871 |
| Log TIMP-2 | 0.07, 0.436 | −0.15, 0.099 | −0.08, 0.366 | −0.14, 0.127 |
| Log TIMP-3 | 0.02, 0.786 | −0.10, 0.271 | −0.10, 0.270 | −0.15, 0.090 |
| Log TIMP-4 | −0.07, 0.399 | −0.08, 0.412 | −0.02, 0.815 | −0.01, 0.893 |
| MMP-2/TIMP-1 | 0.11, 0.235 | −0.16, 0.082 | −0.22, 0.017 | −0.19, 0.031 |
| MMP-2/TIMP-4 | 0.13, 0.148 | −0.10, 0.295 | −0.19, 0.038 | −0.14, 0.105 |
| MMP-9/TIMP-1 | 0.02, 0.792 | −0.10, 0.255 | −0.08, 0.413 | −0.01, 0.973 |
| MMP-9/TIMP-4 | 0.01, 0.904 | −0.04, 0.693 | −0.02, 0.813 | 0.00, 0.957 |
n, number or observations.
DISCUSSION
The three major findings of this current study are as follows: 1) in contrast to our hypothesis, a plasma MMPs and TIMPs profile that would favor increased ECM proteolysis was not observed in those with a high lifelong exercise dose despite exhibiting lower levels of cardiac and central arterial stiffness, 2) there was not a clear dose-response relationship for the MMP and TIMP profile as previously reported for cardiac and central arterial stiffness, and 3) there were only weak relationships between some MMPs and TIMPs with central PWV and no significant relationships with cardiac stiffness.
These observations lead to several possible conclusions. First, the ECM proteolytic state may have nothing to do with the changes in CV stiffness associated with sedentary aging, and its modification by life-long exercise training. However, the ECM is a complex entity that includes non-structural proteins and signalling molecules, and therefore it is possible that unlike those associated with severe CV disease, lifelong exercise-related changes in ECM structure/composition/function are not strongly reflected in plasma MMP and TIMP profiles in humans. Moreover, the substrate portfolios of MMPs is diverse, and thus while the absolute MMP proteolytic profiles are unaffected with lifelong exercise dose, the underlying substrates processed by this family of enzymes may have been distinctly different. Finally, LV and arterial function may be influenced by cellular and extracellular factors including calcium homeostasis, sympathetic adrenergic tone as well as other local and systemic neurohormonal factors not assessed in this study. There is evidence that prolonged and consistent exercise can influence some of these factors and thus may in part have contributed to the overall lower levels of CV structural stiffness observed in our older athletes (Ellison and others 2012; Kemi and Wisloff 2010; Roh and others 2016).
The relationship between MMPs, TIMPs, and cardiovascular structure and function in young and older sedentary human adults
When examined in young and older sedentary adults, we found that the relationship between individual MMPs and TIMPs and age was either weak or inconsistent suggesting that these parameters are not profoundly influenced by sedentary aging. Likewise, except for MMP-2/TIMP-1, MMPs or TIMPs levels were not significantly influenced by aging.
In a slightly larger cohort of apparently healthy individuals ranging from 20-90 years, certain MMPs/TIMPs ratios (MMP-2/TIMP-4, MMP-9/TIMP-1, −4) were significantly lower in older compared to young healthy participants, a finding which was interpreted as indicating a state of reduced cardiac ECM proteolysis (Bonnema and others 2007). Conversely, TIMP-1, −2 were lower, while MMP-2, −9 were unaffected by age, a result that potentially indicates an increase in ECM proteolysis with aging (Tayebjee and others 2005). Likewise, TIMP-1 levels were higher with aging in a large community-based cohort (Sundstrom and others 2004b), a result interpreted by the authors to reflect an increased ECM degradation state.
The reason for these discrepancies between study findings are unclear, but may be due to methodological differences in determining circulating MMPs and TIMPs or potentially to the health and lifestyle factors of the participants, including the absence or presence of CV disease risk factors (Sundstrom and others 2004b) and medication usage (Andrade and others 2013; Sundstrom and others 2004a). Thus, these findings highlight that the relationship between circulating MMPs and TIMPs with human aging is highly complex and the potential interaction of co-morbidities and medications are important methodological considerations.
Several studies have examined the relationship between MMPs and TIMPs and LV and arterial structure and function in healthy aging adults (Bonnema and others 2007; Vlachopoulos and others 2007; Yasmin and others 2005). In adults aged 20-90 years, circulating MMP-7 and TIMP-1, −4 were negatively associated with concentric LV remodelling (a reduction in volume-to-mass ratio) and E/A ratio (Bonnema and others 2007); two commonly reported CV consequences of aging (Carrick-Ranson and others 2012; Lakatta and Levy 2003a; Lakatta and Levy 2003b). Two investigations (Vlachopoulos and others 2007; Yasmin and others 2005) showed that age was an important determinant of the relationship between MMP-2, −9 and central PWV; however, similar to this current study, the relationship between these variables were only weak-to-moderate. Thus, the current findings confirm that other non-ECM factors or alternative ECM regulatory pathways that those previously and currently examined need to be considered when examining the age-related changes in CV structure and stiffness.
The relationship between MMPs, TIMPs, and cardiovascular structure and function in endurance-trained and untrained animals and humans
Experimental studies have reported lower amounts of cardiac ECM fibrosis and collagen cross-linked products (advanced glycation end-products) in aged exercise-trained animals. In older (31 months) rodents, 12 weeks of treadmill exercise prevented an age-related reduction in myocardial tissue MMP activity (MMP-1, −2), while reducing TIMP-1, −2 levels (Kwak and others 2010). In slightly younger rodents (29 months) myocardial TIMP-1 expression and MMP-1, −2 gene and protein expression were not significantly altered by exercise (Wright and others 2014). Importantly, despite these differential findings regarding MMP and TIMP expression, both studies reported lower levels of collagen and collagen cross-linked products in the exercise-trained heart compared to age-similar sedentary heart. Differences in the age when exercise is initiated, and/or the length of the exercise training stimulus may potentially explain these divergent findings, and additionally highlights the complex interaction between exercise and MMP and TIMP regulation and expression and how these processes influence overall ECM homeostasis and remodelling in the heart remain unclear.
Contrary to our hypothesis, endurance exercise even when performed near daily over several decades did not result in an MMP or TIMP profile that would favor increased ECM proteolysis. Moreover, we did not establish a clear or consistent dose-dependent relationship between lifelong exercise frequency and MMPs and TIMPs as reported for LV and central arterial stiffness (Bhella and others 2014; Shibata and others 2018). Vianello and colleagues showed that MMP-2, −9 and TIMP-2 had a weak-to-moderate relationship with LV structure (LVMI, LV posterior wall and septal thickness, and LV end-diastolic diameter) in veteran marathoners and age-similar healthy sedentary controls (Vianello and others 2009). Together, the findings of Vianello and colleagues and the current study suggest that ECM remodelling may only partially explain the marked cardiac and arterial adaptations including structural stiffness that is exhibited in endurance trained older adults.
The reason for this apparent lack of difference in circulating MMPs and TIMPs despite prominent changes in cardiac and arterial stiffness in our athletic older adults is unclear. The timing of the measurement may be an important factor, as the overall response of MMPs and TIMPs to injury and pathological LV remodelling is a dynamic and time-dependent process (Hirohata and others 1997; Spinale and others 1998). Circulating levels of certain MMPs and TIMPs are altered immediately after exercise in untrained and endurance-trained humans (Rullman and others 2013; Suhr and others 2010; Tayebjee and others 2005) and in response to relatively short-term exercise training (days-to-months) in human and animal models (Donley and others 2014; Gatta and others 2012; Kadoglou and others 2010; Kwak and others 2010). Nevertheless, it is possible that circulating markers of ECM remodelling will be altered acutely but return to baseline values after a prolonged period (years). Nevertheless, we cannot exclude the possibility that favourable ECM remodelling may play a contributory role in enhancing LV stiffness in humans, and this effect may not be detectable with the current techniques.
Study limitations
First, the examination of MMPs and TIMPs in plasma may be considered a significant limitation as these biomarkers are not direct evidence of ECM remodelling, nor do they provide a specific indication of the source of matrix metabolism (i.e. cardiovascular versus non-cardiovascular tissue). While the most convincing evidence would be obtained from cardiac or arterial tissue biopsies, this method is not practical in healthy volunteers; therefore, future studies should aim to address this limitation by the addition of the assessment of several other biomarkers of ECM homeostasis and collagen regulation (procollagen type I (PIP); procollagen III N-terminal propeptide (PIIINP); carboxy-terminal telopeptide of collagen type I (CITP)) (Laviades and others 1998; Lindsay and others 2002; Querejeta and others 2000) combined with specialized cardiac MRI imaging (delayed enhancement imaging or T-1 times) which would provide a more detailed examination of cardiac fibrosis.
Second, the exercise-related modulation of ECM structural proteins with exercise training is complex and may involve several other signalling pathways and proteases to those currently investigated (Kwak 2013). For example, Wright and colleagues showed that ECM remodelling (cardiac fibrosis and cross-linking) occurred without a significant change in intramyocardial MMPs and TIMPs in middle-aged rats after exercise training (Wright and others 2014). Thus, we cannot exclude the possibility that these other pathways and proteases are critical in physiologic remodelling of the ECM and consequently influence lifelong exercise training-related changes in cardiac and arterial stiffness. Moreover, we did not directly assess local or global activity of MMPs, which would further the understanding of the biological processes that underpin the changes in cardiac and arterial stiffness with lifelong exercise in humans.
Third, the current cross-sectional design limits the ability to demonstrate a cause-and-effect relationship between MMPs and TIMPs expression and CV structure and function. Moreover, the processes that contribute to aging and lifelong exercise adaptations in cardiac and arterial stiffness likely occur over several months/years/decades and thus may not be captured with a one-off measurement of MMPs and TIMPs expression. Therefore, a longitudinal study involving serial measurement of biomarkers of ECM homeostasis and ventricular and arterial structural and functional measures with habitual exercise in healthy individuals is warranted but such an approach would be extremely difficult to implement over a lifetime.
Fourth, while we examined several CV disease risk factors in our subjects, others that could significantly influence ECM remodelling if elevated over a prolonged period (cholesterol, glucose) were not comprehensively examined or controlled. The effects of aging and exercise training on CV disease risk factors are well established and thus may have influenced LV and arterial stiffness in our participants.
Fifth, the number of young and older sedentary participants was relatively small, primarily because this study was part of two larger investigations that involved a rigorous exclusion criterion for co-morbidities and CV disease risk factors (hypertension, obesity, smoking, and diabetes) and very high-resolution phenotyping, including invasive measures of cardiac compliance. Thus, future studies should aim to examine a larger sample of carefully screened adults that reflect a wide chronological age range to further elucidate the effect of sedentary but otherwise healthy aging on the MMPs/TIMPs profile.
Sixth, we did not include a young trained group; therefore, we are unable to determine whether plasma MMPs and TIMPs are associated with exercise training-related changes in LV and arterial stiffness when vigorous, sustained exercise is performed early in adulthood. Moreover, given the small number of women in the lifelong exercise groups, the present study was not adequately powered to address whether there is was an interaction effect between age, sex and lifelong exercise dose on the MMPs and TIMPs profile, which may be important to examine sex-related differences in CV adaptations to long-term exercise.
Lastly, statistically significant results need to be carefully interpreted given the number of statistical comparisons conducted, which increases the likelihood of a type 1 error.
CONCLUSION
In summary, the plasma MMPs and TIMPs profiles were not consistently or substantially affected at any frequency of lifelong endurance exercise, despite marked improvements in cardiac and arterial stiffness in the older athletes compared to sedentary older adults. There were only weak relationships between MMPs or TIMPs and central arterial stiffness, and there were no significant relationships for cardiac stiffness. Thus, alternative and/or additional factors that regulate the ECM need to be considered when examining potential mechanisms that underpin the beneficial effects of lifelong exercise on cardiac and arterial stiffness.
Highlights.
Plasma matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs) profiles were not significantly influenced by lifelong (>25 years) endurance exercise “dose” as indicated by weekly exercise sessions despite lower cardiac and central arterial stiffness in older committed exercisers or competitive athletes.
Some MMPs and TIMPs were weakly associated with indices of cardiac structure and central arterial stiffness but there was no significant relationship with cardiac stiffness.
Therefore, non-extracellular matrix (ECM) factors or alternative ECM regulatory pathways should be considered when examining the beneficial effects of lifelong endurance exercise on cardiac and central arterial stiffness in humans.
Acknowledgments
Funding support: This project was supported by the National Institutes of Health (ref#AG17479).
Footnotes
Disclosures: The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH; Clark LL; Pennington WR; Webb CS; Bonnema DD; Leonardi AH; McClure CD; Spinale FG; Zile MR Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 113:2089–2096; 2006 [DOI] [PubMed] [Google Scholar]
- Andrade VL; Valle IB; Sandrim VC Simvastatin therapy decreases MMP-9 levels in obese women. J Clin Pharmacol. 53:1072–1077; 2013 [DOI] [PubMed] [Google Scholar]
- Arbab-Zadeh A; Dijk E; Prasad A; Fu Q; Torres P; Zhang R; Thomas JD; Palmer D; Levine BD Effect of aging and physical activity on left ventricular compliance. Circulation. 110:1799–1805; 2004 [DOI] [PubMed] [Google Scholar]
- Aronson D Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 21:3–12; 2003 [DOI] [PubMed] [Google Scholar]
- Bhella P; Hastings J; Fujimoto N; Shibata S; Carrick-Ranson G; Adams-Huet B; Palmer M; Levine B The Impact of Lifelong Exercise “Dose” on Left Ventricular Compliance and Distensibility. J Am Coll Cardiol 64:1257–1266; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnema DD; Webb CS; Pennington WR; Stroud RE; Leonardi AE; Clark LL; McClure CD; Finklea L; Spinale FG; Zile MR Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). J Card Fail. 13:530–540; 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick-Ranson G; Hastings JL; Bhella PS; Fujimoto N; Shibata S; Palmer MD; Boyd K; Livingston S; Dijk E; Levine BD The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol (1985). 116:736–745; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick-Ranson G; Hastings JL; Bhella PS; Shibata S; Fujimoto N; Palmer MD; Boyd K; Levine BD Effect of healthy aging on left ventricular relaxation and diastolic suction. Am J Physiol Heart Circ Physiol. 303:H315–322; 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY; Chang HJ; Choi SI; Kim KI; Cho YS; Youn TJ; Chung WY; Chae IH; Choi J; Kim HS; Kim CH; Oh BH; Kim MH Long-term exercise training attenuates age-related diastolic dysfunction: association of myocardial collagen cross-linking. J Korean Med Sci. 24:32–39; 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donley DA; Fournier SB; Reger BL; DeVallance E; Bonner DE; Olfert IM; Frisbee JC; Chantler PD Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol (1985). 116:1396–1404; 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison GM; Waring CD; Vicinanza C; Torella D Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 98:5–10; 2012 [DOI] [PubMed] [Google Scholar]
- Fujimoto N; Hastings JL; Bhella PS; Shibata S; Gandhi NK; Carrick-Ranson G; Palmer D; Levine BD Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol. 590:1871–1880; 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatta L; Armani A; Iellamo F; Consoli C; Molinari F; Caminiti G; Volterrani M; Rosano GM Effects of a short-term exercise training on serum factors involved in ventricular remodelling in chronic heart failure patients. Int J Cardiol. 155:409–413; 2012 [DOI] [PubMed] [Google Scholar]
- Hansson J; Lind L; Hulthe J; Sundstrom J Relations of serum MMP-9 and TIMP-1 levels to left ventricular measures and cardiovascular risk factors: a population-based study. Eur J Cardiovasc Prev Rehabil. 16:297–303; 2009 [DOI] [PubMed] [Google Scholar]
- Hirohata S; Kusachi S; Murakami M; Murakami T; Sano I; Watanabe T; Komatsubara I; Kondo J; Tsuji T Time dependent alterations of serum matrix metalloproteinase-1 and metalloproteinase-1 tissue inhibitor after successful reperfusion of acute myocardial infarction. Heart. 78:278–284; 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoglou NP; Vrabas IS; Sailer N; Kapelouzou A; Fotiadis G; Noussios G; Karayannacos PE; Angelopoulou N Exercise ameliorates serum MMP-9 and TIMP-2 levels in patients with type 2 diabetes. Diabetes Metab. 36:144–151; 2010 [DOI] [PubMed] [Google Scholar]
- Kemi OJ; Wisloff U Mechanisms of exercise-induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol (Oxf). 199:425–439; 2010 [DOI] [PubMed] [Google Scholar]
- Kwak HB Aging, exercise, and extracellular matrix in the heart. J Exerc Rehabil. 9:338–347; 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HB; Kim JH; Joshi K; Yeh A; Martinez DA; Lawler JM Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 25:1106–1117; 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG; Levy D Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a "set up" for vascular disease. Circulation. 107:139–146; 2003a [DOI] [PubMed] [Google Scholar]
- Lakatta EG; Levy D Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 107:346–354; 2003b [DOI] [PubMed] [Google Scholar]
- Laviades C; Varo N; Fernandez J; Mayor G; Gil MJ; Monreal I; Diez J Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 98:535–540; 1998 [DOI] [PubMed] [Google Scholar]
- Li YY; Feldman AM; Sun Y; McTiernan CF Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 98:1728–1734; 1998 [DOI] [PubMed] [Google Scholar]
- Lindsay MM; Maxwell P; Dunn FG TIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension. 40:136–141; 2002 [DOI] [PubMed] [Google Scholar]
- Liu JH; Chen Y; Zhen Z; Ho LM; Tsang A; Yuen M; Lam K; Tse HF; Yiu KH Relationship of biomarkers of extracellular matrix with myocardial function in Type 2 diabetes mellitus. Biomark Med. 11:569–578; 2017 [DOI] [PubMed] [Google Scholar]
- McNulty M; Mahmud A; Spiers P; Feely J Collagen type-I degradation is related to arterial stiffness in hypertensive and normotensive subjects. Journal of Human Hypertension. 20:867–873; 2006 [DOI] [PubMed] [Google Scholar]
- Querejeta R; Varo N; Lopez B; Larman M; Artinano E; Etayo JC; Martinez Ubago JL; Gutierrez-Stampa M; Emparanza JI; Gil MJ; Monreal I; Mindan JP; Diez J Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation. 101:1729–1735; 2000 [DOI] [PubMed] [Google Scholar]
- Roh J; Rhee J; Chaudhari V; Rosenzweig A The Role of Exercise in Cardiac Aging: From Physiology to Molecular Mechanisms. Circ Res. 118:279–295; 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rullman E; Olsson K; Wagsater D; Gustafsson T Circulating MMP-9 during exercise in humans. Eur J Appl Physiol. 113:1249–1255; 2013 [DOI] [PubMed] [Google Scholar]
- Shibata S; Fujimoto N; Hastings JL; Carrick-Ranson G; Bhella PS; Hearon CM Jr.; Levine BD The effect of lifelong exercise frequency on arterial stiffness. J Physiol. 596:2783–2795; 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S; Levine BD Biological aortic age derived from the arterial pressure waveform. J Appl Physiol. 110:981–987; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S; Levine BD Effect of exercise training on biologic vascular age in healthy seniors. Am J Physiol Heart Circ Physiol. 302:H1340–1346; 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinale FG; Coker ML; Thomas CV; Walker JD; Mukherjee R; Hebbar L Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res. 82:482–495; 1998 [DOI] [PubMed] [Google Scholar]
- Suhr F; Rosenwick C; Vassiliadis A; Bloch W; Brixius K Regulation of extracellular matrix compounds involved in angiogenic processes in short- and long-track elite runners. Scand J Med Sci Sports. 20:441–448; 2010 [DOI] [PubMed] [Google Scholar]
- Sundstrom J; Evans JC; Benjamin EJ; Levy D; Larson MG; Sawyer DB; Siwik DA; Colucci WS; Sutherland P; Wilson PW; Vasan RS Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 109:2850–2856; 2004a [DOI] [PubMed] [Google Scholar]
- Sundstrom J; Evans JC; Benjamin EJ; Levy D; Larson MG; Sawyer DB; Siwik DA; Colucci WS; Wilson PW; Vasan RS Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study. Eur Heart J. 25:1509–1516; 2004b [DOI] [PubMed] [Google Scholar]
- Tayebjee MH; Lip GY; Blann AD; Macfadyen RJ Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and −9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and −2. Thromb Res. 115:205–210; 2005 [DOI] [PubMed] [Google Scholar]
- Vianello A; Caponi L; Franzoni F; Galetta F; Rossi M; Taddei M; Malvaldi G; Pietrini P; Santoro G Role of matrix metalloproteinases and their tissue inhibitors as potential biomarkers of left ventricular remodelling in the athlete's heart. Clin Sci (Lond). 117:157–164; 2009 [DOI] [PubMed] [Google Scholar]
- Victor RG; Haley RW; Willett DL; Peshock RM; Vaeth PC; Leonard D; Basit M; Cooper RS; Iannacchione VG; Visscher WA; Staab JM; Hobbs HH The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 93:1473–1480; 2004 [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C; Aznaouridis K; Dima I; Ioakeimidis N; Vasiliadou C; Zervoudaki A; Gialernios T; Stefanadis C Negative association between serum levels of matrix metalloproteinases-2 and −9 and aortic stiffness in healthy adults. Int J Cardiol. 122:232–238; 2007 [DOI] [PubMed] [Google Scholar]
- Wei M; Kampert JB; Barlow CE; Nichaman MZ; Gibbons LW; Paffenbarger RS Jr.; Blair SN Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 282:1547–1553; 1999 [DOI] [PubMed] [Google Scholar]
- Woodiwiss AJ; Oosthuyse T; Norton GR Reduced cardiac stiffness following exercise is associated with preserved myocardial collagen characteristics in the rat. Eur J Appl Physiol Occup Physiol. 78:148–154; 1998 [DOI] [PubMed] [Google Scholar]
- Wright KJ; Thomas MM; Betik AC; Belke D; Hepple RT Exercise training initiated in late middle age attenuates cardiac fibrosis and advanced glycation end-product accumulation in senescent rats. Exp Gerontol. 50:9–18; 2014 [DOI] [PubMed] [Google Scholar]
- Yasmin; McEniery CM; Wallace S; Dakham Z; Pulsalkar P; Maki-Petaja K; Ashby MJ; Cockcroft JR; Wilkinson IB Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 25:372; 2005 [DOI] [PubMed] [Google Scholar]
- Zile MR; Desantis SM; Baicu CF; Stroud RE; Thompson SB; McClure CD; Mehurg SM; Spinale FG Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Heart Fail. 4:246–256; 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]