Abstract
Human enteroviruses are a major cause of neurological and other diseases. Over 100 serotypes are known that exhibit unexplained complex patterns of incidence, from regular cycles to more irregular patterns, and new emergences. Using 15 years of surveillance data from Japan (2000-2014) and a stochastic transmission model with accurate demography, we show that acquired serotype-specific immunity can explain the diverse patterns of 18 of the 20 most common serotypes (including Coxsackieviruses, Echoviruses and Enterovirus-A71). The remaining two serotypes required a change in viral characteristics, including an increase in pathogenicity for Coxsackievirus-A6, consistent with its recent global rise in incidence. Based on our findings, we are able to predict outbreaks two years ahead of time (2015-2016). These results have implications for the impact of vaccines under development.
Over 100 enterovirus serotypes infect humans, and can cause a wide range of diseases, including meningitis, encephalitis, paralysis, myocarditis, haemorrhagic conjunctivitis, upper respiratory infections, herpangina and hand-foot-and-mouth disease (HFMD) (1). This group contains the Coxsackieviruses A (CV-A) and B (CV-B), the Echoviruses (E), and other serotypes simply called Enterovirus (EV), as well as the three poliovirus serotypes, which are the target of a global eradication program. The epidemiology of enteroviruses is characterised by frequent asymptomatic infections, and a peak of disease in summer. Despite the well-defined annual seasonality of enterovirus-associated diseases, individual serotypes show different long-term patterns of circulation, from regular annual or multi-annual cycles, to more irregular patterns. For example, enterovirus A71 (EV-A71) has a 3-year cycle in Malaysia (2) and Japan, but a 1-year cycle in China (3). Some serotypes also sporadically emerge as significant causes of diseases. For example, CV-A6 has emerged during recent years as the main cause of HFMD worldwide (4), and EV-D68 emerged in 2014 in the USA as an important cause of severe respiratory illnesses (5). The increased incidence of HFMD in South-East Asia during the past two decades (with >1 million cases reported in China annually (3)), the large proportion of viral meningitis attributed to non-polio enteroviruses (>75% (6)), and the sporadic emergence of new serotypes attest their clinical and public health importance.
A fundamental challenge for research on enteroviruses and other endemic and antigenically diverse pathogens (e.g., rotavirus, influenza, pneumococcus, and dengue), is to understand what causes the changes in incidence over time of different serotypes. This has direct implications for prediction of future outbreaks of disease and the impact of serotype-specific vaccines, such as licensed monovalent EV-A71 vaccines (7) and other single and multivalent vaccines under development (8). There is currently no accepted mechanistic explanation for the complex long-term patterns of circulation of enterovirus serotypes, including why different serotypes predominate in different years. Homotypic (i.e. serotype-specific) population immunity, heterotypic (i.e. cross serotype) immunity, and virus evolution (including antigenic drift and recombination) could all contribute
In most countries, enterovirus surveillance is passive and thus, mainly captures the serotypes isolated from severe cases suspected of having an enterovirus infection (e.g., neonatal infections and clusters of aseptic meningitis, encephalitis or paralysis). Serotypes isolated from mild diseases are rarely reported. Furthermore, variation in clinical testing and participation of laboratories results in irregular reporting rates over time. In contrast with most countries, Japan has a national disease sentinel-based surveillance system that isolates and identifies enteroviruses from patients attending sentinel sites for four target diseases representative of enterovirus infections: aseptic meningitis (mostly caused by Echoviruses, CV-Bs and CV-A9), HFMD (mostly caused by EV-A71 and CV-A6, 10 and 16), herpangina (mostly caused by CV-As) and acute haemorrhagic conjunctivitis (mostly caused by a variant of CV-A24 and EV-D70) (9). Enterovirus surveillance in Japan therefore captures the circulation of a broad range of serotypes, and does it in a consistent and systematic way. Here, we use time-series analysis and mathematical modeling of the high-quality surveillance data from Japan collected between 2000 and 2016 to identify mechanisms that can explain the transmission dynamics of enterovirus serotypes.
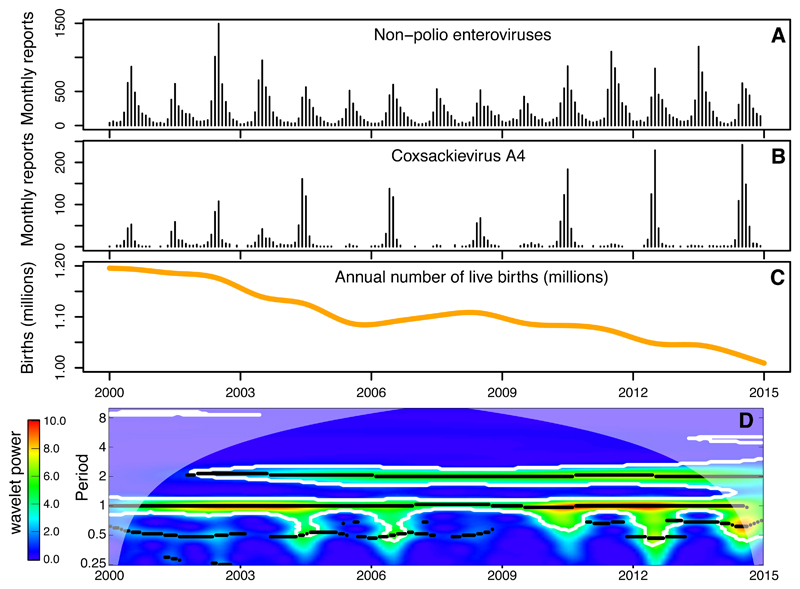
Non-polio enteroviruses show strong seasonality in Japan, with aggregate case numbers showing a peak every summer between January 2000 and December 2014 (Fig. 1A). The incidence of serotypes with >500 isolations during that period (total numbers in Table S1) exhibited diverse patterns (red line in Fig. 2, and Figs. S4–S6), including serotypes with regular cycles of 1 year (e.g. CV-B4), 2 years (e.g. CV-A4), 3 years (e.g. EV-A71), 4 years (e.g. CV-B3), and less regular patterns (e.g. E25). A wavelet analysis (text S1.3) identified these periodicities and their changes over time, i.e. regime shifts (Figs. S7–S9). In particular, we observed that CV-A4 switched from a one-year cycle between 2000 and 2004 to a two-year cycle from 2004 onwards (Figs. 1B and 1D). We hypothesized that if CV-A4 behaved as a highly immunizing infection this temporal pattern could be explained by the general decrease in births that Japan has experienced during the past two decades (Fig. 1C). In this case, only births would replenish the susceptible pool, and consequently determine the chances that an outbreak occurs (10). Similar, but perhaps less obvious signatures in the pattern of other serotypes also indicated this mechanism (e.g. CV-A9, CV-A16). To test this hypothesis, we constructed a simple stochastic transmission model that assumed acquired and life-long homotypic immunity, and that included a seasonal transmission rate, asymptomatic infections, under-reporting, and realistic birth and death rates (text S1.4 and S1.5). Using a maximum-likelihood inference framework based on particle filtering (11), we fitted the model to the number of detections reported each month between January 2000 and December 2014 for each of the 20 most prevalent serotypes with more than one peak of activity. These included seven Coxsackieviruses A, five Coxsackieviruses B, seven Echoviruses, and EV-A71. We then assessed whether the maximum likelihood model was able to predict the 15-year incidence pattern of each serotype when simulating forward from the initial conditions, only varying birth and death rates as observed in Japan. Because demographic stochasticity results in variability between simulated realizations with the same parameter values, we simulated 100 stochastic realizations with the maximum-likelihood estimates (MLE) of the parameters and show here the 10 that most closely match the observed epidemic trajectories (text S2.3). Model failure to reproduce the observed dynamics of specific serotypes guided us to generate hypotheses of extra mechanisms that could explain the observed incidence.
Fig. 1.
Non-polio enterovirus incidence and births in Japan (2000–2014). (A,B) Monthly number of reported enterovirus isolations from January 2000 to December 2014, for: (A) non-polio enteroviruses, and (B) CV-A4. (C) Smoothed annual number of live births. (D) Average wavelet power of the square-root–transformed time-series for CV-A4 showing the emergence of a biennial pattern of incidence.
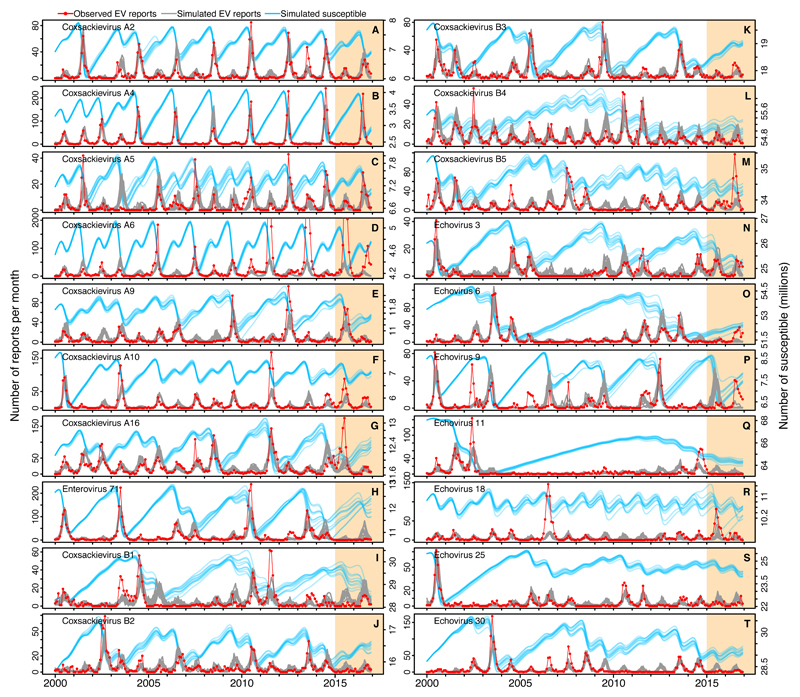
Fig. 2.
Model fit to data. The reported number of monthly isolations (red line) and 10 out of 100 stochastic simulations of the maximum likehood model (gray lines) with the closest match to the data are shown. The estimated number of susceptible individuals (blue lines) is shown in the right y-axis. Maximum likelihood parameter estimation and selection of simulations was based on data for January 2000 to December 2014 (white background), and projections continued out-of-sample for January 2015 to December 2016 (orange background). For CV-A6 and E18, parameter estimation was based on data through to December 2010 and December 2005 respectively, because of the poor fit of the model to the entire period. Parameter values in Table S2.
The model fitted the 15 years of incidence data remarkably well for 18 of the 20 serotypes examined – all, except CV-A6 and E18 (Fig. 2). These included serotypes with periods of 2 years (e.g. CV-A2 and CV-A4), 3 years (e.g. CV-A9 and EV-A71) and even longer (e.g. E3). The model was also able to capture more irregular dynamics, such as those exhibited by E6 and E25, that showed a few years of activity followed by several consecutive years with barely any cases. Underestimation of the largest peaks of cases for a few serotypes could be due to undocumented enhanced sample collection and surveillance efforts during large outbreaks (e.g. 2010 for EV-A71). The model also predicted a few small peaks that were not observed in the data (e.g. 2003 for CV-A5, 2013 for CV-A10, 2005 for CV-B1, 2009–2010 for CV-B5, and 2009–2012 for E30). Possibly these discrepancies reflect gaps in surveillance resulting from the distribution of sentinel sites and spatial clustering of cases, or alternatively, could correspond to local viral extinctions that are not captured by the model. The number of estimated susceptible individuals (blue line in Fig. 2) shows how births progressively increase population susceptibility after each peak of cases, until a threshold is reached and a new outbreak occurs. With decreasing births, it takes longer for this threshold to be reached, increasing the chances of transition to longer cycles (e.g. CV-A4 from 2004 onwards, CV-A9 from 2006, and CV-A16 from 2008), that sometimes result in a bigger outbreak.
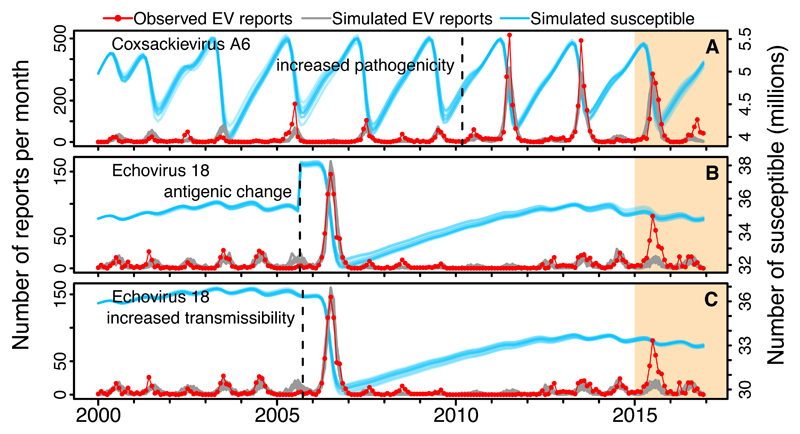
Although the model did not explain the dynamics of CV-A6 and E18 over the entire 15-year period, it fit the data well until the dynamics suddenly changed (2011 and 2006, respectively), suggesting that mechanisms other than homotypic immunity contributed to these dynamics (Fig. 2D and 2R). Virus evolution in antigenic or other regions could perhaps explain these patterns. For example, it has been suggested that diversity in capsid structures of some Echoviruses could have generated different antigenic variants (12), or that recombination in the non-structural proteins could provide a fitness or immunological advantage (13). To test whether a sudden change in transmissibility, pathogenicity or antigenicity alone could explain the pattern of CV-A6 and E18, we fitted three model extensions accounting for those changes (text S1.6). For CV-A6, we estimated that a five-fold increase in pathogenicity in 2010 provided a better fit to the data than models with a change in transmissibility or antigenicity (Fig. 3A). This is consistent with the recent global emergence of this serotype as the main cause of HFMD with atypical symptoms (4), and a change around 2009 in the disease that it caused in Japan (mostly herpangina before, but HFMD since then) (14). For E18, however, either an antigenic change or an increase in transmissibility at the end of 2005 (but not a change in pathogenicity) provided a better fit to the data (Figs. 3B and 3C).
Fig. 3.
Model extensions. Model fit to data for CV-A6 and E18 using the best-fitting model extensions: (A) a five-fold increase in pathogenicity from around 2010 for CV-A6, (B) a change in antigenicity and (C) a 9% increase in transmissibility from around 2006 for E18. Dashed vertical lines indicate the estimated time when the changes occurred. See details in Fig. 2. Parameter values in Table S4.
Parameter estimates revealed interesting differences in transmissibility among the serotypes (Fig. S55 and Table S3). Those associated with HFMD and herpangina (most CV-As and EV-A71) had generally higher estimates of the average basic reproduction number compared with those associated with aseptic meningitis (CV-A9, CV-Bs and Echoviruses). This may reflect higher transmission efficiency of serotypes mainly affecting children and/or differences in transmission routes (fecal-oral vs. respiratory). The estimated reporting probability per infection was between 10-5 and 10-4, which is about 2 orders of magnitude smaller than the probability of paralysis following poliovirus infection (10-3 to 10-3) (15). This may reflect differences in pathogenicity and coverage of the sentinel surveillance system.
Our results have implications for outbreak preparedness. By extending model simulations two years beyond the data used for parameter estimation (i.e. 2015-2016), the magnitude of the epidemics was predicted with reasonable accuracy for most of the serotypes (orange background in Figs. 2 and 3, and Fig. S56). The accuracy of these forecasts was similar to that obtained when simulating forward from 2015 only, using the reconstructed state space variables obtained from the particle-filter (Fig. S57). Inaccurate forecasts for a few serotypes could be due to changes in viral properties during the prediction period, local viral extinctions or inaccurate projections of future number of births. Nevertheless, our simple model could help anticipate years with a large number of cases of a given serotype. These forecasts could be improved by incorporating data on serotype-specific population immunity from seroprevalence surveys.
Similarly, our model could also be used to predict the impact of vaccines under development. For example, we simulated the effect of vaccinating newborns against EV-A71 and CV-A16 from 2008 onwards with a 100% effective vaccine at different coverage levels (Fig. S58). For both serotypes, a coverage of 80% resulted in almost no cases from two years after the introduction of the vaccine, but lower coverage led to continued incidence with altered dynamics and occasionally large outbreaks comparable to those observed pre-vaccination.
Our analysis has some limitations. First, we assumed acquired homotypic immunity against infection is complete and life-long. However, it is likely to be incomplete and wane over time for non-polio enteroviruses, as seen for intestinal immunity to polioviruses (16, 17). Extending our model to include waning immunity (text S1.7) resulted in a similarly good fit to the data (Figs. S62 and S63) and an estimated mean duration of protective immunity that ranged from 8 years to lifelong (Table S5). This relatively long-lasting immunity meant the dynamics were still determined by births, but estimates of the reproduction number were generally lower because infection could be sustained at lower transmission efficiency. Second, we ignored spatial, social and demographic structure of the transmission dynamics. While these simplifications may have affected some of our parameter estimates, they do not affect our main result of the importance of homotypic immunity. Third, although stochastic extinction of a serotype and subsequent reintroduction can occur in our model, it is inherently difficult to predict specific periods where infection may be absent. Finally, smaller changes in pathogenicity, transmissibility or antigenicity may have occurred for serotypes other than CV-A6 and E18, but not led to a strong signature in the incidence dynamics.
Although there is evidence that different enterovirus serotypes share some T- and B-cell epitopes (18, 19), cross-reactive antibodies are not strongly neutralizing and their potential epidemiological implications remain elusive. Our model can explain the temporal dynamics of all the serotypes we examined without the complexity of adding cross-serotype interactions, thus providing evidence that homotypic immunity is the most important driver of the transmission dynamics of enterovirus serotypes. These findings support current development efforts of serotype-specific mono- and multi-valent vaccines, as they point against serotype replacement induced by vaccination. However, two of the serotypes we examined showed dynamics consistent with a change in viral properties (pathogenicity, antigenicity or transmissibility) and emergence as an important cause of disease. A better understanding of this process would support the appropriate choice of serotypes in multivalent vaccines targeting diseases such as HFMD.
Supplementary Material
One sentence summary.
A transmission model with acquired serotype-specific immunity can explain the complex epidemic patterns of diseases caused by human enteroviruses.
Acknowledgements
We thank Hiroyuki Shimizu for providing insightful comments on the enterovirus surveillance system in Japan, Bryan T. Grenfell, Saki Takahashi and Edward P.K. Parker for providing valuable comments to the manuscript, and Natsuko Imai for her help with Japanese.
Funding: M.P.-S. is funded by the Wellcome Trust (grant 106073/Z/14/Z).
Footnotes
Author contributions: M.P.-S. designed research, performed the analyses and wrote the paper. N.C.G. supervised the work and contributed to the writing.
Data and materials availability: All the data used in the manuscript are publicly available in the website of the National Institute of Infectious Diseases (NIID) of Japan: www.niid.go.jp.
References
- 1.Pallansch MA, Oberste MS, Whitton JL. In: Fields Virology. Knipe DM, Howley P, editors. Vol. 2. Lippincott Williams & Wilkins; 2013. chap.17. [Google Scholar]
- 2.Podin Y, et al. Sentinel surveillance for human enterovirus 71 in Sarawak, Malaysia: lessons from the first 7 years. BMC public health. 2006;6:180. doi: 10.1186/1471-2458-6-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xing W, et al. Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis. 2014;14:308–318. doi: 10.1016/S1473-3099(13)70342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bian L, et al. Coxsackievirus A6: a new emerging pathogen causing hand, foot and mouth disease outbreaks worldwide. Expert Rev Anti Infect Ther. 2015;13:1061–1071. doi: 10.1586/14787210.2015.1058156. [DOI] [PubMed] [Google Scholar]
- 5.Midgley MC, et al. Severe respiratory illness associated with enterovirus D68 - Missouri and Illinois, 2014. MMWR. Morbidity and mortality weekly report. 2014;63:798–799. [PMC free article] [PubMed] [Google Scholar]
- 6.Martin GN, et al. Hospital admissions for viral meningitis in children in England over five decades: a population-based observational study. Lancet Infect Dis. 2016;16:1279–1287. doi: 10.1016/S1473-3099(16)30201-8. [DOI] [PubMed] [Google Scholar]
- 7.WHO. China produces world’s first vaccine against virus that causes Hand, Foot and Mouth Disease. 2015 http://www.wpro.who.int/china/mediacentre/releases/2015/20151208/en/
- 8.Mao Q, Wang Y, Bian L, Xu M, Liang Z. EV-A71 vaccine licensure: a first step for multivalent enterovirus vaccine to control HFMD and other severe diseases. Emerg Microbes Infect. 2016;5:e75. doi: 10.1038/emi.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute of Infectious Diseases (NIID) of Japan. Enterovirus Surveillance in Japan, 1982-1999. IASR. 2000;21:212–213. http://idsc.nih.go.jp/iasr/21/248/tpc248.html. [Google Scholar]
- 10.Anderson RM, Grenfell BT, May RM. Oscillatory fluctuations in the incidence of infectious disease and the impact of vaccination: time series analysis. J Hyg (Lond) 1984;93:587–608. doi: 10.1017/s0022172400065177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ionides EL, Nguyen D, Atchade Y, Stoev S, King AA. Inference for dynamic and latent variable models via iterated, perturbed Bayes maps. Proc Natl Acad Sci U S A. 2015;112:719–724. doi: 10.1073/pnas.1410597112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberste MS, Nix WA, Kilpatrick DR, Flemister MR, Pallansch MA. Molecular epidemiology and type-specific detection of echovirus 11 isolates from the Americas, Europe, Africa, Australia, southern Asia and the Middle East. Virus Res. 2003;91:241–248. doi: 10.1016/s0168-1702(02)00291-5. [DOI] [PubMed] [Google Scholar]
- 13.McWilliam Leitch EC, et al. Evolutionary dynamics and temporal/geographical correlates of recombination in the human enterovirus echovirus types 9, 11, and 30. J Virol. 2010;84:9292–9300. doi: 10.1128/JVI.00783-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute of Infectious Diseases (NIID) of Japan. Hand, foot, and mouth disease and herpangina, 2007 to September 2017 (week 38), Japan. IASR. 2017;38 https://www.niid.go.jp/niid/en/iasr-vol38-e/865-iasr/7617-452te.html. [Google Scholar]
- 15.Penttinen K, Patiala R. The paralytic/infected ratio in a susceptible population during a polio type I epidemic. Annales medicinae experimentalis et biologiae Fenniae. 1961;39:195–202. [PubMed] [Google Scholar]
- 16.John J, et al. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: an open-label, randomised controlled trial. Lancet. 2014;384:1505–1512. doi: 10.1016/S0140-6736(14)60934-X. [DOI] [PubMed] [Google Scholar]
- 17.John J, et al. The Duration of Intestinal Immunity After an Inactivated Poliovirus Vaccine Booster Dose in Children Immunized With Oral Vaccine: A Randomized Controlled Trial. J Infect Dis. 2017;215:529–536. doi: 10.1093/infdis/jiw595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hovi T, Roivainen M. Peptide antisera targeted to a conserved sequence in poliovirus capsid VP1 cross-react widely with members of the genus Enterovirus. J Clin Microbiol. 1993;31:1083–1087. doi: 10.1128/jcm.31.5.1083-1087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katrak K, Mahon BP, Minor PD, Mills KH. Cellular and humoral immune responses to poliovirus in mice: a role for helper T cells in heterotypic immunity to poliovirus. J Gen Virol. 1991;72(Pt 5):1093–1098. doi: 10.1099/0022-1317-72-5-1093. [DOI] [PubMed] [Google Scholar]
- 20.National Institute of Infectious Diseases (NIID) of Japan. Infectious Disease Surveillance System in Japan. 2018 https://www.niid.go.jp/niid/ja/nesid-program-summary.html.
- 21.National Institute of Infectious Diseases (NIID) of Japan. The trend of enterovirus isolation in association with aseptic meningitis, 1999-2002. IASR. 2002;23:193–194. http://idsc.nih.go.jp/iasr/23/270/tpc270.html. [Google Scholar]
- 22.National Institute of Infectious Diseases (NIID) of Japan. Herpangina as of July 2005, Japan. IASR. 2005;26:235–236. http://idsc.nih.go.jp/iasr/26/307/tpc307.html. [Google Scholar]
- 23.National Institute of Infectious Diseases (NIID) of Japan. Pathogen surveillance system in Japan and Infectious Agents Surveillance Report (IASR) IASR. 2010;31:69–70. http://idsc.nih.go.jp/iasr/31/361/tpc361.html. [Google Scholar]
- 24.Statistics Bureau: Ministry of Internal Affairs and Communications, Japan. Japan Statistical Yearbook. 2016 http://www.stat.go.jp/english/data/nenkan/65nenkan/index.htm.
- 25.Cazelles B, Chavez M, Magny GC, Guegan JF, Hales S. Time-dependent spectral analysis of epidemiological time-series with wavelets. J R Soc Interface. 2007;4:625–636. doi: 10.1098/rsif.2007.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roesch A, Schmidbauer H. WaveletComp: Computational Wavelet Analysis. 2014 http://CRAN.R-project.org/package=WaveletComp. [Google Scholar]
- 27.Gillespie DT. Approximate accelerated stochastic simulation of chemically reacting systems. The Journal of Chemical Physics. 2001;115:1716. [Google Scholar]
- 28.He D, Ionides EL, King AA. Plug-and-play inference for disease dynamics: measles in large and small populations as a case study. J R Soc Interface. 2010;7:271–283. doi: 10.1098/rsif.2009.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King AA, Nguyen D, Ionides EL. Statistical Inference for Partially Observed Markov Processes via the R Package pomp. Journal of Statistical Software. 2016;69 [Google Scholar]
- 30.Casey AE. Observations on an Epidemic of Poliomyelitis. Science. 1942;95:359–360. doi: 10.1126/science.95.2466.359. [DOI] [PubMed] [Google Scholar]
- 31.Ionides EL, Breto C, Park J, Smith RA, King AA. Monte Carlo profile confidence intervals for dynamic systems. J R Soc Interface. 2017;14 doi: 10.1098/rsif.2017.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.