Abstract
Background/Aim: Increased oxidative stress plays a crucial role in pathogenesis of various diseases. The present study aims to investigate glutathione reductase (GR) and malondialdehyde (MDA) enzymes as markers of oxidative stress mechanisms in lumbar disc degeneration disease (LDDD). Patients and Methods: The study group consisted of 39 patients diagnosed with LDD and 37 healthy individuals in the control group. The enzyme-linked immunosorbent assay (ELISA) method was used to determine serum GR and MDA levels in the two study groups. Results: Serum GR levels were significantly lower (p=0.008), while MDA levels were significantly higher in the patient group compared to the controls (p=0.025). Conclusion: Oxidative stress mechanisms play a crucial role in disc degeneration and GR deficiency could be an eligible risk factor for LDDD.
Keywords: Lumbar disc degeneration, biomarker, glutathione reductase, malondialdehyde
Low back pain and lumbar disc herniation are major problems in industrialized societies especially, hindering the labor force in the modern way of living (1). Despite the advances in treatment, total recovery remains impossible for these disorders. Hence, multidisciplinary diagnostic methods and multimodal treatment are needed in order to better treat low back pain (1,2).
Besides several biomechanical and biochemical changes involved in intervertebral disc degeneration; several intrinsic, extrinsic, and genetic factors play a role in its development (3). Compression, torsional injuries, overloads, and congenital anomalies in the spinal column result in disc degeneration due to over-compression of the intervertebral disc (4). Furthermore, disruption of the nutritional support of the disc may aggravate the degeneration (5). Although there are several available studies on disc degeneration in the literature, the physiopathology and etiology of disc degeneration is not completely understood (4,6).
Found in high concentrations in all prokaryotic and eukaryotic cells; glutathione (GSH) is a tripeptide synthesized from glutamic acid, cysteine, and glycine (7). Glutathione was first discovered in the yeast in 1888 after it was purified and characterized by Hopkins (8). Glutathione protects the cell against the noxious effects of oxidized molecules with the -SH groups in its structure. The main functions of glutathione include: i) detoxification of free radicals and reactive oxygen species (ROS) in the cell, ii) protection of thiol moieties in various enzymes and membrane proteins, such as hemoglobin and spectrin iii) synthesis of DNA and proteins, iv) conjugation of xenobiotics, some antineoplastic drugs, and some metabolic end products for detoxification, v) transport of amino acids, vi) cleavage of disulfide bonds of insulin and some other proteins, leading to alterations in the conformation of their protein structures, vii) storage of intracellular cysteine, and viii) involvement in several enzymatic reactions (9-11). Because these are critical tasks, metabolic impairment may occur due to low intracellular concentration of glutathione (12). Glutathione reductase (GR) was first discovered in 1951. This enzyme catalyzes the transfer of electrons between low- or high-molecular-weight disulfide substrates and reduced pyridine nucleotides (13).
One of the most critical aims of the GR-catalyzed reaction is to maintain the intracellular ratio of reduced glutathione/ oxidized glutathione (GSH/GSSG) (14). Glutathione reductase maintains the intracellular -SH /-SS ratio by increasing the GSH/GSSG ratio (12,15,16). In addition, the GR enzyme is used in the clinical practice for the diagnosis of liver diseases, evaluation of nutritional status, assessing riboflavin deficiency, and diagnosis of genetic disorders (17). At the time of the preparation of this article, no studies were available in the literature investigating the role of glutathione mechanism in disc degeneration. Therefore, we evaluated whether glutathione reductase and malondialdehyde were associated with lumbar disc herniation.
Patients and Methods
Study population and clinical procedures. A total of 76 individuals were included in the study, comprising of 39 patients with lumbar disc degeneration (LDD) and 37 healthy volunteers. This study was approved by the Ethics Committee of the Yeditepe University. The patients admitted to the Yeditepe University, Department of Neurosurgery due to a diagnosis of LDD were included in the study. Patient data and clinical and diagnostic examination findings including: i) age, ii) symptoms, iii) neurological examination findings, iv) scores of Visual Analogue Scale (VAS) for pain, v) the Oswestry Disability Index (ODI) scores, and vi) the lumbar MRI findings, were recorded for statistical analyses (18). It was ensured that the individuals in the control group had never experienced back pain or radiculopathy.
The eligible patients with lower back pain and radiculopathy were included in the study along with the identification of either LDD or disc hernia using lumbar magnetic resonance imaging (MRI). The patients with a history of trauma, recent infections, congenital anomalies, osteoporosis, oncologic diseases, spondylolisthesis, vertebral fractures, or spinal deformities were excluded from the study. The severity of pain was scored using VAS and ODI (18). Informed consent forms on paper were obtained from all patients and controls, who agreed to participate in the study.
The blood samples were collected from the LDDD patients 24-48 hours before the surgery. After the plasma samples were centrifuged, they were frozen at -80°C and were stored until the time of assay. The blood samples of the control group were processed similarly, using the same methods and procedures. The samples were labeled with registration numbers of the patients to enable identification and to prevent any confusion.
Measurement of glutathione reductase enzyme levels. The collected and stored blood samples of the participants were used for quantifying GR and MDA levels. For this purpose, the two-site sandwich ELISA method was employed using Human Malondialdehyde (MDA) ELISA Kit and Human Glutathione Reductase ELISA Kit (Abbkine Scientific Co., Ltd., Wuhan, Hubei, China) GR and MDA levels were determined by reading the color changes compared to the standard curve in photometry.
Statistical analyses. IBM SPSS Statistics version 23 (IBM Corp., Armonk, NY, USA) package program was used for the statistical analyses. The statistical significance was accepted at p<0.05. The collected data were summarized in numbers, percentages, and mean±standard deviation. Chi-square analysis was performed to evaluate the data. The quantitative parameters collected from the LDDD patients and control groups were analyzed using the student t-test.
Results
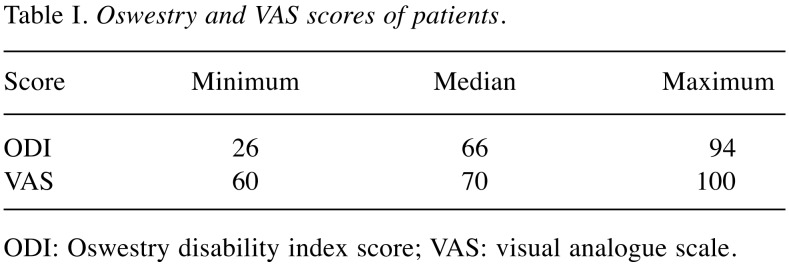
Our study included a total of 76 participants assigned into two groups: i) 39 patients with LDD and ii) 37 healthy individuals. The mean age was 43±10.34 years in the patient group and 45.43±10.01 years in the control group. The mean ages in the two study groups were not significantly different (p=0.301). Of the participants in the patient group, 35.9% were males and 64.1% were females. Of the individuals in the control group, 62.2% were males and 37.8% were females. There was a statistically significant difference in the gender distribution between the study groups. It was found that females were 2.9 times more likely to develop LDD (x2=5.243, p=0.022, OR=2.934, 95%CI=1.155-7.454) compared to males. History of experiencing low back pain at least once and having had a lumbar MRI before the diagnosis was present in 12 out of 39 patients. In the patient group, 23 (59%) had a lumbar disc herniation and 16 (41%) had a “black disc disease”. The mean scores of VAS and ODI in the patient group are presented in Table I.
Table I. Oswestry and VAS scores of patients.
ODI: Oswestry disability index score; VAS: visual analogue scale.
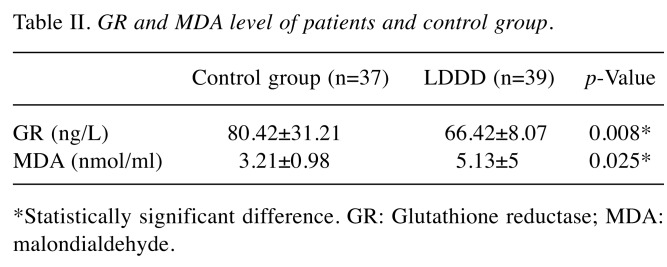
The serum GR and MDA levels in the patient and control groups are presented in Table II. It was found out that serum GR levels were significantly higher in the control group compared to the patient group (p=0.008), however, the MDA levels were significantly higher in the patient group compared to the control group (p=0.025).
Table II. GR and MDA level of patients and control group.
*Statistically significant difference. GR: Glutathione reductase; MDA: malondialdehyde.
Discussion
The intervertebral disc is composed of 3 sections: i) the annulus fibrosus, ii) the nucleus pulposus, and iii) the cartilaginous plaque (19). During childhood, the nucleus pulposus is found in liquid form, however, it progressively dehydrates and undergoes shrinkage over the years (20). Furthermore, over the years, negative changes in matrix integrity, reduced cross-link profile and remodeling of tissue could give rise to LDD (21), the displacement of which may cause disc herniation (22).
Intervertebral disc degeneration is associated with several biomechanical and biochemical changes, along with the involvement of several intrinsic, extrinsic, and genetic factors. Disc degeneration results from over-compression on the intervertebral disc due to compression, torsional injuries, overloads, and congenital anomalies in the spinal column, as well as disruptions to the nutritional support of the disc aggravating the degeneration. Although several studies about disc degeneration are available in the literature, the pathophysiology and etiology of disc degeneration have not been clearly explained yet (6). However, it has been suggested that cell death and extracellular matrix destruction due to oxidative stress is closely associated with the development of disc degeneration (23). Glutathione is a natural peptide found in the cytoplasm of human cells and it plays a critical role as an effective antioxidant in protecting living cells (24).
Oxygen radicals cause reversible or irreversible noxious effects on proteins, free amino acids, lipids, lipoproteins, carbohydrates, and connective tissue molecules. Increased concentrations of superoxide radicals and hydrogen peroxide directly cause cellular injury (25). As a result, the concentrations of reduced NAD, GSH, and ATP become lower, and thus aggravate the injury (26). The superoxide radicals and H2O2 are synthesized in the body as a result of normal metabolic processes. Consequently, they are removed from the environment by superoxide dismutase and glutathione peroxidase (27). These reactions convert GSH to GSSG by means of glutathione peroxidase. GSSG, in turn, is converted to GSH by glutathione reductase (10,11,27). Antioxidant GSSG molecule receives reducing equivalents in a variety of chemical mitochondrial redox system and forms glutathione/oxidized glutathione (GSH/GSSG) system. This system consists of GSH, a γ-Glu-Cys-Gly tripeptide and the pyridine nucleotide disulfide oxidoreductase, GSSG reductase (28). Reduced glutathione (GSH) plays important roles in intracellular and extracellular antioxidant processes by controlling signaling cascades of some xenobiotics and heavy metals detoxification. Furthermore, GSH maintains the intracellular ratio of reduced glutathione/oxidized glutathione (GSH/GSSG) via GR-catalyzed reaction (12,15,16). On the other hand, the GR enzyme is used as a diagnostic marker for several diseases (17). To the best of our knowledge, there is no published study that has investigated the relation between GR level and lumbar disc degeneration.
In our study, when the patients with lumbar disc degeneration were compared to the control group, it was found that the levels of GR were significantly lower; however, MDA values were significantly higher. These findings suggest that GR deficiencies, combined with the involvement of environmental factors, may be the cause of lumbar disc degeneration and lumbar disc herniation. There is a need for further large-scale studies comparing study groups with similar ratios of gender distribution.
Conflicts of Interest
The Authors declare no conflicts of interest with regards to this study.
Authors’ Contributions
Tİ contributed to the design and implementation of the research, approval of the final version, CKY, AHK, SO were responsible for the recruitment of study participants and the clinical investigation, data collection. SGY was responsible for the statistical analysis, study design, and for writing the manuscript. SDB was responsible for the study design, performed the experiments and thestatistical analysis, and wrote the manuscript. DB performed the experiments. All authors read and approved the final manuscript.
Acknowledgements
None.
References
- 1.Allegri M, Montella S, Salici F, Valente A, Marchesini M, Compagnone C, Baciarello M, Manferdini ME, Fanelli G. Mechanisms of low back pain: A guide for diagnosis and therapy. F1000Res. 2016;5 doi: 10.12688/f1000research.8105.1. PMID: 27408698. DOI: 10.12688/f1000research.8105.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it. Spine (Phila Pa 1976) 2006;31(18):2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. PMID: 16915105. DOI: 10.1097/01.brs.000 0231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 3.Chan D, Song Y, Sham P, Cheung KMC. Genetics of disc degeneration. Eur Spine J . 2006;15(Suppl 3):317–325. doi: 10.1007/s00586-006-0171-3. PMID: 16819621. DOI: 10.1007/s00586-006-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi YS. Pathophysiology of degenerative disc disease. Asian Spine J. 2009;3(1):39–44. doi: 10.4184/asj.2009.3.1.39. PMID: 20404946. DOI: 10.4184/ asj.2009.3.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urban JPG, Robert S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–130. doi: 10.1186/ar629. PMID: 12723977. DOI: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willems N, Tellegen AR, Bergknut N, Creemers LB, Wolfswinkel J, Freudigmann C, Benz K, Grinwis GC, Tryfonidou MA, Meij BP. Inflammatory profiles in canine intervertebral disc degeneration. BMC Vet Res. 2016;12:10–10. doi: 10.1186/s12917-016-0635-6. PMID: 26757881. DOI: 10.1186/s12917-016-0635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rana SV, Allen T, Singh R. Inevitable glutathione, then and now. Indian J Exp Biol. 2002;40(6):706–716. PMID: 12587718. [PubMed] [Google Scholar]
- 8.Aoyama K, Nakaki T. Impaired Glutathione Synthesis in Neurodegeneration. Int J Mol Sci. 2013;14(10):21021–21044. doi: 10.3390/ijms141021021. PMID: 24145751. DOI: 10.3390/ijms141021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. PMID: 22228951. DOI: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta. 2013;1830(5):3217–3266. doi: 10.1016/j.bbagen.2012.09.018. PMID: 23036594. DOI: 10.1016/ j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar B, Kulharia M, Mantha AK. Understanding human thiol dioxygenase enzymes: Structure to function, and biology to pathology. Int J Exp Pathol. 2017;98(2):52–66. doi: 10.1111/iep.12222. PMID: 28439920. DOI: 10.1111/iep.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knapen MF, Zusterzeel PL, Peters WH, Steegers EA. Glutathione and glutathione-related enzymes in reproduction. A review. Eur J Obstet Gynecol Reprod Biol. 1999;82(2):171–184. doi: 10.1016/s0301-2115(98)00242-5. PMID: 10206412. DOI: 10.1016/s0301-2115(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 13.Temel Y, Bozkuş T, Karagözoğlu Y, Çiftçi M. Purification and Characterization of Glutathion Reductase Enzyme from Japanese Quail (Coturnix coturnix japanica) Erythrocytes. Igdır Univ J Inst Sci & Tech. 2017;7(3):143–150. DOI: 10.21597/jist.2017.172. [Google Scholar]
- 14.Toribio F, Martinez-Lara E, Pascual P, Lopez-Barea J. Methods for purification of glutathione peroxidase and related enzymes. J Chromatogr B Biomed Appl. 1996;684(1-2):77–97. doi: 10.1016/0378-4347(95)00504-8. PMID: 8906467. DOI: 10.1016/0378-4347(95)00504-8. [DOI] [PubMed] [Google Scholar]
- 15.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. PMID: 20045723. DOI: 10.1016/j.freeradbiomed. 2009. 12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Circu ML, Aw TY. Glutathione and modulation of cell apoptosis. Biochim Biophys Acta. 2012;1823(10):1767–1777. doi: 10.1016/j.bbamcr.2012.06.019. PMID: 22732297. DOI: 10.1016/j.bbamcr.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulherin DM, Thurnham DI, Situnayake RD. Glutathione reductase activity, riboflavin status, and disease activity in rheumatoid arthritis. Ann Rheum Dis. 1996;55(11):837–840. doi: 10.1136/ard.55.11.837. PMID: 8976642. DOI: 10.1136/ard.55.11.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz FK, Bohl DD, Webb ML, Russo GS, Grauer JN. Oswestry disability index is a better indicator of lumbar motion than the visual analogue scale. Spine J. 2014;14(9):1860–1865. doi: 10.1016/j.spinee.2013.10.027. PMID: 24216395. DOI: 10.1016/j.spinee.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Wright AC, Yoder JH, Vresilovic EJ, Elliott DM. Theory of MRI contrast in the annulus fibrosus of the intervertebral disc. MAGMA. 2016;29(4):711–722. doi: 10.1007/s10334-015-0522-3. PMID: 26755061. DOI: 10.1007/s10334-015-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiler C, Schietzsch M, Kirchner T, Nerlich AG, Boos N, Wuertz K. Age-related changes in human cervical, thoracal and lumbar intervertebral disc exhibit a strong intra-individual correlation. Eur Spine J Suppl. 2012;6:810–818. doi: 10.1007/s00586-011-1922-3. PMID: 21837413. DOI: 10.1007/s00586-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–780. doi: 10.1038/nrm3904. PMID: 25415508, DOI: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amin RM, Andrade NS, Neuman BJ. Lumbar disc herniation. Curr Rev Musculoskelet Med. 2017;10(4):507–516. doi: 10.1007/s12178-017-9441-4. PMID: 28980275. DOI: 10.1007/s12178-017-9441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou G, Lu H, Chen M, Yao H, Zhao H. Oxidative stress participates in age-related changes in rat lumbar intervertebral discs. Arch Gerontol Geriatr. 2014;59(3):665–669. doi: 10.1016/j.archger.2014.07.002. PMID: 25081833. DOI: 10.1016/j.archger.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Yang D, Wang D, Shimer A, Shen FH, Li X, Yang X. Glutathione protects human nucleus pulposus cells from cell apoptosis and inhibition of matrix synthesis. Connect Tissue Res. 2014;55(2):132–139. doi: 10.3109/03008207.2013.876421. PMID: 24409809. DOI: 10.3109/0300 8207.2013.876421. [DOI] [PubMed] [Google Scholar]
- 25.Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30(1):11–26. doi: 10.1007/s12291-014-0446-0. PMID: 25646037. DOI: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halliwell B, Gutteridge JMC. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. PMID: 3908871. DOI: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 27.Halliwell B, Gutteridge JMC. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch Biochem Biophys. 1986;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. PMID: 3010861. DOI: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- 28.Prast-Nielsen S, Huang HH, Williams DL. Thioredoxin glutathione reductase: its role in redox biology and potential as a target for drugs against neglected diseases. Biochim Biophys Acta. 2011;1810(12):1262–1271. doi: 10.1016/j.bbagen.2011.06.024. PMID: 21782895. DOI: 10.1016/j.bbagen.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]