Abstract
Individuals with impulsive and addictive disorders, including drug addiction, binge eating/obesity, and problem gambling, exhibit both impaired control over behavior and heightened sensitivity to reward. However, it is not known whether such deviation in inhibitory and reward circuitry among clinical populations is a cause or consequence of the disorders. Recent evidence suggests that these constructs may be related at the neural level, and together, increase risk for engaging in maladaptive behaviors. The current study examined the degree to which brain function during inhibition relates to brain function during receipt of reward in healthy young adults who have not yet developed problem behaviors. Participants completed the stop signal task to assess inhibitory control and the doors task to assess reactivity to monetary reward (win vs loss) during functional magnetic resonance imaging (fMRI). Brain activation during response inhibition was negatively correlated with brain activation during reward. Specifically, less brain activation in right prefrontal regions during inhibition, including the right inferior frontal gyrus, middle frontal gyrus, and supplementary motor area, was associated with greater brain activation in left ventral striatum during receipt of monetary reward. Moreover, these associations were stronger in binge drinkers compared to non-binge drinkers. These findings suggest that the systems are related even before the onset of impulsive or addictive disorders. As such, it is possible that the association between inhibitory and reward circuitry may be a prospective marker of risk.
Keywords: inhibitory control, reward, fMRI, ventral striatum, inferior frontal gyrus, binge drinker
1. Introduction
Impulsive and addictive behaviors, including drug and alcohol addiction, binge eating/obesity, and problem gambling are characterized by both impaired control over behavior and heightened reward sensitivity. These two constructs are often considered as independent risk factors for problem behaviors, but neurobiological theories propose that the two may be related, perhaps exerting reciprocal influences on each other to promote inappropriate or maladaptive behavior (Goldstein and Volkow 2002; 2011; Jentsch and Taylor 1999; Kaye et al. 2013). This hypothesis is supported by evidence that inhibition and reward share overlapping neural circuitry, and that both are strongly linked to the dopamine system (Goldstein and Volkow 2002; Jentsch and Pennington 2014). Importantly, this relative imbalance between hypoactive inhibitory functioning and hyperactive reward functioning may be present before the onset of a disorder. If so, this neural profile could serve as an important marker of risk, as well as provide a potential target for prevention mechanisms.
Functional neuroimaging studies provide consistent evidence implicating altered inhibition and reward circuitry in addiction and impulse control disorders. For instance, individuals who abuse drugs show hypoactivation of prefrontal regions involved in inhibitory control, including the right inferior frontal gyrus (IFG), supplementary motor area (SMA), and dorsolateral prefrontal cortex (DLPFC), during response inhibition (Luijten et al. 2014; Spechler et al. 2016). Similar hypoactivation of frontal regions during inhibition has also been observed in problem gamblers, individuals with internet gaming disorder, and adults and adolescents with bulimia nervosa, compared to healthy controls (Chen et al. 2015; de Ruiter et al. 2012; Liu et al. 2014; Marsh et al. 2011; Marsh et al. 2009). Individuals with addictive and impulse control disorders also display heightened activation of reward regions, including the ventral striatum (VS) and medial prefrontal cortex (mPFC), in response to disorder-specific rewarding stimuli. For instance, individuals who abuse drugs display heightened activation in reward circuitry while viewing images of drugs and drug cues (Jasinska et al. 2014; Schacht et al. 2012). Problem gamblers and individuals with internet gaming disorder also show heightened activation of reward circuitry when viewing images related to gambling or gaming, respectively (Fauth-Buhler and Mann 2017), and obese individuals show greater reward response to food cues (Tomasi and Volkow 2013). Interestingly, there is also evidence that individuals with addictive disorders display a blunted response to non-disorder-related reward, including monetary and sexual reward, and pleasant emotional stimuli (Dunning et al. 2011; Garavan et al. 2000; Balodis and Potenza 2015). This suggests that the relative values of different rewards may change as the disorders develop. In sum, individuals with addiction and impulse control disorders display hypoactivation of inhibitory circuitry, coupled with hyperactivation of reward circuitry to disorder-specific stimuli, compared to healthy controls.
Several recent imaging studies suggest that inhibition and reward circuits are closely linked, particularly in addicted populations. Compared to healthy controls, individuals with alcohol dependence show less functional connectivity between DLPFC (a region strongly implicated in inhibitory control) and VS during monetary reward (Becker et al. 2017; Forbes et al. 2014). Moreover, lower frontal-striatal connectivity in alcohol dependent individuals is associated with greater activation of VS during reward (Becker et al. 2017). These findings suggest that greater behavioral sensitivity to reward could be due in part to dampened prefrontal regulation of striatal reward responses.
It is not known whether aberrant functioning of inhibition and reward circuitry in these disorders is a cause or consequence of the disorder. Preliminary prospective studies show that individuals with less engagement of inhibitory control regions during response inhibition (Mahmood et al. 2013; Norman et al. 2011; Wetherill et al. 2013) as well as greater activation in reward regions during monetary reward (Heitzeg et al. 2015; Whelan et al. 2014) are more likely to exhibit substance use later in life, suggesting a potential causal relationship. Another way to investigate the relationships between these processes before problematic behaviors develop is to examine engagement of prefrontal regions during response inhibition in relation to engagement of reward circuitry during receipt of reward in healthy, non-disordered populations. That is, a risk profile for future problematic behaviors may be poor prefrontal regulation of reward circuitry.
The current study examined the degree to which brain function during inhibition relates to brain function during reward in healthy young adults with no history of addictive or impulse control disorders. Specifically, we used fMRI to examine associations between brain activation during response inhibition and during monetary reward. Brain activation during inhibition was assessed using the stop signal task. This task activates right-lateralized frontal regions known to be involved in inhibitory control, including DLPFC, IFG, and SMA, and less frontal activation is associated with poor inhibition (Aron and Poldrack 2006; Congdon et al. 2010). Brain activation during receipt of monetary reward was assessed using the doors task (Carlson et al. 2011), which reliably activates regions involved in reward, including VS (Carlson et al. 2011; Crane et al. 2017). We hypothesized that less prefrontal activation during response inhibition would be associated with greater activation of reward circuitry during monetary reward. Additionally, we conducted exploratory analyses to examine these associations separately in binge and non-binge drinkers. As binge drinkers are a population at risk for developing substance use disorders, these analyses provided some insight as to whether associations between brain function during inhibition and reward related to risk for addictive and impulse control disorders.
2. Methods
2.1. Participants
Volunteers age 21–29 were recruited from the community through online and printed advertisements. This age range was selected to target young adults, as the study aimed to identify biomarkers of addiction vulnerability, and young adulthood is a known period of risk. Individuals younger than 21 were excluded to limit variability due to ongoing brain maturation in prefrontal regions of interest (Silveri 2012). Inclusion criteria were as follows: age 21–29, BMI between 19 and 26, at least a high school education, fluency in English, no current or past year DSM-IV diagnosis, no lifetime history of substance dependence or ADHD, no serious medical conditions, and no night shift work. Participants were excluded if they reported smoking more than five cigarettes per day or daily use of any medications other than birth control, contraindication for fMRI (e.g., metal in the body, claustrophobia), or left-handedness, or if they were pregnant, lactating, or planning to become pregnant in the next 3 months.
2.2. Measures
2.2.1. Stop Signal Task
Participants performed the stop signal task (Logan et al. 1997; Kareken et al. 2013) during blood oxygen level dependent (BOLD) fMRI to assess brain activation during response inhibition. Participants were instructed to respond as quickly as possible to go signals (left- or right-pointing blue arrows), and to inhibit responses on trials in which a stop signal (up-pointing red arrow superimposed on the go arrow) occurred. The duration of the delay between presentation of the go and stop signal (i.e., the stop signal delay) was adjusted to target a 50% successful inhibition rate. Stop signal reaction time (SSRT) was calculated by subtracting the mean stop signal delay from the participant’s mean go reaction time. Longer SSRT indicates a longer time needed to inhibit a response, and thus poorer inhibitory control. Supplemental Figure S1 presents a schematic of the task and the main task parameters (go reaction time, stop signal delay, and SSRT). Behavioral task performance was considered valid if participants had a mean Go reaction time of 800ms or less, Go accuracy of at least 75%, and Stop accuracy between 25% and 75%. Participants completed three task runs (80 go and 40 stop trials each), and each run required approximately 6 minutes to complete.
2.2.2. Doors Task
Participants completed the doors task (Carlson et al. 2011) during BOLD fMRI to assess brain activation during monetary reward and loss. Task procedures are described in detail in Crane et al. (2017). Briefly, two doors are presented on the screen, and participants are told that behind one of the doors is a monetary prize of $.50 (signaled by ‘↑’) and behind the other door is a loss of $.25 (signaled by ‘↓’). For each trial, participants are instructed to use a button box to choose one of the two doors. They are told that they will either win or lose money based on their choices, and that they had a chance of winning between $0 and $15 total based on their performance. In actuality, the subjects’ performance had no impact on outcomes. The task consisted of 30 predetermined Wins and 30 Losses presented in a pseudorandom order over two runs. The task required 15 minutes to complete, and all subjects earned $10.
2.2.3. Timeline Follow-Back (TLFB)
Participants completed a retrospective timeline calendar of their alcohol consumption for the past month to assess daily patterns of drinking, including number of binge episodes (Sobell and Sobell 1992). For each day, participants estimated the number of standard drinks they consumed, and days on which they consumed 5 (men) or 4 (women) or more drinks were considered binge episodes. From the TLFB we calculated subjects’ total number of binge-drinking days and average number of drinks per week over the past month.
2.3. Procedure
Participants were screened in the Human Behavioral Pharmacology Laboratory at the University of Chicago and provided informed consent. As a separate part of the parent study, they completed four drug-challenge sessions in which they received amphetamine or placebo (presented elsewhere: Crane et al. 2018; Weafer et al. 2017a; Weafer et al. 2017b). Subjects then completed an fMRI session at the University of Illinois at Chicago. They abstained from drugs, including alcohol, for 24h prior to the fMRI session, as verified by self-report, breath alcohol, and urine screens. Upon arrival at the laboratory, they completed a scanner safety questionnaire and a practice round of the stop signal task. They were then placed in the scanner, where they completed a battery of tasks, including the stop signal and doors tasks. All participants completed the stop signal task prior to the doors task. Upon completion of all sessions, participants were debriefed and paid for their time. The study was approved by the IRB at each institution.
2.3.1. Imaging acquisition and processing
Participants were imaged using a 3T GE scanner with an 8-channel head coil array at the UIC Center for Magnetic Resonance Research. T1-weighted high-resolution anatomical images (BRAVO sequence with 1 × 1 × 1 mm3 voxels; repetition time, 9.3ms; echo time, 3.8ms; 220 × 220 mm field of view; flip angle, 13°) were acquired for co-registration and normalization to the MNI coordinate system. Whole-brain functional imaging was performed with a standard T2*-sensitive echo planar imaging sequence (gradient-echo; repetition time, 2000ms; echo time, 22.2 ms; 64 × 64 matrix; 220 × 220 mm field of view; flip angle, 90°; 3 mm slice thickness with no gap, 44 axial slices).
Images were processed using SPM12 (Wellcome Trust Centre for Neuroimaging). Standard preprocessing of functional images for both tasks included slice-time correction, spatial realignment to correct for head motion, coregistration to the participant’s T1 image and warping to MNI space, resampling to 2mm/side voxels and smoothing with an 8mm FWHM isotropic Gaussian kernel. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and included a 128s high-pass filter. Condition effects were modeled with event-related regressors: correct go (Go) and stop (StopInh) trials, and incorrect go and stop trials for the stop signal task, and Win, Loss, and Fixation for the doors task. Effects were estimated at each voxel, and for each subject. Individual contrast maps were created for each participant (StopInh>Go for the stop signal task, and Win>Loss for the doors task). Volumes were identified as motion outliers based on image intensity difference (dvars) or framewise displacement (fd; >0.5mm) using FSL’s motion outlier tool (Power et al. 2012). Six head motion parameters from the SPM realignment and the FSL-tagged motion outlier files were included as regressors-of-no-interest.
2.4. Data Analyses
2.4.1. Stop Signal Task
We conducted a second-level, random effects one sample t-test for StopInh>Go. As our hypotheses were specific to frontal regions, statistical inferences were made based on peak voxel significance within a frontal-insular-subcortical (FIS) mask previously used for analyses with this task (Weafer et al. 2015; Weafer et al. 2017a). This 382,584 mm3 (47,823 voxels) mask included the following structural regions from AAL library (Tzourio-Mazoyer et al. 2002) available in MarsBar: medial and lateral frontal and orbital regions, bilateral precentral gyri, anterior and middle cingulate cortex, anterior insula, and subcortical motor regions consisting of bilateral putamen, pallidum, and caudate. Peak voxel activation (pFWE < 0.05) within the FIS mask was used as the statistical threshold.
2.4.2. Doors Task
We conducted a second-level, random effects one sample t-test for Win>Loss. The doors task does not include a Neutral condition, and so we were not able to compare Win to Neutral. It is important to note, however, that the Win>Loss contrast is a more sensitive indicator of neural activation during reward processing than examining Win or Loss individually (Carlson et al. 2011; Forbes et al. 2014; Foti et al. 2011). As our hypotheses were specific to regions implicated in reward, statistical inferences were made based on peak voxel significance within a functional reward mask downloaded from Neurosynth (Yarkoni et al. 2011). The mask was based on a meta-analysis of 532 studies with 100 terms relating to ‘reward’ and displaying regions that are reported more often in studies that load highly on ‘reward’ (http://neurosynth.org/analyses/topics/v4-topics-100/99). Peak voxel activation (pFWE < 0.05) within the reward mask was used as the statistical threshold.
2.4.3. Associations between brain activation during inhibition and monetary reward
We extracted parameter estimates/β-weights representing BOLD response activation in arbitrary units averaged across all voxels within a 10-mm radius sphere surrounding peak areas of activation (pFWE < 0.05) for both tasks. For the stop signal task, we focused our analyses on significant peaks within well-established inhibitory control regions, including right IFG, MFG, insula, and SMA. For the doors task, we focused our analyses on significant peaks within VS, given this region’s well-established role in reward (see Supplementary Table 3 for correlations among all significant regions of activation). We also extracted significant peaks in visual regions to serve as control regions. We first examined the activation distributions to identify any potential outliers, defined as >3 standard deviations above or below the mean. We then conducted bivariate correlational analyses to determine the degree to which BOLD activation in inhibitory regions during response inhibition was associated with BOLD activation in reward regions during monetary reward.
2.4.4. Association between inhibition-reward relationships in binge and non-binge drinkers
To examine the degree to which associations between brain activity during inhibition and reward related to risk for addictive behaviors, we tested associations separately in binge and non-binge drinkers. Binge drinkers were defined as participants who reported at least one binge episode in the last month on the TLFB and non-binge drinkers were defined as participants who reported no binge episodes in the past month.
3. Results
3.1. Sample characteristics
A total of 67 healthy young adults (33 men and 34 women) took part in this study. Demographic and substance use data for the sample are presented in Table 1.
Table 1.
Sample characteristics (n=44)
| Mean (SD) | |
|---|---|
| Sex (M:F) | 22:22 |
| Age | 24.7 (2.6) |
| Education (years) | 15.7 (1.4) |
| Race | |
| Caucasian | 29 |
| More than one race | 6 |
| Asian | 5 |
| African-American | 3 |
| American Indian/Alaskan | 1 |
| Alcohol consumption (past 28 days) | |
| Binge episodes | 2.0 (3.0) |
| Drinks per week | 7.0 (9.0) |
3.2. Brain activation during response inhibition
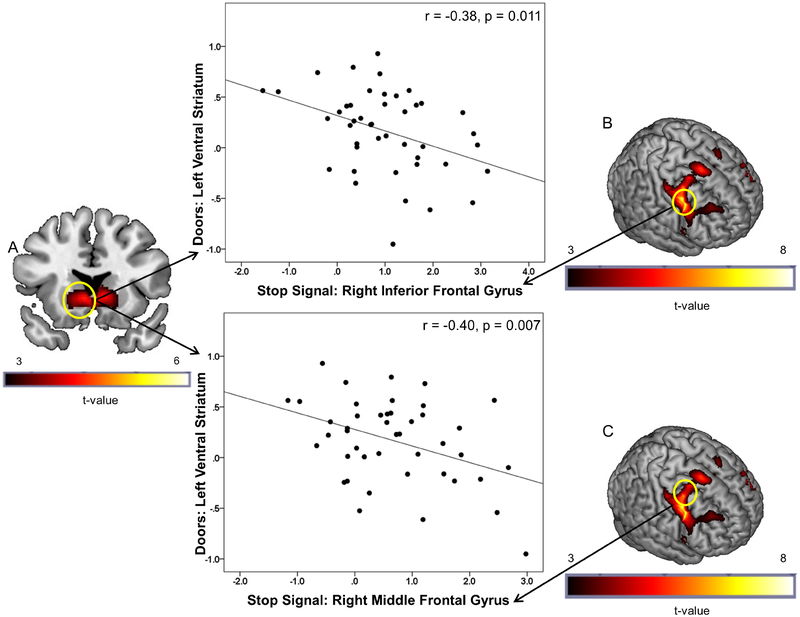
Behavioral data for the stop signal task were lost from 11 subjects due to computer error, and 3 additional subjects had incomplete behavioral or fMRI data. Five participants were excluded from stop signal task fMRI analyses due to invalid behavioral performance (mean Go reaction time > 800ms, n=3; Go accuracy < 75%, n=1; Stop Accuracy < 25%, n=1), resulting in a final sample size of n=48. Behavioral task performance measures were as follows: mean SSRT = 305.7 ms; mean inhibition rate = 52.7%; mean go accuracy = 96.0%; and mean go RT = 462.1 ms. As previously reported in this sample (Weafer et al. 2017a), the StopInh>Go contrast activated inhibitory control regions, including right IFG, insula, MFG, and SMA (Figure 1B and 1C; Supplementary Table 1).
Figure 1.
(A) Brain activation during receipt of monetary reward (Doors task: Win>Loss) in left VS. Significant peak activation was observed at the [−6 18 −2] MNI coordinate after correcting for family wise error (pFWE < 0.05) within the reward mask. (B) Brain activation during successful response inhibition (StopInh>Go) within the frontal-insular-subcortical (FIS) mask. Significant (pFWE < 0.05) peak activation was observed in a large right prefrontal cluster encompassing precentral, middle, and inferior gyri and anterior insula. The top scatter plot presents the negative relationship between extracted BOLD signal from a 10 mm radius spherical region centered at the [−6 18 −2] peak in the left VS (A) during receipt of monetary reward and extracted BOLD signal from a 10 mm radius spherical region centered at the [42 4 32] peak in the right IFG during response inhibition (B). The bottom scatter plot presents the negative relationship between extracted BOLD signal from the left VS (A) during receipt of monetary reward and extracted BOLD signal from a 10 mm radius spherical region centered at the [28 2 50] peak in the right MFG during response inhibition (C). The negative relationships indicate that less right frontal activation during response inhibition was associated with greater left VS activation during reward.
3.3. Brain activation during monetary reward
As previously reported in this sample (Crane et al. 2017), fMRI analyses of the doors task showed that the Win>Loss contrast activated bilateral VS (Figure 1A; Supplementary Table 2), as well as bilateral middle and inferior occipital gyri (Supplementary Table 2).
3.4. Associations between brain activation during inhibition and monetary reward
We extracted parameter estimates/β-weights representing BOLD activation within a 10 mm-radius sphere surrounding the following regions of significant peak activation for the stop signal task: right IFG [42 4 32], insula [34 24 4], MFG [28 2 50], and SMA [14 12 64]. No outliers were present. For the doors task, we extracted activation surrounding the following peaks: bilateral VS [−6 18 −2] and [10 22 −2], as well as bilateral visual cortex [−30 −86 −8] and [34 −86 −6] to serve as control regions. Outlier analyses (activation exceeding 3 standard deviations from the mean) identified two outliers for the [−6 18 −2] peak and two outliers for the [10 22 −2] peak. After confirming that the second level model remained unchanged after removing these four participants, we excluded them from subsequent analyses. As we tested correlations for each region of activation from the doors task with four inhibitory control regions, we applied a Bonferroni correction of p < 0.0125 (.05/4). Bivariate correlational analyses showed a negative association between brain activation during response inhibition and receipt of monetary reward. Specifically, less brain activation in right prefrontal regions during inhibition, including right IFG, MFG, and SMA, was associated with greater brain activation in left, but not right, VS during receipt of monetary reward (Figure 1; Table 2). By contrast, right insula activation during inhibition was not related to VS activation during monetary reward. We also examined correlations between brain activation during inhibition and activation in visual cortex during reward processing to serve as a control region. Table 2 shows that no significant associations were observed between prefrontal and visual regions (ps > 0.27), suggesting that prefrontal activation during inhibition was specifically correlated with regions known to be implicated in reward.
Table 2.
Correlations between brain activation during response inhibition and receipt of monetary reward (n=44)
| SST (StopInh>Go) | Doors (Win>Loss) | |||
|---|---|---|---|---|
| L VS | R VS | L Occipital | R Occipital | |
| R IFG | −0.382* | −0.337 | 0.060 | 0.167 |
| R MFG | −0.400* | −0.294 | 0.026 | 0.024 |
| R Insula | −0.111 | −0.075 | 0.002 | −0.099 |
| R SMA | −0.400* | −0.322 | −0.020 | −0.047 |
Note.
p<0.0125.
SST = stop signal task; L = left; R = right; VS = ventral striatum; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; SMA = supplementary motor area
No significant associations were detected between the in-scanner behavioral measure of inhibition (SSRT) and brain activation during inhibition or reward (ps > 0.05).
3.5. Association between inhibition-reward relationships in binge and non-binge drinkers
Twenty-three participants were classified as binge drinkers (at least one binge episode in the last month) and 21 were classified as non-binge drinkers (no binge episodes in the past month). Among the binge drinkers, greater brain activation in left VS during reward was associated with less brain activation in right SMA (r = −0.60, p = 0.003) and IFG (r = −0.53; p = 0.009), but not the MFG (r = −0.26, p = 0.23) during inhibition. By contrast, no significant correlations were observed at the corrected p-value of 0.0125 among the non-binge drinkers (SMA: r = −0.22, p = 0.34; IFG: r = −0.12, p = 0.62; MFG: r = −0.46; p = 0.03).
4. Discussion
This study revealed a novel association between brain activation underlying two seemingly independent constructs: response inhibition and reward sensitivity. Both inhibition and reward sensitivity have long been linked to propensity for impulsive and addictive behaviors; however, few studies have examined how the two risk factors might be related to one another. The current study provides the first evidence of a negative correlation between brain activation during inhibition and reward. Specifically, individuals with less prefrontal engagement during response inhibition displayed greater engagement of reward circuitry during monetary reward. Additionally, these associations were more pronounced in binge drinkers, a population at risk for addictive disorders, compared to non-binge drinkers. These findings have important implications for understanding interactions between brain engagement during inhibition and reward processing, and how a potential imbalance between the two might confer increased risk for problematic and maladaptive behaviors.
Our finding that underactive inhibitory engagement is coupled with overactive reward circuitry among healthy adults, and that the association is more pronounced in binge drinkers compared to non-binge drinkers, has important implications for understanding disrupted inhibitory and reward processing in addiction and other impulse control disorders. Specifically, the imbalance observed in individuals with these disorders may not be entirely a consequence of the disorder. Instead, it may be a prospective risk factor and could even play a causal role in the onset of problematic behaviors. Indeed, prospective studies have shown that neural circuitry underlying inhibitory control and reward sensitivity both predict the development of drug-related problems independently (Heitzeg et al. 2015; Mahmood et al. 2013; Norman et al. 2011; Wetherill et al. 2013; Whelan et al. 2014). It will be important for future longitudinal studies to assess the relative balance between inhibition and reward functioning as a predictor of addictive and other impulse control disorders, as individuals who experience both poor inhibition and overactive reward are likely at greater risk.
It is important to note that the current analyses are correlational, and thus do not directly speak to the mechanisms underlying the negative association between prefrontal inhibitory and sub-cortical reward processing observed here. Although speculative, one potential explanation is that prefrontal functioning could serve a regulatory role in reward processing, similar to its regulatory role in motor inhibition. In the context of motor inhibition, frontal regions are thought to exert ‘top-down’ executive control over lower level, sub-cortical regions, including the striatum (Bari and Robbins 2013). This frontal-striatal circuitry primarily encompasses dorsal striatal regions involved in motor control, and deficits in prefrontal engagement and weakened frontal-dorsal striatal connectivity are associated with impaired response inhibition (Courtney et al. 2012; Ghahremani et al. 2012). It is possible that such downstream inhibitory projections could extend to ventral striatal regions implicated in reward as well. This idea is consistent with the current findings, as well as reports in both healthy and addicted populations showing that weakened frontal-ventral striatal connectivity during reward processing is associated with greater striatal activation during reward (Becker et al. 2017; Behan et al. 2015; Forbes et al. 2014). In order to test whether prefrontal inhibitory functioning does indeed exert top-down regulation over striatal reward regions, future studies should assess reward reactivity following direct manipulations of prefrontal functioning. For example, engagement of inhibitory brain regions can be acutely enhanced using transcranial magnetic stimulation (TMS; Watanabe et al. 2015; Xu et al. 2016) or pharmacotherapy (Schmaal et al. 2013). These methods could be used to test the causal effects of increased inhibitory functioning on activation of reward circuitry. If, as hypothesized, enhanced prefrontal functioning dampens reward activation, then targeting inhibitory functioning could be a promising means of preventing drug abuse problems in at-risk individuals.
A second potential explanation for the association between neural correlates of inhibition and reward is that the two are connected by an as-yet unidentified common mechanism, such as fewer striatal dopamine D2 receptors, which have been separately linked to both inhibition and reward. Animals with fewer striatal D2 receptors commit more inhibitory errors on response inhibition tasks (Jentsch et al. 2014) and self-administer greater amounts of stimulant drugs (Dalley et al. 2007; Diergaarde et al. 2008; Nader et al. 2008), suggesting a link between D2 receptor number and drug reward sensitivity. In humans, individuals with fewer striatal D2 receptors exhibit poorer inhibitory control and less brain activation during response inhibition (Ghahremani et al. 2012; Robertson et al. 2015) and report greater positive subjective response to stimulant drugs and sweet tastes (Pepino et al. 2016; Volkow et al. 1999; Volkow et al. 2002). Thus, it could be that low levels of D2 receptors contribute to both low levels of brain engagement during inhibition and heightened engagement during reward. It will be important for future multimodal imaging studies to combine PET and fMRI to directly test associations between striatal D2 receptor availability and neural correlates of inhibition and reward.
This study has some limitations. First, this sample was comprised of healthy adults without addictive or impulse control disorders. Without longitudinal follow-up data, we can only speculate about the associations between inhibition and reward as a predictor of addictive behaviors. Second, while our finding that the association is stronger in binge drinkers supports the idea that associations between inhibition and reward may be a prospective marker of risk, this finding also raises the possibility that the association in binge drinkers is a result of heavy alcohol consumption. Again, longitudinal studies are needed to assess associations between inhibition and reward before the onset of any substance use to directly address this question. Third, we probed activation of neural reward circuitry using monetary reward, yet hyperactivation of reward circuitry in addiction is often specific to drug- or disorder-related cues (Garavan et al. 2000; Dunning et al. 2011; Berridge and Robinson 2016). As such, it is not clear if our findings of responses to monetary reward generalize to responses to drug reward in addiction. Fourth, we did not include a behavioral measure of appetitive motivation, and the behavioral measure of response inhibition (SSRT) did not correlate with brain activity during response inhibition or reward. However, previous studies have also failed to observe robust correlations between SSRT and brain activation, likely due to the need for very large samples and sufficient power for voxelwise analyses (Congdon et al. 2010). Finally, this study did not include a resting state scan. Future studies are needed that examine associations between inhibitory and reward circuitry when subjects are at rest, to determine whether these networks are coupled in the absence of any task manipulation.
In sum, this is the first study to show that individual differences in prefrontal engagement during response inhibition are related to individual differences in engagement of reward circuitry during receipt of monetary reward. These findings have important implications for understanding risk for impulsive and addictive behaviors, particularly in individuals with compromised inhibitory functioning. It will be important for future longitudinal studies to examine interactions between inhibitory and reward functioning as prospective predictors of the onset and escalation of these disorders later in life. If, as hypothesized, poor inhibitory control mechanisms result in heightened sensitivity to reward, this will serve as an important target for prevention and treatment efforts in at-risk populations.
Supplementary Material
Acknowledgements
This research was supported by National Institute on Drug Abuse (NIDA) Grant R01 DA002812 (HdW and KLP). JW was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) grant K01 AA024519. SMG was supported by NIAAA grant K23 AA025111. The funding agencies had no involvement in the research other than financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Aron AR, Poldrack RA (2006) Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. J Neurosci 26: 2424–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108: 44–79 [DOI] [PubMed] [Google Scholar]
- Balodis IM, Potezna MN (2015) Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biol Psychiatry 77: 434–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Kirsch M, Gerchen MF, Kiefer F, Kirsch P (2017) Striatal activation and frontostriatal connectivity during non-drug reward anticipation in alcohol dependence. Addiction Biology 22: 833–843 [DOI] [PubMed] [Google Scholar]
- Behan B, Stone A, Garavan H (2015) Right prefrontal and ventral striatum interactions underlying impulsive choice and impulsive responding. Human Brain Mapping 36: 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (2016) Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol 71: 670–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G (2011) Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. Neuroimage 57: 1608–1616 [DOI] [PubMed] [Google Scholar]
- Chen CY, Huang MF, Yen JY, Chen CS, Liu GC, Yen CF, Ko CH (2015) Brain correlates of response inhibition in Internet gaming disorder. Psychiatry and Clinical Neurosciences 69: 201–209 [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Aron AR, Xue G, Miller E, Poldrack RA (2010) Engagement of large-scale networks is related to individual differences in inhibitory control. Neuroimage 53: 653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA (2012) Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol 18: 593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, Phan KL (2017) Preliminary evidence for disrupted nucleus accumbens reactivity and connectivity to reward in binge drinkers. Alcohol and Alcoholism 52: 647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Gorka SM, Weafer J, Langenecker SA, de Wit H, Phan KL (2018) Neural activation to monetary reward is associated with amphetamine reward sensitivity. Neuropsychopharmacology 43: 1738–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315: 1267–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter MB, Oosterlaan J, Veltman DJ, van den Brink W, Goudriaan AE (2012) Similar hyporesponsiveness of the dorsomedial prefrontal cortex in problem gamblers and heavy smokers during an inhibitory control task. Drug Alcohol Depend 121: 81–9 [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63: 301–8 [DOI] [PubMed] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, et al. (2011) Motivated attention to cocaine and emotional cues in abstinent and current cocaine users-an ERP study. Eur J Neurosci 33: 1716–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth-Buhler M, Mann K (2017) Neurobiological correlates of internet gaming disorder: Similarities to pathological gambling. Addictive Behaviors 64: 349–356 [DOI] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R (2014) Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS One 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G (2011) Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping 32: 2207–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. (2000) Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 157: 1789–98 [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED (2012) Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci 32: 7316–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159: 1642–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Reviews Neuroscience 12: 652–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Cope LM, Martz ME, Hardee JE (2015) Neuroimaging risk markers for substance abuse: recent findings on inhibitory control and reward system functioning. Curr Addict Rep 2: 91–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y (2014) Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev 38: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT (2014) Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Pennington ZT (2014) Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology 76: 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146: 373–90 [DOI] [PubMed] [Google Scholar]
- Kareken DA, Dzemidzic M, Wetherill L, Eiler W 2nd, Oberlin BG, Harezlak J, Wang Y, O’Connor SJ (2013) Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology (Berl) 228: 335–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Wagner A, Bischoff-Grethe A (2013) Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biological Psychiatry 73: 836–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GC, Yen JY, Chen CY, Yen CF, Chen CS, Lin WC, Ko CH (2014) Brain activation for response inhibition under gaming cue distraction in internet gaming disorder. Kaohsiung Journal of Medical Sciences 30: 43–51 [DOI] [PubMed] [Google Scholar]
- Logan GD, Schachar RJ, Tannock R (1997) Impulsivity and inhibitory control. Psychol Sci 8:60–64 [Google Scholar]
- Luijten M, Machielsen MWJ, Veltman DJ, Hester R, de Haan L, Franken IHA (2014) Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. Journal of Psychiatry & Neuroscience 39: 149–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood OM, Goldenberg D, Thayer R, Migliorini R, Simmons AN, Tapert SF (2013) Adolescents’ fMRI activation to a response inhibition task predicts future substance use. Addictive Behaviors 38: 1435–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Horga G, Wang Z, Want P, Klahr KW, Berner LA, Walsh BT, Peterson BS (2011) An fMRI study of self-regulatory control and conflict resolution in adolescents with bulimia nervosa. Am J Psychiatry 168: 1210–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R, Steinglass JE, Gerber AJ, O’Leary KG, Wang Z, Murphy D, Walsh T, Peterson BS (2009) Deficient activity in the neural systems that mediate self-regulatory control in bulimia nervosa. Archives of General Psychiatry 66: 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV (2008) Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philosophical Transactions of the Royal Society B-Biological Sciences 363: 3223–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF (2011) Neural activation during inhibition predicts initiation of substance use in adolescence. Drug and Alcohol Dependence 119: 216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Eisenstein SA, Bischoff AN, Klein S, Moerlein SM, Perlmutter JS, Black KJ, Hershey T (2016) Sweet dopamine: sucrose preferences relate differentially to striatal D2 receptor binding and age in obesity. Diabetes 65: 2618–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59: 2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CL, Ishibashi K, Mandelkern MA, Brown AK, Ghahremani DG, Sabb F, Bilder R, Cannon T, Borg J, London ED (2015) Striatal D1- and D2-type dopamine receptors are linked to motor response inhibition in human subjects. J Neurosci 35: 5990–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H (2012) Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18: 121–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE (2013) Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biological Psychiatry 73: 211–218 [DOI] [PubMed] [Google Scholar]
- Silveri MM (2012) Adolescent brain development and underage drinking in the United States: identifying risks of alcohol use in college populations. Harv Rev Psychiatry 20: 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back: A technique for assessing self-reported alcohol consumption In Litten RZ & Allen JP (Eds.) Measuring alcohol consumption: Psychosocial and biochemical methods (pp. 41–72). Totowa, NJ: Humana Press [Google Scholar]
- Spechler PA, Chaarani B, Hudson KE, Potter A, Foxe JJ, Garavan H (2016) Response inhibition and addiction medicine: from use to abstinence. Prog Brain Res 223: 143–64 [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2013) Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Critical Reviews in Biochemistry and Molecular Biology 48: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N (1999) Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry 156: 1440–1443 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N (2002) Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse 46: 79–82 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, Tsutsumi R, Shimizu T, Hayashi T, Terao Y, Ugawa Y, Katsura M, Kunimatsu A, Ohtomo K, Hirose S, Miyashita Y, Konishi S (2015) Effects of rTMS of pre-supplementary motor area on fronto basal ganglia network activity during stop-signal task. Journal of Neuroscience 35: 4813–4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Dzemidzic M, Eiler W, Oberlin BG, Wang Y, Kareken DA (2015) Associations between regional brain physiology and trait impulsivity, motor inhibition, and impaired control over drinking. Psychiatry Res 233: 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Gorka SM, Hedeker D, Dzemidzic M, Kareken DA, Phan KL, de Wit H (2017a) Associations between behavioral and neural correlates of inhibitory control and amphetamine reward sensitivity. Neuropsychopharmacology 42: 1905–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, Lyon N, Hedeker D, de Wit H (2017b) Sweet taste liking is associated with subjective response to amphetamine in women but not men. Psychopharmacology 234: 3185–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF (2013) A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology 230: 663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Carvalho FM, Conrod PJ, Flor H, Fauth-Buhler M, Frouin V, Gallinat J, Gan G, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Martinot JL, Nees F, Ortiz N, Paillere-Martinot ML, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Strohle A, Schumann G, Garavan H (2014) Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature 512: 185–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Sandrini M, Wang WT, Smith JF, Sarlls JE, Awosika O, Butman JA, Horwitz B, Cohen LG (2016) PreSMA stimulation changes task-free functional connectivity in the fronto-basal-ganglia that correlates with response inhibition efficiency. Human Brain Mapping 37: 3236–3249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011) Large-scale automated synthesis of human functional neuroimaging data. Nature Methods 8: 665–U95 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.