Abstract
Aim: To report results from the first phase I study of napabucasin plus paclitaxel in Japanese patients with pre-treated unresectable/recurrent gastric cancer. Patients and Methods: Patients received napabucasin (480 mg bid) plus paclitaxel [80 mg/m2 on days 3, 10 and 17 (cycles 1 and 2) and on days 1, 8 and 15 (cycle 3 and subsequent cycles)] until disease progression or unacceptable toxicity. Primary objectives were tolerability, safety and pharmacokinetics of napabucasin plus paclitaxel. Trial registration ID: JapicCTI-142420. Results: Six patients were enrolled. Paclitaxel had a minimal effect on napabucasin pharmacokinetics and median plasma paclitaxel concentrations were similar in combination and monotherapy. No dose-limiting toxicities were observed. There were no grade 4/5 adverse events. Partial response, stable disease and progressive disease were reported in two patients each. Conclusion: Napabucasin plus paclitaxel was well-tolerated in Japanese patients with gastric cancer.
Keywords: Napabucasin, cancer stem cells, gastric cancer
Gastric cancer (GC) is the third most common cause of cancer-related deaths worldwide (1). Chemotherapy is the standard of care for the majority of patients with advanced, unresectable or metastatic disease (2) with standard first-line treatment being fluoropyrimidine plus platinum combinations (3); patients who have human epidermal growth factor 2 (HER2)-positive tumours should also receive trastuzumab. Second-line treatment options include chemotherapy (docetaxel, paclitaxel or irinotecan), and ramucirumab, a monoclonal antibody vascular endothelial growth factor receptor-2, as monotherapy or in combination with paclitaxel (2,4). However, the prognosis for patients with advanced or recurrent GC is still poor, and new therapies are needed (5).
Accumulating evidence suggests that cancer stem cells (CSCs) play a key role in metastasis, relapse and resistance to treatment in a number of cancer types, including GC (6). Therefore, targeting CSCs might improve patient outcomes compared with current standards of care (7).
Napabucasin is an investigational, orally administered agent which inhibits cancer stemness pathways (8). It is a small-molecule inhibitor of the signal transducer and activator of transcription 3 (STAT3) pathway, which has been shown to target expression of cancer stemness-related genes, block tumor cell sphere formation and kill CSCs isolated from various cancer types (9). Furthermore, a phase I dose-escalation study reported that napabucasin monotherapy was well-tolerated and showed signs of clinical activity in patients with advanced cancer (10). In this phase I study, we assessed the safety, tolerability, pharmacokinetics (PK) and efficacy of napabucasin plus weekly paclitaxel in Japanese patients with pre-treated advanced or recurrent gastric cancer.
Patients and Methods
Study design. This was an open-label phase I study in patients with unresectable/recurrent GC. Patients received oral napabucasin (Boston Biomedical, Cambridge, MA, USA) at 480 mg twice daily (bid) and a 1-hour infusion of paclitaxel (80 mg/m2 on days 3, 10 and 17 in cycles 1 and 2, and on days 1, 8 and 15 in cycle 3 and subsequent cycles) after taking the first dose of napabucasin. Each cycle was defined as 28 days.
Three patients were scheduled to receive napabucasin 480 mg bid in combination with paclitaxel. If one out of three patients experienced a dose-limiting toxicity (DLT) in cycle 1, or if the principal investigator and the sponsor considered the addition of patients necessary for the assessment of PK or efficacy of napabucasin, then a further three additional patients would be enrolled.
Primary study objectives were to investigate the safety, tolerability and PK of napabucasin with paclitaxel. The secondary objective was to investigate preliminary antitumour activity. An exploratory objective was to assess the relationship between biomarkers and the antitumour activity of napabucasin.
The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization and Good Clinical Practice guidelines. An Ethics Committee or Institutional Review Board approved the final protocol at the study site (IRB approval number: K0366). All patients provided their written, informed consent. This study was registered with JapicCTI, number JapicCTI-142420.
Patients. Eligible patients had histologically confirmed advanced or recurrent GC; disease progression after one or more chemotherapy regimen and sufficient organ function. Treatment was continued until disease progression, unacceptable toxicity, or withdrawal of patient consent.
PK assessments. Blood samples for the determination of plasma concentration of napabucasin were collected at cycle 1, day 1: 0 hours (pre-morning dose), and 2, 4, 6, 8, 10, 12 and 24 hours after the morning dose; cycle 1, days 3 and 17: 0 hours (pre-morning dose), and 3, 4 and 24 hours after the morning dose; and cycle 2, day 1: 0 hours (pre-morning dose), and 2, 4, 6, 8, 10 and 12 hours after the morning dose. Blood samples for the determination of plasma concentration of paclitaxel were collected at 3, 4 and 24 hours after the morning dose on days 3 and 17 in cycle 1.
Safety and ant-tumour assessments. Patients were evaluable for DLT assessment if they had received at least one paclitaxel dose and ≥80% of their napabucasin doses in cycle 1. Safety was assessed during cycles 1 and 2 on days 1, 3, 10 and 17; and during cycle 3 and subsequent cycles on days 1, 8 and 15. Adverse events (AEs)were monitored during the study and graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (11), including laboratory testing for blood haematology and serum chemistry.
Efficacy endpoints were the objective tumour response (ORR), progression-free survival (PFS) and overall survival (OS). Tumour assessment was performed on day 1 in cycle 3, and every 8 weeks thereafter until radiographic-confirmed disease progression or the end of treatment scan. Disease progression and tumour response were assessed by investigators in accordance with RECIST 1.1 (12). PFS was defined as the time from the date of the first dose of napabucasin to the date of progressive disease (PD), or death, whichever occurred first. OS was defined as the time from the date of the first dose of napabucasin to the date of death from any cause.
Immunohistochemical (IHC) analysis. Staining of tumour cells for nuclear phosphorylated (p)-STAT3 and β-catenin was performed on archival tumour tissue samples and biopsy samples. IHC for p-STAT3 and β-catenin were performed using rabbit monoclonal antibody to p-STAT3 (Tyr705) (D3A7; XP®Cell Signalling, Danvers, MA, USA) and mouse antibody to β-catenin (BD Biosciences, San Jose, CA, USA). Samples were evaluated by a pathologist and scored using the following intensity scale: 0+ Negative, 1+ weak, 2+ intermediate, 3+ strong.
Results
Patients. A total of six patients were enrolled from March to May 2014. Their median age was 66.0 years and the majority of patients had an Eastern Cooperative Oncology Group performance score of 0 (5/6; 83.3%), and four patients had previously received two or more prior lines of therapy. All patients had received prior chemotherapy with fluoro-pyrimidines and platinum; three patients had received prior taxanes and two patients had received prior irinotecan (13). A total of three patients had undergone gastrectomy.
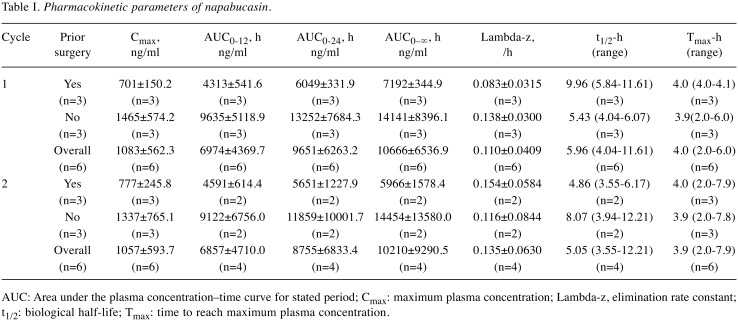
Pharmacokinetics. Despite inter-patient variability in PK analyses, the maximum plasma concentration of napabucasin was similar after single doses and repeated doses (Table I). Plasma concentration of napabucasin was also similar in patients receiving napabucasin alone (cycle 1, day 1) or in combination with paclitaxel (cycle 1, days 3 and 17), suggesting that paclitaxel did not affect the PK profile of napabucasin. The plasma concentration of napabucasin in single doses and repeated doses was similar in patients with previous gastrectomy and those without.
Table I. Pharmacokinetic parameters of napabucasin.
AUC: Area under the plasma concentration–time curve for stated period; Cmax: maximum plasma concentration; Lambda-z, elimination rate constant; t1/2: biological half-life; Tmax: time to reach maximum plasma concentration.
Safety and tolerability. No DLTs were observed. The most common AEs were diarrhoea (n=6), and decreased neutrophil count, decreased white blood cell count and alopecia (all n=4). Diarrhea (grade 1/2, n=6) was considered to be related to napabucasin and the majority of cases were grade 1 (83.3%). Grade 3 AEs were anaemia in one patient, decreased neutrophil count in one patient, decreased white blood cell count in one patient and peripheral neuropathy in one patient (none considered to be related to treatment with napabucasin). There were no grade 4/5 AEs.
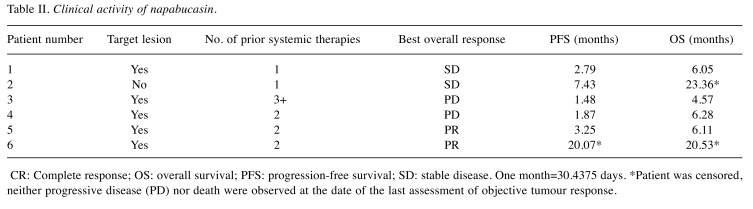
Antitumour activity. Among the six patients, five had measurable disease (Table II). In total, two patients achieved partial responses (PR), two had stable disease and two had PD. Patient 6 achieved PR then discontinued paclitaxel treatment at cycle 7 because of peripheral sensory neuropathy; PR was maintained until cycle 22 with napabucasin monotherapy.
Table II. Clinical activity of napabucasin.
CR: Complete response; OS: overall survival; PFS: progression-free survival; SD: stable disease. One month=30.4375 days. *Patient was censored, neither progressive disease (PD) nor death were observed at the date of the last assessment of objective tumour response.
IHC. Tissue samples were obtained from two patients. A sample was also obtained by biopsy from patient 2 post-napabucasin treatment. In patient 2, p-STAT3 and β-catenin were positive at baseline and β-catenin became negative post-treatment. In contrast, in patient 6, both p-STAT3 and β-catenin were negative in tumour nuclei.
Discussion
In this phase I trial, napabucasin at 480 mg bid plus weekly paclitaxel was well tolerated in Japanese patients with pre-treated advanced or recurrent GC. AEs related to napabucasin were gastrointestinal, generally mild in nature and controlled with concomitant administration of loperamide. The safety profile is also in line with that reported in other phase I clinical trials of napabucasin monotherapy (14,15) and there were no specific AEs reported due to the combination of napabucasin with weekly paclitaxel. Furthermore, the PK results suggested that paclitaxel did not affect the PK profile of napabucasin and that the napabucasin PK profiles were similar between patients with previous gastrectomy and those without. Although antitumour activity was not the primary endpoint of this study, preliminary signs of clinical activity were observed in two patients who achieved PR. One of these patients maintained PR until cycle 21 with napabucasin monotherapy, even after the discontinuation of paclitaxel at cycle 7.
As napabucasin inhibits the STAT3 pathway, which is linked to β-catenin (16,17), a biomarker analysis was performed in patients with long-term survival. A post-napabucasin treatment sample from one patient showed weak pSTAT3 staining in the tumour microenvironment and negative β-catenin staining in tumour nuclei. As there were only two samples, further exploratory studies are needed to clarify the usefulness of pSTAT3 and β-catenin as predictive biomarkers. However, it should be noted that a recent multicentre, phase III trial on colorectal cancer reported that patients with pSTAT3-positive tumours had longer OS with napabucasin compared with placebo (18).
However, it is difficult to draw meaningful conclusions here due to the small sample size of the study. Unfortunately, a subsequent randomised phase III trial comparing paclitaxel plus napabucasin with paclitaxel monotherapy failed to achieve an improvement in OS in pre-treated gastric cancer (19). However, additional phase III studies of napabucasin plus chemotherapeutic agents in other cancer types are currently ongoing (20,21).
Conflicts of Interest
K.S. received personal fees from AbbVie, Astellas Pharma, Bristol-Myers Squibb, Eli Lilly, Ono Pharmaceutical, Novartis, Pfizer, Takeda and Yakult and grants from Eli Lilly, Ono Pharmaceutical, Sumitomo Dainippon Pharma, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharma and MSD. YY and S.I are employees of Sumitomo Dainippon Pharma.
Authors’ Contributions
All Authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published.
Acknowledgements
Medical writing support, under the direction of the Authors, was provided by Mark Holland Ph.D. of CMC CONNECT, a division of McCann Health Medical Communications Ltd., Manchester, UK, and Molly MacFadyen M.Sc., of CMC CONNECT, a division of McCann Health Medical Communications Ltd., Glasgow, UK, funded by Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan, in accordance with Good Publication Practice (GPP3) guidelines. This study was funded by Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan.
References
- 1.Wang C, Zhang J, Cai M, Zhu Z, Gu W, Yu Y, Zhang X. DBGC: A database of human gastric cancer. PLoS One. 2015;10(11):e0142591–e0142591. doi: 10.1371/journal.pone.0142591. PMID: 26566288. DOI: 10.1371/journal. pone.0142591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) J Natl Compr Canc Netw. 2018;16:1–115. doi: 10.6004/jnccn.2018.0001. [DOI] [PubMed] [Google Scholar]
- 3.Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki T, Esaki T, Nagase M, Fujitani K, Yamaguchi K, Ura T, Hamamoto Y, Morita S, Okamoto I, Boku N, Hyodo I. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31(35):4438–4444. doi: 10.1200/JCO.2012.48.5805. PMID: 24190112. DOI: 10.1200/ jco.2012.48.5805. [DOI] [PubMed] [Google Scholar]
- 4.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;2(1):1–19. doi: 10.1007/s10120-016-0622-4. PMID: 27342689. DOI: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lordick F, Allum W, Carneiro F, Mitry E, Tabernero J, Tan P, Van Cutsem E, van de Velde C, Cervantes A. Unmet needs and challenges in gastric cancer: the way forward. Cancer Treat Rev. 2014;40(6):692–700. doi: 10.1016/j.ctrv.2014.03.002. PMID: 24656602. DOI: 10.1016/ j.ctrv.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V. Concise review: Cancer cells, cancer stem cells and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med. 2017;6(12):2115–2125. doi: 10.1002/sctm.17-0138. PMID: 29072369. DOI: 10.1002/sctm.17-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchihara T, Ishimoto T, Yonemura A, Baba H. Therapeutic targets against gastric cancer stem cells interacting with tumor microenvironment. J Cancer Metastasis Treat. 2018;4:9–9. DOI: 10.20517/2394-4722.2017.81. [Google Scholar]
- 8.Boston Biomedical. Boston Biomedical Oncology Pipeline. 2018. Available at: https://www.bostonbiomedical.com/pipeline/. Last accessed on 22nd August 2018.
- 9.Li Y, Rogoff HA, Keates S, Gao Y, Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB, Li CJ. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci USA. 2015;112(6):1839–1844. doi: 10.1073/pnas.1424171112. PMID: 25605917. DOI: 10.1073/pnas.1424171112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langleben A, Supko JG, Hotte SJ, Batist G, Hirte HW, Rogoff H, Li Y, Li W, Kerstein D, Leggett D, Hitron WJ, Li C. A dose-escalation phase I study of a first-in-class cancer stemness inhibitor in patients with advanced malignancies. J Clin Oncol. 2013;31(15 Suppl). Abstract 2542 DOI: 10.1200/jco.2013. 31.15_suppl.2542. [Google Scholar]
- 11.National Cancer Institute. CTCAE v4.0. Available at:https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40. Last accessed on 14th March 2019.
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. PMID: 19097774. DOI: 10.1016/ j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Shitara K, Kuboki Y, Nakamura Y, Doi A, Harada K, Kawazoe A, Doi T, Yoshino T, Yodo Y, Yoshikawa R, Ohtsu A. A phase I study of BBI608, a cancer stemness inhibitor, administered with paclitaxel (PTX) as combination therapy (Rx) for pretreated unresectable or recurrent gastric cancer. J Clin Oncol. 2015;33(15_suppl):e15089. DOI: 10.1200/ jco.2015.33.15_suppl.e15089. [Google Scholar]
- 14.Bekaii-Saab TS, Starodub A, El-Rayes BF, Shahda S, O’Neil BH, Noonan AM, Shaib WL, Hanna WT, Mikhail S, Neki AS, Chang Y, Dai X, Li W, Brooks E, Oh C, Borodyansky L, Li C. Phase 1b/2 trial of cancer stemness inhibitor napabucasin (NAPA) + nab-paclitaxel (nPTX) and gemcitabine (Gem) in metastatic pancreatic adenocarcinoma (mPDAC) J Clin Oncol. 2018;36(Suppl). Abstract 411 DOI: 10.1200/JCO.2018. 36.15_suppl.4110. [Google Scholar]
- 15.Larson T, Feliu Ortuzar W, Bekaii-Saab TS, Becerra C, Ciombor KK, Hubbard JM, Edenfield WJ, Shao SH, Grothey A, Borodyansky L, Xu B, Li W, Li Y, Li C, Khan W. BBI608-224: A phase Ib/II study of cancer stemness inhibitor napabucasin (BBI-608) administered with panitumumab in KRAS wild-type patients with metastatic colorectal cancer. J Clin Oncol. 2017;35(4 Suppl). Abstract 677 DOI: 10.1200/ JCO.2017.35.4_suppl.677. [Google Scholar]
- 16.Anand M, Lai R, Gelebart P. β-catenin is constitutively active and increases STAT3 expression/activation in anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Haematologica. 2011;96(2):253–261. doi: 10.3324/haematol.2010.027086. PMID: 20971814. DOI: 10.3324/haematol.2010.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armanious H, Gelebart P, Mackey J, Ma Y, Lai R. STAT3 upregulates the protein expression and transcriptional activity of ß-catenin in breast cancer. Int J Clin Exp Pathol. 2010;3(7):654–664. PMID: 20830236. [PMC free article] [PubMed] [Google Scholar]
- 18.Jonker DJ, Nott L, Yoshino T, Gill S, Shapiro J, Ohtsu A, Zalcberg J, Vickers MM, Wei AC, Gao Y, Tebbutt NC, Markman B, Price T, Esaki T, Koski S, Hitron M, Li W, Li Y, Magoski NM, Li CJ, Simes J, Tu D, O’Callaghan CJ. Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3(4):263–270. doi: 10.1016/S2468-1253(18)30009-8. PMID: 29397354. DOI: 10.1016/s2468-1253(18) 30009-8. [DOI] [PubMed] [Google Scholar]
- 19.Shah MA, Shitara K, Lordick F, Bang Y-J, Tebbutt NC, Metges J-P, Muro K, Shen L, Tjulandin S, Hays JL, Xu R-H, Fontaine M, Brooks E, Xu B, Li W, Li C, Borodyansky L, Van Cutsem E. The BRIGHTER trial: a phase 3 randomized double-blind study of napabucasin (NAPA) plus paclitaxel (PTX) versus placebo (PBO) plus PTX in patients (pts) with pretreated advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2018;36(15 Suppl). Abstract 4010 DOI: 10.1200/JCO.2018.36.15_suppl.4010. [Google Scholar]
- 20.Grothey A, Shah MA, Yoshino T, Van Cutsem E, Taieb J, Xu R, Tebbutt NC, Falcone A, Cervantes A, Borodyansky L, Li C. CanStem303C trial: A phase III study of napabucasin (BBI-608) in combination with 5-fluorouracil (5-FU), leucovorin, irinotecan (FOLFIRI) in adult patients with previously treated metastatic colorectal cancer (mCRC) J Clin Oncol. 2017;35(suppl). Abstract TPS361 DOI: 10.1200/JCO.2017.35.15_suppl.TPS3619. [Google Scholar]
- 21.Bekaii-Saab TS, Li C-P, Okusaka T, O’Neil BH, Reni M, Tabernero J, Qin S, Van Cutsem E, Borodyansky L, Li C. CanStem111P trial: A phase III study of napabucasin (BBI-608) plus nab-paclitaxel (nab-PTX) with gemcitabine (gem) in adult patients with metastatic pancreatic adenocarcinoma (mPDAC) J Clin Oncol. 2017;35(suppl). Abstract TPS4148 DOI: 10.1200/ JCO.2017.35.15_suppl.TPS4148. [Google Scholar]