Abstract
Background:
Steroids used to treat acute graft-versus-host-disease (GVHD) are thought to blunt clinical symptoms of infection. We aimed to assess the value of weekly surveillance blood cultures (SBCs) drawn in an outpatient setting from hematopoietic cell transplant (HCT) patients receiving high-dose steroids. We hypothesized that most positive outpatient surveillance cultures would be low-pathogenicity gram positive organisms and would lead to excess vancomycin therapy.
Methods:
We conducted a retrospective review of blood cultures collected from a cohort of adult HCT patients enrolled in a clinical trial of acute GVHD therapy with high-dose steroids (prednisone-equivalent doses ≥ 0.5 mg/kg/day) between April 2009 and May 2013. SBCs were defined as those collected weekly from central venous catheters (CVCs) in the outpatient setting while patients were receiving high-dose steroids. Cultures obtained as part of a symptom work-up or as follow-up for documented bacteremia were excluded. Clinical data were collected using center databases supplemented by medical record review.
Results:
A total of 127 HCT recipients were eligible for inclusion in the study. A total of 1015 SBCs were obtained, with a median of 8 cultures (interquartile range [IQR]; 5–10) per patient. Forty-two organisms were isolated from 36 of 1015 cultures (3.5%) in 30 unique patients, or 1 positive culture per 28 blood cultures drawn. The most frequently detected organism was coagulase-negative Staphylococcus (25/1015 [2.5%]). Gram negative organisms were rare (4/1015 [0.4%]. Antibiotics were administered the majority of patients with positive surveillance cultures (33/36 [92%]). Six were admitted to the hospital for treatment; none needed intensive care or died from their bacteremia. Vancomycin was the most frequently administered antibiotic, comprising 256 of 376 (68%) total days of antibiotic received by the cohort with a median duration of 10 days (IQR: 7, 14).
Conclusions:
Weekly outpatient surveillance blood cultures obtained from asymptomatic patients on high-dose glucocorticoids for treatment of acute GVHD after allogeneic HCT were infrequently positive, and the majority of organisms were low-pathogenicity organisms. Surveillance blood cultures also led to excess antibiotic exposure and costs, suggesting benefits of such ambulatory screening may be of limited value in this setting.
Keywords: Infectious Diseases, Bacteremia, Surveillance, Graft-versus-host disease, Glucocorticoids
INTRODUCTION
Bloodstream infections are a frequent cause of morbidity and mortality in patients undergoing hematopoietic cell transplantation (HCT).1 HCT recipients are at high risk for bacterial infections caused by gram negative (GNR) organisms due to underlying neutropenia, immunosuppressive therapy, and mucosal disruption from chemotherapy and acute graft-versus-host-disease (GVHD).1,2 While the majority of cancer/transplant centers utilize antibiotic prophylaxis during neutropenia to prevent GNR bacteremia and associated mortality in these patients, acute GVHD primarily occurs outside the window of typical neutropenic prophylaxis. Glucocorticoids, which are used during the treatment of GVHD, are known to inhibit the synthesis and function of certain cytokines, thereby limiting classical responses to infection such as tachycardia, flushing and fever.3,4 The limited symptoms while on steroid therapy has been thought to potentially delay diagnosis of bacteremia, which is associated with serious complications such as septic shock, intensive care unit (ICU) admission, organ dysfunction and death.5
Concern over missed and/or delayed detection of these life-threatening infections has led some centers to collect blood cultures over periodic intervals in an attempt to identify bacteremia events prior to symptom onset for high-risk HCT patients, such as those on high-dose glucocorticoids,3,4,6 with neutropenia,7,8 and/or those with a central venous catheter.9,10 Studies of the value of such surveillance blood cultures (SBCs) have produced variable results.2–4,6–10 Our center has performed weekly outpatient SBC for HCT patients treated with glucocorticoids for GVHD for over 10 years, but the utility of this policy had not yet been formally evaluated. Here, we quantified and characterized outpatient surveillance cultures, subsequent antibiotic treatment, and patient outcomes in a cohort of HCT recipients from a clinical trial of patients treated with glucocorticoids for active acute GVHD.11 We hypothesized that the majority of positive cultures from outpatient surveillance cultures would be low-pathogenicity gram positive organisms and would lead to excess vancomycin therapy.
MATERIALS & METHODS
Study Design, Population & Data Collection
The study population consisted of a cohort of patients who underwent an allogeneic HCT between April 2009 and May 2013 and were enrolled in a randomized trial comparing efficacy and safety of different doses of glucocorticoids for initial treatment of acute GVHD.11 As per study protocol, decisions to begin glucocorticoid therapy were at the discretion of the attending physician, and initial therapy varied according to treatment arm (methylprednisolone equivalent doses: 0.5 mg/kg/day vs. 1 mg/kg/day vs. 2 mg/kg/day) based on GVHD grade at symptom onset.11 In the present study of SBCs, the study period began at the time of initiation of high-dose glucocorticoids. HCT patients at our center have tunneled double lumen CVCs for a minimum of 90 days post-transplant. Patients were excluded from the primary analysis if they were less than 18 years of age or did not have any outpatient surveillance cultures performed. Demographic, laboratory and clinical outcome data were extracted from prospectively collected institutional databases. Clinical and microbiology data were collected by abstraction from electronic medical records.
Definitions:
Per our current center-based guidelines, SBCs were defined as once weekly outpatient blood cultures for allogeneic HCT patients receiving high-dose glucocorticoids (≥ 0.5 mg/kg/day) for GVHD. GVHD was defined as per established international criteria.12 SBCs were identified by either surveillance labels in microbiology records or as blood cultures collected weekly in the outpatient department while patients were asymptomatic per chart review. Cultures drawn due to other clinical symptoms, were considered non-SBC cultures. Labeled SBCs were excluded if they were obtained as part of a symptom work-up (e.g. fevers, chills or rigors) or if obtained within 7 days following a documented bacteremia. Since such cultures were often a single set, positive cultures were defined as positive with the detection of any bacterial species.
Infectious Diseases Prophylaxis Post-HCT:
HCT recipients are given pre and post-HCT prophylaxis for Pneumocystis jirovecii with Bactrim, dapsone, or atovaquone; those not on Bactrim who have a known history of a splenectomy are also placed on daily oral Penicillin VK after count recovery. All patients with GVHD treated with ≥0.5 mg/kg of steroids are placed on posaconazole prophylaxis, unless already on antifungal therapy for a prior diagnosis or suspected filamentous mold infection, or until steroid dose drops to <0.5 mg/kg. All patients are given levofloxacin 750 mg daily for bacterial prophylaxis during periods of post-transplant neutropenia. Antiviral prophylaxis is given as has previously been described.13
Outcomes of interest were antibiotic days of therapy, hospital admission, intensive care unit (ICU) admission and death within 30 days if deemed directly related to a positive culture based on review of the clinical data. Each antibiotic administered specifically for a positive blood cultures was documented. If multiple antibiotics were administered on any given day (e.g. broad-spectrum antibiotics while speciation and sensitivities were pending), each antibiotic was counted as a separate day regardless of how many doses were administered.
RESULTS
A total of 127 allogeneic HCT patients were eligible for study inclusion, and Table 1 shows the characteristics of the patient population. Acute leukemias were the most common underlying malignancies at 47%, followed by lymphoma and myelodysplastic syndromes representing 13% and 11%, respectively. The average age of the cohort was 52 years old (interquartile range [IQR] 40.5, 59) with a male predominance (61%). All patients were on high-dose glucocorticoids to treat acute GVHD, with 26/127 (21%) receiving an initial methylprednisolone equivalent dose of 2mg/kg/day, 65 (51%) receiving 1mg/kg/day, and 36 (28%) receiving 0.5mg/kg/day.
Table 1:
Patient Demographics (n = 127)*
| Characteristic | n (%) |
|---|---|
| Age – median (IQR) | 52 (41, 59) |
| Gender | |
| Male | 78 (61) |
| Female | 49 (39) |
| Underlying Disease | |
| Acute leukemia | 60 (47) |
| Lymphoma | 16 (13) |
| Myelodysplastic Syndrome | 14 (11) |
| Chronic leukemia | 12 (9) |
| Myelofibrosis | 8 (6) |
| Multiple myeloma | 8 (6) |
| Myeloproliferative disorder | 3 (2) |
| Aplastic anemia | 3 (2) |
| Other | 3 (2) |
| Donor | |
| Unrelated | 88 (69) |
| Related Sibling | 31 (24) |
| Haploidentical | 8 (6) |
| Graft | |
| PBSC | 110 (87) |
| Cord blood | 9 (7) |
| Bone Marrow | 8 (6) |
| Conditioning | |
| Myeloablative | 79 (62) |
| Non-Myeloablative | 48 (48) |
| CMV Status | |
| Recipient positive | 58 (46) |
| Recipient negative | 69 (54) |
| GVHD prevention | |
| Tacrolimus/MTX | 55 (43) |
| Cyclosporine/MMF | 41 (32) |
| Tacrolimus/MMF | 8 (6) |
| Combination with Rapamycin | 6 (5) |
| Other combination | 17 (13) |
| GVHD grade 2 | 108 (85) |
| grade 3 | 17 (13) |
| grade 4 | 2 (2) |
| GVHD location | |
| Skin and Gut | 68 (54) |
| Gut only | 46 (36) |
| Skin only | 13 (10) |
| Initial Glucocorticoid Dose | |
| 2 mg/kg/day | 26 (21) |
| 1 mg/kg/day | 65 (51) |
| 0.5 mg/kg/day | 36 (28) |
Abbreviations: IQR – interquartile range; GVHD – graft-versus-host disease; MTX – methotrexate; MMF – mycophenolate; CMV – cytomegalovirus
percentages may not equal 100% due to rounding
Among these 127 patients, 1015 SBCs were obtained in the outpatient department for a median of 8 cultures (IQR; 5–10) per patient. The vast majority of patients (97 [76%]) had no positive cultures, while 26 (20%) had one positive culture, and 4 (3%) had >1 positive culture. Bacteria were isolated from 36 of 1015 blood cultures (3.5%) from 30 unique patients, or 1 positive culture per 28 blood cultures drawn. Median day for positive surveillance culture was 59 days after HCT (interquartile range [IQR]: 46–89 days) and 29 days after start of steroid therapy for acute GVHD (IQR: 20–50 days).
More than 1 bacterial organism was isolated in 5 blood cultures; 2 of these isolated >1 species of coagulase negative Staphylococcus. When we compared SBCs to non-SBC obtained in the same cohort for symptomatic work-up, SBCs isolated significantly fewer organisms (36/1015 [3.5%] vs. 12/86 [14%], p<0.001). There were no differences in the number of positive cultures based on glucocorticoid dose: 5 of 201 (2.5%) in patients initiated at 2 mg/kg/day methylprednisolone equivalent, 18 of 542 (3.3%) initiated at 1 mg/kg/day, and 13 of 272 (4.8%) initiated at 0.5 mg/kg/day (p=0.38). Mean cumulative prednisone-equivalent dose from day 0 to day 42 post initiation of steroids were similar when comparing patients with to those without positive surveillance blood cultures (Figure 1). Only one patient was neutropenic at the time of positive blood culture (4%), and an additional 2 had an absolute neutrophil count between 500 and 1000 neutrophils per uL. In addition to steroids, the most frequent immunosuppressive agents used at the time of positive outpatient surveillance blood cultures were calcineurin inhibitors (27/30 [90%) and anti-metabolites (e.g. mycophenolate (12 [40%]). A total of 16 (53%) were also receiving concomitant oral beclomethasone/budesonide (16 [53%]) for gastrointestinal GVHD. Two patients with positive outpatient surveillance cultures had received antithymocyte globulin (7%), one alemtuzumab, and another was receiving extracorporeal photopheresis (ECP) prior to their positive cultures.
Figure 1.
Cumulative steroid dose (mg/kg) over time in patients with and without positive surveillance cultures

Data demonstrating first 42 days of follow-up after initiating steroid therapy; blue line = patients with bacteremia on surveillance cultures, blue circles = bacteremia episodes; black line = patients without bacteremia. Mean cumulative prednisone-equivalent dose at day 42 after initiating steroids when comparing patients with (n=23; 31.1 mg/kg [SD=14.9]) or without positive surveillance cultures (n=98; 35.5 mg/kg [SD=15.5], p=0.24).
The most frequently detected bacteria were gram positive organisms, dominated by coagulase-negative Staphylococcus (25/1015 [2.5%] SBCs), which represented 60% of the organisms isolated (Table 2); median time to report of positive cultures for those with this pathogen [coagulase negative Staphylococcus] (at time when GPCs were first noted) was 26 hours post collection (IQR 24–30). One patient had vancomycin-resistant enterococcus (VRE), one vancomycin-sensitive enterococcus and one Staphylococcus aureus. GNRs were rare (4/1015 [0.4%], and included Serratia marcescens, Klebsiella pneumoniae, Pseudomonas putida, and Stenotrophomonas maltophilia. Case reviews of patients with blood cultures positive for high pathogenicity organisms including GNRs, S. aureus and Enterococci are provided in Table 3. One SBC set was drawn for each of these patients, and average time from blood culture to administration of antibiotics was >24 hours (average = 32.9 hours [range 17.5 – 45 hours] from culture to antibiotic administration, Table 3). Half of these blood cultures were obtained from patients whose steroid dose was below the threshold outlined by our center’s standard practice guidelines. Repeat blood cultures among those with high risk pathogens were drawn prior to antibiotic administration in only 2 patients (Table 3); one yielded the same organisms while the other repeat culture was negative.
Table 2:
Microbiology Identified from Positive Surveillance Blood Cultures (n=42)*
| IDENTIFIED ORGANISMS | n (%) |
|---|---|
| GRAM POSITIVE | |
| Coagulase negative Staphylococcus | 25 (60) |
| Bacillus spp. | 2 (4.8) |
| Diphtheroids | 2 (4.8) |
| Enterococcus faecium† | 2 (4.8) |
| Alpha hemolytic Streptococcus | 2 (4.8) |
| Non-hemolytic Streptococcus | 1 (2.4) |
| Rothia mucilaginosa | 1 (2.4) |
| Viridans group Streptococcus | 1 (2.4) |
| Methicillin-sensitive Staphylococcus aureus | 1 (2.4) |
| Gram positive cocci (NOS) | 1 (2.4) |
| GRAM NEGATIVE | |
| Serratia marcescens | 1 (2.4) |
| Klebsiella pneumonia | 1 (2.4) |
| Pseudomonas putida | 1 (2.4) |
| Stenotrophomonas maltophilia | 1 (2.4) |
| TOTAL | 42 |
Among 36/1015 (3.5%) surveillance blood cultures from 30/127 (28%) total patients in the cohort, which includes patients with polymicrobial infections.
Includes one vancomycin-resistant and one vancomycin-sensitive strain;
Abbreviations: spp – species; NOS – not otherwise specified
Table 3:
Case Review of Surveillance Cultures with High-Pathogenicity Organisms
| Patient | Days Post-Transplant | Steroid dosea | Organism(s) | Hours from blood culture collection to positivity | Hours to 1st antibiotic(s) after notificationb | Blood culture repeated prior to antibiotics? | Admit | ICU | 30-day Mortality |
|---|---|---|---|---|---|---|---|---|---|
| 1a | 53 | 0.8 | Serratia marcescens | 19.5 | 2.5 | No | Yes | No | No |
| 1b | 78 | 0.4 | Pseudomonas putida & Enterococcus faeciumc | 14.5 | 3 | Yes | Yes | No | No |
| 2 | 265 | 0.34 | Stenotrophomon as maltophilia | 30 | N/A (<24) | No | No | No | No |
| 3 | 55 | 0.4 | Klebsiella pneumoniae | 42 | 3 | Yes | Yes | No | No |
| 4 | 56 | 1 | Enterococcus faeciumd | 19 | 2 | No | No | No | No |
| 5 | 85 | 0.6 | MSSA | 24 | 5 | No | No | No | No |
N/A: not available; MSSA: methicillin-susceptible Staphylococcus aureus
Dose in milligrams per kilogram per day.
Time at which lab reported positive culture results
vancomycin resistant
vancomycin sensitive
Figure 2 illustrates days of antibiotic therapy for patients with positive SBCs. Thirty-three of 36 (92%) positive SBCs were treated with a sum total of 376 antibiotic days. Vancomycin comprised a total of 256 days (68% of all antibiotic days); the median duration of vancomycin was 10 days (IQR: 7, 14). Together, antibiotics targeting GPC pathogens (vancomycin, daptomycin and linezolid) made up 302 or 80% of all antibiotic days. Only 6 patients were admitted to the hospital for treatment (2 GNR, 3 GP, and 1 polymicrobial). None required ICU-level care or died from their documented bacteremia within 30 days.
Figure 2.
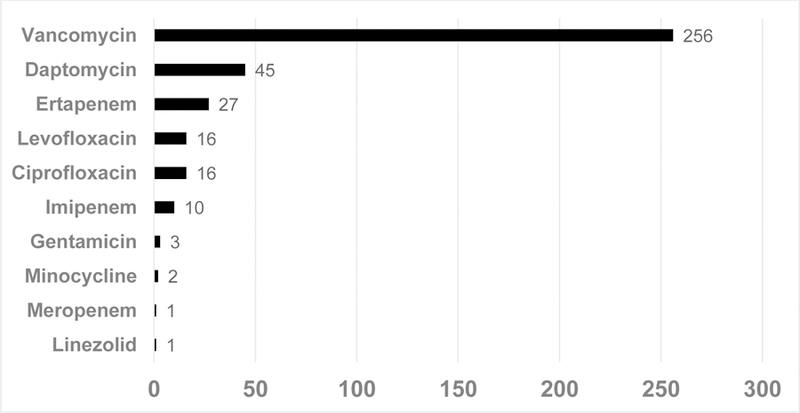
Antibiotic Days Received in Patients with Positive Surveillance Blood Cultures*
*Represents 37 total antibiotic courses. Individual patients may have received more than one agent during the course of their therapy
DISCUSSION
Weekly outpatient surveillance blood cultures in asymptomatic allogeneic HCT patients on high dose glucocorticoids were infrequently positive (3.5%) and high-pathogenicity organisms accounted for less than 1% of all cultures. Coagulase-negative Staphylococcus was the most common organism recovered, which led to excess vancomycin use within this cohort.
Previous studies have evaluated the utility of SBCs in various high-risk populations of oncology and transplant patients with mixed results. The term surveillance blood cultures in these studies refer to periodic collection of blood cultures for defined patient populations but frequency of collection, inpatient vs outpatient setting, and presence of clinical symptoms are variable (Supplemental Table 1). For instance, Chizuka et al. found that weekly blood cultures in 69 afebrile allogeneic HCT patients who were on >0.5 mg/kg prednisolone equivalent yielded 110/968 positive blood cultures in 36 patients and were the only “diagnostic clue for occult bloodstream infection;”3 however, patients’ courses were not reviewed for other symptoms beyond fever. In contrast, when studies are limited strictly to asymptomatic HCT patients, SBCs appear to provide limited value (Table 4).2,6,9,10 The largest study of surveillance blood cultures to date was performed by Nesher et al. at the University of Texas MD Anderson Cancer Center on a cohort of 776 allogeneic hematopoietic cell transplant patients, who had weekly surveillance blood cultures from central venous catheters whether or not they were on high dose glucocorticoids.9 The general conclusions from studies of asymptomatic HCT patients reflected that surveillance cultures were of limited value, not cost effective, and had a weak correlation for predicting subsequent septicemia.2,6,9,10 Ghazal et al. evaluated the utility of weekly SBCs in asymptomatic hospitalized HCT recipients vs those who were symptomatic at time of blood cultures to detect catheter-related bloodstream infection. In their cohort of 205 HCT patients, SBCs did not identify any bloodstream infections and resulted in overdiagnosis and overtreatment for over 10% of their cohort.2
Table 4:
Review of Previous Studies Examining the Utility of Surveillance Blood Cultures from Asymptomatic HCT Patients
| Study | Number of patients | Median age (y) | Study year(s) | Proportion of positive SBCs | Proportion of SBCs with high pathogenicity organismsa | Conclusions / Comments |
|---|---|---|---|---|---|---|
| Colombier et al. | 82 | 52 | 2013 | 103/1450 (7%) accounting for 73 infectious episodesb in 33 patients | 22/73 (30%) episodes | Daily SBCs rarely identified BSI. Clear benefit could not be demonstrated. |
| Ghazal et al. | 205 | 49 | 2010–11 | NA/2474 SBCs accounting for 55 episodesc | 13/55 (24%) Episodes | SBCs did not identify BSI. 22/55 episodes were treated with antibiotics for at least 10 days/episode |
| Nesher et al. | 776 | 53 | 2010–11 | 211/6801 (3%) in 187 patients | 21/211 (10%) | Frequency of clinically significant SBCs is very low and leads to unnecessary medical interventions and added costs. |
| Rigby et al. | 43 | 7 | 1999–2005 | NA/316 accounting for 3 episodes in 3 patients | 2/3 (66%) | SBCs is low yield and significant cost. Unclear whether SBCs contribute to improved patient outcomes. |
| This study | 127 | 52 | 2009–13 | 36/1015 (3.5%) in 30 patients | 10/36 (27%) | _ _ |
SBC: surveillance blood culture; BSI: bloodstream infection; NA: not available
High pathogenicity organisms included any gram-negative rod, S. aureus, Enterococci, Streptococcus sp., and Candida sp.
Blood cultures growing the same organism over a 7-day period were considered as part of a single episode.
Episode defined as a set or group of successive positive culture sets done within one week, irrespective of their number or whether the patient was classified as infected or not.
Our results suggest that the practice of obtaining weekly surveillance blood cultures among asymptomatic patients in the outpatient department may be of limited value due to the low frequency of positive blood cultures. When we compared SBCs to blood cultures obtained in the same cohort for symptomatic work-up, SBCs isolated significantly fewer organisms (36/1015 [3.5%] vs. 12/86 [14%], p<0.001).
Furthermore, the majority of positive cultures ultimately grew coagulase-negative Staphylococcus, a common skin commensal.14 Distinguishing true bloodstream infection from contamination may be difficult in this patient population given their medically vulnerable state, the lack of a simultaneously collected peripheral blood culture in most cases, and a provider’s sense of urgency to treat when a blood culture returns positive. Adherence to CVC care maintenance practices by nursing staff, patients and caregivers should be another consideration. Thirty three of 36 positive SBCs were treated, but only 11 (33%) patients had repeat blood cultures before being started on antibiotics. While documentation of blood culture clearance for non-S. aureus bacteremia may not always be necessary, particularly for GNRs,15,16 in this cohort with largely GP organisms from single positive blood cultures obtained from central venous catheters, repeat cultures may help to distinguish contamination from true infections.
The practice of surveillance blood cultures led to vancomycin use in 27 of the 36 (75%) positive blood cultures, with a median duration of therapy of 10 days, mainly for low-risk pathogens (e.g. coagulase negative Staphylococcus), or pathogens almost universally considered contaminants (e.g. Diptheroids). While difficult to ascertain whether these courses are warranted in a retrospective study, chart review revealed sparse documentation regarding rationale for vancomycin duration, oversight in dosing, and drug-level monitoring. As programs begin to focus on antimicrobial stewardship among immunocompromised patients, this study reinforces the importance of monitoring antibiotic use in the ambulatory setting. Since this study completed, a number of changes have been made at our center. An antimicrobial stewardship pharmacist performs prospective audit and feedback on vancomycin administered among outpatients and assesses appropriateness of dosing, indication, duration and identifies opportunities for de-escalation.
Given the association of GNR bacteremia with mortality in highly immunocompromised patients,17–19 early detection and treatment of GNR bacteremia may arguably be the most important target of surveillance blood cultures. In our cohort, occult GNR bacteremia was rare; surveillance blood cultures identified 4 GNRs in 3 unique patients. The number of weekly cultures needed to identify one occult GNR bacteremia in this cohort was 254. For three of these bacteremia events, the patient was admitted; none required ICU care and there were no deaths. As glucocorticoids for GVHD are gradually tapered, prednisone doses in these cases were reviewed and interestingly only one patient was still on high-dose glucocorticoids when cultures were drawn. Therefore, strict adherence to our center’s standard practice guidelines for surveillance blood cultures would have identified only one occult GNR bacteremia, suggesting less benefit from these efforts. Since limiting morbidity and mortality among these high-risk patients remains crucial, each center must rely on their own observational data on SBCs until additional randomized-controlled trials can document the utility of this approach.
Costs of weekly blood cultures, microbiology work up, IV antibiotic administration, and days of hospitalization should be considered potential burdens of such policies. Additionally, the effect of antibiotics, particularly how they may lead to other adverse side effects are important to consider. Furthermore, antibiotic effects on the microbiome in allogeneic HCT patients with GVHD are highly dynamic20 and may potentially worsen GVHD outcomes.21 Low microbiome diversity from antibiotic exposure has been associated with multi-drug resistant pathogen colonization/disease22 and progression to lower respiratory tract disease in allogeneic HCT patients.23
Our study is limited by the retrospective observational design at a single cancer center. However, our focus on the practice of surveillance blood cultures for patients on high-dose glucocorticoids in the outpatient setting is unique. Additionally, clinical information, decision-making and lab monitoring for outpatient antibiotics were infrequently documented in the outpatient setting. The risk profile of hospitalized patients receiving glucocorticoid treatment for acute GVHD may be different and was not evaluated in the present study. Finally, we cannot assess the role additional antibiotic therapy could have on late GVHD complications. While these data highlight the need for diagnostic and antimicrobial stewardship, it also precedes implementation of rapid molecular diagnostics, review of all positive blood cultures by our stewardship team, and outpatient vancomycin pharmacy review, which likely have influenced subsequent antibiotic use since the time period in which this study was conducted.
CONCLUSION
In conclusion, our study examined the utility of outpatient ambulatory SBCs to identify occult bacteremia in patients on high-dose glucocorticoids for treatment of acute GVHD after allogeneic HCT. SBCs were infrequently positive (3.5%), and the majority of identified organisms were coagulase-negative Staphylococcus for which vancomycin was administered. Our results do not support the practice of obtaining weekly SBCs in asymptomatic outpatients, although a randomized trial would be needed to confirm this recommendation. Ambulatory diagnostic stewardship and antibiotic administration are important areas that need renewed focus for future stewardship efforts in high-risk immunocompromised populations.
Supplementary Material
Highlights.
Steroids used to treat acute GVHD increase the risk and blunt symptoms of infection
Surveillance cultures are thought to detect occult bacteremia but are poorly studied
We found weekly outpatient surveillance blood cultures were rarely positive
Coagulase negative Staphylococcus were the most frequently identified pathogens
Surveillance blood cultures led to excess antibiotic exposure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts of Interest: S.A.P. has served as a site investigator for Merck and Chimerix.
Presentation of material in submitted manuscript: Data from this manuscript have been presented in part at the ASBMT/CIBMTR Tandem Meeting in San Diego, CA February 2015.
REFERENCES
- 1.Mikulska M, Del Bono V, Raiola AM, et al. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: reemergence of Gram-negative rods and increasing antibiotic resistance. Biol Blood Marrow Transplant 2009;15(1):47–53. [DOI] [PubMed] [Google Scholar]
- 2.Ghazal SS, Stevens MP, Bearman GM, Edmond MB. Utility of surveillance blood cultures in patients undergoing hematopoietic stem cell transplantation. Antimicrob Resist Infect Control 2014;3(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chizuka a, Kami M, Kanda Y, et al. Value of surveillance blood culture for early diagnosis of occult bacteremia in patients on corticosteroid therapy following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2005;35(6):577–582. [DOI] [PubMed] [Google Scholar]
- 4.Joosten A, Maertens J, Verhaegen J, Lodewyck T, Vermeulen E, Lagrou K. High incidence of bloodstream infection detected by surveillance blood cultures in hematology patients on corticosteroid therapy. Support Care Cancer 2012;20(11):3013–3017. [DOI] [PubMed] [Google Scholar]
- 5.Marín M, Gudiol C, Ardanuy C, et al. Factors influencing mortality in neutropenic patients with haematologic malignancies or solid tumours with bloodstream infection. Clin Microbiol Infect 2015;21(6):583–590. [DOI] [PubMed] [Google Scholar]
- 6.Colombier M-A, Lafaurie M, de Fontbrune FS, et al. Usefulness of daily surveillance blood cultures in allogeneic hematopoietic stem cell transplant recipients on steroids: a 1-year prospective study. Transpl Infect Dis 2016;18(4):504–511. [DOI] [PubMed] [Google Scholar]
- 7.Penack O, Keilholz U, Thiel E, Blau IW. Value of surveillance blood cultures in neutropenic patients--a pilot study. Jpn J Infect Dis 2005;58(3):171–173. [PubMed] [Google Scholar]
- 8.Penack O, Rempf P, Eisenblätter M, et al. Bloodstream infections in neutropenic patients: early detection of pathogens and directed antimicrobial therapy due to surveillance blood cultures. Ann Oncol 2007;18(11):1870–1874. [DOI] [PubMed] [Google Scholar]
- 9.Nesher L, Chemaly RF, Shah DP, Mulanovich VE, Hosing C, Rolston KVI. Utility of routine surveillance blood cultures in asymptomatic allogeneic hematopoietic stem cell transplant recipients with indwelling central venous catheters at a comprehensive cancer center. Am J Infect Control 2014;42(10):1084–1088. [DOI] [PubMed] [Google Scholar]
- 10.Rigby H, Fernandez CV, Langley J, Mailman T, Crooks B, Higgins A. Routine surveillance for bloodstream infections in a pediatric hematopoietic stem cell transplant cohort: Do patients benefit? Can J Infect Dis Med Microbiol 2007;18(4):253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mielcarek M, Furlong T, Storer BE, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: A randomized controlled trial. Haematologica 2015;100(6):842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995;15(6):825–828. [PubMed] [Google Scholar]
- 13.Pergam SA, Xie H, Sandhu R, et al. Efficiency and risk factors for CMV transmission in seronegative hematopoietic stem cell recipients. Biol Blood Marrow Transplant 2012;18(9):1391–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth RR, James WD. Microbial ecology of the skin. Annu Rev Microbiol 1988;42:441–464. [DOI] [PubMed] [Google Scholar]
- 15.Canzoneri CN, Akhavan BJ, Tosur Z, Andrade PEA, Aisenberg GM. Follow-up Blood Cultures in Gram-Negative Bacteremia: Are They Needed? Clin Infect Dis 2017;65(11):1776–1779. [DOI] [PubMed] [Google Scholar]
- 16.Wiggers JB, Xiong W, Daneman N. Sending repeat cultures: Is there a role in the management of bacteremic episodes? (SCRIBE study). BMC Infect Dis 2016;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collin BA, Leather HL, Wingard JR, Ramphal R. Evolution, Incidence, and Susceptibility of Bacterial Bloodstream Isolates from 519 Bone Marrow Transplant Patients. Clin Infect Dis 2001;33(7):947–953. [DOI] [PubMed] [Google Scholar]
- 18.Miles-Jay A, Butler-Wu S, Rowhani-Rahbar A, Pergam S a. Incidence Rate of Fluoroquinolone-Resistant Gram-Negative Rod Bacteremia among Allogeneic Hematopoietic Cell Transplantation Patients during an Era of Levofloxacin Prophylaxis. Biol Blood Marrow Transplant 2015;21(3):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010;363(22):2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golob JL, Pergam SA, Srinivasan S, et al. Stool Microbiota at Neutrophil Recovery Is Predictive for Severe Acute Graft vs Host Disease After Hematopoietic Cell Transplantation. Clin Infect Dis 2017; 65(12):1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shono Y, Docampo MD, Peled JU, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med 2016; 8(339):339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 2010;120(12):4332–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogimi C, Krantz EM, Golob JL, et al. Antibiotic Exposure Prior to Respiratory Viral Infection Is Associated with Progression to Lower Respiratory Tract Disease in Allogeneic Hematopoietic Cell Transplant Recipients. Biol Blood Marrow Transplant 2018; 24(11):2293–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frère P, Hermanne J-P, Debouge M-H, de Mol P, Fillet G, Beguin Y. Bacteremia after hematopoietic stem cell transplantation: incidence and predictive value of surveillance cultures. Bone Marrow Transplant 2004;33(7):745–749. [DOI] [PubMed] [Google Scholar]
- 25.Kanathezhath B, Shah A, Secola R, Hudes M, Feusner JH. The utility of routine surveillance blood cultures in asymptomatic hematopoietic stem cell transplant patients. J Pediatr Hematol Oncol 2010;32(4):327–331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


