Abstract
Purpose: The aim of this pooled analysis was to evaluate the clinical efficacy and safety of transarterial radioembolization (TARE) with yttrium-90 (90Y) microspheres for the treatment of unresectable intrahepatic cholangiocarcinoma (ICC).
Methods: We searched the Cochrane Library, Embase, PubMed, SCI with the English language from inception to October 2018. A pooled analysis was conducted using Stata software.
Results: There were 16 eligible studies included in this pooled analysis. The pooled median overall survival (OS) from 12 studies was 14.3 (95% CI: 11.9–17.1) months. Based on Response Evaluation Criteria in Solid Tumors (RECIST), no complete response was reported, and the median of partial response, stable disease and progressive disease were 11.5% (range: 4.8–35.3%), 61.5% (range: 42.9–81.3%) and 22.7% (range: 12.5–52.4%) respectively. The pooled disease control rate (DCR) from nine studies was 77.2% (95% CI: 70.2–84.2%). According to the type of microspheres, subgroup analysis was performed, the median OS in the glass microspheres group was 14.0 (95% CI: 9.1–21.4) months, and 14.3 (95% CI: 11.5–17.8) months in the resin microspheres group. The DCR was 77.3% (95% CI: 63.5–91.1%) and 77.4% (95% CI: 66.8–87.9%) in the glass and resin microspheres groups respectively. Most of the side effects reported in the included studies were mild and did not require intervention.
Conclusion: TARE with 90Y microspheres is safe and effective for patients with unresectable ICC with acceptable side effects. And it seems that the type of microsphere has no influence on therapeutic efficacy.
Keywords: transarterial radioembolization, yttrium-90 microspheres, intrahepatic cholangiocarcinoma, pooled analysis
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a highly invasive malignancy of the biliary tract with high mortality due to its infiltrative nature, propensity for advanced disease presentation and resistance to chemotherapy.1,2 From diagnosis, the median overall survival (OS) of ICC without treatment is about 4.5 months. Surgical resection may be the only potentially curative treatment, however, only 30–40% of ICC patients are the surgical candidate when the diagnosis is first confirmed.3,4 Systemic chemotherapy with cisplatin plus gemcitabine is also limited by poor response rates.5,6 Transarterial radioembolization (TARE) with yttrium-90 (90Y)-labeled glass or resin microspheres are being used increasingly in primary and secondary liver malignancies, which provides an advantage to the median OS with good tolerance.7,8 Al-Adra et al9 reviewed 12 studies regarding TARE with 90Y microspheres for the treatment of unresectable ICC in 2014; there are emerging studies on TARE with 90Y microspheres for the treatment of ICC, so it is necessary to further systematically evaluate the outcomes of TARE with 90Y microspheres in these patients. The aim of this pooled analysis was to comprehensively evaluate the therapeutic efficacy and safety of TARE with 90Y microspheres for the treatment of unresectable ICC.
Material and methods
Search strategy
We searched the Cochrane Library, Embase, PubMed, SCI with English language from inception to October 2018. Relevant documents were supplemented by references of retrieved articles. The terms we used to search were related to intrahepatic cholangiocarcinoma, intrahepatic bile duct carcinoma, cholangiocellular carcinoma, neoplasms of the biliary tract, cholangiohepatoma, yttrium-90, Y90, 90Y, SIR-Spheres, TheraSphere, radiation lobectomy.
Inclusion and exclusion criteria
Clinical trials or studies
Studies that described TARE with 90Y microspheres in the treatment of unresectable ICC
Exclusion criteria
Review articles, animal studies, abstracts, case reports
Duplicated clinical studies
Studies with fewer than 10 cases
The quality of the studies was independently evaluated by two reviewers based on the Downs and Black quality assessment checklist.10
Data extraction
Two authors extracted the data and a third one resolved any disagreements. The extracted data included details of type of researches (prospective or retrospective cohort), number of patients, age, sex, Eastern Cooperative Oncology Group (ECOG) score, extrahepatic metastases, pre- and postchemotherapy, type of microspheres, dosimetric calculation, follow-up time, median OS, 1-year survival, evaluation criteria, tumor response, side effects (eg clinical toxicities such as fatigue, abdominal pain, nausea, and biochemical toxicities such as decreased albumin, elevated bilirubin, alkaline phosphatase, etc).
Statistical analysis
Only median OS and disease control rate (DCR) were pooled analysis by Stata 11.0 (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP.), while other outcomes were analyzed in descriptive statistics. The I2 measure was used to show the inconsistency between studies. An Egger test was used to assess publication bias, and Metaninf was used for sensitivity analysis, a two-sided P<0.05 was regarded as significant.
Results
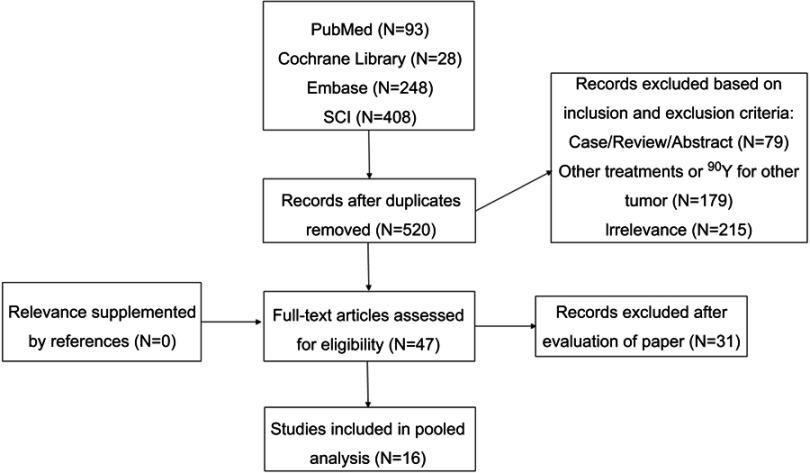
According to the inclusion and exclusion criteria, 16 eligible studies11–26 were identified that reported the TARE with 90Y microspheres for unresectable ICC (Figure 1). Five prospective and 11 retrospective studies were included. There were 472 patients included in this pooled analysis. Patient characteristics were presented in Table 1. Extrahepatic metastases were observed in a median of 48.7% (range: 8.7–57.9%). A median of 71.9% (range: 0.0–100.0%) patients received systemic chemotherapy before TARE with 90Y microspheres, and a median of 12.3% (range: 7.1–28.0%) received postoperative chemotherapy.
Figure 1.
A flowchart of study identification and selection.
Table 1.
Study design and baseline characteristics
| Author | Year | Study design | Patient | Mean age | Male, N (%) | ECOG score | Extrahepatic metastases N (%) |
Evidence level | Prechemotherapy N (%) | Postchemotherapy N (%) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||||||||
| Saxena et al26 | 2010 | PC | 25 | 57 | 13 (52.0) | 15 | 7 | 3 | 0 | 12 (48.0) | Moderate | 18 (72.0) | 7 (28.0) |
| Mosconi et al25 | 2016 | RC | 23 | 65 | 14 (60.9) | 18 | 5 | 0 | 0 | 2 (8.7) | Low | 12 (52.2) | 4 (17.4) |
| Rafi et al24 | 2013 | PC | 19 | 63.3 | 7 (36.8) | 1 | 14 | 4 | 0 | 11 (57.9) | Moderate | 19 (100.0) | N/A |
| Mouli et al23 | 2013 | PC | 46 | 68a | 25 (54.3) | 24 | 21 | 1 | 0 | 16 (34.8) | Moderate | 16 (34.8) | N/A |
| Hoffmann et al22 | 2012 | RC | 33 | 65.2 | 18 (54.5) | 17 | 7 | 9 | 0 | 8 (24.2) | Moderate | 27 (78.8) | N/A |
| Jia et al21 | 2017 | RC | 24 | 61.8 | 8 (33.3) | 16 | 8 | 0 | 0 | 3 (12.5) | Moderate | 24 (100.0) | N/A |
| Soydal et al20 | 2016 | RC | 16 | 55.4 | 8 (50.0) | N/A | N/A | N/A | N/A | 5 (31.3) | Moderate | 9 (56.3) | N/A |
| Swinburne et al19 | 2017 | RC | 29 | 66 | 14 (48.3) | 11 | 13 | 5 | 0 | 11 (37.9) | Moderate | 15 (51.7) | N/A |
| Reimer et al18 | 2018 | RC | 21 | 69.5 | 12 (57.1) | 0 | 3 | 16 | 2 | 3 (14.3) | Moderate | 0 (0.0) | N/A |
| Orwat et al17 | 2017 | RC | 16 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Moderate | N/A | N/A |
| Paprottka et al16 | 2017 | RC | 35 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Moderate | N/A | N/A |
| Gangi et al13 | 2018 | RC | 85 | 73.4 | 41 (48.2) | 35 | 22 | 28 | 0 | 36 (42.4) | Moderate | 61 (71.8) | 6(7.1) |
| Filippi et al11 | 2015 | PC | 17 | 59.4 | 6 (35.3) | N/A | N/A | N/A | N/A | 4 (23.5) | Moderate | 15 (88.2) | N/A |
| Beuzit et al12 | 2016 | RC | 45 | 64a | 24 (53.3) | 25 | 20 | 0 | N/A | Moderate | 41 (91.1) | N/A | |
| Shaker et al14 | 2018 | RC | 17 | 69.3 | 7 (41.2) | N/A | N/A | N/A | N/A | 7 (41.2) | Moderate | 5 (29.4) | 3 (17.6) |
| Camacho et al15 | 2014 | PC | 21 | 62.7a | 13 (62.0) | 9 | 8 | 3 | 0 | N/A | Moderate | 21 (100.0) | N/A |
Note: aMedian age.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; RC, Retrospective cohort; PC, Prospective cohort; N/A, not available.
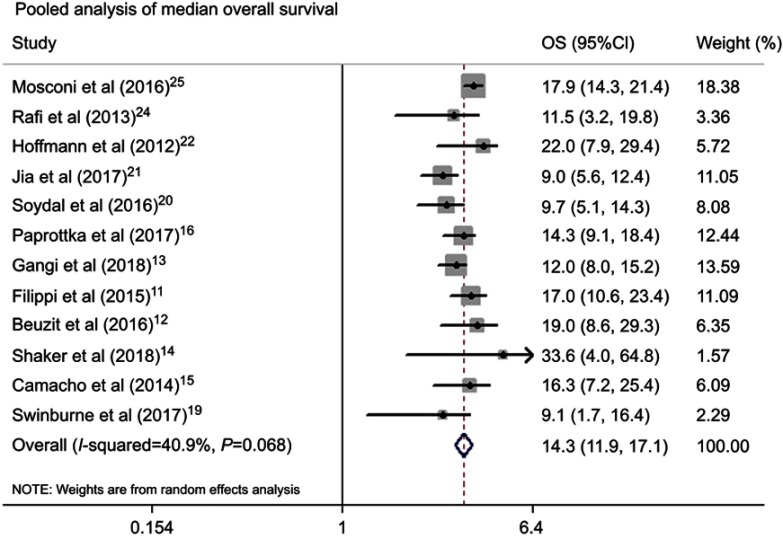
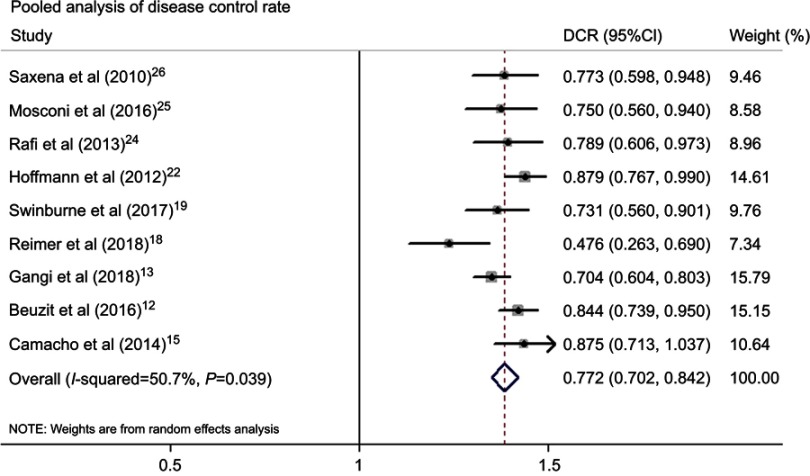
Table 2 summarized information about the therapeutic outcomes of TARE with 90Y microspheres for ICC. The pooled median OS from 12 studies was 14.3 (95%CI: 11.9–17.1) months (Figure 2). The tumor response at 3 months after TARE with 90Y microspheres was evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST), no complete response was reported, and the median of partial response, stable disease, progressive disease was 11.5% (range: 4.8–35.3%), 61.5% (range: 42.9–81.3%), 22.7% (range: 12.5–52.4%) respectively. The pooled DCR from available studies was 77.2% (95%CI: 70.2–84.2%) (Figure 3). Subgroup analysis was conducted by microspheres type, the median OS in the glass microspheres group was 14.0 (95%CI: 9.1–21.4) months, and 14.3 (95%CI: 11.5–17.8) months in the resin microspheres group. The DCR was 77.3% (95%CI: 63.5–91.1%) and 77.4% (95%CI: 66.8–87.9%) in the glass and resin microspheres group respectively. There were six studies reporting 1-year survival rate with a median of 51.5% (range: 32.6–67.9%).
Table 2.
Treatment characteristics and efficacy
| Author | Year | Microsphere | Dosimetric calculation | Follow-up (months) | Median OS (months) | 1-year survival | Evaluation criteria | Recist | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | PR | SD | PD | ||||||||
| Saxena et al26 | 2010 | Resin | BSA | 8.1 | 9.3 | 40.0% | RECIST | 0 | 6 | 11 | 5 |
| Mosconi et al25 | 2016 | Resin | BSA | 16.0 | 17.9 | 67.9% | RECIST | 0 | 4 | 11 | 5 |
| mRECIST | 1 | 13 | 3 | 3 | |||||||
| EASL | 1 | 11 | 5 | 3 | |||||||
| Rafi et al24 | 2013 | Resin | BSA | 15.0 | 11.5 | 56.0% | RECIST | 0 | 2 | 13 | 4 |
| Mouli et al23 | 2013 | Glass | N/A | 29.0 | N/A | N/A | WHO | 0 | 11 | 33 | 1 |
| EASL | 4 | 28 | - | 0 | |||||||
| Hoffmann et al22 | 2012 | Resin | BSA | 10 | 22.0 | N/A | RECIST | 0 | 12 | 17 | 5 |
| Jia et al21 | 2017 | Resin | BSA | 11.3d | 9.0 | 32.6% | mRECIST | - | 8 | 10 | 4 |
| Soydal et al20 | 2016 | Resin | BSA | 8.1 | 9.7 | N/A | RECIST | - | - | - | - |
| Swinburne et al19 | 2017 | Resin/glass | BSA; Othera | 8.4d | 9.1 | N/A | RECIST | 0 | 3 | 16 | 7 |
| Reimer et al18 | 2018 | Resin | BSA | N/A | 15.0 | N/A | RECIST | 0 | 1 | 9 | 11 |
| Orwat et al17 | 2017 | Resin/glass | BSA | N/A | 5.2 | N/A | N/A | N/A | N/A | N/A | N/A |
| Paprottka et al16 | 2017 | Resin | mBSA | N/A | 14.3 | N/A | RECIST | N/A | N/A | N/A | N/A |
| Gangi et al13 | 2018 | Glass | Otherb | 9.8 | 12.0 | 49.0% | RECIST | 0 | 5 | 52 | 24 |
| Filippi et al11 | 2015 | Resin | BSA | N/A | 17.0 | N/A | PERCIST | 0 | 14 | 3 | 0 |
| Beuzit et al12 | 2016 | Glass | N/A | N/A | 19.0 | 54.0% | RECIST | 0 | 6 | 32 | 7 |
| Choi | - | 37 | 2 | 6 | |||||||
| Shaker et al14 | 2018 | Resin/glass | N/A | 21.3d | 33.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| Camacho et al15 | 2014 | Resin | BSA; otherc | N/A | 16.3 | N/A | RECIST | 0 | 1 | 13 | 2 |
| mRECIST | 2 | 7 | 5 | 2 | |||||||
| EASL | 2 | 6 | 6 | 2 | |||||||
Notes: aTheraSphere dosimetry was calculated based upon a desired radiation treatment dose for a targeted portion of the liver. bDosimetry was calculated based on the treated liver volume, the administered activity, and the lung shunt fraction. cDosimetry was based on tumor volumetry and then adjusted by the pulmonary shunt fraction. dMean follow-up.
Abbreviations: RECIST, Response Evaluation Criteria in Solid Tumors; PERCIST, Positron Emission Tomography Response Criteria in Solid Tumors; mRECIST, modified RECIST; EASL, the European Association for the Study of the Liver; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; OS, overall survival; N/A, not available; BSA, body surface area.
Figure 2.
Pooled analysis of median overall survival.
Abbreviation: OS, overall survival.
Figure 3.
Pooled analysis of disease control rate.
Abbreviation: DCR, disease control rate.
Side effects and the proportion of grade III–IV toxicities were listed in Table 3. Clinical toxicities mainly included fatigue (median: 31.7%; range: 0.0–87.5%), anorexia (median: 10.0%; range: 0.0–79.2%), abdominal pain (median: 30.0%; range: 0.0–85.0%), nausea (median: 16.0%; range: 0.0–62.5%), vomiting (median: 9.0%; range: 0.0–27.0%), ascites (median: 10.5%; range: 0.0–21.7%). Biochemical toxicities were decreased albumin (median: 2.0%; range: 0.0–9.0%), elevated bilirubin (median: 5.7%; range: 0.0–70.0%), elevated alkaline phosphatase (median: 1.7%; range: 0.0–46.0%). The incidence of gastroduodenal ulceration was a median of 4.0% (range: 0.0–5.0%) in 5 studies reporting side effects. A median of 7.8% (range: 0.0–25.0%) grade III–IV toxicities (including gastroduodenal ulceration) was reported in 10 studies.
Table 3.
Clinical and biochemical toxicities
| Author | Year | Clinical toxicities, N (%) | Biochemical Toxicities, N (%) | Severity, N (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatigue | Anorexia | Abdominal pain | Nausea | Vomiting | Ascites | Gastroduodenal ulceration | Abumin | Bilirubin | Alkaline phosphatase | Grade III–IVa | ||
| Saxena et al26 | 2010 | 16 (64.0) | 4 (16.0) | 10 (40.0) | 4 (16.0) | 2 (8.0) | 4 (16.0) | 1 (4.0) | 1 (4.0) | 1 (4.0) | 1 (4.0) | 4 (16.0) |
| Mosconi et al25 | 2016 | 2 (8.7) | N/A | 5 (21.7) | N/A | N/A | 5 (21.7) | N/A | N/A | 1 (4.3) | N/A | 2 (8.6) |
| Rafi et al24 | 2013 | 4 (21.0) | N/A | 6 (32.0) | N/A | N/A | N/A | 0 | N/A | 3 (15.8) | N/A | 2 (11.0) |
| Mouli et al23 | 2013 | 25 (54.0) | 2 (4.0) | 13 (28.0) | 6 (13.0) | 4 (9.0) | 7 (15.0) | 1 (2.0) | 4 (9.0) | 3 (7.0) | 0 | 8 (18.0) |
| Hoffmann et al22 | 2012 | N/A | N/A | 28 (85.0) | 20 (61.0) | 9 (27.0) | N/A | N/A | N/A | 23 (70.0) | N/A | N/A |
| Jia et al21 | 2017 | 21 (87.5) | 19 (79.2) | 10 (58.3) | 15 (62.5) | 4 (16.7) | N/A | 1 (4.2) | 0 | 0 | 0 | 6 (25.0) |
| Soydal et al20 | 2016 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Swinburne et al19 | 2017 | 2 (6.7) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 4 (13.3) | 1 (3.3) | 0 |
| Reimer et al18 | 2018 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5.0) | 0 | 0 | 0 | 1 (5.0) |
| Orwat et al17 | 2017 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Paprottka et al16 | 2017 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Gangi et al13 | 2018 | 36 (42.3) | N/A | 16 (18.8) | N/A | N/A | 5 (5.9) | N/A | N/A | N/A | 39 (46.0) | 6 (7.0) |
| Filippi et al11 | 2015 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1 (4.0) |
| Beuzit et al12 | 2016 | N/A | N/A | N/A | N/A | N/A | 1 (2.0) | N/A | N/A | N/A | N/A | 1 (2.0) |
| Shaker et al14 | 2018 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Camacho et al15 | 2014 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
Note: aGrade III–IV toxicities including gastroduodenal ulceration.
Abbreviation: N/A, not available.
Mild and moderate heterogeneity was shown in pooled median OS and DCR. These estimates were robust in the sensitivity analysis. No significant publication bias was identified in pooled analysis.
Discussion
The pooled analysis showed that TARE with 90Y microspheres can be an effective treatment for unresectable ICC with a pooled median OS of 14.3 (95%CI: 12.0–17.1) months. According to RECIST, the pooled DCR was 77.2% (95%CI: 70.2–84.2%). Subgroup analysis was conducted by microsphere type, it seems that there were similar median OS and DCR in the glass and resin microspheres group. In addition, it was associated with mild clinical and biochemical toxicities, and often these symptoms were relieved over time.
With the increasing incidence of ICC and impossibility of surgical resection, more and more people are exploring new treatments. TARE with 90Y microspheres has gradually become an effective treatment by using an intra-arterial injection of microspheres loaded with 90Y microspheres as the source of internal radiation.27Al-A dra et al9reported the OS of 15.5 months in the pooled analysis for the treatment of ICC with 90Y radioembolization. However, seven abstracts were included in the pooled analysis, which provided limited information regarding treatment and follow-up outcomes. In the current pooled analysis, we excluded abstracts and added literature published in recent years, which provided more comprehensive information. We came to a similar median OS of 14.3 months. Subgroup analysis was performed based on the type of microspheres, and the median OS was similar in the resin and glass microspheres groups (14.0 vs 14.3 months). Unfortunately, due to the heterogeneity of studies in each group, the random effects model was performed, which failed to compare the differences between groups. Nezami et al28 compared the dose of radiation delivered through glass and resin-based 90Y microspheres to ICC and concluded that 90Y both glass and resin-based microspheres radioembolization were feasible and safe in the treatment of ICC, while glass microsphere delivers a higher dose of 90Y to the targeted tumors. However, it remains to be further studied whether the two types of microspheres affect the prognosis of ICC patients. Ray et al29 reported that the pooled median OS of transarterial chemoembolization (TACE) for unresectable ICC was 13.4 months. Boehm et al30 conducted a pooled median OS of 12.4 months for the treatment of TACE. It seems that median OS of TARE with 90Y microspheres was generally consistent with TACE. However, further randomized controlled trials are needed to confirm these results.
In the current pooled analysis, most of the studies (11/16) evaluated tumor response according to RECIST, and the pooled DCR was 77.2%, which indicated that TARE was an effective treatment for ICC. However TARE with 90Y microspheres usually leads to necrosis without an actual decrease of tumor size, RECIST31,32 only considers the change in the size of target lesions, and the association between RECIST and survival still needs further to be investigated. PET can evaluate the change of tumor volume through the difference of standardized uptake value, which is valuable in assessing the activity of cancer therapies that stabilize diseases.33 Zerizer et al34 reported that 18F-FDG PET-CT was superior to RECIST in evaluating early response of TARE and predicting progression free survival in patients with liver metastases. Therefore, PET-based approaches are expected to be effective evaluation criteria in tumor response after TARE with 90Y microspheres.
In addition, TARE with 90Y microspheres is associated with some side effects. In the current pooled analysis, the common clinical toxicities mainly included fatigue, abdominal pain, nausea, vomiting, ascites, and biochemical toxicities had decreased albumin, elevated bilirubin and alkaline phosphatase, etc. These side effects were usually mild and acceptable, and could be resolved without medical therapy. Moreover, gastroduodenal ulceration is a relatively common serious side effect of TARE with 90Y microspheres,35 which is caused by nontargeted microsphere distribution, so it is necessary to clarify the vascular anatomy and undergo prophylactic arterial embolization; in addition, microspheres must be carefully injected during the treatment process to avoid nontargeted embolization.
There are several limitations in the current pooled analysis. First, in the pooled analysis, not all studies reported the population and treatment characteristics that were meta-analyzed, thus not allowing a complete analysis of heterogeneity sources. Second, meta-regression was not performed in the current analysis because the pooled results were robust in the sensitivity analysis, which suggested the source of heterogeneity may not exist in studies, but in individuals. Third, side effects were summarized only as descriptive words, standardized methodology needs to be used. Fourth, the current results failed to help define the best population for TARE, but this pooled analysis included the best available evidence and provided valuable information on the therapeutic efficacy and safety of TARE with 90Y microspheres for unresectable ICC.
Conclusion
TARE with 90Y microspheres is a promising therapeutic option for patients with unresectable ICC with acceptable side effects. The different microspheres seem to have no influence on therapeutic efficacy, and TARE with 90Y microspheres has a similar OS compared with TACE reported in previous studies. A large sample of randomized controlled trial is warranted to confirm the above results.
Acknowledgments
We would like to thank all our colleagues and authors who cooperated with us by preparing the full text of the papers. The authors received no financial support for the research, authorship, and/or publication of this article.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma. Ann Surg. 2008;248(1):84–96. doi: 10.1097/SLA.0b013e318176c4d3 [DOI] [PubMed] [Google Scholar]
- 2.Hogdall D, O'Rourke CJ, Taranta A, Oliveira DV, Andersen JB. Molecular pathogenesis and current therapy in intrahepatic cholangiocarcinoma. Dig Dis. 2016;34(4):440–451. doi: 10.1159/000444562 [DOI] [PubMed] [Google Scholar]
- 3.Ellis MC, Cassera MA, Vetto JT, Orloff SL, Hansen PD, Billingsley KG. Surgical treatment of intrahepatic cholangiocarcinoma: outcomes and predictive factors. Hpb. 2011;13(1):59–63. doi: 10.1111/j.1477-2574.2010.00242.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong K, Geschwind J-FH. Locoregional intra-arterial therapies for unresectable intrahepatic cholangiocarcinoma. Semin Oncol. 2010;37(2):110–117. doi: 10.1053/j.seminoncol.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 5.Yang L, Shan J, Shan L, Saxena A, Bester L, Morris DL. Trans-arterial embolisation therapies for unresectable intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Oncol. 2015;6(5):570–588. doi: 10.3978/j.issn.2078-6891.2015.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2013;25(2):391–398. doi: 10.1093/annonc/mdt540 [DOI] [PubMed] [Google Scholar]
- 7.Koay EJ, Odisio BC, Javle M, Vauthey J-N, Crane CH. Management of unresectable intrahepatic cholangiocarcinoma: how do we decide among the various liver-directed treatments? Hepatobiliary Surg Nutr. 2017;6(2):105–116. doi: 10.21037/hbsn.2017.01.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, Irani Z, Lawrence D, Flaherty K, Arellano RS. Combined effects of Yttrium-90 transarterial radioembolization around immunotherapy for hepatic metastases from uveal melanoma: a preliminary retrospective case series. J Vasc Interv Radiol. 2018;29(10):1369–1375. doi: 10.1016/j.jvir.2018.04.030 [DOI] [PubMed] [Google Scholar]
- 9.Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau -S-S. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol (EJSO). 2015;41(1):120–127. doi: 10.1016/j.ejso.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sara H, Downs NB. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Filippi L, Pelle G, Cianni R, Scopinaro F, Bagni O. Change in total lesion glycolysis and clinical outcome after (90)Y radioembolization in intrahepatic cholangiocarcinoma. Nucl Med Biol. 2015;42(1):59–64. doi: 10.1016/j.nucmedbio.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 12.Beuzit L, Edeline J, Brun V, et al. Comparison of Choi criteria and response evaluation criteria in solid tumors (RECIST) for intrahepatic cholangiocarcinoma treated with glass-microspheres Yttrium-90 selective internal radiation therapy (SIRT). Eur J Radiol. 2016;85(8):1445–1452. doi: 10.1016/j.ejrad.2016.05.020 [DOI] [PubMed] [Google Scholar]
- 13.Gangi A, Shah J, Hatfield N, et al. Intrahepatic cholangiocarcinoma treated with transarterial Yttrium-90 glass microsphere radioembolization: results of a single institution retrospective study. J Vasc Interventional Radiol. 2018;29(8):1101–1108. doi: 10.1016/j.jvir.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaker TM, Chung C, Varma MK, et al. Is there a role for Ytrrium-90 in the treatment of unresectable and metastatic intrahepatic cholangiocarcinoma? Am J Surg. 2018;215(3):467–470. doi: 10.1016/j.amjsurg.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 15.Camacho JC, Kokabi N, Xing M, Prajapati HJ, El-Rayes B, Kim HS. Modified response evaluation criteria in solid tumors and European Association for the study of the liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following Yttrium-90 radioembolization. J Vasc Interventional Radiol. 2014;25(2):256–265. doi: 10.1016/j.jvir.2013.10.056 [DOI] [PubMed] [Google Scholar]
- 16.Paprottka KJ, Schoeppe F, Ingrisch M, et al. Pre-therapeutic factors for predicting survival after radioembolization: a single-center experience in 389 patients. Eur J Nucl Med Mol Imaging. 2017;44(7):1185–1193. doi: 10.1007/s00259-017-3646-z [DOI] [PubMed] [Google Scholar]
- 17.Orwat KP, Beckham TH, Cooper SL, et al. Pretreatment albumin may aid in patient selection for intrahepatic Y-90 microsphere transarterial radioembolization (TARE) for malignancies of the liver. J Gastrointest Oncol. 2017;8(6):1072–1078. doi: 10.21037/jgo.2017.06.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reimer P, Virarkar MK, Binnenhei M, Justinger M, Schön MR, Tatsch K. Prognostic factors in overall survival of patients with unresectable intrahepatic cholangiocarcinoma treated by means of Yttrium-90 radioembolization: results in therapy-naive patients. Cardiovasc Intervent Radiol. 2018;41(5):744–752. doi: 10.1007/s00270-017-1871-2 [DOI] [PubMed] [Google Scholar]
- 19.Swinburne NC, Biederman DM, Besa C, et al. Radioembolization for unresectable intrahepatic cholangiocarcinoma: review of safety, response evaluation criteria in solid tumors 1.1 imaging response and survival. Cancer Biother Radiopharm. 2017;32(5):161–168. doi: 10.1089/cbr.2017.2189 [DOI] [PubMed] [Google Scholar]
- 20.Soydal C, Kucuk ON, Bilgic S, Ibis E. Radioembolization with (90)Y resin microspheres for intrahepatic cholangiocellular carcinoma: prognostic factors. Ann Nucl Med. 2016;30(1):29–34. doi: 10.1007/s12149-015-1026-y [DOI] [PubMed] [Google Scholar]
- 21.Jia Z, Paz-Fumagalli R, Frey G, Sella DM, McKinney JM, Wang W. Resin-based Yttrium-90 microspheres for unresectable and failed first-line chemotherapy intrahepatic cholangiocarcinoma: preliminary results. J Cancer Res Clin Oncol. 2017;143(3):481–489. doi: 10.1007/s00432-016-2291-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann RT, Paprottka PM, Schon A, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35(1):105–116. doi: 10.1007/s00270-011-0142-x [DOI] [PubMed] [Google Scholar]
- 23.Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. 2013;24(8):1227–1234. doi: 10.1016/j.jvir.2013.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36(2):440–448. doi: 10.1007/s00270-012-0463-4 [DOI] [PubMed] [Google Scholar]
- 25.Mosconi C, Gramenzi A, Ascanio S, et al. Yttrium-90 radioembolization for unresectable/recurrent intrahepatic cholangiocarcinoma: a survival, efficacy and safety study. Br J Cancer. 2016;115(3):297–302. doi: 10.1038/bjc.2016.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17(2):484–491. doi: 10.1245/s10434-009-0777-x [DOI] [PubMed] [Google Scholar]
- 27.Cristina Mosconi AC, Ascanio S. Yttrium-90 microsphere radioembolization in unresectable intrahepatic cholangiocarcinoma. Future Oncol. 2017;13(15):1301–1310. doi: 10.2217/fon-2017-0022 [DOI] [PubMed] [Google Scholar]
- 28.Nezami N, Kokabi N, Camacho JC, Schuster DM, Xing M, Kim HS. (90)Y radioembolization dosimetry using a simple semi-quantitative method in intrahepatic cholangiocarcinoma: glass versus resin microspheres. Nucl Med Biol. 2018;59:22–28. doi: 10.1016/j.nucmedbio.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 29.Ray CE Jr., Edwards A, Smith MT, et al. Metaanalysis of survival, complications, and imaging response following chemotherapy-based transarterial therapy in patients with unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2013;24(8):1218–1226. doi: 10.1016/j.jvir.2013.03.019 [DOI] [PubMed] [Google Scholar]
- 30.Boehm LM, Jayakrishnan TT, Miura JT, et al. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J Surg Oncol. 2015;111(2):213–220. doi: 10.1002/jso.23781 [DOI] [PubMed] [Google Scholar]
- 31.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl_1):122S–150S. doi: 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MN, Kim BK, Han KH, Kim SU. Volution from WHO to EASL and mRECIST for hepatocellular carcinoma: considerations for tumor response assessment. Expert Rev Gastroenterol Hepatol. 2015;9(3):335–348. doi: 10.1586/17474124.2015.959929 [DOI] [PubMed] [Google Scholar]
- 33.Haug AR, Heinemann V, Bruns CJ, et al. 18F-FDG PET independently predicts survival in patients with cholangiocellular carcinoma treated with 90Y microspheres. Eur J Nucl Med Mol Imaging. 2011;38(6):1037–1045. doi: 10.1007/s00259-011-1736-x [DOI] [PubMed] [Google Scholar]
- 34.Zerizer I, Al-Nahhas A, Towey D, et al. The role of early 18F-FDG PET/CT in prediction of progression-free survival after 90Y radioembolization: comparison with RECIST and tumour density criteria. Eur J Nucl Med Mol Imaging 2012;39(9):1391–1399. doi: 10.1007/s00259-012-2149-1 [DOI] [PubMed] [Google Scholar]
- 35.Lam MGEH, Banerjee S, Louie JD, et al. Root cause analysis of gastroduodenal ulceration after Yttrium-90 Radioembolization. Cardiovasc Intervent Radiol. 2013;36(6):1536–1547. doi: 10.1007/s00270-013-0579-1 [DOI] [PubMed] [Google Scholar]