Abstract
Cyanobacteria can form dense blooms under specific environmental conditions, and some species produce harmful secondary metabolites known as cyanotoxins, which present significant risks to public health and the environment. Identifying toxins produced by cyanobacteria present in surface water and fish is critical to ensuring high quality food and water for consumption, and recreation. Current analytical screening methods typically focus on one class of cyanotoxins in a single matrix and rarely include saxitoxin. Thus, a cross-class screening method for microcystins, nodularin, anatoxin-a, cylindrospermopsin, and saxitoxin was developed to examine target analytes in environmental water and fish tissue. This was done, due to the broad range of cyanotoxin physicochemical properties, by pairing two extraction and separation techniques to improve isolation and detection. For the first time a zwitterionic hydrophilic interaction liquid chromatography column was evaluated to separate anatoxin-a, cylindrospermopsin, and saxitoxin, demonstrating greater sensitivity for all three compounds over previous techniques. Further, the method for microcystins, nodularin, anatoxin-a, and cylindrospermopsin were validated using isotopically labeled internal standards, again for the first time, resulting in improved compensation for recovery bias and matrix suppression. Optimized extractions for water and fish tissue can be extended to other congeners in the future. These improved separation and isotope dilution techniques are a launching point for more complex, non-targeted analyses, with preliminary targeted screening.
Keywords: RPLC, HILIC, nontarget analysis, harmful algal bloom, water quality, food safety
1. Introduction
Cyanobacteria are prokaryotic microorganisms found globally in both inland waters and coastal and marine systems [1, 2]. Across varying environmental conditions, cyanobacteria can produce harmful secondary metabolites called cyanotoxins, which possess various physiochemical properties, structures, and toxicological mechanisms of action [2, 3]. Microcystins (MCs) and nodularin (NOD) are hepatotoxic cyclic peptides [1, 2]. Cylindrospermopsin (CLD) is a cytotoxic, dermotoxic, and hepatotoxic cyclic guanidinic alkaloid [4]. Anatoxin-a (ANA) is a neurotoxic bicyclic secondary amine [1, 2]. Saxitoxin (SAX) is a neurotoxic guanadinium derivative with two amine functional groups [1, 2]. Occurrence of these cyanotoxins in the environment, resulting from cyanobacterial Harmful Algal Blooms (HABs), have been reported globally in surface waters [2].
Cyanobacterial HABs result in various water quality problems and severe economic damage by impairing water supplies, recreational activities and fisheries [1, 5]. Further, exposures to cyanotoxins through food and water can be fatal to both humans and wildlife [1–3]. Despite the complexity of environmental exposures and economic losses caused by these compounds, few water quality criteria and regulations for exposure to cyanotoxins exist, especially in the developing world [1, 6]. Many countries have regulatory values for exposure to microcystin-LR that are in agreement with the recommended exposure level (1.0 μg L−1) provided by the World Health Organization [1, 7]. In the United States, the Environmental Protection Agency (EPA) has revised the Unregulated Contaminant Monitoring Rule (UCMR 4) for Public Water Systems to add 10 cyanotoxins [8], has added several toxins to the Contaminant Candidate List 3 and 4 (CCL3&4) [9], and has proposed the Draft Human Health Recreational Ambient Water Quality Criteria or Swimming Advisories for MCs (4 μg L−1) and CLD (8 μg L−1) [10]. However, a lesser studied pathway to exposure in humans is through the consumption of contaminated food, such as dietary supplements, invertebrates, and fish [2]. Thus, a multi-toxin screening method for cyanotoxins in water and fish tissue is necessary to support environmental evaluations of cyanotoxins in surface waters and aquatic organisms that are consumed by human populations.
Recent developments in reverse phase chromatography (RPLC) stationary phases have resulted in separation methods that allow for the simultaneous detection of ANA, CLD, MCs, and NOD in water and fish tissue [11–15]. However, resolving all toxin classes on a single RPLC column is challenging due to the diverse range of physiochemical properties, charge states, and structures exhibited by cyanotoxins [16]. For example, coelution of ANA and d-phenylalanine (DPA), which are isobaric and produce similar fragment ions [17, 18], can lead to misidentification of ANA [13] on RPLC columns. Additionally, the high water solubility of some cyanotoxins requires use of ion-pairing agents to achieve sufficient retention on an RPLC column, which increases background noise, decreases ionization efficiency, and results in higher detection limits [16]. Alternatively, other multi-toxin screening methods obtain successful retention and separation of the polar cyanotoxins, ANA, CLD, and SAX using hydrophilic interaction liquid chromatography (HILIC) [16, 19–22]. The advantage of HILIC includes functionality similar to traditional normal phase chromatography with the compatibility of solvents suitable for RPLC, allowing the same mobile phases to be used for both separation techniques [23]. Thus, use of HILIC separation in addition to RPLC separation would allow for the regular incorporation of SAX in analytical screening methods.
Another challenge for the development of cyanotoxin analytical methods is finding reliable strategies that account and correct for the influence of matrix effect on ionization efficiency, a frequent problem when using electrospray ionization (ESI), and during extraction recovery. Robust methods to correct for matrix effect and recovery involve use of a surrogate/internal standards that shares physiochemical and structural properties with that of the target compound, resulting in similar column retention, ionization efficiency, and extraction recovery [24, 25]. To date, several compounds have been used as internal standards for cyanotoxin method development [11, 15, 17, 19, 26]. Unfortunately, no compounds have been generally accepted as suitable internal standards because robust evaluation in terms of observed variation in relative response and recoveries compared to the target compounds has not been performed [15]. Ideally, an isotopically labeled version of all target analytes could be used in an isotope dilution method to correct for recovery bias and matrix effects because variations in retention, ionization efficiency, and recovery would be rendered negligible [24, 25]. However, commercially available isotopically labeled internal standards for cyanotoxins were not available until recently.
Herein, we report the first multi-toxin screening method to incorporate the use of commercially available isotopically labeled internal standards for anatoxin-a, cylindrospermopsin, microcystin-LA, LR, RR, and YR to correct for matrix effect and extraction bias of MCs. This approach further evaluates use of a zwitterionic HILIC analytical column to separate ANA, CLD, and SAX simultaneously, for the first time. Use of optimized HILIC and RPLC separation methods allows for rapid detection of ANA, CLD, SAX, MCs, and NOD in water and fish tissue. Method development of solid phase extraction (SPE) for water and liquid-liquid extraction (LLE) for fish tissue involved the optimization of extraction techniques where highly polar cyanotoxins (ANA, CLD, and SAX) were isolated separately from moderately polar cyanotoxins (MCs and NOD). SPE extraction methods in the present study were built from previously existing methods to incorporate SAX. Multiple solvent systems were evaluated for optimized extraction from fish tissue, followed by cleanup of extracted tissue samples using the SPE methods developed for water. This method was subsequently used to screen target analytes in water and fish from a caged fish study staged in a hypereutrophic impoundment located in Waco, TX, USA.
2. Methods and Materials
2.1. Chemicals and reagents
All chemicals were reagent grade or better, obtained from various commercial vendors, and used as received. The cyanotoxin standards microcystin-LA (M-LA), microcystin-LR (M-LR), microcystin-LY (M-LY), microcystin-RR (M-RR), microcystin-YR (M-YR), nodularin, anatoxin-a, anatoxin-a-13C4 (ANA-13C), cylindrospermopsin, cylindrospermopsin-15N5 (CLD-15N) and saxitoxin (Figure S4) were purchased from Abraxis (Warminster, PA, USA). Isotopically labeled 15N internal standards (IS) microcystin-LA-15N7 (M-LA-15N), microcystin-LR-15N10 (M-LR-15N), microcystin-RR-15N13 (M-RR-15N), microcystin-YR-15N10 (M-YR-15N), and D-phenylalanine-d5 (DPA-d5) were obtained from Cambridge Isotope Laboratory (Tewksbury, MA, USA). Methanol (MeOH), acetonitrile (MeCN), and dichloromethane (DCM) were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Formic acid (FA) was purchased from VWR Scientific (Radnor, PA, USA). Ammonium formate was purchased from Sigma-Aldrich (St. Louis, MO, USA). A Thermo Barnstead™ Nanopure™ (Dubuque, IA, USA) Diamond UV water purification system was used throughout sample analysis to provide 18 MΩ nanopure water.
2.1. Liquid chromatography – tandem mass spectrometry
Chromatographic separation was carried out using an Agilent HPLC system consisting of an Agilent 1260 Quaternary Pump (G1311B), Agilent 1260 Infinity Standard Autosampler, and Agilent 1260 Infinity Thermostatted Column Compartment (G1316A). The HPLC system was interfaced with an Agilent G6420 Triple Quadrupole Mass Spectrometer. The goal of the current method was to incorporate SAX in the screening methodology; thus, we decided to employ complimentary RPLC and HILIC separation. ANA, CLD, and SAX were separated using a Poroshell HILIC-Z column (2.1 × 150 mm, 2.7 μm, 120Å), produced by Agilent Technologies (Santa Clara, CA, USA), with a binary gradient consisting of water as solvent B, and 5:95 water:MeCN (v v−1) as solvent A, both buffered with 5mM NH4OOCH and 3.6 mM HCOOH (pH 3.7). Flow rate was held constant at 0.5 mL min−1 with column temperature maintained at 30 °C. MCs and NOD were separated using a Poroshell SB-C18 RPLC column (2.1 × 100 mm, 2.7 μm, 120Å), produced by Agilent Technologies (Santa Clara, CA, USA), with a binary gradient method consisting of water as solvent A, and 5:95 water:MeCN (v v−1) as solvent B, both buffered with 5mM NH4OOCH and 3.6 mM HCOOH (pH 3.7). Flow rate was held constant at 0.5 mL min−1 with column temperature maintained at 60 °C. Both methods used an injection volume of 50 μL and monitored for target analytes ionized in positive mode via ESI. Cycle time was adjusted to 200 ms for the dynamic MRM acquisition mode.
2.2. Nontarget mass spectrometry
Nontarget analysis was carried out using the above chromatographic separations ported to a Dionex Ultimate 3000 RS HPLC system (Thermo Scientific, Waltham, Massachusetts, USA) coupled with a Thermo Scientific Q Exactive™ Focus Hybrid Quadrupole-Orbitrap™ Mass Spectrometer. An injection volume of 20 μL and a flow rate of 0.5 mL min−1 was used in both separation methods. The first minute of each separation was diverted to waste to keep the detector free of salts. All samples were run in triplicate. Eluting analytes were ionized using heated electrospray ionization (HESI) with a spay voltage of 4000 V and a capillary temperature of 350 °C. The spectrometer was operated in positive full-scan mode with spectra ranging from 200 to 1200 m/z and 150 to 1000 m/z for RPLC and HILIC separations, respectively. The full width at half maximum resolution was set to 70,000 with the automatic gain control (AGC) at 1×106. After nontarget analysis, samples were rerun on the Q Exactive™ with an inclusion list using confirmation mode (dd-MS2). MS2 spectra were collected with a resolution of 17,500 m/z with a 1.0 m/z isolation window. A stepped normalized collision energy (NCE) of 30, 60, and 90 was used with the AGC set to 2×104. To minimize data size, all spectra were collected in centroid mode.
Nontarget data was analyzed using the open-source software Mzmine 2 version 2.32 [27, 28]. The general Mzmine 2 nontarget workflow consists of five steps: mass detection, chromatogram builder, chromatogram deconvolution, retention time normalization, peak list alignment. The complete Mzmine 2 nontarget workflow used in the analysis of environmental water samples is shown in Table S2. A comprehensive custom database containing exact masses and common salt adducts (adapted from Bogialli et al. 2017 [29]) was used to screen samples. The database contains 366 compounds including the targeted cyanotoxins and is provided as an excel file in the supplemental material. MS2 data and the custom database were used to acquire nontarget data; however, settings were optimized for peak lists before further analysis was done. The open source software Mzmine 2 provides superior control over peak list settings, and future nontarget analyses should modify the settings (Table S2) to fit the chromatographic data. The minimum peak height and minimum time span in the chromatogram builder setting control the number of features present in a peak list. This is important for minimizing false positives, and even after background correction peak lists contained approximately 20,000 different features. Peak lists were filtered using the following criteria: 1) features for a specific sample must be present in the triplicate LC-MS runs and 2) features must contain at least 2 m/z peaks in the isotope pattern. After filtering and database screening, approximately 50 to 100 features were left depending on the given sample. The remaining features were manually inspected and added to the inclusion list for further confirmation measurements using the Q Exactive™ if the isotope patterns were within 10 ppm of the theoretical patterns.
2.3. Solid phase extraction (SPE)
Due to the variety of physiochemical properties that cyanotoxins possess, isolation of target analytes from water was split into two SPE extractions methods, corresponding to the HILIC or RPLC separation methods. SPE extraction methodology and optimization were developed from a previously reported study [15], with modifications for individual SPE extraction and the incorporation of SAX. Water samples were filtered through two filters: a glass fiber prefilter (1.0-μm pore size, 47 mm, Pall Corporation, Port Washington, NY, USA) and a nitrocellulose filter (0.45-μm pore size, 47 mm, GE Healthcare, Little Chalfont, BUX, UK). 2 Ls were separated into 2 – 1 L volumetric flasks for extraction. ANA, CLD, and SAX were extracted on a Supelclean ENVI-carb (6cc, 500mg, Supelco Inc., Bellefonte, PA, USA). MCs and NOD were extracted using an Oasis HLB (6 cc, 200 mg, Waters Corporation, Milford, MA, USA). Prior to extraction all samples were spiked with 100 μg L−1 of corresponding Iss. HLB cartridges were pretreated with 6 mL of MeOH and 6 mL of nanopure water. ENVI-carb cartridges were pretreated with 6 mL of DCM, 6 mL of MeOH, and 6 mL of nanopure H2O (Barnstead™ Nanopure™). 1 L of sample was loaded on each cartridge separately at ~10 mL/min (visible dripping) via a 24 port Visiprep™ vacuum manifold (Supelco Inc., Bellefonte, PA, USA). Cartridges were then blown dry under nitrogen. HLB cartridges were eluted with 10 mL of MeOH (0.5% formic acid) into 20 mL test tubes. ENVI-carb cartridges were eluted with 10 mL 60:40 (v v−1) MeOH:DCM (0.5% formic acid) into 20 mL test tubes. Eluates were blown to dryness under a gentle stream of nitrogen in a Turbovap (Zynmark, Hopkinton, MA, USA) set to 55°C. HLB samples were reconstituted with 1 mL 90:10 (v v−1) H2O:MeCN buffered with 5mM NH4OOCH and 3.6 mM HCOOH (pH 3.7). ENVI-carb samples were reconstituted with 1 mL 10:90 (v v−1) H2O:MeCN buffered with 5mM NH4OOCH and 3.6 mM HCOOH (pH 3.7). All reconstituted samples were syringe filtered using a BD 1 mL TB syringe (BD, Franklin Lakes, NJ, USA) and Acrodisc® hydrophobic Teflon Supor membrane syringe filters (13-mm diameter; 0.2-μm pore size, Pall Corporation, Port Washington, NY, USA) and placed in 2 mL analytical vials (Agilent Technologies, Santa Clara, CA, USA) for analysis.
2.4. Fish tissue extraction
Similar to water extractions, isolation of target analytes from fish tissue was split into two extractions methods, corresponding to the HILIC or RPLC separation methods. Fish whole body homogenates were prepared for extraction by grinding frozen samples to a paste. Two – 1g w/w aliquots were placed in separate preweighed 20 mL borosilicate glass vial (Wheaton; VWR Scientific, Rockwood, TN, USA). Samples were lyophilized for 72 hours using a VirTis BenchTop Pro freeze dryer (SP Scientific, Garnider, NY, USA) and the dry weight of each sample was determined. Samples were then spiked with 100 μg kg−1 of corresponding Iss. ANA, CLD, and SAX were extracted with 10 mL of 25:75 (v v−1) MeCN:NP water added to each vial. MCs and NOD were extracted using 10 mL of 75:25 (v v−1) MeCN:aqueous 0.1% formic acid added to each vial. Vials were inverted by hand for 30 seconds to mix the contents prior to sonicating for 30 minutes on a CPHX ultrasonicator (Fisher Scientific, Fair Lawn, NJ, USA). Samples were then placed on a rotating table at 40 rpm for 30 minutes. After rotation, samples were centrifuged at 25,000 rpm for 20 minutes using an Avanti JXN-26 centrifuge (Beckman Coulter, Brea, CA, USA). Following centrifugation, supernatant was collected, and syringe filtered using a Kendall monoject 6 mL luer lock syringe (Tyco, Milwaukee, WI, USA) and Whatman puradisc 25 polypropylene filter (0.45-μm pore size, 47 mm, GE Healthcare, Little Chalfont, BUX, UK). Supernatants were blown down under a gentle stream of nitrogen in a Turbovap (Zynmark, Hopkinton, MA, USA) set to 55°C for 1 hour to remove the organic solvent (MeCN). Samples were then diluted to 20 mL with nanopure water and loaded to SPE cartridges following the same protocols described above for water extractions.
2.5. Extraction recovery
To calculate absolute extraction recoveries two groups of samples were prepared for each matrix. Group 1 samples were spiked with corresponding IS and each target analyte for the given extraction method, while Group 2 samples were not spiked. Both groups were carried through complete and identical extraction procedures. Following reconstitution, group 2 samples were spiked with each target analyte and IS at the same concentration previously used in group 1 samples. Samples were analyzed, and absolute recoveries were calculated using the following equation:
where AX1, AIS1, AX2, and AIS2 are the peak areas of the analyte (X) and internal standard (IS) for groups 1 and 2, respectively.
2.6. Analysis of environmental samples
The developed method was applied to water and fish samples collected during two seven-day caged fish studies executed in Lake Waco, Waco, Texas, USA during September (19th-26th) 2017 and January (7th-14th) 2018. Naïve channel catfish (Ictalurus punctatus) housed at Baylor University were caged in a sheltered cove (31°35’40.48”N, 97°13’54.41”W). Caged fish were sampled every 24 hours (n=3) by anesthetization using MS-222, bagged onsite, then frozen at Baylor University until analysis. Duplicate water samples for targeted cyanotoxins analysis were collected using acetone cleaned 4 L amber glass bottles. Water samples were split into two 1 L subsamples where one sample (filtered) was filtered immediately, representing freely available dissolved toxins, and the second sample (seston) was frozen and thawed three times to lyse algal cells present in the sample, representing intracellular toxins [30]. Nontarget analysis utilizing the same sample preparation and chromatographic separations, was developed to screen environmental water samples for cyanotoxins not present in the targeted method. Analysis software for nontarget applications follow similar workflows and ultimately provide a list of features (peak lists) that are ran against chemical databases to provide the chemical formulas and names. Typically, large databases (e.g. ChemSpider or PubChem) provide more than one compound per feature in a peak list. Specific custom databases or further confirmation via MS2 can reduce the number of suspected compounds per given feature and has been used in previous cyanotoxin nontarget studies [29, 30].
3. Results and Discussion
3.1. LC-MS/MS methodology
Compound specific mass spectrometry parameters were automatically determined using MassHunter Optimizer (Agilent Technologies, Santa Clara, CA, USA) by flow injection analysis. Optimized MS/MS transitions and instrument parameters are provided in Table 1. Typically, MCs containing a single arginine residue will form [M+H]+ precursor ions at the arginine moiety [31]. Similarly, M-RR will form [M+2H]2+ precursor ions due to the presence of two arginine residues [32]. In contrast, MCs without an arginine residue typically form [M+H]+ precursors ions where protonation occurs on the methoxy group present within the ADDA side chain [32, 33]. However, in the current study, the doubly protonated precursor ions [M+2H]2+ for M-LR, M-LY, M-RR, M-YR, M-LR-15N, M-RR-15N, and M-YR-15N were found to be the most abundant, while the single protonated [M+H]+ precursor was most abundant for M-LA and M-LA-15N, and NOD. The observed precursor ions used in this work are in agreement with those observed in previous studies [32–36]. For all MCs and NOD the most abundant fragment ion, used as the quantitation ion, was m/z 135, except for M-LA where m/z 776 was the most abundant fragment. The qualification ion employed for all MCs and NOD was m/z 103, except for M-LA where m/z 135 was used. Observed formation of [M+2H]2+ precursor ions for M-LR and M-YR is not surprising due to the ubiquitous use of ammonium formate, added to the mobile phases, which actively minimized sodium replacement ions causing an increased abundance of the doubly protonated species [32, 35, 37]. M-LR and M-YR form [M+2H]2+ precursor ion when protonation occurs on both the arginine residue and methoxy residue of the ADDA side chain [32]. Further, previous studies have demonstrated the utility of selecting the unconventional [M+2H]2+ precursor for M-LR, M-RR, and M-YR noting that a higher abundance of m/z 135 fragments are produced, resulting in lower overall detection limits [32, 34]. Formation of the [M+2H]2+ precursor ion for M-LY is surprising and suggests a second protonation site other than the methoxy residue of the ADDA side chain [33]. ANA formed a [M+H]+ precursor ion of m/z 166 with major fragment ions of m/z 131, used as the quantitation ion, and m/z 91, employed as the qualification ion. The [M+H]+ precursor ion of m/z 416 formed for CLD and produced fragment ions of m/z 194, used as the quantitation ion, and m/z 176, for the qualification ion. The [M+H]+ precursor ion of m/z 300 was observed for SAX and major fragment ions of m/z 204, employed as the quantitation ion, and m/z 138, used as the qualification ion, were produced.
Table 1.
Target analyte mass spectrometry parameters
| Method | Analyte | Formula | Precurser ion (m/z) | Product ions (m/z) | Fragmentor (V) | Collision Energy (V) | tR(mins) | |
|---|---|---|---|---|---|---|---|---|
| HILIC | ANA | C10H15NO | 166 | [M+H]+ | 91.1, 131 | 103 | 29, 17 | 1.5 |
| ANA-13C (IS) | C413C6H15NO | 170 | [M+H]+ | 93.1, 135 | ||||
| CLD | C15H21N5O7S | 416 | [M+H]+ | 194.1, 176 | 152 | 37, 41 | 4.0 | |
| CLD-15N (IS) | C15H2115N5O7S | 421 | [M+H]+ | 197.1, 176 | ||||
| SAX | C10Hi7N7O4 | 300 | [M+H]+ | 204.1, 138 | 152 | 25, 33 | 5.2 | |
| DPA-d5 (IS) | C9D5H6NO2 | 171 | [M+H]+ | 125 | 103 | 17 | 2.0 | |
| RPLC | M-LA | C46H67N7Ol2 | 911 | [M+H]+ | 776.4, 135 | 200 | 21, 69 | 5.3 |
| M-LA-15N (IS) | C46H6715N7O12 | 917 | 783.4, 135 | |||||
| M-LR | C49H74N10O12 | 498 | [M+2H]2+ | 135.1, 103 | 103 | 9, 69 | 3.7 | |
| M-LR-15N (IS) | C49H7415Nl0O12 | 503 | ||||||
| M-LY | C52H71N7O13 | 502 | [M+2H]2+ | 135.1, 103 | 103 | 13, 69 | 5.7 | |
| M-RR | C49H75N13O12 | 520 | [M+2H]2+ | 135.1, 103 | 152 | 33, 69 | 2.5 | |
| M-RR-15N (IS) | C49H7515N13O12 | 526 | ||||||
| M-YR | C52H72N10Ol3 | 523 | [M+2H]2+ | 135.1, 103 | 103 | 13, 69 | 3.4 | |
| M-YR-15N (IS) | C52H7215N10O13 | 528 | ||||||
| NOD | Q41H60NO10 | 825 | [M+H]+ | 135.1, 103 | 201 | 69, 137 | 2.4 | |
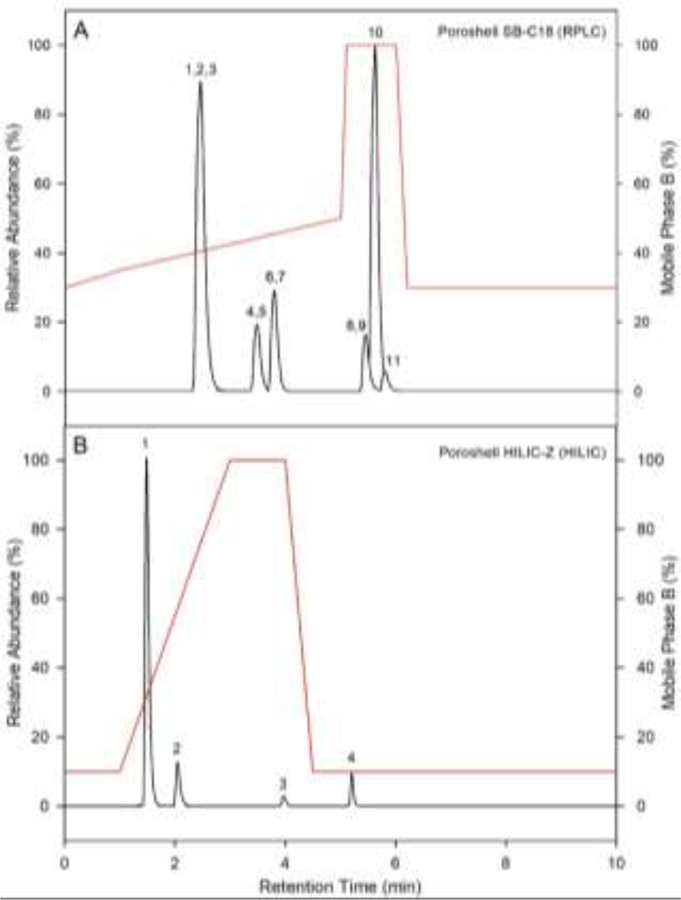
Separation of MCs and NOD was achieved using RPLC (Figure 1A), with retention order similar to previous reports [11, 14, 15, 19, 26, 31, 35]. Overlap of M-RR and NOD does not interfere with positive identification of either compound due the large difference in parent ion mass. Further, observation of overlapping retention times for MC 15N Iss with unlabeled counterparts demonstrates minimalized isotope effect (Figure S1), consistent with previous studies reporting reduced isotope effect from 15N labeled IS [38]. Initial development of a HILIC separation method was performed on a TSKgel amide-80 due to previous reports and used for ANA, ANA-13C, CLD, CLD-15N, and SAX [16, 19–23, 39, 40]. Complete separation of ANA and ANA-13C, DPA-d5, CLD and CLD-15N, and SAX was achieved using HILIC (Figure S2), solving the issue of ANA and DPA coelution [13, 18], and providing excellent retention of the polar cyanotoxins, similar to previous results [16, 19–22]. Further, in agreement with previous work, the current study found that target analyte peak shape and retention when using HILIC were greatly affected by mobile phase buffer conditions [16]; thus, a previously reported optimized buffer with 5mM NH4OOCH and 3.6 mM HCOOH (pH 3.7) was utilized [19].
Figure 1.
LC-MS/MS total ion chromatograms for compounds separated by the RPLC method on the Agilent Poroshell SB-C18 (A) and the HILIC method on the Agilent Poroshell HILIC-Z (B), monitored in a 10 ng mL−1 standard solution. Peak identifications for the RPLC method (A) are as follows: (1) microcystin-RR, (2) microcystin-RR-15N13, (3) nodularin, (4) microcystin-YR, (5) microcystin-YR-15N10, (6) microcystin-LR, (7) microcystin-LR-15N10, (8) microcystin-LA, (9) microcystin-LR-15N7, (10) clarithromycin-d3, (11) microcystin-LY. Peak identifications for the HILIC method (B) are as follows: (1) anatoxin-a & anatoxin-a-13C4 (2) D-phenylalanine-d5, (3) cylindrospermopsin & cylindrospermopsin-15N5 (4) saxitoxin. The red line represents the mobile phase gradient conditions in percentage of the strong solvent B for each separation respectively.
Whereas excellent separation was observed on the TSKgel amide-80, amide HILIC columns are designed to target polar compounds with hydroxyl groups (weak acids). However, ANA and SAX have ionizable amine groups [17, 39], while CLD is zwitterionic at relevant pH [4]. Therefore, the use of a zwitterionic HILIC column may improve retention, method sensitivity, matrix interference, and separation of the polar cyanotoxins [23]. This has previously been demonstrated for SAX [41], though no such studies have been performed for ANA or CLD to date [23]. Recently, a new zwitterionic HILIC column, the Agilent Poroshell HILIC-Z, which demonstrated excellent separation of small polar molecules [42], became commercially available and was tested using the current method. Complete separation of all target analytes was observed on the HILIC-Z (Figure 1B & S3).
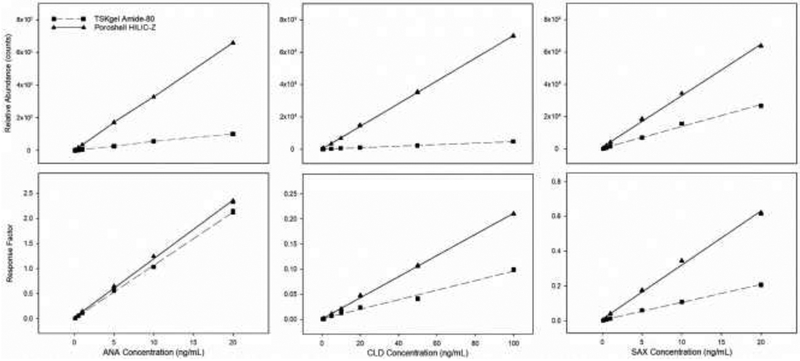
To evaluate analytical differences between each column calibration curves for the TSKgel amide-80 and HILIC-Z are plotted side by side using target analyte peak areas and response factors (RF). Differences in the slope are statistically evaluated as a measure of sensitivity (Figure 2) [24, 25]. The HILIC-Z produced greater peak areas for all target analytes along the range of the calibration curve and calibration slopes were significantly (p<0.05) greater on the HILIC-Z, resulting in greater method sensitivity. Interestingly, slope of the RF calibration curves is significantly (p<0.05) greater for ANA and CLD, but significantly (p<0.05) lower for SAX. The observed decrease in slope for the SAX RF calibration curve is due to a 6-fold increase in the abundance of DPA-d5 on the HILIC-Z column, while SAX abundance only increased 2.5-fold. Likewise, abundance of ANA increased 6-fold and CLD increased 11-fold, resulting in the observed differences in RF calibration slope.
Figure 2.
Comparison of calibration curves across the linear range for ANA, CLD, and SAX on the TSKgel Amide-80 and Poroshell HILIC-Z.
3.2. Solid phase extraction
The current study provides the first report of matched IS corrected recoveries for ANA, CLD, MCs, and NOD. Mean recoveries for MCs and NOD on the Oasis HLB ranging from 73–98% (Table 2). Recoveries of MCs and NOD in the current study were in agreement with previous reports [14, 15, 31] demonstrating high recoveries when using the Oasis HLB. Mean recoveries on the ENVI-carb were 53% for SAX, 90% for CLD, 97% for ANA (Table 2) and 80% (± 4.4) for DPA-d5 (IS). Recoveries of CLD in the current study are similar to previous reports that used graphitic carbon SPE [14, 15, 26]. Higher recoveries of ANA and some MCs from water samples which have been modified to pH 10.5 have been previously reported with no IS correction [15]. In the current study, filtered surface water samples from local Texas streams and lakes were pH modified and a soapy residue formed in the sample due to the high organic content. This material clogged SPE cartridges and produced unacceptable recoveries. Use of IS correction for recovery bias was tested by comparing water samples modified to pH 10.5 without IS correction and unmodified water samples with IS correction in laboratory water. Similar results were obtained. Thus, extraction of water was carried out under neutral conditions with IS correction. Higher ANA recoveries in the current method extracted at neutral conditions are attributed to IS correction. Recoveries of SAX utilizing current methods represent a useful first step toward development of greater all-in-one extraction protocols.
Table 2.
Validation data for target analytes in water and fish tissue.
| Linear Range | Correlation | Recovery (%, CV) | Precision (%, CV) | Detection Limits (pg) | MDL | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | (ngmL−1) | Coefficient (r2) | Water | Fish | Water | Fish | LOD | LOQ | Water (ng L−1) | Fish (μg kg−1) |
| ANA | 0.1–20 | 0.999 | 97 (1.2) | 103 (1.3) | 1.9 | 3.4 | 4.0 | 10 | 0.08 | 0.14 |
| CLD | 0.5–100 | 0.998 | 90 (2.6) | 90 (1.8) | 5.0 | 8.2 | 70 | 230 | 0.43 | 0.12 |
| SAX | 0.1–20 | 0.995 | 53 (2.7) | 45 (4.4) | 1.6 | 5.6 | 10 | 40 | 0.22 | 0.04 |
| M-LA | 0.5–100 | 0.998 | 97 (1.8) | 96 (5.7) | 0.8 | 5.7 | 20 | 70 | 0.60 | 0.61 |
| M-LR | 0.1–100 | 0.998 | 94 (2.4) | 97 (5.1) | 0.6 | 5.1 | 40 | 130 | 0.38 | 0.31 |
| M-LY | 0.5–100 | 0.998 | 73 (7.3) | 75 (7.1) | 7.2 | 4.7 | 40 | 140 | 0.83 | 0.35 |
| M-RR | 0.1–100 | 0.999 | 97 (3.0) | 90 (5.9) | 2.4 | 6.3 | 60 | 220 | 0.91 | 0.28 |
| M-YR | 0.5–100 | 0.999 | 93 (4.6) | 77 (4.7) | 3.9 | 1.2 | 80 | 280 | 0.80 | 0.70 |
| NOD | 0.5–100 | 0.996 | 98 (3.1) | 90 (7.9) | 2.9 | 4.7 | 50 | 180 | 0.96 | 0.57 |
Limit of detection (LOD) was defined as LOD = (3σ/b) where σ is the standard deviation of the quantified value from replicate blank samples (n=8), and b is the slope of the calibration for the target analyte. Limit of quantification (LOQ) was defined as LOQ = (10σ/b). Method detection limits (MDL) were defined as MDL = t (n-1,0.99) × SD, where t (n-1,0.99) is the one-sided Student’s t-statistic at the 99% confidence limit for n-1 degrees of freedom, (2.998 for n = 8), and SD is the standard deviation of replicate spiked matrix sample (spiking level ≤ 10 × MDL).
3.3. Fish tissue extraction
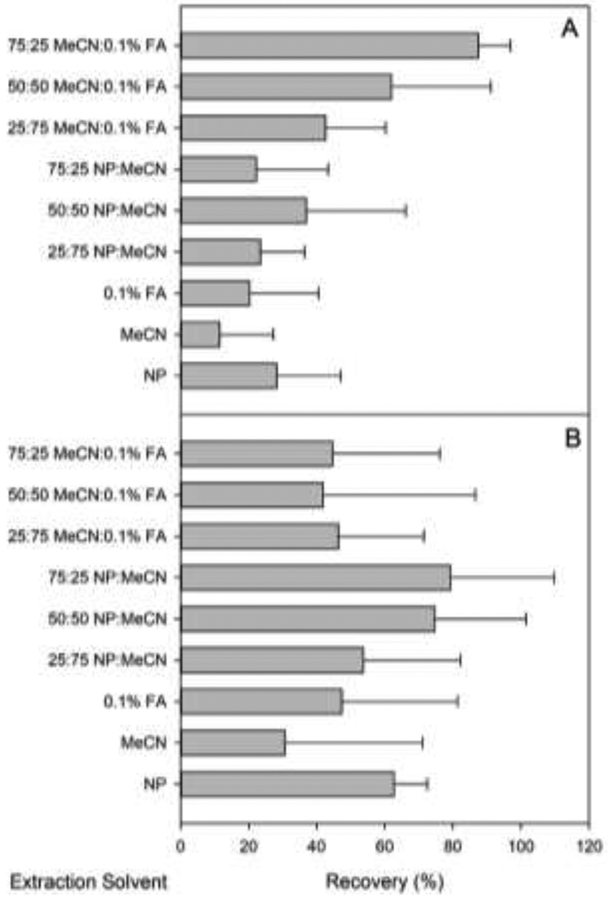
The large variation in pKa and lipophilicity of cyanotoxins led to a systematic study of extraction behaviors using different solvent systems to optimize extraction from fish tissue. Varying ratios of MeCN, nanopure water, and aqueous 0.1% formic acid were tested side by side. Mean recoveries (n = 3) for each solvent system are given in Table S1. Individual target analyte recoveries were averaged for each method and plotted for comparison (Figure 3). Data in each graph have not been statistically examined but provide a convenient visual metric for comparing overall solvent performance, where the most effective solvents are those displaying a maximum recovery and minimum error. Error bars represent the standard deviation from the mean of recoveries and provide an assessment of variability among mean recoveries for individual analytes. Recoveries for MCs and NOD were highest in solvent systems that utilized a mixture of MeCN and aqueous 0.1% formic acid, with the overall highest recoveries observed for 75:25 MeCN:aqueous 0.1% formic acid ranging from 75% to 97%, in agreement with previous reports utilizing the same or similar solvent systems [12, 13, 44]. Recoveries for ANA, CLD, and SAX were highest using 75:25 nanopure:MeCN ranging from 45% to 103%, with recovery of DPA-d5 measured at 92 ± 2.3%. Whereas a higher recovery for SAX (99%) was observed using 50:50 nanopure:MeCN (Table S1), 75:25 nanopure:MeCN was used because of higher overall recoveries for ANA and CLD.
Figure 3.
Average recoveries for extraction of ANA, CLD, and SAX (A) and M-LA, M-LR, M-LY M-RR, M-YR, and NOD (B) from clean whole-body homogenates of fish tissue. Extraction solvents were prepared by combining the noted ratios of solvents in a binary mixture equal to 10 mL.
3.4. Matrix effect
Absolute matrix effect was quantified by the addition of standards to post-extraction samples. Water and fish tissue were carried through the extraction procedures detailed above then spiked with both internal standard and target analytes immediately prior to analysis. Peak areas of target analytes from post-extraction samples and standard solution were compared to calculate absolute matrix effect. To examine IS compensation for matrix effect, matched 15N labeled CLD, M-LA, M-LR, M-RR, and M-YR were used for CLD, MCs, and NOD, while 13C labeled ANA was used for ANA. Relative matrix effect (IS corrected) was calculated from an internal standard calibration curve. Absolute matrix effect and relative matrix effect for water and fish tissue (n = 3) are presented in Table 3, where positive values indicate ion enhancement while negative values indicate ion suppression. No pair matched 15N standards were available for MLY or NOD, however M-LA-15N was used for M-LY and M-RR-15N was used for NOD with acceptable results. An ion suppression ranging from −22% to −77% was observed for MCs and NOD in water, with IS corrections showing improvement where ion suppression and enhancement range from −4.2% to +5.4%. Similar to water, ion suppression for fish tissue ranged from −26% to −58%, which was improved with IS correction, where relative values ranged from −17% to +17%. Percent CV of relative matrix values for water and fish tissue are all <15% demonstrating acceptable reproducibility. ANA, CLD, and SAX showed ion suppression in water ranging from −44% to −50%, with IS correction showing improvement ranging from −16% to +10%. Percent CV was <15% for ANA CLD, and SAX. The greatest ion suppression was observed for ANA, CLD, and SAX in fish tissue ranging from −60% to −81%. Ion suppression was corrected to ion enhancement of +1.4 for ANA and +3.4 for CLD with a CV of 6.7% for ANA and 18% for CLD. Ion enhancement was observed for SAX (+26%) when DPA-d5 was used to correct for matrix effect, with percent CV of 46%. While DPA-d5 is structurally similar to ANA[17], use as an IS to correct for matrix effect in SAX may be limited, but at present represents an acceptable option until commercially available isotopically labeled standards are available.
Table 3.
Absolute and relative (IS corrected) matrix effect (n=3) for target analytes in water and fish tissue.
| Absolute matrix effect (%, CV) | Relative matrix effect (%, CV) | |||
|---|---|---|---|---|
| Analyte | Water | Fish | Water | Fish |
| ANA | −50 (12) | −81 (15) | −3.4 (14) | +1.4 (6.7) |
| CLD | −45 (23) | −61 (27) | +1.0 (14) | +3.4 (18) |
| SAX | −44 (14) | −60 (7.0) | −16 (14) | +26 (46) |
| M-LA | −55 (18) | −53 (41) | +2.9 (12) | +2.5 (5.1) |
| M-LR | −45 (11) | −49 (36) | +5.4 (12) | −1.3 (4.4) |
| M-LY | −77 (28) | −50 (45) | −4.2 (10) | −17 (7.8) |
| M-RR | −35 (6.0) | −26 (33) | +0.5 (11) | −2.5 (2.3) |
| M-YR | −48 (12) | −58 (36) | +5.3 (13) | + 17 (7.2) |
| NOD | −22 (7.0) | −49 (24) | +3.6 (10) | +16 (2.7) |
3.5. Method validation
Method validation results are presented in Table 2. Method linearity and range of measurement for each target analyte was examined with an eight-point internal standard calibration curves ranging from 0.1–100 ng mL−1, run in triplicate. Linear range was confirmed with plots of sensitivity (i.e., relative response factor; RRF) versus analyte concentration. Our criterion for linearity required that the percent CV of RRFs spanning the calibration range was ≤ 15%. Linear range calibration data were fit to a linear regression with a minimum of six points to determine correlation coefficients (r2), which were greater than ≥0.995 for all target analytes. Method precision was evaluated in terms of repeatability calculated as the percent CV of the quantified values in spiked water (10 ng L−1) and fish tissue (10 μg kg−1) replicates (n=3), and were <9.0% for all target analytes indicating excellent reproducibility in water and fish tissue. Method trueness was measured as the IS corrected absolute recovery (% ± CV) in spiked water (10 ng L−1) and fish tissue (10 μg kg−1) replicates (n=3), with the results discussed previously. Method LODs ranged from 4.0 to 80 pg (0.004–0.08 ng mL−1), LOQs ranged from 10 to 280 pg (0.01–0.28 ng mL−1), and MDLs ranged from 80 to 960 pg (0.08–0.96 ng L−1) in water and 120 to 700 pg (0.12–0.70 μg kg−1) in fish tissue. While LOD and LOQ are recognized performance metrics, MDL is more appropriate as a threshold in environmental analyses [45]. LOD and LOQ are calculated from laboratory blank samples, while MDL is derived from replicate matrix spikes following an accepted US EPA regulatory protocol (40 CFR Part 136, Appendix B). In most cases, matrix specific MDLs for all target analytes were an order of magnitude higher than reported LODs or LOQs demonstrating the effect sample matrix and the extraction process has on detection of target analytes. Using LOD or LOQ values developed in laboratory blanks as a reporting threshold could lead to the reporting of questionable environmental detections. Thus, MDLs were used in the current study as the detection and quantitation threshold for reporting cyanotoxin concentrations in water and fish tissue.
3.6. Analysis of environmental samples
Only M-LA and M-LR were detected in all water samples from both sampling periods (Table 4); however, no cyanotoxins were observed in fish tissue. As expected, concentrations of M-LA and M-LR were higher in water samples subjected to freezing and thawing to release bound intercellular components. M-LA levels were higher in September than in January, when conditions for cyanobacterial growth were predicted to be optimal. Surprisingly, M-LR concentrations did not differ to a high degree from September to January in either filtered or seston water samples. Two MCs not present in the targeted analysis were identified in the September seston samples (Table 5). The m/z 103 and 135 peaks in MS2 spectra were used for confirmation of MCs. Further, the nontarget method identified MC-LR in September and January samples as expected from the results from the targeted method. However, due to lower instrument sensitivity, MC-LA was identified by the nontarget method when the minimum peak height setting was adjusted to a lower value. However, this produced more features in the peak lists which lead to more false positives. To avoid this the minimum peak height was set to 1000, which did not allow for a positive identification of MC-LA given the parameters specified in Table S2. There were no observed features in the nontarget method using the HILIC separation.
Table 4.
Concentrations of target analytes detected in water samples collected during September and January from Lake Waco, Waco, TX, USA.
| M-LA (ng L−1) | M-LR (ng L−1) | |||
|---|---|---|---|---|
| Sample Type | range | mean | range | mean |
| September filtered | 7.0–26 | 13 | 11–38 | 23 |
| September seston | 43–81 | 59 | 43–120 | 86 |
| January filtered | 0.5–6.3 | 3.5 | 4.0–27 | 13 |
| January seston | 2.3–28 | 17 | 10–160 | 92 |
Table 5.
List of nontarget compounds found in environmental water samples.
| Compound | Adduct | m/z | RT (min) | Sample Type |
|---|---|---|---|---|
| MC-LR | [M+H]+ | 995.5553 | 3.56 | SF, SS, JS |
| MC-LM | [M+H]+ | 970.4951 | 5.84 | SS |
| MC-LL | [M+H]+ | 952.5385 | 6.78 | SS |
SS – September seston; SF – September filtered; JS – January seston
In the current study clean fish from laboratory cultures were caged in Lake Waco and subsampled daily to examine potential uptake of cyanotoxins detected in the filtered and seston water samples. It is not surprising that cyanotoxins were not detected in the fish samples because the major route of exposure for aquatic organisms to MCs is via diet [46], with very little accumulation expected from freely dissolved MCs [47]. Fish were caged in wire mesh cages, partially exposed to the sediment, to allow for the free movement of water and potential feeding of fish on native organisms. However, the current study was conducted for 7 days, while previous caged fish studies in natural environments were conducted for 60 days and even then bioconcentration factors of MCs in fish ranged from 0.6 to 13.3 [48] with elimination half-life calculated to be 0.7 to 8.4 days−1. The findings of the current study reinforce previous observations of diet as the major route of exposure for MCs to fish [46] because no uptake of M-LA or M-LR was measured in fish, though water concentrations were similar to a recent study conducted in aquaculture ponds from Southeast Asia, where accumulation of MCs in fish was observed [13]. It is also important to note that these improved separation and isotope dilution techniques can be extended to other congeners in the future and further represent a launching point for more complex, non-targeted analyses, with preliminary targeted screening in fish tissue.
Supplementary Material
Highlights:
Optimized extraction and separation of cyanotoxins for water and fish tissue.
Novel use of a zwitterionic hydrophilic interaction liquid chromatography column.
Novel characterization of matrix effect using isotopically labeled standards.
Targeted and nontargeted analysis using the same extraction and separation methods.
Novel data mining techniques for use with nontargeted screening.
Acknowledgement
Funding for this work was provided by the United States Department of Agriculture (USDA), National Institute of Food and Agriculture (NIFA) (#20166900725093) to JLC and BWB and the National Institute of Environmental Health Science (NIEHS) (#1P01ES028942–01) to BWB. We thank Agilent Technologies for providing the Poroshell HILIC-Z column used in the current study, and Gavin Saari, Bekah Burket, Baylor Steele and Bridgett Hill for aid maintaining channel catfish cultures. Additional thanks to Dr. Alejandro Ramirez and the Baylor University Mass Spectrometry Core Facility for technical support, training, and aid with instrument maintenance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Merel S, Walker D, Chicana R, Snyder S, Baures E, Thomas O, State of knowledge and concerns on cyanobacterial blooms and cyanotoxins, Environ. Int, 59 (2013) 303–327. [DOI] [PubMed] [Google Scholar]
- [2].Buratti FM, Manganelli M, Vichi S, Stefanelli M, Scardala S, Testai E, Funari E, Cyanotoxins: producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation, Arch. Toxicol, 91 (2017) 1049–1130. [DOI] [PubMed] [Google Scholar]
- [3].Pearson LA, Dittmann E, Mazmouz R, Ongley SE, D’Agostino PM, Neilan BA, The genetics, biosynthesis and regulation of toxic specialized metabolites of cyanobacteria, Harmful Algae, 54 (2016) 98–111. [DOI] [PubMed] [Google Scholar]
- [4].de la Cruz AA, Hiskia A, Kaloudis T, Chernoff N, Hill D, Antoniou MG, He X, Loftin K, O’Shea K, Zhao C, Pelaez M, Han C, Lynch TJ, Dionysiou DD, A review on cylindrospermopsin: the global occurrence, detection, toxicity and degradation of a potent cyanotoxin, Environmental Science: Processes & Impacts, 15 (2013) 1979–2003. [DOI] [PubMed] [Google Scholar]
- [5].Brooks BW, Lazorchak JM, Howard MD, Johnson MV, Morton SL, Perkins DA, Reavie ED, Scott GI, Smith SA, Steevens JA, Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems?, Environ Toxicol Chem, 35 (2016) 6–13. [DOI] [PubMed] [Google Scholar]
- [6].Brooks BW, Lazorchak JM, Howard MDA, Johnson MVV, Morton SL, Perkins DAK, Reavie ED, Scott GI, Smith SA, Steevens JA, In some places, in some cases, and at some times, harmful algal blooms are the greatest threat to inland water quality, Environ. Toxicol. Chem, 36 (2017) 1125–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].WHO, Guidelines for drinking-water quality, World Health Organization, Geneva, Switzerland, 2011. [Google Scholar]
- [8].U.S.EPA, Revisions to the Unregulated Contaminant Monitoring Rule (UCMR 4) for public water systems and announcement of a public meeting. 40 CFR Part 121. Fed Reg 80:76897–76923. [cited 2018 April 7], https://federalregister.gov/a/2015-30824, (2015). [Google Scholar]
- [9].U.S.EPA, Drinking Water Contaminant Candidate List (CCL) and Regulatory Determination, https://www.epa.gov/ccl, (2016).
- [10].U.S.EPA, Human Health Recreational Ambient Water Quality Criteria or Swimming Advisories for Microcystins and Cylindrospermopsin. EPA 822-P-16–002. [cited 2018 April 7]. https://www.epa.gov/sites/production/files/2016-12/documents/draft-hh-rec-ambient-water-swimming-document.pdf, (2016).
- [11].Oehrle SA, Southwell B, Westrick J, Detection of various freshwater cyanobacterial toxins using ultra-performance liquid chromatography tandem mass spectrometry, Toxicon, 55 (2010) 965–972. [DOI] [PubMed] [Google Scholar]
- [12].Chrıstophorıdıs C, Argyropoulos I, Mpampourıs V, Kaloudıs T, Trıantıs TM, Hıskıa A, Analysis of multi-class cyanotoxins in fish tissue. Application to fish from Greek lakes, in: 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 2017. [Google Scholar]
- [13].Greer B, Maul R, Campbell K, Elliott CT, Detection of freshwater cyanotoxins and measurement of masked microcystins in tilapia from Southeast Asian aquaculture farms, Anal. Bioanal. Chem, 409 (2017) 4057–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Greer B, McNamee SE, Boots B, Cimarelli L, Guillebault D, Helmi K, Marcheggiani S, Panaiotov S, Breitenbach U, Akçaalan R, Medlin LK, Kittler K, Elliott CT, Campbell K, A validated UPLC–MS/MS method for the surveillance of ten aquatic biotoxins in European brackish and freshwater systems, Harmful Algae, 55 (2016) 31–40. [DOI] [PubMed] [Google Scholar]
- [15].Zervou SK, Christophoridis C, Kaloudis T, Triantis TM, Hiskia A, New SPE-LC MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins, J. Hazard. Mater, 323 (2017) 56–66. [DOI] [PubMed] [Google Scholar]
- [16].Dell’Aversano C, Eaglesham GK, Quilliam MA, Analysis of cyanobacterial toxins by hydrophilic interaction liquid chromatography–mass spectrometry, J. Chromatogr, 1028 (2004) 155–164. [DOI] [PubMed] [Google Scholar]
- [17].Dimitrakopoulos IK, Kaloudis TS, Hiskia AE, Thomaidis NS, Koupparis MA, Development of a fast and selective method for the sensitive determination of anatoxin-a in lake waters using liquid chromatography-tandem mass spectrometry and phenylalanine-d5 as internal standard, Anal. Bioanal. Chem, 397 (2010) 2245–2252. [DOI] [PubMed] [Google Scholar]
- [18].Furey A, Crowley J, Hamilton B, Lehane M, James KJ, Strategies to avoid the mis-identification of anatoxin-a using mass spectrometry in the forensic investigation of acute neurotoxic poisoning, J. Chromatogr, 1082 (2005) 91–97. [DOI] [PubMed] [Google Scholar]
- [19].Lajeunesse A, Segura PA, Gelinas M, Hudon C, Thomas K, Quilliam MA, Gagnon C, Detection and confirmation of saxitoxin analogues in freshwater benthic Lyngbya wollei algae collected in the St. Lawrence River (Canada) by liquid chromatography-tandem mass spectrometry, J. Chromatogr, 1219 (2012) 93–103. [DOI] [PubMed] [Google Scholar]
- [20].Ballot A, Fastner J, Wiedner C, Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in northeast Germany, Appl. Environ. Microbiol, 76 (2010) 1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hollingdale C, Thomas K, Lewis N, Bekri K, McCarron P, Quilliam MA, Feasibility study on production of a matrix reference material for cyanobacterial toxins, Anal. Bioanal. Chem, 407 (2015) 5353–5363. [DOI] [PubMed] [Google Scholar]
- [22].Heussner AH, Mazija L, Fastner J, Dietrich DR, Toxin content and cytotoxicity of algal dietary supplements, Toxicol. Appl. Pharmacol, 265 (2012) 263–271. [DOI] [PubMed] [Google Scholar]
- [23].Salas D, Borrull F, Fontanals N, Marcé RM, Hydrophilic interaction liquid chromatography coupled to mass spectrometry-based detection to determine emerging organic contaminants in environmental samples, TrAC, Trends Anal. Chem, 94 (2017) 141–149. [Google Scholar]
- [24].Kruve A, Rebane R, Kipper K, Oldekop ML, Evard H, Herodes K, Ravio P, Leito I, Tutorial review on validation of liquid chromatography-mass spectrometry methods: part I, Anal. Chim. Acta, 870 (2015) 29–44. [DOI] [PubMed] [Google Scholar]
- [25].Kruve A, Rebane R, Kipper K, Oldekop ML, Evard H, Herodes K, Ravio P, Leito I, Tutorial review on validation of liquid chromatography-mass spectrometry methods: part II, Anal. Chim. Acta, 870 (2015) 8–28. [DOI] [PubMed] [Google Scholar]
- [26].Yen HK, Lin TF, Liao PC, Simultaneous detection of nine cyanotoxins in drinking water using dual solid-phase extraction and liquid chromatography-mass spectrometry, Toxicon, 58 (2011) 209–218. [DOI] [PubMed] [Google Scholar]
- [27].Pluskal T, Castillo S, Villar-Briones A, Oresic M, MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data, BMC Bioinformatics, 11 (2010) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Myers OD, Sumner SJ, Li SZ, Barnes S, Du XX, One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks, Anal. Chem, 89 (2017) 8696–8703. [DOI] [PubMed] [Google Scholar]
- [29].Bogialli S, Bortolini C, Di Gangi IM, Di Gregorio FN, Lucentini L, Favaro G, Pastore P, Liquid chromatography-high resolution mass spectrometric methods for the surveillance monitoring of cyanotoxins in freshwaters, Talanta, 170 (2017) 322–330. [DOI] [PubMed] [Google Scholar]
- [30].Ortiz X, Korenkova E, Jobst KJ, MacPherson KA, Reiner EJ, A high throughput targeted and non-targeted method for the analysis of microcystins and anatoxin-A using on-line solid phase extraction coupled to liquid chromatography-quadrupole time-of-flight high resolution mass spectrometry, Anal. Bioanal. Chem, 409 (2017) 4959–4969. [DOI] [PubMed] [Google Scholar]
- [31].Kaloudis T, Zervou SK, Tsimeli K, Triantis TM, Fotiou T, Hiskia A, Determination of microcystins and nodularin (cyanobacterial toxins) in water by LC-MS/MS. Monitoring of Lake Marathonas, a water reservoir of Athens, Greece, J. Hazard. Mater, 263 Pt 1 (2013) 105–115. [DOI] [PubMed] [Google Scholar]
- [32].Yuan M, Namikoshi M, Otsuki A, Rinehart KL, Sivonen K, Watanabe MF, Low-energy collisionally activated decomposition and structural characterization of cyclic heptapeptide microcystins by electrospray ionization mass spectrometry, J. Mass Spectrom, 34 (1999) 33–43. [DOI] [PubMed] [Google Scholar]
- [33].Hiller S, Krock B, Cembella A, Luckas B, Rapid detection of cyanobacterial toxins in precursor ion mode by liquid chromatography tandem mass spectrometry, J. Mass Spectrom, 42 (2007) 1238–1250. [DOI] [PubMed] [Google Scholar]
- [34].Li CM, Chu RY, Hsientang Hsieh DP, An enhanced LC-MS/MS method for microcystin-LR in lake water, J. Mass Spectrom, 41 (2006) 169–174. [DOI] [PubMed] [Google Scholar]
- [35].Draper WM, Xu D, Behniwal P, McKinney MJ, Jayalath P, Dhoot JS, Wijekoon D, Optimizing LC-MS-MS determination of microcystin toxins in natural water and drinking water supplies, Analytical Methods, 5 (2013) 6796. [Google Scholar]
- [36].Maizels M, Budde WL, A LC/MS method for the determination of cyanobacteria toxins in water, Anal. Chem, 76 (2004) 1342–1351. [DOI] [PubMed] [Google Scholar]
- [37].Draper WM, Xu D, Perera SK, Electrolyte-Induced Ionization Suppression and Microcystin Toxins: Ammonium Formate Suppresses Sodium Replacement Ions and Enhances Protiated and Ammoniated Ions for Improved Specificity in Quantitative LC-MS-MS, Anal. Chem, 81 (2009) 4153–4160. [DOI] [PubMed] [Google Scholar]
- [38].Szarka S, Prokai-Tatrai K, Prokai L, Application of screening experimental designs to assess chromatographic isotope effect upon isotope-coded derivatization for quantitative liquid chromatography-mass spectrometry, Anal. Chem, 86 (2014) 7033–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dell’Aversano C, Hess P, Quilliam MA, Hydrophilic interaction liquid chromatography–mass spectrometry for the analysis of paralytic shellfish poisoning (PSP) toxins, J. Chromatogr, 1081 (2005) 190–201. [DOI] [PubMed] [Google Scholar]
- [40].Kikuchi S, Kubo T, Kaya K, Cylindrospermopsin determination using 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES) as the internal standard, Anal. Chim. Acta, 583 (2007) 124–127. [DOI] [PubMed] [Google Scholar]
- [41].Diener M, Erler K, Christian B, Luckas B, Application of a new zwitterionic hydrophilic interaction chromatography column for determination of paralytic shellfish poisoning toxins, J. Sep. Sci, 30 (2007) 1821–1826. [DOI] [PubMed] [Google Scholar]
- [42].Huang Y, Li W, Keller A, Anumol T, Bivens Adam, Quantitative Analysis of Underivatized Amino Acids in Plant Matrix by Hydrophilic Interaction Chromatography (HILIC) with LC/MS Detection, in: Application Note, Agilent Technologies, Inc., https://www.agilent.com/cs/library/applications/5991-8922EN_Plant_Amino_Acids_Quant_Poroshell_120_HILICZ_Application.pdf, 2018. [Google Scholar]
- [43].Meriluoto J, Spoof L, Codd GA, Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis, Wiley, 2017. [Google Scholar]
- [44].Geis-Asteggiante L, Lehotay SJ, Fortis LL, Paoli G, Wijey C, Heinzen H, Development and validation of a rapid method for microcystins in fish and comparing LC MS/MS results with ELISA, Anal. Bioanal. Chem, 401 (2011) 2617–2630. [DOI] [PubMed] [Google Scholar]
- [45].Ramirez AJ, Mottaleb MA, Brooks BW, Chambliss CK, Analysis of pharmaceuticals in fish using liquid chromatography-tandem mass spectrometry, Anal. Chem, 79 (2007) 3155–3163. [DOI] [PubMed] [Google Scholar]
- [46].Ferrao-Filho Ada S, Kozlowsky-Suzuki B, Cyanotoxins: bioaccumulation and effects on aquatic animals, Mar. Drugs, 9 (2011) 2729–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].de Maagd PG-J, Hendriks AJ, Seinen W, Sijm DTHM, pH-Dependent hydrophobicity of the cyanobacteria toxin microcystin-LR, Water Res, 33 (1999) 677–680. [Google Scholar]
- [48].Adamovský O, Kopp R, Hilscherová K, Babica P, Palíková M, Pašková V, Navrátil S, Maršálek B, Bláha L, Microcystin kinetics (bioaccumulation and elimination) and biochemical responses in common carp (Cyprinus carpio) and silver carp (Hypophthalmichthys molitrix) exposed to toxic cyanobacterial blooms, Environ. Toxicol. Chem, 26 (2007) 2687–2693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.