Abstract
Background/Aim: Silk is a natural biomaterial with several superior features for applications in regenerative medicine. In the present study an optimized process for manufacturing porous scaffolds out of the silk protein fibroin was developed. Materials and Methods: The silk protein fibroin was dissolved in Ajisawa’s reagent and the resulting fibroin solution was used to produce scaffolds by means of freeze-thawing cycling. Porosity, pressure and stab resistance as well as degradation behavior were assessed in order to characterize the physical properties of the resulting scaffolds. Results: The resulting sponge-like fibroin scaffolds were highly porous while the porosity correlated inversely with the concentration of the starting fibroin solution. Increased initial fibroin concentrations of the scaffolds resulted in increased compressive and cannulation resistance. The majority of the fibroin scaffolds were digested by 1 mg/ml protease XIV in 3 weeks, indicating their biodegradability. Conclusion: The production of scaffolds made of varying fibroin concentrations by means of freeze-thawing, following dissolution using Ajisawa’s reagent, provides a simple and straightforward strategy for adjusting the physical and chemical properties of fibroin scaffolds for various medical applications.
Keywords: Silk, fibroins, biomaterials, regeneration
Silk is a promising biomaterial in tissue engineering and regenerative medicine. Its advantageous features include high biocompatibility, simple biochemical composition, smooth surface, mechanical strength and controllable degradation (1,2). Silk can be manufactured into various forms and structures according to the application demands and therefore provides a favorable basic material in reconstructive medicine (3,4). Mainly composed of fibroin (70-80%) and sericin (20-30%), silk is produced by the silkworm bombyx mori to form cocoons (5). The silk fibers are made of fibroins which are bundled by sericin. Solid silk can be brought into solution by means of removing sericin using acidic or alkaline solutions at a high temperature and subsequently dissolving the fibroin fiber in acidic or highly concentrated ion solutions (6-8). An alternative to the conventional concentrated and highly toxic lithium bromide solution is Ajisawa’s reagent composed of CaCl2–EtOH–H2O, which is inexpensive, simple and much safer (9). The dissolved fibroin can be further processed into various types of materials for medical applications including membranes, thin films, hydrogel, fiber, matrix and sponges (10-12). For example, tube-shaped fibroin sponges held by fibroin membranes can serve as scaffolds for stem cells to differentiate into adipocytes (13). Sponges made of fibroin may provide an attractive alternative to conventional materials, like xenogenous collagen for hemostasis after tooth extraction (14).
So far, fibroin scaffolds have only been produced from fibroin solutions obtained using highly concentrated lithium bromide solutions or formic acid. The present study aimed to establish and optimize a process for producing porous sponge-like scaffolds using the less expensive and safer Ajisawa’s reagent. The resulting scaffolds were characterized for their structural, mechanical and degradation properties. In addition, the effect of concentration of the initial fibroin solution on the properties of the scaffolds was evaluated.
Materials and Methods
Dissolution of fibroin and production of scaffolds. Silk cocoons (Source: Cantiere della provvidenza, Belluno, Italy) were used for fibroin extraction. Forty-five g of cocoons were immersed in 9 l of 0.02 M Na2CO3 solution and boiled at 95±5˚C for 120 min. After washing out the sericin residues using completely desalted water, the released fibroin fibers were placed on a supporting-mesh and air-dried for 24 h. Subsequently, the dried fibroin was mixed with Ajisawa’s reagent which was composed of CaCl2, ethanol and water (1:2:8) at a ratio of 3 g to 27 ml, and kept in a water bath at 78˚C for 1 h. After cooling down, the dissolved fibroin solution was centrifuged at 4,000 rpm (Rotofix 32, Hettich, Tuttlingen, Germany) for 30 min to remove debris and undissolved silk. Desalination of the solution was carried out by dialysis against water for two days to reach the final electrical conductivity of less than 15 μScm/cm. The resulting fibroin solution in a concentration of approximately 6% can either be diluted in distilled H2O or concentrated by evaporating ethanol and water at 60-70˚C.
Fibroin solutions of various concentrations of 1.5-15% were used to produce porous fibroin sponge-like scaffolds using freeze-thawing. Four ml of a fibroin solution and 2 ml of 2% EtOH were mixed in each well of a 12-well cell culture plate. The plate was then sealed and placed into a –20˚C freezer for 24 h. On the next day, the plate was taken out of the freezer and thawed for one h at room temperature. Fibroin attached to the wall of the well was detached using a scalpel. The freezing-thawing process was repeated three times. The resulting fibroin sponges were carefully removed from the wells and stored in water.
Characterization of the scaffolds
Electron microscopy. Surface structure of the fibroin scaffolds was evaluated using a scanning electron microscope (Philips XL30 CP, Philips GmbH, Hamburg, Germany). Scaffolds were centrifuged at 500 rpm (Rotofix 32, Hettich, Tuttlingen, Germany) for 60 min to remove the water. After air-drying for 24 h, samples were sputtered with gold-particles for 90 sec in vacuum (3×10–1 bar) using argon as carrier gas (Sputter Coater S150B, Edwards, London, UK).
Porosity. A measuring cylinder was filled with 50 ml pure hexane. Hexane penetrates the sponge-like scaffolds without changing the pore structure and without causing swelling or shrinking. For measuring porosity, a sponge was left in the measuring cylinder for 5 min for the gas to penetrate into the pores. The volume of the hexane was measured before and after taking out the sponge from the measuring cylinder. Porosity in percent was defined as [volume of hexane in the measuring cylinder after filling – residual volume of hexane after taking out the sponge]/[volume of hexane which was displaced by the sponge – residual volume of hexane after taking out the scaffold] * 100.
Pressure and stab resistance. The pressure resistance of moist scaffolds was measured for compressive strength and stab-resistance using a Zwick/Roell Z010 XForceK (Zwick GmbH & Co KG, Ulm, Germany) with an initial force of 0.2 N and pressing speed of 5 mm/min. Compressive strength was determined by the pressure module (gradient of the compression). Penetration resistance was measured by the depth of a needle stabbing into the sponge under a given force. Stabbing speed was set at 0.5 mm/min with an initial force of 0.2 N. The gradient was determined by force and penetration depth.
Degradation. Degradation of fibroin scaffolds was examined by placing a test group of 4 scaffolds in a solution of 1 mg/ml protease XIV in PBS at 37˚C in a humified atmosphere of 95% air and 5% CO2 for 3 weeks using a HERAcell incubator (HERAcell 150i, Thermo Scientific, Waltham, MA, USA). Four scaffolds placed in PBS without protease XIV were used as a control group. A specially designed probe mount was placed inside the incubator in order to keep the sample flasks in motion during incubation.
Results
Production of fibroin scaffolds. Cylindrical sponge-like scaffolds were successfully produced from fibroin solutions of various concentrations between 1.5 and 15% by means of repeated freezing and thawing (Figure 1). Higher concentration of fibroin led to faster sponge formation, likely due to a more pronounced beta-sheet structure and faster crystallization of the protein. Scaffolds from a higher concentration of fibroin solution developed an intensified yellowish color.
Figure 1. Porous fibroin sponge made after dissolving silk using Ajisawa’s reagent and freeze-thawing.

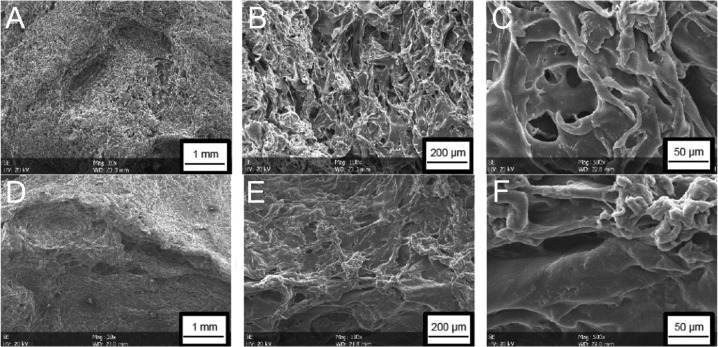
Electron microscopy. The surface of the scaffolds was porous (Figure 2). The porosity was subjectively more prominent in the scaffolds of 6% fibroin solution than in those of 10% fibroin solution. In scaffolds with reduced fibroin concentration, deep holes were visible, while pores of various forms and sizes interleaved each other, resulting in a complex structure. By contrast, the surface of the 10% fibroin scaffolds appeared rather flat and the beta-sheet formations of the fibroin were more compact. Porous structures were also observed in cross-sections of the scaffolds complying with the expectation that the pores pass through the whole sponge.
Figure 2. Electron microscopy of the surface structure of the porous fibroin sponges. Upper row, sponge made of 6% fibroin concentration in solution; lower row, sponge made of 10% fibroin solution. Magnification 20× (A; D), 100× (B; E) and 500× (C; F).
Porosity. Porosity measurement, using hexane gas, revealed that pores occupied 50%±1.6% of the volume of a 6% fibroin sponge. For a 7% fibroin sponge, the ratio of pore volume to total volume was 10% less (40%±2.1%). Prolonged treatment with ethanol led to further crystallization of the fibroin resulting in hardening through enhanced beta-structures of the protein and reduced pore sizes. Subsequently, these ethanol hardened scaffolds were mechanically characterized.
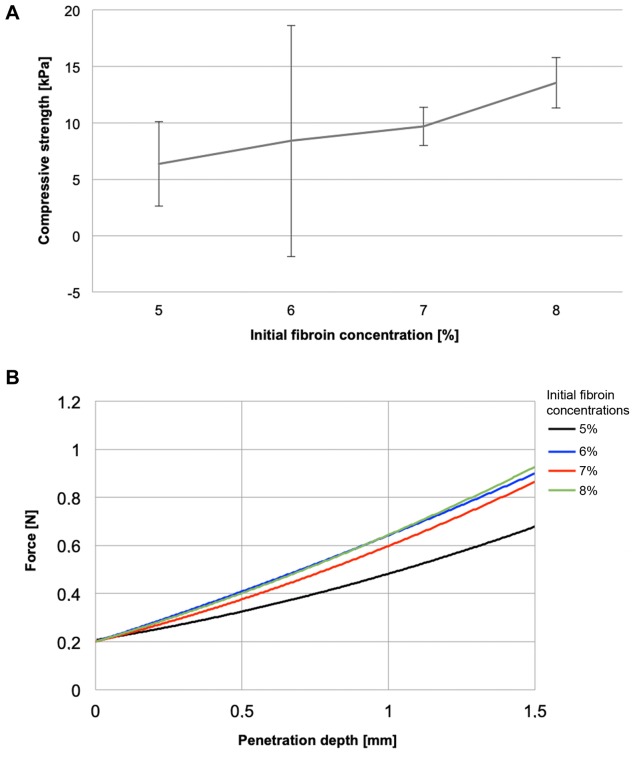
Pressure and penetration resistance. Compressive strength of the scaffolds correlated nearly linearly with the concentration of the initial fibroin solution (Figure 3A). Maximum average compressive strengths of 6.4±3.7 and 13.5±2.2 kPa were measured in the 5% and 8% fibroin scaffolds, respectively. The penetration resistance of the scaffolds correlated positively with the initial fibroin concentrations. The mean force-penetration depth ratio was 0.3 and 0.5 mm/N for scaffolds from 0.5 and 0.8% fibroin solutions, respectively (Figure 3B).
Figure 3. Mechanical characterization of the sponges. A: Compressive strength in correlation with fibroin concentration. B: Penetration resistance.
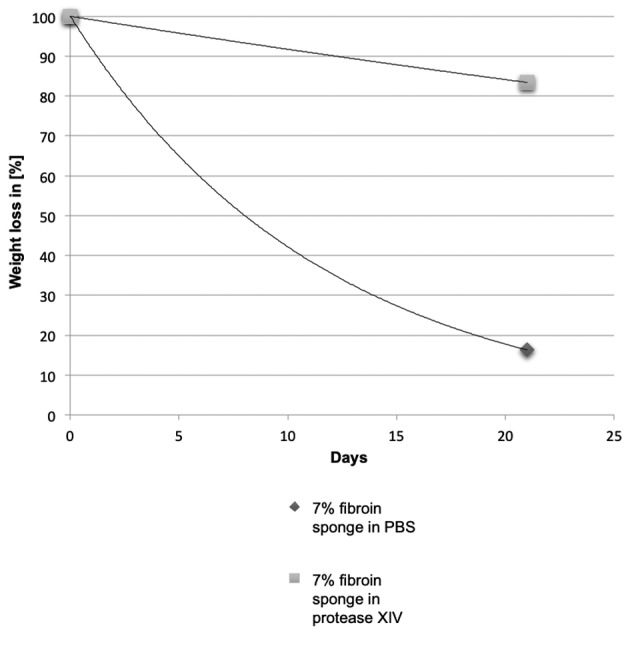
Degradation. When incubated in a solution of 1 mg/ml protease XIV, 4% fibroin scaffolds were completely digested in 3 weeks. Scaffolds containing 7% of fibroin lost approximately 83% of the initial weight after 3 weeks of incubation in protease XIV (Figure 4). In the absence of protease, fibroin scaffolds lost approximately 16% of their initial weight, regardless of the fibroin concentration.
Figure 4. Digestion of 7% fibroin sponges by protease XIV.
Discussion
Many advances in biomaterials science have been made through the past decades (15-17). However, the treatment of extensive defects in soft and hard tissue is still challenging. Silk as biomaterial has been investigated extensively in several studies (18,19). Silk based fibroin scaffolds may be favorable as biomaterial due to their low price, sufficient availability and improved tissue recovery (2). However, conventional manufacturing methods include dissolving in high molarity chaotropic salt solutions such as lithium bromide (LiBr) or ionic liquids (20).
In the present work, an optimized protocol for producing fibroin scaffolds was established. Ajisawa’s reagent, which is inexpensive and harmless unlike the conventional reagents, was used to dissolve fibroin from silk fibers. The freeze-thaw method that is a saltless alternative to conventional methods was used to create sponge-like structures in order to use them as scaffolds for medical applications, e.g. in reconstructive medicine (3,10). The resulting scaffolds are, therefore, free of solvent and metal which is a key issue for application in human body.
Three-dimensional structures of fibroin may be favorable in tissue engineering because they can mimic a physiological environment more precisely than two-dimensional structures (21,22). Three-dimensional fibroin scaffolds can be obtained by salt leaching, gas foaming or freeze-thawing (23). Among them, freeze-thawing, which was first described by Tamada, is the simplest and cheapest way to create 3D-fibroin scaffolds (24). Fibroin scaffolds formed by this process are cytocompatible and demonstrate good mechanical characteristics (25). In this study, cylindrical scaffolds were successfully produced from fibroin solutions of various concentrations using the freeze-thaw method after dissolving procedures with Ajisawa’s reagent. They displayed high porosity that might be a favorable characteristic for cells. Several studies suggested that interconnected pores within fibroin scaffolds may support cell attachment, proliferation and differentiation (26,27). However, this study only covers the physical characterization of the scaffolds and their behavior in vitro still has to be investigated. Mechanical properties of the created scaffolds correlated positively with the initial fibroin concentration of the solutions. Fibroin scaffolds showed sufficient pressure and penetration resistance that allows their usage as material for tissue engineering. However, the freeze-thaw method has the disadvantage of creating pores of varying sizes, uneven surfaces of the scaffolds, and sticking of the fibroin to the well. The latter problems may be overcome to some extent by using other types of well-plates and non-stick coatings. However, caution has to be taken to ensure that no traces of substances will remain in the final products intended for medical application. Rotating the well-plate during the freeze-thaw process may enable more homogeneous porosity in the scaffolds.
Fibroin concentrations influence many features of the resulting scaffolds, including the structure, porosity, mechanical strength, and degradation behavior (28). Therefore, varying fibroin concentration provides a simple and straightforward strategy for tailoring physical and chemical properties of the fibroin scaffolds for various medical needs. Depending on the tissue of application, for example bone, muscle or fatty tissues, scaffolds with the most suitable pore-size can be produced by selecting the appropriate fibroin concentration. However, the accordance between biodegradation of the scaffolds and growth rate of new tissues has to be considered (29). Three-dimensional bioprinting for layer-by-layer fabrication of objects and scaffolds is one topic of current research (30). A recent study described tissue integration of 3D-printed fibroin-based implants in a mouse model (31). Due to the complexity of 3D-printing procedures, currently only simple structures can be printed. However, as printing techniques advance steadily, the construction of complex and multifunctional structures will be possible in the near future (32).
To conclude, this project has established and optimized a simple procedure for dissolving fibroin and producing porous fibroin scaffolds, which also enables tailoring of their physical and mechanical features for various medical applications. Further studies are in progress to assess cytocompatibility and biocompatibility of the developed fibroin scaffolds.
Conflicts of Interest
The Authors declare no conflicts of interests and received no financial funding for this study.
Authors’ Contributions
The work presented here was carried out in collaboration between all authors. AK, RS and AH designed the study. SF, MeB and MiB collected and analyzed the data. SF, RR and SB interpreted the data. LK and RF wrote the first draft of the article. AK, RS and MG drafted the article. MG, RF and AH revised the article. The research consortium completed the final manuscript. All Authors read and approved the final manuscript.
References
- 1.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi: 10.1016/s0142-9612(02)00353-8. PMID: 12423595. [DOI] [PubMed] [Google Scholar]
- 2.Guan G, Bai L, Zuo B, Li M, Wu Z, Li Y, Wang L. Promoted dermis healing from full-thickness skin defect by porous silk fibroin scaffolds (psfss) Biomed Mater Eng. 2010;20(5):295–308. doi: 10.3233/BME-2010-0643. PMID: 21084741. DOI: 10.3233/BME-2010-0643. [DOI] [PubMed] [Google Scholar]
- 3.Smeets R, Knabe C, Kolk A, Rheinnecker M, Grobe A, Heiland M, Zehbe R, Sachse M, Grosse C Siestrup, Woltje M, Hanken H. Novel silk protein barrier membranes for guided bone regeneration. J Biomed Mater Res B Appl Biomater. 2017;105(8):2603–2611. doi: 10.1002/jbm.b.33795. PMID: 27731930. DOI: 10.1002/ jbm.b.33795. [DOI] [PubMed] [Google Scholar]
- 4.Smeets R, Vorwig O, Woltje M, Gaudin R, Luebke AM, Beck-Broichsitter B, Rheinnecker M, Heiland M, Grupp K, Grobe A, Hanken H. Microvascular stent anastomosis using n-fibroin stents: Feasibility, ischemia time, and complications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(5):e97–e103. doi: 10.1016/j.oooo.2016.01.009. PMID: 27068318. DOI: 10.1016/j.oooo.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Sobajo C, Behzad F, Yuan XF, Bayat A. Silk: A potential medium for tissue engineering. Eplasty. 2008;8:e47–e47. PMID: 18997857. [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Ge Z, Wang Y, Toh SL, Sutthikhum V, Goh JC. Modification of sericin-free silk fibers for ligament tissue engineering application. J Biomed Mater Res B Appl Biomater. 2007;82(1):129–138. doi: 10.1002/jbm.b.30714. PMID: 17318818. DOI: 10.1002/ jbm.b.30714. [DOI] [PubMed] [Google Scholar]
- 7.Lovett ML, Cannizzaro CM, Vunjak-Novakovic G, Kaplan DL. Gel spinning of silk tubes for tissue engineering. Biomaterials. 2008;29(35):4650–4657. doi: 10.1016/j.biomaterials.2008.08.025. PMID: 18801570. DOI: 10.1016/j.biomaterials.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lv Q, Feng Q. Preparation of 3-D regenerated fibroin scaffolds with freeze drying method and freeze drying/foaming technique. J Mater Sci Mater Med. 2006;17(12):1349–1356. doi: 10.1007/s10856-006-0610-z. PMID: 17143767. DOI: 10.1007/s10856-006-0610-z. [DOI] [PubMed] [Google Scholar]
- 9.Wang HY, Zhang YQ. Processing and characterisation of a novel electropolymerized silk fibroin hydrogel membrane. Sci Rep. 2014;4:6182–6182. doi: 10.1038/srep06182. PMID: 25154713. DOI: 10.1038/srep06182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etienne O, Schneider A, Kluge JA, Bellemin-Laponnaz C, Polidori C, Leisk GG, Kaplan DL, Garlick JA, Egles C. Soft tissue augmentation using silk gels: An in vitro and in vivo study. J Periodontol. 2009;80(11):1852–1858. doi: 10.1902/jop.2009.090231. PMID: 19905955. DOI: 10.1902/jop.2009.090231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosetti M, Boccafoschi F, Calarco A, Leigheb M, Gatti S, Piffanelli V, Peluso G, Cannas M. Behaviour of human mesenchymal stem cells on a polyelectrolyte-modified hema hydrogel for silk-based ligament tissue engineering. J Biomater Sci Polym Ed. 2008;19(9):1111–1123. doi: 10.1163/156856208785540145. PMID: 18727855. DOI: 10.1163/156856208785540145. [DOI] [PubMed] [Google Scholar]
- 12.Frauchiger DA, May RD, Bakirci E, Tekari A, Chan SCW, Woltje M, Benneker LM, Gantenbein B. Genipin-enhanced fibrin hydrogel and novel silk for intervertebral disc repair in a loaded bovine organ culture model. J Funct Biomater. 2018;9(3) doi: 10.3390/jfb9030040. PMID: 18727855. DOI: 10.3390/jfb9030040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanken H, Gohler F, Smeets R, Heiland M, Grobe A, Friedrich RE, Busch P, Blessmann M, Kluwe L, Hartjen P. Attachment, viability and adipodifferentiation of pre-adipose cells on silk scaffolds with and without co-expressed FGF-2 and VEGF. In Vivo. 2016;30(5):567–572. PMID: 27566073. [PubMed] [Google Scholar]
- 14.Saran K, Shi P, Ranjan S, Goh JC, Zhang Y. A moldable putty containing silk fibroin yolk shell particles for improved hemostasis and bone repair. Adv Healthc Mater. 2015;4(3):432–445. doi: 10.1002/adhm.201400411. PMID: 25296961. DOI: 10.1002/adhm.201400411. [DOI] [PubMed] [Google Scholar]
- 15.Hartjen P, Hoffmann A, Henningsen A, Barbeck M, Kopp A, Kluwe L, Precht C, Quatela O, Gaudin R, Heiland M, Friedrich RE, Knipfer C, Grubeanu D, Smeets R, Jung O. Plasma electrolytic oxidation of titanium implant surfaces: Microgroove-structures improve cellular adhesion and viability. In Vivo. 2018;32(2):241–247. doi: 10.21873/invivo.11230. PMID: 29475905. DOI: 10.21873/invivo.11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartjen P, Nada O, Silva TG, Precht C, Henningsen A, Holthaus MG, Gulow N, Friedrich RE, Hanken H, Heiland M, Zwahr C, Smeets R, Jung O. Cytocompatibility of direct laser interference-patterned titanium surfaces for implants. In Vivo. 2017;31(5):849–854. doi: 10.21873/invivo.11138. PMID: 28882950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vollkommer T, Henningsen A, Friedrich RE, Felthaus OH, Eder F, Morsczeck C, Smeets R, Gehmert S, Gosau M. Extent of inflammation and foreign body reaction to porous polyethylene in vitro and in vivo. In Vivo. 2019;33(2):337–347. doi: 10.21873/invivo.11479. PMID: 30804110. DOI: 10.21873/invivo.11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Y, Wang B. Biodegradation of silk biomaterials. Int J Mol Sci. 2009;10(4):1514–1524. doi: 10.3390/ijms10041514. PMID: 19468322. DOI: 10.3390/ijms10041514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan G, Wang L, Li M, Bai L. In vivo biodegradation of porous silk fibroin films implanted beneath the skin and muscle of the rat. Biomed Mater Eng. 2014;24(1):789–797. doi: 10.3233/BME-130870. PMID: 24211965. DOI: 10.3233/BME-130870. [DOI] [PubMed] [Google Scholar]
- 20.Qi Y, Wang H, Wei K, Yang Y, Zheng RY, Kim IS, Zhang KQ. A review of structure construction of silk fibroin biomaterials from single structures to multi-level structures. Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030237. PMID: 28273799. DOI: 10.3390/ijms18030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–441. doi: 10.1038/nature08602. PMID: 19940913. DOI: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung O, Hanken H, Smeets R, Hartjen P, Friedrich RE, Schwab B, Grobe A, Heiland M, Al-Dam A, Eichhorn W, Sehner S, Kolk A, Woltje M, Stein JM. Osteogenic differentiation of mesenchymal stem cells in fibrin-hydroxyapatite matrix in a 3-dimensional mesh scaffold. In Vivo. 2014;28(4):477–482. PMID: 24982212. [PubMed] [Google Scholar]
- 23.Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5(3):718–726. doi: 10.1021/bm034327e. PMID: 15132652. DOI: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 24.Tamada Y. New process to form a silk fibroin porous 3-D structure. Biomacromolecules. 2005;6(6):3100–3106. doi: 10.1021/bm050431f. PMID: 16283733. DOI: 10.1021/bm050431f. [DOI] [PubMed] [Google Scholar]
- 25.Nourmohammadi J, Roshanfar F, Farokhi M, Haghbin Nazarpak M. Silk fibroin/kappa-carrageenan composite scaffolds with enhanced biomimetic mineralization for bone regeneration applications. Mater Sci Eng C Mater Biol Appl. 2017;76:951–958. doi: 10.1016/j.msec.2017.03.166. PMID: 28482612. DOI: 10.1016/j.msec.2017.03.166. [DOI] [PubMed] [Google Scholar]
- 26.Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009;30(15):2956–2965. doi: 10.1016/j.biomaterials.2009.02.006. PMID: 19249094. DOI: 10.1016/j.biomaterials.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro M, de Moraes MA, Beppu MM, Garcia MP, Fernandes MH, Monteiro FJ, Ferraz MP. Development of silk fibroin/nanohydroxyapatite composite hydrogels for bone tissue engineering. Eur Polym J. 2015;67:66–77. DOI: 10.1016/ j.eurpolymj.2015.03.056. [Google Scholar]
- 28.Koh LD, Cheng Y, Teng CP, Khin YW, Loh XJ, Tee SY, Low M, Ye E, Yu HD, Zhang YW, Han MY. Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci. 2015;46:86–110. DOI: 10.1016/j.progpolymsci.2015.02.001. [Google Scholar]
- 29.Boyd M, Flasza M, Johnson PA, Roberts JS, Kemp P. Integration and persistence of an investigational human living skin equivalent (icx-skn) in human surgical wounds. Regen Med. 2007;2(4):363–370. doi: 10.2217/17460751.2.4.363. PMID: 17608606. DOI: 10.2217/17 460751.2.4.363. [DOI] [PubMed] [Google Scholar]
- 30.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. PMID: 25093879. DOI: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez MJ, Brown J, Giordano J, Lin SJ, Omenetto FG, Kaplan DL. Silk based bioinks for soft tissue reconstruction using 3-dimensional (3D) printing with in vitro and in vivo assessments. Biomaterials. 2017;117:105–115. doi: 10.1016/j.biomaterials.2016.11.046. PMID: 27940389. DOI: 10.1016/j.biomaterials.2016.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Han G, Yan S, Zhang Q. 3D printing of silk fibroin for biomedical applications. Materials (Basel) 2019;12(3) doi: 10.3390/ma12030504. PMID: 30736388. DOI: 10.3390/ma12030504. [DOI] [PMC free article] [PubMed] [Google Scholar]