Abstract
Human neuroimaging studies have consistently reported changes in cerebellar function and integrity in association with obesity. To date, however, the nature of this link has not been studied directly. Emerging evidence suggests a role for the cerebellum in higher cognitive functions through reciprocal connections with the prefrontal cortex. The purpose of this exploratory study was to examine appetite changes associated with noninvasive prefronto-cerebellar neuromodulation in obesity. 12 subjects with class I obesity (mean BMI 32.9 kg/m2) underwent a randomized, single-blinded, sham-controlled, crossover study, during which they received transcranial direct current stimulation (tDCS; active/sham) aimed at simultaneously enhancing the activity of the prefrontal cortex and decreasing the activity of the cerebellum. Changes in appetite (state and food-cue-triggered) and performance in a food-modified working memory task were evaluated. We found that active tDCS caused an increase in hunger and desire to eat following food-cue exposure. In line with these data, subjects also tended to make more errors during the working memory task. No changes in basic motor performance occurred. This study represents the first demonstration that prefronto-cerebellar neuromodulation can influence appetite in individuals with obesity. While preliminary, our findings support a potential role for prefronto-cerebellar pathways in the behavioral manifestations of obesity.
Introduction
Obesity is associated with brain changes and impaired performance in laboratory measures of neurocognitive functioning.1–3 These alterations may contribute to the development and maintenance of maladaptive eating behaviors, but the specific mechanisms remain largely unknown. The cerebellum is one of the regions most consistently associated with body mass index (BMI) and obesity. A number of functional neuroimaging studies has identified cerebellar activation in response to hunger/satiation4, gastric distension5, and food cues.6 Obesity and obesity risk status impact the structure of the cerebellum, with a high degree of heritability.7 Additionally, the cerebellum is an important target for leptin action8 and its gray matter volume is inversely associated with abdominal obesity and related inflammatory processes.9 The animal literature also supports an important role for the cerebellum in homeostatic control of feeding and body weight.10 Altogether, these data suggest an inverse association between BMI/obesity and cerebellar function and integrity, but no study has provided direct demonstration for such link in humans to date.
Notably, the cerebellum is well positioned to exert a broad coordinating role in the regulation of appetite and food intake, namely via access to the hypothalamus, reward centers and cognitive circuits.10,11 Current models posit that the cerebellum may act as a multi-domain integrator, fine-tuning the quality of behavioral outputs and providing optimized shortcuts.11–14 Emerging data suggest that beyond the well-recognized role of the cerebellum in motor control, this area can also contribute to cognition, learning, reward processing, habit formation and craving.11–15 In particular, the human cerebellum has a highly developed system of contralateral, reciprocal connections (via thalamus and pons) with high-order brain regions, including the prefrontal cortex.16 Prefronto-cerebellar interactions are believed to coordinate and temporally synchronize multiple cognitive representations with external stimuli and voluntary actions12,14,17; however, the extension of these functions to cognitive processes that support adaptive behavioral regulation of food intake is currently unknown.
In the present study we preliminarily examined acute effects of experimental manipulation of prefronto-cerebellar pathways in individuals with obesity. We used transcranial direct current stimulation (tDCS), a noninvasive neuromodulation technique that delivers weak direct currents to the brain via scalp electrodes18, with the purpose of enhancing the activity of the left dorsolateral prefrontal cortex (DLPFC) and reducing the activity of the right cerebellum. Previous studies with tDCS in obesity have focused on the DLPFC19,20; however, the effects of modulating DLPFC-cerebellum interactions have not yet been explored. We selected a left DLPFC/right cerebellum tDCS montage based on past work in obesity (target: left DLPFC)19, and some neuroimaging data pointing more specifically to the right cerebellum in association with BMI.7 We hypothesized that this tDCS approach would facilitate prefronto-cerebellar interactions, by increasing the influence of the DLPFC on the cerebellum, leading to a reduction in appetite and an improvement of cognitive performance under the presence of food cues.
Materials and Methods
Twelve tDCS naïve participants (9 female, 3 male) with class I obesity (BMI 32.7 ± 1.9 Kg/m2) aged 33–47 years (41.6 ± 4.8) took part in this pilot study. Participants were recruited from Clinica Sagrada Familia and Universitat Oberta de Catalunya (Barcelona, Spain). Exclusion criteria included BMI < 30 or > 35 Kg/m2, unstable body weight (defined as ± 5% change within 6 months prior to participation), any history of neurological disorder, psychiatric illness, alcohol or drug abuse, and any known cause of secondary obesity (self-reported). Subjects gave written informed consent to participate at the beginning of the study. The study was approved by the Institutional Review Board of Universitat Oberta de Catalunya.
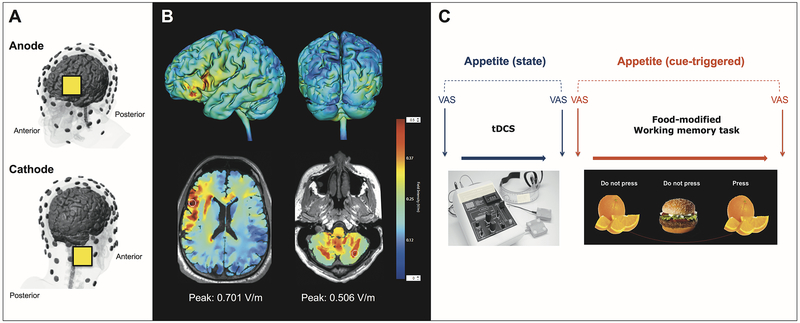
The study protocol involved two visits. In each visit, a different stimulation condition (active or sham) was applied in a randomized and counterbalanced order. Subjects were unaware of stimulation condition. Visits took place on two consecutive days, at the same time of the day, and within a postprandial period of 4 hours. tDCS (2 mA, 20 min) was administered with the cathode over the right cerebellum (1 cm below and 4 cm lateral to the inion, i.e. centered within the posterior cerebellar lobe21,22) and the anode over the left DLPFC (F3) (Fig. 1A). This montage, guided by our own computational modeling data (Fig. 1B), was planned with the intention of modulating prefronto-cerebellar pathways by simultaneously enhancing the activity of the left DLPFC and decreasing the activity of the right cerebellum. We used a Soterix Medical 1×1 tDCS device (Soterix Medical, New York, NY) equipped with 5×5 cm sponge electrodes soaked in 0.9% sodium chloride solution. During tDCS sessions, participants were awake, relaxed and seated in a comfortable chair. All technical aspects of tDCS application adhered to recent recommendations for safe and replicable use of this technique.23
Figure 1.
A. tDCS montage used in the present study. 5×5 cm electrode pads were placed over right cerebellum (cathode) and F3 (anode). B. Computational model of the tDCS montage used. Peak electric field magnitude is shown at the approximate location of the electrodes (axial images, white circles). The scale bar on the right shows the color code for current density values (V/m). C. Study diagram showing the time course of measurements for each of the study visits. VAS: visual analogue scale.
Subjects were evaluated in three domains: a) subjective appetite, b) food-related cognitive performance, and c) general effects on motor performance and working memory. Fig. 1C depicts the time course of assessments for each session. For a) we evaluated both state and cue-induced changes in appetite using visual analogue scales (VAS) with questions on hunger, fullness, desire to eat and prospective consumption.24 State appetite was defined as VAS scores obtained immediately before and after receiving tDCS. Cue-triggered appetite was defined as VAS scores obtained immediately before and after exposure to food cues. For b) we used a food-modified N-back task with 3 levels of cognitive load (1-back, 2-back, and 3-back). For c) we used a finger tapping task and a digit span test. Additionally, we evaluated tDCS adverse effects in each session and, at the end of the study, subjects also filled in questionnaires on personality, eating behavior and food craving. For more details about methods see Supplementary material section on IJO website.
Statistical analyses were performed as indicated, using α=0.05, and two-tailed hypotheses. Normality was examined using Shapiro-Wilk test. All analyses were conducted in SPSS software (IBM SPSS Statistics 23, Chicago, IL).
Results
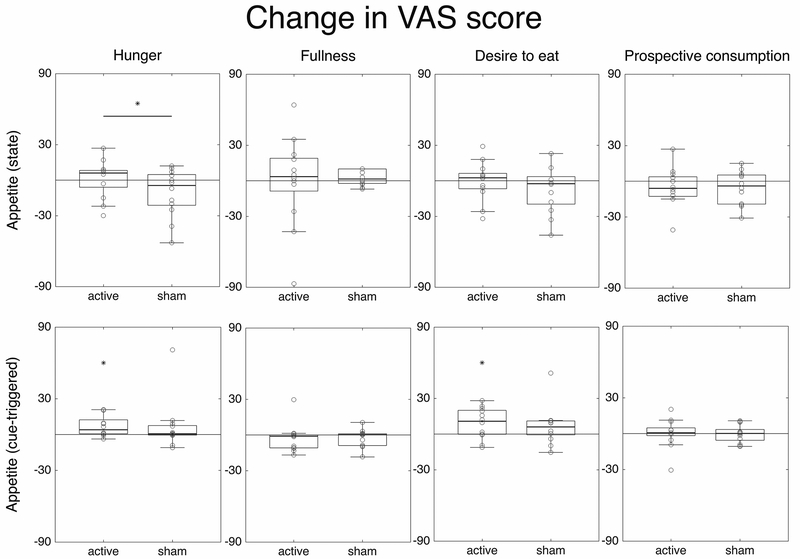
Fig. 2 shows all changes in appetite VAS scores. Repeated measures ANOVA of appetite state revealed an interaction effect time x stimulation condition for hunger (F(1,11)=5.041, p=0.046). Post hoc analyses using t-test with Bonferroni correction showed a decrease in score after sham stimulation (pre=42.5, post=31.67) nearly significant (p=0.094) but not after active stimulation (pre=39.45, post=40.33, p=0.903), indicating a relative increase in hunger following active tDCS. There were no other differences pre vs. post stimulation.
Figure 2.
Box and dot plots representing changes in the four VAS scores (Δ, columns) for the two appetite measurements: state and cue-triggered (rows). The horizontal line represents a significant main effect of stimulation condition (active vs. sham) and the asterisks (*) over one condition represent significant pre-post differences within that condition.
In the case of cue-triggered appetite (VAS scores pre/post cue exposure), there was a main effect of time on desire to eat, indicating a significant increase when comparing pre vs. post task scores (F(1, 11)=5.919, p=0.033). Even though this interaction was not significant, the increase was greater in the active stimulation condition (difference: active=9.13, p=0.034; sham=6.5, p=0.202). Paired-sample t-test comparing pre vs. post scores also revealed a significant increase in hunger for the active condition (t(11)=−2.75, p=0.019), but not for the sham condition (t(11)=−1.019, p=0.299).
Regarding performance in the n-back food task, paired-sample t test revealed a tendency towards more errors committed after active stimulation, compared to sham (5.25 % more, t(11)=1.892, p=0.085). No differences were found in reaction times (speed).
We found no effects on the finger tapping task. Digit span ANOVAs revealed a main effect of time on digit backward span (F(1, 11)=10.385, p=0.008) with higher scores the second time participants performed the task, both in active and sham sessions (mean 5.08 vs. 5.62). Paired-sample t test also revealed an increase in backward digit span only after sham stimulation (t(11)=−2.345, p=0.039). Evidence for a possible contribution of individual characteristics (personality factors and eating behavior trait) was also observed (Supplementary Material, Table S1). Only few -expected- side effects were reported at the end of each stimulation session, but with no differences between sham and active conditions (Fisher’s exact test) (Table S2).
Discussion
In this study, we examined acute effects on appetite and food-related cognitive performance associated with noninvasive prefronto-cerebellar neuromodulation in obesity for the first time. Contrary to our hypothesis, we found that active tDCS caused a relative elevation in the general state of hunger, compared with sham tDCS. Additionally, there was an increase in cue-triggered desire to eat and hunger, and a trend suggesting impairment of performance in a food-specific working memory task. While preliminary and limited by the small sample size, our results support the notion that prefronto-cerebellar pathways may contribute to appetite regulation and mechanisms related to behavioral control over external food cues.
A number of scenarios could explain our unexpected findings. First, tDCS may have caused a more dominant impact on the cerebellum (reduced activity) than on the DLPFC (increased activity). The association between reduced activity in the cerebellum and increased hunger is compatible with the inverse relationship between cerebellar function/integrity and BMI that has been reported in the neuroimaging literature.3 Furthermore, previous studies with tDCS that showed decreases in appetite and food craving, i.e. opposite effects from our findings, used montages with the same anodal DLPFC location, but different positioning of the cathode, which here was placed over the cerebellum, versus the supraorbital/prefrontal region in prior studies.19,20 The relative increase in hunger state that we found could also fit with a modulatory role of the cerebellum in basic appetite sensations driven by homeostatic and visceral regulation, conveyed by cerebellar-hypothalamic circuits10, and more selectively related to the vermis sector.25 Abnormalities in cerebellar-hypothalamic connectivity have recently been associated with obesity and difficulty achieving successful weight-loss.26 A second scenario to explain our findings is that the prefronto-cerebellar tDCS montage that we used may have engaged a more ventral sector of the prefrontal cortex, or even reached components of the orbitofrontal cortex, which are more prominently involved in reward processing (see current density peaks predicted by computational modeling, Fig. 1B), and thus could have contributed to the observed increase in hunger. A third scenario to interpret our results is that the anodal DLPFC/cathodal cerebellum tDCS montage could have disrupted, rather than facilitated, the function of prefronto-cerebellar pathways, e.g. due to functional decoupling between DLPFC and cerebellum as a result of tDCS simultaneously increasing and decreasing the activity of these interconnected areas, or a reversal in the flow of information (DLPFC to cerebellum versus cerebellum to DLPFC). This third possibility is particularly intriguing, as prior research with a similar tDCS montage in patients with stable mood disorders showed improvements in neurocognitive performance.21 Last, we also observed that active tDCS caused an increase in cue-triggered appetite (hunger and desire to eat) and a trend-level impairment of food-related working memory performance. These effects could be accounted for by the above scenarios, by an impact on cerebellar connections to reward centers -known to be altered in obesity27, or simply as a result of elevated homeostatic motivation to eat.
Our study has a number of limitations. The prefronto-cerebellar tDCS montage that we used has poor topographic resolution, making it impossible to explain effects based on specific cerebellar subregions or brain circuits. Future studies should combine tDCS with fMRI, allowing for a detailed topographical characterization of the effects and their association with specific mechanisms. Also, we only examined the impact of tDCS on the left DLPFC/right cerebellum pathway. Whether the observed effects can be extended to the homologous pathway, i.e. right DLPFC/left cerebellum, remains unknown. We selected this specific side as a first investigation, but there is no clear evidence of lateralization, based on the available neuroimaging data.22
Given that our results were in the opposite direction as hypothesized, we cannot make conclusions on the potential of prefronto-cerebellar neuromodulation for the treatment of obesity. Our findings call for alternative strategies to influence prefronto-cerebellar pathways in the direction of appetite reduction and improvement of behavioral control over food cues. Future studies should examine the effects of reversing the polarity of the tDCS montage that we used here, and other higher resolution approaches to simultaneously enhance DLPFC and cerebellum activity. If a benefit can be confirmed, clinical trials evaluating the effect of repeated tDCS sessions on body weight are warranted. It is also unclear whether the effects that we found here are specific of obesity or, rather, can be extended to individuals with healthy weight or undereating conditions. Notwithstanding these limitations, our study represents the first direct evidence that the human cerebellum, possibly via prefronto-cerebellar pathways, may be involved in the regulation of appetite and food cue reactivity, uncovering a role in processes that are central to the behavioral manifestations of obesity.
Supplementary Material
Acknowledgements
Dr. Alonso-Alonso is a recipient of grants from the Boston Nutrition and Obesity Research Center, P30 DK046200, the Nutrition Obesity Research Center at Harvard, P30 DK040561, and the Center for Nutritional Research Charitable Trust. We thank Universitat Oberta de Catalunya for its support in this research and Clinica Sagrada Familia for its involvement in the study. We also thank all participants who took part in this study for their time and help.
Footnotes
Conflict of Interest
Drs. Abhishek Datta and Marom Bikson are co-founders of Soterix Medical. The City University of New York has patent applications in Drs. Datta and Bikson’s name on brain stimulation. The rest of the authors declare no conflict of interest.
References
- 1.Vainik U, Dagher A, Dubé L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: a systematic review. Neurosci Biobehav Rev 2013; 37: 279–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev 2012; 13: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-García I, Michaud A, Dadar M, Zeighami Y, Neseliler S, Collins DL et al. Neuroanatomical differences in obesity: meta-analytic findings and their validation in an independent dataset. Int J Obes 2018. doi: 10.1038/s41366-018-0164-4. [DOI] [PubMed] [Google Scholar]
- 4.Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M et al. Differential brain responses to satiation in obese and lean men. Diabetes 2000; 49: 838–846. [DOI] [PubMed] [Google Scholar]
- 5.Tomasi D, Wang G-J, Wang R, Backus W, Geliebter A, Telang F et al. Association of body mass and brain activation during gastric distention: implications for obesity. PloS One 2009; 4: e6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noori HR, Cosa Linan A, Spanagel R. Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: A comprehensive meta-analysis. Eur Neuropsychopharmacol 2016; 26: 1419–1430. [DOI] [PubMed] [Google Scholar]
- 7.Weise CM, Piaggi P, Reinhardt M, Chen K, Savage CR, Krakoff J et al. The obese brain as a heritable phenotype: a combined morphometry and twin study. Int J Obes 2017; 41: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman SM, Paz-Filho G, Wong M-L, Kohno M, Licinio J, London ED. Effects of leptin deficiency and replacement on cerebellar response to food-related cues. Cerebellum 2013; 12: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raschpichler M, Straatman K, Schroeter ML, Arelin K, Schlögl H, Fritzsch D et al. Abdominal fat distribution and its relationship to brain changes: the differential effects of age on cerebellar structure and function: a cross-sectional, exploratory study. BMJ Open 2013; 3. doi: 10.1136/bmjopen-2012-001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J-N, Wang J-J. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol 2008; 28: 469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmahmann JD. The Cerebellum and Cognition. Academic Press: San Diego, CA, 1997. [Google Scholar]
- 12.D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits 2012; 6: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 2013; 80: 807–815. [DOI] [PubMed] [Google Scholar]
- 14.Koziol LF, Budding D, Andreasen N, D’Arrigo S, Bulgheroni S, Imamizu H et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum 2014; 13: 151–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno-Rius J, Miquel M. The cerebellum in drug craving. Drug Alcohol Depend 2017; 173: 151–158. [DOI] [PubMed] [Google Scholar]
- 16.Palesi F, De Rinaldis A, Castellazzi G, Calamante F, Muhlert N, Chard D et al. Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci Rep 2017; 7: 12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 2018; 19: 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimulat 2016; 9: 641–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinitz S, Reinhardt M, Piaggi P, Weise CM, Diaz E, Stinson EJ et al. Neuromodulation directed at the prefrontal cortex of subjects with obesity reduces snack food intake and hunger in a randomized trial. Am J Clin Nutr 2017; 106: 1347–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Val-Laillet D, Aarts E, Weber B, Ferrari M, Quaresima V, Stoeckel LE et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage Clin 2015; 8: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bersani FS, Minichino A, Bernabei L, Spagnoli F, Corrado A, Vergnani L et al. Prefronto-cerebellar tDCS enhances neurocognition in euthymic bipolar patients. Findings from a placebo-controlled neuropsychological and psychophysiological investigation. J Affect Disord 2017; 209: 262–269. [DOI] [PubMed] [Google Scholar]
- 22.Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R et al. Non-invasive cerebellar stimulation--a consensus paper. Cerebellum 2014; 13: 121–138. [DOI] [PubMed] [Google Scholar]
- 23.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016; 127: 1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blundell JE, De Graaf K, Finlayson G, Halford JCG, Hetherington M, King NA et al. Measuring food intake, hunger, satiety and satiation in the laboratory In: Allison DB, Baskin ML (eds). Handbook of Assessment Methods for Eating Behaviours and Weight-Related Problems : Measures, Theory and Research [2nd. ed.]). Sage: Newbury Park, California, 2009, pp 283–325. [Google Scholar]
- 25.Demirtas-Tatlidede A, Freitas C, Pascual-Leone A, Schmahmann JD. Modulatory effects of theta burst stimulation on cerebellar nonsomatic functions. Cerebellum 2011; 10: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contreras-Rodríguez O, Vilar-López R, Andrews ZB, Navas JF, Soriano-Mas C, Verdejo-García A. Altered cross-talk between the hypothalamus and non-homeostatic regions linked to obesity and difficulty to lose weight. Sci Rep 2017; 7: 9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carnell S, Benson L, Pantazatos SP, Hirsch J, Geliebter A. Amodal brain activation and functional connectivity in response to high-energy-density food cues in obesity. Obesity 2014; 22: 2370–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.