Abstract
Some compositional and structural features of mature bone mineral particles remain unclear. They have been described as calcium-deficient and hydroxyl-deficient carbonated hydroxyapatite particles in which a fraction of the PO43− lattice sites are occupied by HPO42− ions. The time has come to revise this description since it has now been proven that the surface of mature bone mineral particles is not in the form of hydroxyapatite but rather in the form of hydrated amorphous calcium phosphate. Using a combination of dedicated solid-state nuclear magnetic resonance techniques, the hydrogen-bearing species present in bone mineral and especially the HPO42− ions were closely scrutinized. We show that these HPO42− ions are concentrated at the surface of bone mineral particles in the so-called amorphous surface layer whose thickness was estimated here to be about 0.8 nm for a 4-nm thick particle. We also show that their molar proportion is much higher than previously estimated since they stand for about half of the overall amount of inorganic phosphate ions that compose bone mineral. As such, the mineral-mineral and mineral-biomolecule interfaces in bone tissue must be driven by metastable hydrated amorphous environments rich in HPO42− ions rather than by stable crystalline environments of hydroxyapatite structure.
Subject terms: Biomineralization, Solid-state NMR
Introduction
Bone is a natural composite material whose main components are mineral and organic matrices1,2. The mature mineral matrix is in the form of nanosized, elongated platelet-like carbonated calcium phosphate particles whose elongated direction is preferentially aligned along the long axis of type I collagen fibrils3. Those collagen fibrils compose the organic matrix, together with different types of biomacromolecules, including proteoglycans and non-collagenous proteins (NCPs)4–6. Bone mineral is known to possess compositional and structural similarities with synthetic stoichiometric hydroxyapatite (HA) mineral, Ca10(PO4)6(OH)2. However, in contrast to stoichiometric HA, bone mineral is structurally disordered, and compositionally nonstoichiometric due to the presence of a substantial amount of anionic (e.g., HPO42−, CO32−, Cl−) and cationic (e.g., Na+, Mg2+) species, together with the presence of ion vacancies into the crystal lattice. For instance, CO32− ions, whose weight proportion can reach up to ∼5–9% in bone mineral, can occupy the PO43− (called B-type substitution - major) and/or OH− (A-type substitution - minor) sites within the hydroxyapatite’s crystal lattice. In addition, it was reported that the PO43− lattice sites may also be occupied by a fraction of monohydrogen-phosphate (HPO42−) ions. As a consequence, it is generally accepted that “the deficit in negative charge caused by the replacement of PO43− by either CO32− or HPO42− can be compensated by the loss of positive charge, as through removal of Ca2+ from the lattice”7. Furthermore, beside their presence within the hydroxyapatite’s crystal lattice, HPO42− ions were also proposed to be present in the so-called amorphous surface layer that coats both biological8–11 (bone, dentin) and biomimetic11–16 nanocrystalline hydroxyapatite particles. Lastly, HPO42− ions in bone could also originate from the presence of octacalcium phosphate (OCP), Ca8(HPO4)2(PO4)4·5H2O and OCP-like domains. Indeed, OCP was identified as a possible transient precursor phase of bone hydroxyapatite17–19, whilst OCP-like domains were also proposed as a component of bone mineral20–22.
The presence of HPO42− ions was initially proposed and quantified in synthetic hydroxyapatites based on spectroscopic analysis23, together with bulk chemical analysis of pyrolyzed samples24 for which the pyrophosphate ions created by the condensation of two HPO42− ions after dehydration upon heating were titrated. The latter method was also used to quantify HPO42− ions incorporated into bone mineral25,26. A combination of chemical and spectroscopic analysis of bone mineral samples of different age and origin (rat, calf and cow) was further undertaken. It lead to the average chemical formula27 (neglecting the most minor substitutions) proposed by Legros et al. in 1987 for mature cortical bone mineral which is still considered as a reference formula to this day: Ca8.3□1,7(PO4)4.3(HPO4 or CO3)1.7(OH or ½ CO3)0,3□1.7. Since then, the presence of HPO42− ions in bone mineral was also proposed based on vibrational spectroscopic analyses25–27. Indeed, thanks to the comparison with hydrogen-phosphate-containing calcium phosphate mineral standards such as brushite (Dicalcium Phosphate Dihydrate, DCPD; CaHPO4.2H2O) and octacalcium phosphate, Fourier Transform-Infrared (FT-IR) analyses of bone (chicken) and enamel (pig) have revealed that biological hydroxyapatites exhibit characteristic adsorption bands that were attributed to HPO42−ions28,29. Solid-state Nuclear Magnetic Resonance (ssNMR) spectroscopy has also been used to study bone mineral and, in particular, its chemical structure30,31, its hydrophilicity32, and its interaction with bioorganic molecules33–37. The 31P NMR chemical environments have been probed; and early studies on bone tissue samples have also suggested the presence of HPO42− ions in bone mineral based on the measurement of chemical shift anisotropy (CSA) parameters38: they were found to differ from those of apatitic PO43− ions but to be similar with those found for brushite. Similarly, 1H-31P dipolar-based ssNMR experiments39,40 revealed two different behaviours in various bone tissue samples (chicken, bovine and rabbit) attributed to HPO42− vs PO43−. Lastly, the presence of HPO42− ions in bone mineral was also proposed based on 1H NMR chemical shift considerations: two-dimensional (2D) {1H}31P heteronuclear correlation (HetCor) NMR experiments were performed to indirectly detect the 1H NMR chemical environments of bone41–43 and dentine9 mineral that were then compared with the 1H NMR chemical environments of brushite, monetite (Dicalcium Phosphate Anhydrous, DCPA; CaHPO4) and octacalcium phosphate44.
Many efforts have therefore been made over the years to identify, localize and quantify HPO42− ions within bone mineral. While their presence in bone mineral is now accepted, both their localisation and quantification are still being debated45. This prevents the design of an accurate chemical and structural model of mature bone mineral particles which will not only provide design principles for the next generation of alloplasts for bone regeneration, but will also facilitate the understanding of bone mineral chemistry and reactivity in vivo. Hence, the present study aims to more precisely assess the identification, the localisation, and the quantification of HPO42− ions in bone mineral. To this end, we used a combination of dedicated ssNMR techniques from intact mature bone tissue samples together with synthetic reference samples. In particular, the {1H-31P}1H double cross polarization (CP) ssNMR experiment was used to suppress the proton signal from the bone organic matrix, and, therefore, to selectively record the 1H NMR spectrum of bone mineral. The variation of the 31P → 1H contact time in the {1H-31P}1H double CP experiment, followed by numerical modelling and calculations allowed us to determine the H•••P distances within the HPO42− ions identified in bone mineral. These distances were subsequently compared with those determined for known inorganic POH groups found in HPO42− ions from monetite. Further ssNMR investigations including 1H-1H double quantum-Single quantum experiments have also been undertaken to study the localization of the HPO42− ions present in a bone mineral proxy sample. Lastly, a single pulse 31P ssNMR spectrum of bone mineral recorded under quantitative conditions was recorded to quantify these HPO42− ions with respects to the overall amount of inorganic phosphate ions that compose bone mineral.
Methods
Samples preparation
Cortical bone tissue samples were harvested from healthy 2-year-old sheep; and were extracted from the distal femoral metaphysis. The animal experiments were approved by the IMMR’s Institutional Animal Care and Use committee (IACUC) and performed in accordance with relevant guidelines and regulations. The IMMR received an agreement (n° 75-14-01) on September 08th, 2008 for period of 5 years by the “Sous-Direction de la protection Sanitaire” of the French Authorities. Fresh bone tissue samples were analysed within two hours following their extraction from the animal. This delay corresponds to the trip from the hospital where the bone tissue samples were harvested to our lab. In the meantime, the bone samples were conserved intact in a sealed vial at ambient temperature. The dry bone tissue samples were obtained once the fresh bone tissue samples were dehydrated at ambient temperature in a laminar flow hood for one night.
Monetite (DCPA) was obtained by mixing calcium carbonate (CaCO3) and an aqueous phosphoric acid solution (H3PO4, 85 wt. %) in water (ratio Ca/P = 1). The mixture was placed in an autoclave at 150 °C for 48 h. The resulting precipitate was then washed with ethanol and dried at 37 °C.
OctaCalcium Phosphate (OCP) was prepared according to the protocol of Bigi et al. (2004)46. Briefly, 500 mL of an aqueous solution containing 40 mM Ca(CH3COO)2 was added dropwise to 1500 mL of an aqueous solution containing 6.6 mM of Na2HPO4 and 6.6 mM of NaH2PO4 with a starting pH of 5. The reaction was performed at 70 °C, and no stirring was applied. Fifteen minutes after the end of the addition, the precipitate was centrifuged, washed three times with deionized water, and then dried at 37 °C
A biomimetic Carbonated HydroxyApatite (CHA-SBF) sample was precipitated directly from a modified simulated body fluid solution (SBF) inspired from human blood plasma. A solution 1.5 times more concentrated compared to standard SBF was prepared (1.5 × SBF)47. Briefly, 1 L of this solution was frozen at −20 °C for 1 night. Then, the solution was thawed and conserved at 5 °C for 1 month. The resulting nanoparticles were recovered by centrifugation and finally dried at 37 °C. To study CHA-SBF in wet conditions, about 10 µL of deionized water has been directly added to the rotor prior to NMR analysis.
A well-crystallized hydroxyapatite (HA) sample which, unlike CHA-SBF, is exempt of amorphous surface layer, but which is composed of HPO42− ions that only occupy the PO43− sites within the hydroxyapatite’s crystal lattice was also prepared. This sample, named here HPO42−-substituted HA, corresponds to so-called apatitic tricalcium phosphate, with the following chemical formula: Ca9(PO4)5(HPO4)(OH). It thus contains 1/6th (~ 17%) of protonated HPO42− ions out of the total phosphate content48. This sample was obtained by hydrothermal treatment by converting β−tricalcium phosphate Ca3(PO4)2, of particle size <125 µm, with water vapor at 160 °C for 3 days in an autoclave.
FT-IR analyses
Fourier Transform-Infrared (FT-IR) spectra were recorded at room temperature using a Nicolet Magna FT-IR spectrometer in ATR mode, in the range of 650–4000 cm−1 and at a resolution of 4 cm−1. The weight proportion of CO32− ions in bone mineral from our 2-year-old sheep bone tissue sample was evaluated according to the FT-IR analysis procedure described by Grunenwald et al.49.
Solid-state NMR
Solid-state Nuclear Magnetic Resonance (ssNMR) experiments were performed on an Avance 300 Bruker spectrometer (7.0 T) using a 4 mm double resonance magic angle spinning (MAS) probe head. 3-4mm-thick pieces of cortical bone samples were packed into a 4 mm (O.D.) NMR zirconia rotor and spun at a Magic Angle Spinning (MAS) frequency νMAS = 14 kHz. The temperature in the NMR probe was kept at 25 °C during all analysis periods. The recycle delays in the one-dimentional (1D) {1H}31P cross polarization (CP), two-dimentional (2D) {1H}31P Heteronuclear Correlation (HetCor), 1D {1H-31P}1H double CP; and in the 2D 1H-1H double quantum-Single quantum experiments, were set to 2 sec (bone and CHA-SBF), 10 sec (monetite) and 30 sec (HPO42−-substituted HA). The 2D {1H}31P HetCor experiments were recorded with a contact time of 1000 µs and 80 scans for each 100 t1 increments. The radio frequency (RF) field (B1) applied during the CP steps was νRF(1H) = 70 kHz and νRF(31P) = 50 kHz. The {1H-31P}1H double CP experiments were recorded with identical RF fields. The sequence consists of two consecutive CP transfers, and is schematically described in Fig. S1. Following the first transfer (during tCP1), the 31P magnetization is flipped back to the z direction through a 90° pulse. The 1H residual signal is eliminated by two low power pulses phase shifted by 90° at νRF = νMAS/2 = 7 kHz (HORROR condition50). The length of each pulse corresponds to the length of the 1H free induction decay (∼ 10 ms). This saturation step not only enables suppression of all unwanted 1H signals; but it also, within the model of thermal reservoirs51, enables transformation of the proton bath into a hot reservoir into which the 31P magnetization can be back-transferred from the cold reservoir. After this step, the 31P magnetization is then flipped back into the transverse plane through a 90° pulse and a second CP transfer is then applied (during tCP2) prior to 1H acquisition. In regard to the CP dynamics experiments, the Hartmann-Hahn condition of the first CP 1H → 31P transfer was set through a tangential ramp on the 1H channel in order to maximize the 31P signal52. The Hartmann-Hahn condition of the second CP 31P → 1H transfer was set through square pulses on both channels. The 1H and 31P radio frequency field strengths were matched to the first spinning sidebands (n = ±1) of the Hartmann-Hahn matching profile (Fig. S2). High power 1H decoupling was applied during acquisition (60 kHz of RF field strength, spinal 64). Regarding the NMR signal processing, no line broadening (LB) was employed to process the {1H-31P}1H double CP free induction decay (FID); while a line broadening of 30 and 100 Hz was employed for the 2D {1H}31P HetCor experiments in the F2 and F1 dimensions, respectively. Two dimensional 1H-1H double quantum-Single quantum NMR spectra were recorded using the Back-to-Back (BABA) excitation scheme based on the recoupling of 1H homonuclear dipolar couplings53. The 1H RF field was 70 kHz. The recoupling delay was synchronized to the rotor rotation period (71.4 µs). 1H chemical shifts were referenced to TetraMethylSilane (TMS) at δ1H = 0.0 ppm, whereas 31P chemical shifts were referenced to H3PO4 (85% w/w aqueous solution) at δ31P = 0.0 ppm.
Results and Discussion
Identification of HPO42− ions in bone mineral
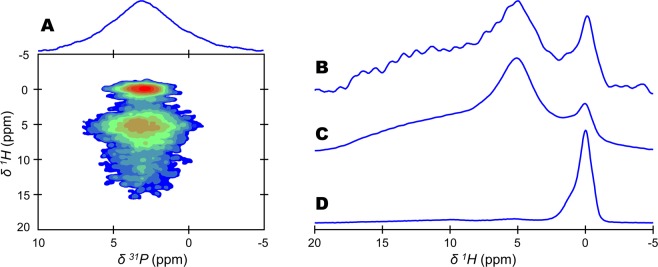
The direct solid-state Nuclear Magnetic Resonance (ssNMR) detection of the protons localized in bone mineral from an intact bone tissue sample is not possible. This is due to the presence of the extracellular organic matrix whose different signals dominate the 1H single pulse (SP) ssNMR spectrum of a bone tissue sample54. However, the possibility to reveal atomic-scale spatial proximities among hydrogen and phosphorus nuclei in the two-dimensional (2D) {1H}31P Heteronuclear Correlation (HetCor) ssNMR experiment allows for probing bone mineral hydrogen environments through the analysis of the F1 dimension (Fig. 1A). Unfortunately, this experiment is time consuming and gives rise to a 1H projection of the vertical (F1) dimension with a relatively poor signal-to-noise ratio (S/N) and a low digital resolution (Fig. 1B). To overcome these limitations, we used the one-dimensional (1D) {1H-31P}1H double cross polarization (CP) ssNMR experiment. It consists of a double CP transfer conducted in a “there-and-back” manner (1H→31P→1H) (Fig. S1). First, this experiment allowed us to obtain 31P-filtered 1H ssNMR spectra of bone mineral from an intact, cortical 2-year-old sheep bone tissue sample with an excellent S/N despite a relatively short acquisition time (i.e., 9 hours) (Fig. 1C,D). The different 1H chemical environments from bone mineral are now readily observable and can be safely analyzed with precision. With regard to the internal crystalline core of bone mineral particles, the hydroxyl ions present in the hydroxyapatite’s crystal lattice are observed in the form of a complex resonance centred at δ(1H) = 0.0 ppm. Regarding their amorphous surface layer, structural water molecules and acidic phosphate species present in non-apatitic environments are observable in the form of a single resonance centred at δ(1H) = 5.2 ppm and a broad resonance ranging from δ(1H) = 7 to 17 ppm32, respectively.
Figure 1.
Detection of hydrogen-bearing species in bone mineral. 1H-31P cross polarization (CP) based magic angle spinning (MAS) solid-state Nuclear Magnetic Resonance (ssNMR) spectra of a dry 2-year-old sheep bone tissue sample. (A) two-dimensional (2D) {1H}31P Heteronuclear Correlation (HetCor) spectrum (contact time, tCP = 1000 µs). Signal intensity increases from blue to red. (B) 1H projection of the vertical (F1) dimension of the 2D {1H}31P HetCor spectrum shown in (A). {1H-31P}1H double CP MAS spectra recorded with the following contact times: (C) tCP1 = tCP2 = 1000 µs; and, (D) tCP1 = tCP2 = 15000 µs. The total experimental time was the same in each experiment (i.e., 9 hours).
Second, this experiment allows the investigation of the 1H-detected CP dynamics to selectively reveal the nature of the 1H nuclei nearby 31P nuclei. To this end, the contact time 1 (tCP1) was kept fixed at 1000 µs, while the contact time 2 (tCP2) was varied from 75 µs up to 1000 µs (Fig. 2). A uniform increase of the magnetization is observed for both the resonances centred at δ(1H) = 0.0 and δ(1H) = 5.2 ppm (see the black dashed lines), previously attributed to OH− ions and structural H2O molecules according to their respective 1H NMR chemical shift. In contrast, the evolution of the broad signal in the range of δ(1H) = 7–17 ppm initially shows a rapid increase of its magnetization (up to tCP2 = 300 µs) and is followed by the presence of an oscillatory behaviour (up to tCP2 = 1250 µs - see the black dashed line). This oscillatory behaviour is characteristic of 1H-31P dipolar (DP-H) oscillations55,56. The fitting of the corresponding {1H-31P}1H ssNMR spectra at various tCP2 is not straightforward due to the overlapping of various resonances. Whereas synthetic HA samples usually exhibit a symmetric OH- resonance32; we show here that the OH− resonance of bone mineral is particularly complex and can be fitted as follows: a main peak at δ(1H) = 0.0 ppm surrounded by two shoulders peaks at δ(1H) = −0.7 and 0.9 ppm (Fig. S3). The residual structural water resonance can be properly fitted with a single peak centred at δ(1H) = 5.2 ppm (Fig. S4). In contrast, the broad signal from the acidic phosphate species observable in the range of δ(1H) = 7–17 ppm cannot be satisfactorily fitted with a single peak with fixed position and line width, especially at short contact times (see the best fitting results for the various tCP2 values in Fig. S4 – left column). However, the fitting results are accurate when two different peaks are used with fixed positions [at δ(1H) = 9.8 ± 0.1 and 14.0 ± 0.1 ppm] and fixed line widths (6.2 ± 0.1 and 5.0 ± 0.1 ppm, respectively) (Fig. S4 – right column). We cannot claim that this broad signal is only composed of two peaks corresponding to two distinct proton environments, but it is probably composed of a wide distribution of chemical environments leading to a distribution of NMR chemical shifts. Accordingly, Fig. 3A shows the four peaks that were used to analyse the 31P-filtered 1H ssNMR spectra of bone mineral for each tCP2 values: (i) a composite peak centred at δ(1H) = 0.0 (purple) and a single peak centred at δ(1H) = 5.2 (grey) ppm both characterized by a relatively slow and uniform growth of their magnetizations; and (ii) two peaks at δ(1H) = 9.8 (blue) and 14.0 (green) ppm both displaying dipolar oscillations (Fig. 3B). In regard to these latter two peaks, the precise match of the Hartmann-Hahn (H-H) profile (Fig. S2) enables numerical modelling of the CP build-up curves according to the single spin pair model accounting for dipolar oscillations resulting from coherent polarization transfer and the impact of 1H spin diffusion57:
| 1 |
in which M0, tCP, Tsd, T1ρ(1H) and DPH’ are the CP intensity, the contact time, the 1H spin-diffusion rate constant, the spin-lattice relaxation time in the rotating frame, and the apparent 1H-31P dipolar coupling, respectively. The internuclear distance between 1H and 31P nuclei is then readily extracted from DPH:
| 2 |
in which DPH = DPH’ × √2, dPH is the internuclear distance between the two spins, γ1H and γ31P are the gyromagnetic ratios of the coupled spins, µ0 is the vacuum permeability, and h is the Planck constant. A dipolar constant (DPH) of 4050 Hz was found for the peak centred at δ(1H) = 9.8, while DPH = 4695 Hz for the peak centred at δ(1H) = 14.0 ppm. In addition, the corresponding H•••P internuclear distances were calculated: 2.24 Å for the former peak and 2.14 Å for the latter peak (Fig. 3C,D, and Table 1). The estimated precision is ± 490 Hz for DPH and ± 0.07 Å for dPH. Lastly, in regard to the evolutions of the magnetization of the resonances at δ(1H) = 0.0 ppm (OH-) and δ(1H) = 5.2 ppm (H2O); they are readily fitted within the classical I-S model. This is an analytical extension of Eq. 1 for an extended spin system in which the behaviour of the magnetization is dominated by an incoherent transfer from 31P to 1H as follows57:
| 3 |
in which M0, tCP, THP, T1ρ(1H) are the CP intensity, the contact time, the CP rate constant and the spin-lattice relaxation time in the rotating frame, respectively. A THP value of 795 µs was found for the OH- resonance, while a THP value of 522 µs was found for the H2O resonance (Table 1).
Figure 2.
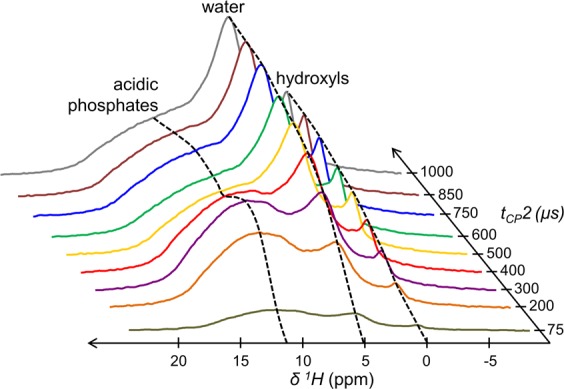
Cross-polarization dynamics of the 1H resonances from bone mineral. {1H-31P}1H double cross polarization (CP) magic angle spinning (MAS) solid-state Nuclear Magnetic Resonance (ssNMR) dynamics of a dry 2-year-old sheep bone tissue sample. Contact time 1 (tCP1) was fixed at 1000 µs, while contact time 2 (tCP2) was varied from 75 µs to 1000 µs. Black dashed lines are guidelines for the eyes.
Figure 3.
Determination of 31P-1H internuclear distances within the acidic phosphate species present in bone mineral. (A) {1H-31P}1H double cross polarization (CP) magic angle spinning (MAS) solid-state Nuclear Magnetic Resonance (ssNMR) spectrum of a dry 2-year-old sheep bone tissue sample (blue line) and its corresponding fitting (red dashed line). Contact times 1 (tCP1) was 1000 µs, while contact time 2 (tCP2) was 500 µs. (B) Numerical modelling of the evolution of the magnetization of the δ(1H) = 0 (purple, hydroxyl ions); 5.2 (grey, structural water molecules); 9.8 (blue, acidic phosphate species); and, 14.0 (green, acidic phosphate species) ppm peaks shown in (A). The acidic phosphate species peaks were simulated according to Eq. (1), while the hydroxyl ions and structural water molecules resonances were simulated according to Eq. (3). Numerical modelling according to Eq. (1) of the magnetization evolution of the δ(1H) = 14.0 (C) and 9.8 (D) ppm peaks attributed to acidic phosphate species; together with the calculations of their respective dipolar constant (DPH) and P•••H distance (dPH).
Table 1.
Nuclear Magnetic Resonance (NMR) parameters associated with the different proton (1H) species detected in the various samples (bone mineral, CHA-SBF and Monetite): 1H chemical shifts (δ1H); dipolar coupling constants for the 31P-1H interaction (DPH); P•••H internuclear distances (dPH); cross-polarization (CP) rate constants (THP); spin-diffusion rate constants (Tdf); spin-lattice relaxation times in the rotating frame (T1ρ1H). awas calculated according to Equation 3; whereas bwas calculated according to Equation 1.
| Sample | 1H species | δ(1H) (ppm) | DPH (Hz) | dPH (Å) | THPa (µs) | Tdf b (µs) | T1ρ (1H) (µs). |
|---|---|---|---|---|---|---|---|
| Bone mineral | OH− | 0.0 | — | — | 795 | — | ∞ |
| H2O | 5.2 | — | — | 522 | — | ∞ | |
| HPO42− | 9.8 | 4050 ± 500 | 2.24 ± 0.07 | — | 788 | 1485 | |
| HPO42− | 14.0 | 4695 ± 500 | 2.14 ± 0.07 | — | 624 | 1224 | |
| CHA-SBF | HPO42− | 7.0–17.0 | 4960 ± 500 | 2.10 ± 0.07 | — | 665 | 3531 |
| Monetite | HPO42− | 13.0 | 5275 ± 500 | 2.10 ± 0.07 | — | 626 | ∞ |
| HPO42− | 15.8 | 6863 ± 900 | 1.92 ± 0.14 | — | 691 | ∞ |
The same ssNMR-based approach was also applied to monetite (CaHPO4) as a reference sample which is a hydrogen-phosphate-containing calcium phosphate mineral standard (Figs S5 and S7). Dipolar oscillations are also observed for which our numerical modelling and calculations reveal H•••P internuclear distances of 1.92 ± 0.07 and 2.10 ± 0.07 Å within the two inequivalent HPO42− groups present in the monetite’s crystal lattice. Such distances are close to those found for the acidic phosphate species within our 2-year-old sheep bone tissue sample, and are in agreement with the distances measured in monetite58 and brushite59 through neutron diffraction analysis (i.e., dPH ranging from 2.08 to 2.25 in the HPO4− ions of monetite; and dPH = 2.19 Å in the HPO42− ions of brushite). Since previous spectroscopic analyses have shown that the presence of H2PO4− ions in carbonated hydroxyapatite was unlikely12, we can now safely attribute the broad signal in the range of δ(1H) = 7–17 ppm observable in bone mineral solely to HPO42− ions.
In addition, the same ssNMR-based approach was also applied to a biomimetic Carbonated HydroxyApatite (CHA-SBF) sample that was precipitated from a modified simulated body fluid solution (SBF) devoid of organic additives. This synthetic bone-like mineral sample displays a similar {1H-31P}1H double CP ssNMR spectrum than bone mineral: a resonance centred at δ(1H) = 0.0 ppm attributed to hydroxyl ions; a resonance centred at δ(1H) = 5.2 ppm attributed to structural water molecules; and last, a broad signal in the range of δ(1H) = 7–17 ppm attributed to acidic phosphate species (Fig. S7). The variation of the second contact time (tCP2) also leads to the observation of dipolar oscillations in the acidic phosphate species region. Our corresponding numerical modelling and calculations reveal a dipolar constant (DPH) of 4960 ± 500 Hz, and a distance (dPH) of 2.10 ± 0.07 Å. These values are similar to those calculated for the acidic phosphate species found in bone mineral (Fig. 3 and Table 1). These results demonstrate that the acidic phosphate species detected in bone mineral from our 2-year-old sheep bone tissue sample are purely inorganic (HPO4− ions) and do not originate from POH groups present in bioorganic molecules such as phosphorylated NCPs and phospholipids.
Localisation of the HPO42− ions in bone mineral
The exact localisation of the structural water molecules and the HPO42− ions within bone mineral particles is still unclear. To date, they are suggested to be present both in the hydroxyapatite’s crystal lattice (internal crystalline core) and in the non-apatitic environments (amorphous surface layer)45. We first investigated their exact localisation through the examination of 31P-filtered 1H ssNMR spectra of hydrated (fresh) vs dry 2-year-old sheep bone tissue samples (Fig. S8A). The comparison of the two spectra demonstrates that all the protons from the HPO42− ions undergo fast chemical exchanges with the protons from free water molecules present in the extracellular fluid. Indeed, in the case of the fresh bone tissue sample, the structural water resonance at δ(1H) = 5.2 ppm together with the broad signal in the range of δ(1H) = 7–17 ppm now safely attributed to HPO4− ions, are no longer detected. Instead, a sharp and intense signal of strongly bound water molecules is now observable at δ(1H) = 4.8 ppm. A similar behaviour was observed for the biomimetic Carbonated HydroxyApatite (CHA-SBF) sample that was studied in dry conditions and soaked in water (Fig. S8B). These observations make clear that all the HPO42− ions are easily accessible to H2O molecules and, therefore, are located near the surface of the particles within the so-called amorphous surface layer that coats bone mineral particles11,16.
Second, we have also investigated whether or not HPO42− ions are present in the hydroxyapatite’s crystal lattice of bone mineral particles. To this end, we studied a synthetic HPO42−-substituted HA sample. This sample, named HPO42−substituted HA, does not possess non-apatitic environments in the form of an amorphous surface layer so that all its HPO42− ions are allegedly localized within the hydroxyapatite’s crystal lattice. The 2D {1H}31P HetCor ssNMR spectrum of HPO42−-substituted HA reveals that the 31P NMR chemical shift of its HPO42− ions is quite different than in the case of bone mineral: δ(31P) = 1.5 ppm vs δ(31P) = 3.2 ppm, respectively (Fig. S9). Further, the presence of HPO42− ions in the hydroxyapatite’s crystal lattice of HPO42−substituted HA was confirmed with the help of an 1H homonuclear dipolar coupling-based ssNMR experiment: the 2D 1H–1H Single Quantum–Double Quantum (SQ–DQ) correlation experiment (Fig. S10A) which can reveal short 1H-1H spatial proximities (a few Å). A cross-peak on the left side of the diagonal is observable. It spatially correlates the HPO42− ions region in the range of δ(1H) = 7–12 ppm with the OH− ions region at around δ(1H) = 0.0 ppm. Such off-diagonal signal demonstrates the incorporation of HPO42− ions within the hydroxyapatite’s crystal lattice of HPO42−-substituted HA. In particular, a spatial correlation of those HPO42− ions with a resonance at around δ(1H) = 1.0 ppm is highlighted (see red dotted lines); it corresponds to OH− ions close to HPO42− ions that were also observed in the 1H single pulse (SP) MAS ssNMR spectrum, see Fig. S9A. Importantly, the same experiment recorded on the biomimetic Carbonated HydroxyApatite (CHA-SBF) sample does not exhibit such spatial proximity between OH− and HPO42− ions (Fig. S10B). These observations not only demonstrate that the HPO42− ions that compose CHA-SBF are concentrated within the so-called amorphous surface layer, but also suggest that the same scenario applies to bone mineral particles.
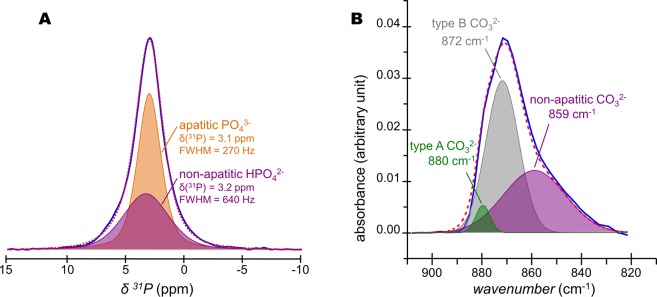
Lastly, since octacalcium phosphate (OCP) has recently been proposed as a component of bone mineral20, the question whether the HPO42− ions detected in the bone mineral of our 2-year-old sheep bone tissue sample originate from OCP environments needs to be raised. In this direction, the 2D {1H}31P HetCor MAS ssNMR spectrum of a fresh and intact 2-year-old sheep bone tissue sample is shown in Fig. 4A. Again, the upper correlation peak corresponds to the OH− and PO43− containing apatitic environments that compose the internal crystalline core of bone mineral particles. The lower correlation peak corresponds to H2O and HPO42−-containing non-apatitic environments whose individual 31P NMR signal is revealed in Fig. 4B (blue line). This signal is in the form of a single broad resonance whose maximum intensity is at δ(31P) = 3.2 ppm, and whose lineshape and linewidth are practically identical to those of the signal of a synthetic sample of amorphous calcium phosphate (ACP) recorded in similar experimental conditions11. In contrast, the 31P NMR signal of OCP recorded in similar conditions is in the form of three resolved resonances from δ(31P) ∼ 3.6 ppm to δ(31P) ∼ −0.2 ppm (Fig. 4C). The latter upfield resonance is here the most intense and arises from two overlapping peaks at δ(31P) ∼ −0.1 and ∼ −0.3 ppm. They correspond to the two HPO42− groups (P5 and P6) and one of the two PO43− groups (P3) present in the hydrated layer of the OCP crystal lattice60. It is worth emphasizing that such upfield signal appears to be absent from the 31P NMR signal of the H2O and HPO42−-containing non-apatitic environments of bone mineral (Fig. 4B). Further, based on a customized ssNMR experiment that was used to probe the long-range spatial proximities among the hydrogen-bearing species in bone mineral, we previously demonstrated that the H2O and HPO42−-containing non-apatitic environments and the OH−-containing apatitic environments belong to the same particle11. According to these results that were obtained from intact bone tissue samples, we demonstrated that the H2O and HPO42−-containing non-apatitic environments evidenced here do not originate from OCP but can be safely attributed to the amorphous surface layer that coats bone mineral particles.
Figure 4.
Implication of octacalcium phosphate (OCP) in the non-apatitic environments of bone mineral. (A) Two-dimensional (2D) {1H}31P Heteronuclear Correlation (HetCor) magic angle spinning (MAS) solid-state Nuclear Magnetic Resonance (ssNMR) spectrum of a fresh 2-year-old sheep bone tissue sample (contact time, tCP = 1000 µs). Signal intensity increases from blue to red. (B) One-dimensional (1D) individual 31P NMR signal of the H2O and HPO42−-containing non-apatitic environments attributed to the amorphous surface layer that coats bone mineral particles (blue line); and 1D individual 31P NMR signal of the OH−-containing apatitic environments that compose the internal crystalline core of bone mineral particles (orange line). These individual 31P NMR signals were generated from the 2D {1H}31P HetCor ssNMR spectrum shown in (A). To do so, the F2 slices taken at the bound water molecules position [from δ(1H) = 3 to 7 ppm, blue area] and hydroxyl ions position [from δ(1H) = −2 to 2 ppm, orange area] in F1 have been summed. (C) 1D 31P CP MAS ssNMR spectrum (tCP = 1000 µs) of a synthetic octacalcium phosphate (OCP) sample. P1 to P6 correspond to the six different phosphate groups present in the OCP crystal lattice according to the work of Davies et al.60. The red dashed-line marks the most intense resonance in the signal of OCP which is not detected in bone mineral (B).
Quantification of HPO42− ions in bone mineral
The quantification of the HPO42− ions present in bone mineral were undertaken. To this end, the lineshape and linewidth of the individual 31P NMR signals of the OH− and PO43−-containing internal crystalline core [δ(1H) = 3.1 ppm, full width at half maximum (FWHM) = 270 Hz] and the H2O and HPO42−-containing non-apatitic environments (amorphous surface layer) [δ(1H) = 3.2 ppm, FWHM = 640 Hz] that were revealed in Fig. 4B, were used in the fitting of the quantitative 31P single pulse (SP) MAS ssNMR spectrum of a fresh 2-year-old sheep bone tissue sample (Fig. 5A). The molar percentage proportion of HPO42− and PO43− ions in bone mineral were found to be about 50/50 ± 5%. As suggested by our observations in Figs S9 and S10, this calculation was made assuming that the molar proportion of HPO42− ions in the internal crystalline core of the particles was close to 0%. Such a high molar proportion of HPO42− ions present in the amorphous surface layer reflects the small size of bone mineral particles: ∼1–5 nm in thickness, ∼10–40 nm in width, and ∼20–100 nm in length1,61–63. Since we showed that the HPO42− ions are concentrated within the amorphous surface layer, we can now estimate the average thickness of this layer for a 2-year-old sheep bone tissue sample. Here we consider a nanosized platelet with a thickness of 4.0 nm, and we assume that the densities of phosphate atoms present in the hydroxyapatite’s crystal lattice and in the amorphous surface layer are equivalent. In such scenario, the thickness of the internal crystalline core is about 2.4 nm (i.e., which is about twice the size of the hexagonal unit cell of hydroxyapatite64 along the crystallographic axes a and b; a = b = 0.94 nm); whilst the thickness of the outer amorphous surface layer can be estimated to be about 0.8 nm (i.e., which then corresponds to the size of the hexagonal unit cell of hydroxyapatite along a and b, and, hence, is equivalent to the stacking of only two phosphate ions). One should be conscious that these are average values that correspond to the sum of the contributions of all the inorganic phosphate ions present in our 2-year-old sheep bone tissue sample. These results might be different for older specimen in which the proportion of the non-apatitic environments might be less65 due to bone mineral maturation: the progressive transformation of the amorphous surface layer into apatitic environments15. Nevertheless, the thickness of the surface layer determined here (0.8 nm) is in good agreement with the estimated sizes proposed in some previous studies: about the size of one phosphate unit in fluorapatite-gelatine mesocrystals66; and about 1–2 nm in synthetic hydroxyapatites32,67,68.
Figure 5.
Quantification of HPO42− and CO32− ions present in bone mineral. (A) Quantitative 31P single pulse (SP) magic angle spinning (MAS) solid-state Nuclear Magnetic Resonance (ssNMR) spectrum of a fresh 2-year-old sheep bone tissue sample (blue line) and its corresponding fitting (red dashed line) with two peaks. Those two peaks, whose lineshape and linewidth were revealed in Fig. 4B, correspond to the PO43−-containing internal crystalline core in the form of hydroxyapatite (orange peak) and the HPO42−-containing non-apatitic environments in the form of an amorphous surface layer (purple peak). (B) Fourier Transform-Infrared (FT-IR) spectrum of the ν2(CO3) vibration mode for a 2-year-old sheep bone tissue sample (blue line) and its corresponding fitting (red dashed line). Type B CO32− ions occupy the PO43− sites within the hydroxyapatite’s crystal lattice; type A CO32− ions occupy the OH− sites within the hydroxyapatite’s crystal lattice; whereas non-apatitic CO32− are present within the amorphous surface layer that coats bone mineral particles.
Update on bone mineral chemical composition
The results presented here and elsewhere45 suggest that the average chemical composition of mature cortical bone mineral proposed by Legros et al.27, Ca8.3□1,7(PO4)4.3(HPO4 or CO3)1.7(OH or ½ CO3)0,3□1.7, must be reconsidered. Indeed, this formula does not only disregard the presence of the amorphous surface layer whose chemical composition greatly varies with respect to the apatitic environments present in the internal crystalline core of the particles, but also underestimates the molar proportion of HPO42− ions. To propose an up to date formula of our 2-year-old sheep bone tissue sample, we needed firstly to determine the weight proportion of CO32− ions. A value of 4.8% with a major contribution in B-type carbonates was found through FT-IR analyses49 (Fig. 5B), which is in accordance with the values found for other bone mineral samples3. In addition, the following parameters were considered: (i) the particles must remain electrically neutral (both the internal crystalline core and the amorphous surface layer); (ii) the molar proportion of HPO42− ions relative to the overall amount of inorganic phosphate ions is constrained close to 50% according to the present study; (iii) the degree of carbonation should be close to the experimental value (4.8% w/w); (iv) the overall Ca/(P + C) molar ratio should remain acceptable for a bone tissue sample, i.e. in the range of 1.2–1.53 and, last, (v) the proportion of A-type, B-type, and non-apatitic carbonate ions present in the amorphous surface layer should remain in accordance with the FT-IR data. Once all of these constraints have been amalgamated, the average chemical composition of the mature cortical bone mineral from our 2-year-old sheep bone tissue sample can be approximated as follows: Ca7.5(PO4)2.8(HPO4)2.6(CO3)0.6(OH)0,2. One should note that this average chemical composition solely corresponds to our specific bone tissue sample according to our own experimental results. Hence this formula is not universal since variability occurs among bone specimens depending, in particular, on the specie, the age, the food supply and their degree of maturation.
Conclusions
In this work, we determined the H•••P distances (2.1–2.3 Å) within the acidic phosphate ions that compose bone mineral, and, hence, shown that they undeniably correspond to POH groups in inorganic HPO42− ions. The presence of HPO42− ions in bone mineral was therefore demonstrated based on accurate interatomic distance measurements. Further, in contrast to what was previously proposed, our results suggest that these HPO42− ions are concentrated at the surface of bone mineral particles within the so-called amorphous surface layer since they were not detected within the internal crystalline core in the form of hydroxyapatite in our bone mineral proxy sample. Our results also indicate that the amount of HPO42− ions present in bone mineral is higher than previously determined in previous studies. Indeed, our calculations show that at least half of the overall amount of inorganic phosphate ions that compose bone mineral are in the form of monohydrogen-phosphate ions. As a result, the average chemical composition of the mature cortical bone mineral from our 2-year-old sheep bone tissue sample was approximated as follows: Ca7.5(PO4)2.8(HPO4)2.6(CO3)0.6(OH)0,2. According to the similarities between sheep and humans, not only in terms of bone and joint structure, but also in terms of bone regeneration and metabolism69, this methodological and analytical approach may be translatable to human bones for which a comparable quantification attempt has been undertaken70.
In summary, the present study provides unprecedented insights into the chemical composition and structural features of bone mineral at the atomic scale; and, hence, embodies a key step to design an accurate chemical and structural model of mature bone mineral particles in their biological environments (Fig. 6). Such model is of primary importance to predict bone mineral chemistry and reactivity in vivo with an overarching objective of enhancing our understanding of processes involved in healthy and pathological bone formations. In this direction, our results emphasize that the surface chemistry and reactivity of bone mineral are driven by metastable amorphous environments rich in monohydrogen-phosphate ions, rather than by stable crystalline environments with hydroxyapatite structure. As such, the recognition mechanisms at the biomineral-biomolecule (collagen, non-collagenous proteins, etc.) interface, which are long-standing questions in the field of bone biomineralization71,72 for shedding light on nucleation and growth processes, must be reconsidered. Further, our results also show the importance of bone mineral surface chemistry in the control of the homeostasis of phosphate ions (i.e., the second ionic buffer in the human body fluids along with carbonates). Last, the analytical tools reported here could be very advantageous for the study of other mineralized tissues in various organisms, including corals73 and bivalve mollusks74 in which the presence of interfacial monohydrogen-carbonate ions localized in highly-disordered environments have been proposed.
Figure 6.
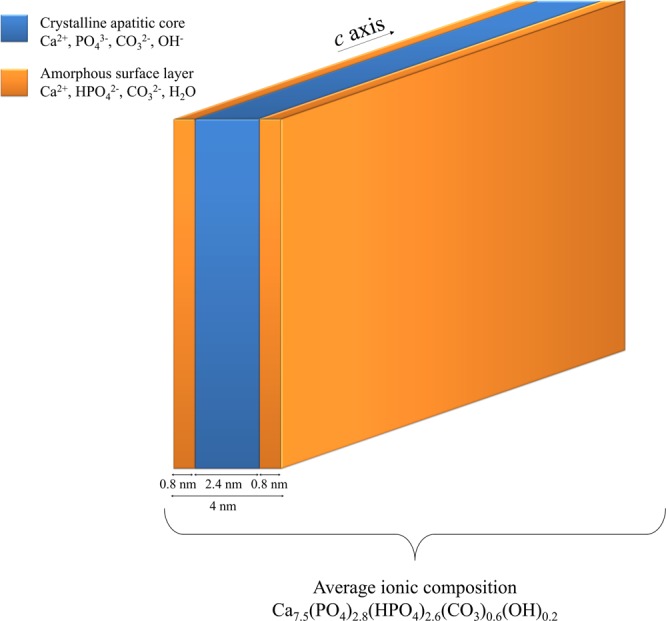
Chemical and structural model of mature bone mineral particles. Schematic representation of platelet-shaped mature bone mineral particles including dimensions and ionic composition according to the results obtained from our 2-year-old sheep bone tissue sample. They are composed by an internal crystalline core in the form of carbonated hydroxyapatite coated by an amorphous layer in which the HPO42− ions are concentrated.
Supplementary information
Acknowledgements
This work was supported by the Agence Nationale de la Recherche (ANR) through the ANR-09-BLAN-0120-01 “NanoShap” program. IMMR in acknowledged and more specifically L. Behr for providing the bone tissue samples.
Author Contributions
S.V.E., Y.W., G.L., C.D. and T.A. performed the research; N. N and T.A. analysed the data; S.V.E, C.D., N.N. and T.A. wrote the main manuscript text; S.V.E., C.D. and T.A. prepared the figures; T.A. designed the research; F.B. looked for financial support.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stanislas Von Euw and Yan Wang contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44620-6.
References
- 1.Reznikov N, Bilton M, Lari L, Stevens MM, Kröger R. Fractal-like hierarchical organization of bone begins at the nanoscale. Science. 2018;360:eaao2189. doi: 10.1126/science.aao2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grandfield K, Vuong V, Schwarcz HP. Ultrastructure of Bone: Hierarchical Features from Nanometer to Micrometer Scale Revealed in Focused Ion Beam Sections in the TEM. Calcif. Tissue Int. 2018;103:606–616. doi: 10.1007/s00223-018-0454-9. [DOI] [PubMed] [Google Scholar]
- 3.Glimcher MJ. Bone: Nature of the Calcium Phosphate Crystals and Cellular, Structural, and Physical Chemical Mechanisms in Their Formation. Rev. Mineral. Geochem. 2006;64:223–282. doi: 10.2138/rmg.2006.64.8. [DOI] [Google Scholar]
- 4.Delmas PD, Tracy RP, Riggs BL, Mann KG. Identification of the noncollagenous proteins of bovine bone by two-dimensional gel electrophoresis. Calcif. Tissue Int. 1984;36:308–316. doi: 10.1007/BF02405335. [DOI] [PubMed] [Google Scholar]
- 5.Boskey AL. Noncollagenous matrix proteins and their role in mineralization. Bone Miner. 1989;6:111–123. doi: 10.1016/0169-6009(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 6.Boskey, A. L. & Robey, P. G. The Composition of Bone. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (ed. Rosen, C. J.) 49–58 (John Wiley & Sons, Inc. 2013).
- 7.Wopenka B, Pasteris JD. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C. 2005;25:131–143. doi: 10.1016/j.msec.2005.01.008. [DOI] [Google Scholar]
- 8.Wu Y, et al. Nuclear magnetic resonance spin-spin relaxation of the crystals of bone, dental enamel, and synthetic hydroxyapatites. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2002;17:472–480. doi: 10.1359/jbmr.2002.17.3.472. [DOI] [PubMed] [Google Scholar]
- 9.Huang S-J, Tsai Y-L, Lee Y-L, Lin C-P, Chan JCC. Structural Model of Rat Dentin Revisited. Chem. Mater. 2009;21:2583–2585. doi: 10.1021/cm9006537. [DOI] [Google Scholar]
- 10.Wang Y, et al. The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat. Mater. 2012;11:724–733. doi: 10.1038/nmat3362. [DOI] [PubMed] [Google Scholar]
- 11.Von Euw S, et al. Amorphous surface layer versus transient amorphous precursor phase in bone - A case study investigated by solid-state NMR spectroscopy. Acta Biomater. 2017;59:351–360. doi: 10.1016/j.actbio.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Rey C, Combes C, Drouet C, Sfihi H, Barroug A. Physico-chemical properties of nanocrystalline apatites: Implications for biominerals and biomaterials. Mater. Sci. Eng. C. 2007;27:198–205. doi: 10.1016/j.msec.2006.05.015. [DOI] [Google Scholar]
- 13.Jäger C, Welzel T, Meyer-Zaika W, Epple M. A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. MRC. 2006;44:573–580. doi: 10.1002/mrc.1774. [DOI] [PubMed] [Google Scholar]
- 14.Nassif N, et al. In Vivo Inspired Conditions to Synthesize Biomimetic Hydroxyapatite. Chem. Mater. 2010;22:3653–3663. doi: 10.1021/cm903596q. [DOI] [Google Scholar]
- 15.Vandecandelaere N, Rey C, Drouet C. Biomimetic apatite-based biomaterials: on the critical impact of synthesis and post-synthesis parameters. J. Mater. Sci. Mater. Med. 2012;23:2593–2606. doi: 10.1007/s10856-012-4719-y. [DOI] [PubMed] [Google Scholar]
- 16.Von Euw S, et al. Organization of Bone Mineral: The Role of Mineral–Water Interactions. Geosciences. 2018;8:466. doi: 10.3390/geosciences8120466. [DOI] [Google Scholar]
- 17.Brown WE. Crystal growth of bone mineral. Clin. Orthop. 1966;44:205–220. doi: 10.1097/00003086-196601000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Crane NJ, Popescu V, Morris MD, Steenhuis P, Ignelzi MA. Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone. 2006;39:434–442. doi: 10.1016/j.bone.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 19.Vyalikh A, Elschner C, Schulz M, Mai R, Scheler U. Early Stages of Biomineral Formation—A Solid-State NMR Investigation of the Mandibles of Minipigs. Magnetochemistry. 2017;3:39. doi: 10.3390/magnetochemistry3040039. [DOI] [Google Scholar]
- 20.Simon P, et al. First evidence of octacalcium phosphate@osteocalcin nanocomplex as skeletal bone component directing collagen triple–helix nanofibril mineralization. Sci. Rep. 2018;8:13696. doi: 10.1038/s41598-018-31983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin R, Leng Y, Wang N. HRTEM Study of the Mineral Phases in Human Cortical Bone. Adv. Eng. Mater. 2010;12:B552–B557. doi: 10.1002/adem.200980080. [DOI] [Google Scholar]
- 22.Davies E, et al. Citrate bridges between mineral platelets in bone. Proc. Natl. Acad. Sci. 2014;111:E1354–E1363. doi: 10.1073/pnas.1315080111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arends J, et al. A calcium hydroxyapatite precipitated from an aqueous solution. J. Cryst. Growth. 1987;84:515–532. doi: 10.1016/0022-0248(87)90284-3. [DOI] [Google Scholar]
- 24.Gee A, Deitz VR. Pyrophosphate Formation Upon Ignition of Precipitated Basic Calcium Phosphates1. J. Am. Chem. Soc. 1955;77:2961–2965. doi: 10.1021/ja01616a009. [DOI] [Google Scholar]
- 25.Pellegrino ED, Biltz RM. Mineralization in the chick embryo. Calcif. Tissue Res. 1972;10:128–135. doi: 10.1007/BF02012542. [DOI] [PubMed] [Google Scholar]
- 26.Termine JD, Eanes ED, Greenfield DJ, Nylen MU, Harper RA. Hydrazine-deproteinated bone mineral. Calcif. Tissue Res. 1973;12:73–90. doi: 10.1007/BF02013723. [DOI] [PubMed] [Google Scholar]
- 27.Legros R, Balmain N, Bonel G. Age-related changes in mineral of rat and bovine cortical bone. Calcif. Tissue Int. 1987;41:137–144. doi: 10.1007/BF02563793. [DOI] [PubMed] [Google Scholar]
- 28.Rey C, Shimizu M, Collins B, Glimcher MJ. Resolution-enhanced fourier transform infrared spectroscopy study of the environment of phosphate ions in the early deposits of a solid phase of calcium-phosphate in bone and enamel, and their evolution with age. I: Investigations in the v4 PO4 domain. Calcif. Tissue Int. 1990;46:384–394. doi: 10.1007/BF02554969. [DOI] [PubMed] [Google Scholar]
- 29.Rey C, Shimizu M, Collins B, Glimcher MJ. Resolution-enhanced fourier transform infrared spectroscopy study of the environment of phosphate ion in the early deposits of a solid phase of calcium phosphate in bone and enamel and their evolution with age: 2. Investigations in the v3 PO4 domain. Calcif. Tissue Int. 1991;49:383–388. doi: 10.1007/BF02555847. [DOI] [PubMed] [Google Scholar]
- 30.Kolodziejski W. Solid-state NMR studies of bone. Top. Curr. Chem. 2005;246:235–270. doi: 10.1007/b98652. [DOI] [PubMed] [Google Scholar]
- 31.Xu J, et al. Natural-Abundance 43Ca Solid-State NMR Spectroscopy of Bone. J. Am. Chem. Soc. 2010;132:11504–11509. doi: 10.1021/ja101961x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Water-mediated structuring of bone apatite. Nat. Mater. 2013;12:1144–1153. doi: 10.1038/nmat3787. [DOI] [PubMed] [Google Scholar]
- 33.Jaeger C, et al. Investigation of the Nature of the Protein−Mineral Interface in Bone by Solid-State NMR. Chem. Mater. 2005;17:3059–3061. doi: 10.1021/cm050492k. [DOI] [Google Scholar]
- 34.Hu Y-Y, Rawal A, Schmidt-Rohr K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. 2010;107:22425–22429. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rai RK, Sinha N. Dehydration-Induced Structural Changes in the Collagen–Hydroxyapatite Interface in Bone by High-Resolution Solid-State NMR Spectroscopy. J. Phys. Chem. C. 2011;115:14219–14227. doi: 10.1021/jp2025768. [DOI] [Google Scholar]
- 36.Nikel O, et al. Solid State NMR Investigation of Intact Human Bone Quality: Balancing Issues and Insight into the Structure at the Organic–Mineral Interface. J. Phys. Chem. C. 2012;116:6320–6331. doi: 10.1021/jp2125312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chow WY, et al. NMR Spectroscopy of Native and in Vitro Tissues Implicates PolyADP Ribose in Biomineralization. Science. 2014;344:742–746. doi: 10.1126/science.1248167. [DOI] [PubMed] [Google Scholar]
- 38.Roufosse AH, Aue WP, Roberts JE, Glimcher MJ, Griffin RG. Investigation of the mineral phases of bone by solid-state phosphorus-31 magic angle sample spinning nuclear magnetic resonance. Biochemistry. 1984;23:6115–6120. doi: 10.1021/bi00320a033. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Glimcher MJ, Rey C, Ackerman JL. A Unique Protonated Phosphate Group in Bone Mineral Not Present in Synthetic Calcium Phosphates: Identification by Phosphorus-31 Solid State NMR Spectroscopy. J. Mol. Biol. 1994;244:423–435. doi: 10.1006/jmbi.1994.1740. [DOI] [PubMed] [Google Scholar]
- 40.Kaflak-Hachulska A, Samoson A, Kolodziejski W. 1H MAS and 1H– > 31P CP/MAS NMR study of human bone mineral. Calcif. Tissue Int. 2003;73:476–486. doi: 10.1007/s00223-002-2111-5. [DOI] [PubMed] [Google Scholar]
- 41.Santos RA, Wind RA, Bronnimann CE. 1H CRAMPS and 1H-31P HetCor Experiments on Bone, Bone Mineral, and Model Calcium Phosphate Phases. J. Magn. Reson. B. 1994;105:183–187. doi: 10.1006/jmrb.1994.1120. [DOI] [PubMed] [Google Scholar]
- 42.Cho G, Wu Y, Ackerman JL. Detection of hydroxyl ions in bone mineral by solid-state NMR spectroscopy. Science. 2003;300:1123–1127. doi: 10.1126/science.1078470. [DOI] [PubMed] [Google Scholar]
- 43.Maltsev S, Duer MJ, Murray RC, Jaeger C. A solid-state NMR comparison of the mineral structure in bone from diseased joints in the horse. J. Mater. Sci. 2007;42:8804–8810. doi: 10.1007/s10853-007-1916-z. [DOI] [Google Scholar]
- 44.Yesinowski JP, Eckert H. Hydrogen environments in calcium phosphates: proton MAS NMR at high spinning speeds. J. Am. Chem. Soc. 1987;109:6274–6282. doi: 10.1021/ja00255a009. [DOI] [Google Scholar]
- 45.Rey C, Combes C, Drouet C, Glimcher MJ. Osteoporos. Int. 2009. Bone mineral: update on chemical composition and structure; pp. 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bigi A, et al. Morphological and Structural Modifications of Octacalcium Phosphate Induced by Poly- l -Aspartate. Cryst. Growth Des. 2004;4:141–146. doi: 10.1021/cg034078d. [DOI] [Google Scholar]
- 47.Rhee S-H, Tanaka J. Hydroxyapatite formation on cellulose cloth induced by citric acid. J. Mater. Sci. Mater. Med. 2000;11:449–452. doi: 10.1023/A:1008992009826. [DOI] [PubMed] [Google Scholar]
- 48.Rey, C., Combes, C., Drouet, C. & Somrani, S. 15 - Tricalcium phosphate-based ceramics. In Bioceramics and their Clinical Applications (ed. Kokubo, T.) 326–366 (Woodhead Publishing 2008).
- 49.Grunenwald A, et al. Revisiting carbonate quantification in apatite (bio)minerals: a validated FTIR methodology. J. Archaeol. Sci. 2014;49:134–141. doi: 10.1016/j.jas.2014.05.004. [DOI] [Google Scholar]
- 50.Nielsen NC, Bildso/e H, Jakobsen HJ, Levitt MH. Double‐quantum homonuclear rotary resonance: Efficient dipolar recovery in magic‐angle spinning nuclear magnetic resonance. J. Chem. Phys. 1994;101:1805–1812. doi: 10.1063/1.467759. [DOI] [Google Scholar]
- 51.Yannoni CS. High-resolution NMR in solids: the CPMAS experiment. Acc. Chem. Res. 1982;15:201–208. doi: 10.1021/ar00079a003. [DOI] [Google Scholar]
- 52.Hediger S, Meier BH, Kurur ND, Bodenhausen G, Ernst RR. NMR cross polarization by adiabatic passage through the Hartmann—Hahn condition (APHH) Chem. Phys. Lett. 1994;223:283–288. doi: 10.1016/0009-2614(94)00470-6. [DOI] [Google Scholar]
- 53.Feike M, et al. Broadband Multiple-Quantum NMR Spectroscopy. J. Magn. Reson. A. 1996;122:214–221. doi: 10.1006/jmra.1996.0197. [DOI] [Google Scholar]
- 54.Rey C, Miquel JL, Facchini L, Legrand AP, Glimcher MJ. Hydroxyl groups in bone mineral. Bone. 1995;16:583–586. doi: 10.1016/8756-3282(95)00101-I. [DOI] [PubMed] [Google Scholar]
- 55.Müller L, Kumar A, Baumann T, Ernst RR. Transient Oscillations in NMR Cross-Polarization Experiments in Solids. Phys. Rev. Lett. 1974;32:1402–1406. doi: 10.1103/PhysRevLett.32.1402. [DOI] [Google Scholar]
- 56.Hester RK, Ackerman JL, Cross VR, Waugh JS. Resolved Dipolar Coupling Spectra of Dilute Nuclear Spins in Solids. Phys. Rev. Lett. 1975;34:993–995. doi: 10.1103/PhysRevLett.34.993. [DOI] [Google Scholar]
- 57.Kolodziejski W, Klinowski J. Kinetics of Cross-Polarization in Solid-State NMR: A Guide for Chemists. Chem. Rev. 2002;102:613–628. doi: 10.1021/cr000060n. [DOI] [PubMed] [Google Scholar]
- 58.Catti M, Ferraris G, Filhol A. Hydrogen bonding in the crystalline state. CaHPO4 (monetite), P 1 or P1? A novel neutron diffraction study. Acta Crystallogr. B. 1977;33:1223–1229. doi: 10.1107/S0567740877005706. [DOI] [Google Scholar]
- 59.Curry NA, Jones DW. Crystal structure of brushite, calcium hydrogen orthophosphate dihydrate: a neutron-diffraction investigation. J. Chem. Soc. Inorg. Phys. Theor. 1971;0:3725–3729. doi: 10.1039/j19710003725. [DOI] [Google Scholar]
- 60.Davies E, Duer MJ, Ashbrook SE, Griffin JM. Applications of NMR Crystallography to Problems in Biomineralization: Refinement of the Crystal Structure and 31 P Solid-State NMR Spectral Assignment of Octacalcium Phosphate. J. Am. Chem. Soc. 2012;134:12508–12515. doi: 10.1021/ja3017544. [DOI] [PubMed] [Google Scholar]
- 61.Eppell SJ, Tong W, Katz JL, Kuhn L, Glimcher MJ. Shape and size of isolated bone mineralites measured using atomic force microscopy. J. Orthop. Res. 2001;19:1027–1034. doi: 10.1016/S0736-0266(01)00034-1. [DOI] [PubMed] [Google Scholar]
- 62.Kim H-M, Rey C, Glimcher MJ. Isolation of calcium-phosphate crystals of bone by non-aqueous methods at low temperature. J. Bone Miner. Res. 1995;10:1589–1601. doi: 10.1002/jbmr.5650101021. [DOI] [PubMed] [Google Scholar]
- 63.Bocciarelli DS. Morphology of crystallites in bone. Calcif. Tissue Res. 1970;5:261–269. doi: 10.1007/BF02017554. [DOI] [PubMed] [Google Scholar]
- 64.Kay MI, Young RA, Posner AS. Crystal Structure of Hydroxyapatite. Nature. 1964;204:1050–1052. doi: 10.1038/2041050a0. [DOI] [PubMed] [Google Scholar]
- 65.Kuhn LT, et al. A Comparison of the Physical and Chemical Differences Between Cancellous and Cortical Bovine Bone Mineral at Two Ages. Calcif. Tissue Int. 2008;83:146–154. doi: 10.1007/s00223-008-9164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vyalikh, A. et al. An NMR Study of Biomimetic Fluorapatite – Gelatine Mesocrystals. Sci. Rep. 5, (2015). [DOI] [PMC free article] [PubMed]
- 67.Nakayama, M. et al. Stimuli-responsive hydroxyapatite liquid crystal with macroscopically controllable ordering and magneto-optical functions. Nat. Commun. 9, (2018). [DOI] [PMC free article] [PubMed]
- 68.Bertinetti L, et al. Surface Structure, Hydration, and Cationic Sites of Nanohydroxyapatite: UHR-TEM, IR, and Microgravimetric Studies. J. Phys. Chem. C. 2007;111:4027–4035. doi: 10.1021/jp066040s. [DOI] [Google Scholar]
- 69.Nunamaker, D. M. Experimental models of fracture repair. Clin. Orthop. S56–65 (1998). [DOI] [PubMed]
- 70.Kaflak A, Chmielewski D, Kolodziejski W. Solid-state NMR study of discrete environments of bone mineral nanoparticles using phosphorus-31 relaxation. J. Appl. Biomed. 2016;14:321–330. doi: 10.1016/j.jab.2016.07.001. [DOI] [Google Scholar]
- 71.Shaw WJ. Solid-state NMR studies of proteins immobilized on inorganic surfaces. Solid State Nucl. Magn. Reson. 2015;70:1–14. doi: 10.1016/j.ssnmr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gilbert PUPA, Abrecht M, Frazer BH. The Organic-Mineral Interface in Biominerals. Rev. Mineral. Geochem. 2005;59:157–185. doi: 10.2138/rmg.2005.59.7. [DOI] [Google Scholar]
- 73.Von Euw S, et al. Biological control of aragonite formation in stony corals. Science. 2017;356:933–938. doi: 10.1126/science.aam6371. [DOI] [PubMed] [Google Scholar]
- 74.Ben Shir I, Kababya S, Katz I, Pokroy B, Schmidt A. Exposed and Buried Biomineral Interfaces in the Aragonitic Shell of Perna canaliculus Revealed by Solid-State NMR. Chem. Mater. 2013;25:4595–4602. doi: 10.1021/cm4028226. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.