Abstract
Affiliative behaviours have evolved many times across animals. Research on the mechanisms underlying affiliative behaviour demonstrates remarkable convergence across species spanning wide evolutionary distances. Shared mechanisms have been identified with genomic approaches analysing genetic variants and gene expression differences as well as neuroendocrine and molecular approaches exploring the role of hormones and signalling molecules. We review the genomic and neural basis of pair bonding and parental care across diverse taxa to shed light on mechanistic patterns that underpin the convergent evolution of affiliative behaviour. We emphasize that mechanisms underlying convergence in complex phenotypes like affiliation should be evaluated on a continuum, where signatures of convergence may vary across levels of biological organization. In particular, additional comparative studies within and across major vertebrate lineages will be essential in resolving when and why shared neural substrates are repeatedly targeted in the independent evolution of affiliation, and how similar mechanisms are evolutionarily tuned to give rise to species-specific variations in behaviour.
This article is part of the theme issue ‘Convergent evolution in the genomics era: new insights and directions'.
Keywords: pair bonding, parental care, convergent evolution, affiliation, social behaviour
1. Introduction
Scientists and non-scientists alike can readily appreciate that diverse species exhibit similar behaviours. Most animals display some form of social behaviour—including reproductive behaviour, parental care, aggression, and sexual and social affiliation—with some classes of social behaviours having evolved independently many times across animals. Yet at the same time that we see similarities in general strategies, we also find remarkable species-specific variations on these general social behaviour themes. Despite the prevalence of social behaviour across taxa, we know little about how broad-scale similarities and species diversity are generated, as exploration of the neural and genomic mechanisms underlying social behaviour in vertebrates remain largely limited to a small number of mammalian species.
A major strength of evolutionary comparisons is the ability to determine whether shared genomic and/or neural mechanisms are associated with similar behaviours across species or if there are many alternative mechanistic ‘solutions’ that can produce similar behaviours. Characterization of these patterns will, in turn, inform our understanding of when and how novel behaviours arise and are shaped by evolutionary constraints. Thus, comparative mechanistic research is crucial to identifying general principles shared across species, as well as mechanistic variations responsible for species-specific adaptations and behavioural diversity. Moreover, comparative work is important from a human health perspective, as much of biomedical research relies on only a few species and expanding this view to find general principles across animals may advance translational insights and improve therapeutic strategies.
Evolutionary biologists have made great strides in uncovering genetic variants associated with adaptive traits, such as linking haemoglobin mutations to altitudinal adaptations [1] or melanocortin receptor mutations to skin/coat colour [2]. However, resolving how specific genetic mutations contribute to complex polygenic traits remains a central challenge. Behavioural traits are particularly challenging in this regard, as the brain is a complex organ where constant integration and coordination of internal and external cues is required across molecular, neuronal and brain region networks to produce context-specific behaviours. Behavioural neuroscientists have made great progress in understanding the hormonal, molecular and neural circuit mechanisms of select social behaviours in a few select species. Comparative mechanistic research within an evolutionary framework is crucial for determining whether similar or distinct mechanisms regulate analogous behaviours across animals, and how and whether these patterns differ across levels of organization in the brain.
We present here a brief comparative summary of work on the mechanisms underlying pair bonding and parental care. These affiliative behaviours are selective and often enduring, facilitating reproduction and survival of oneself and/or offspring. We focus on these two social behaviours because they have evolved repeatedly and independently across animals as remarkable examples of convergent behavioural evolution. Much of this text reviews what is known about neural and molecular mechanisms, although we include insights from genomic studies where possible and highlight needed additional work in this area. We focus on vertebrates for reasons of brevity and our expertise, though many invertebrates exhibit complex affiliative behaviours (see [3,4] for reviews). Furthermore, we emphasize the need to unify strengths and insights across the fields of evolution and neuroscience to expand our understanding of behavioural mechanisms and their evolution.
2. Framework for considering mechanisms of convergent behavioural evolution
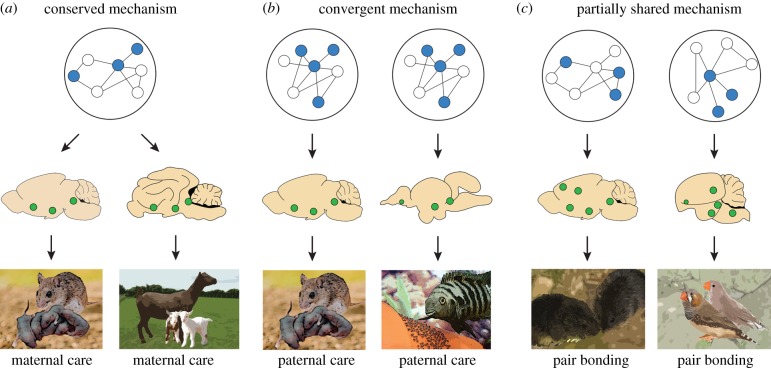
Mechanisms underpinning repeated phenotypic evolution may follow three theoretical evolutionary trajectories. First, are distinct mechanisms, where similar behaviours across species are governed by different genes and neural circuits. In contrast are shared mechanisms, where convergent behaviour between species is governed by the same genes and neural circuits. Shared mechanisms may be classified as conserved if they have been inherited from a common ancestor (figure 1a), or convergent if they have arisen independently (figure 1b). Here, we encourage the inclusion of a third category, partially shared mechanisms (figure 1c). In the independent origins of complex phenotypes like behaviour, the level of mechanistic convergence may depend on the level of organization under investigation (e.g. anatomical, neural and genetic). For example, shared brain regions may promote convergent behaviour but use different neuronal cell types or signalling molecules. While this concept is commonly acknowledged by evolutionary biologists in the context of adaptive phenotypic evolution, it is not often discussed among behavioural neuroscientists, and we know little about how patterns of mechanistic constraint versus flexibility differ across levels of hierarchical organization in the brain. Consideration of this latter category will facilitate discussion of the extent to which underlying anatomical, neural or genetic components are shared and how this informs our understanding of the repeated, independent evolution of complex behaviour.
Figure 1.
Similar phenotypes may rely on different underlying mechanisms. (a) Conserved mechanisms produce similar phenotypes when a common ancestor also expressed the phenotype and the same mechanisms, including the same genes, neuron cell types (top) and brain regions (middle). A behavioural example of this is maternal care in mammals (bottom). (b) Convergent mechanisms are involved when similar phenotypes arise independently but the same genes, neuron cell types (top) and brain regions (middle) are responsible for these similar behaviours. A behavioural example of this is paternal behaviour in mammals and fish. (c) We encourage the use of the category of partially shared mechanisms, where the independent evolution of similar phenotypes likely relies on similar features at one level of biological organization (e.g. same brain regions; middle panel) but different mechanisms at other levels (e.g. different neuronal cell types or gene modulatory networks; top panel). Additionally, this can also apply to partially shared mechanisms within a level, such as the involvement of a subset of brain regions, but not a perfect overlap, in promoting the convergent evolution of behaviour. A behavioural example of this is pair bonding in mammals and birds.
Various factors may influence the extent to which mechanisms underlying behaviour are shared between species. First, evolutionary distance likely has an influence, such that genetic programmes or neural structures are more similar in closely related organisms (e.g. among mammals) compared to organisms separated by wider evolutionary distances (e.g. between mammals and amphibians). Second, similar life histories may predispose species to convergent trait evolution via co-option of shared mechanisms (see example below, in pair bonding section). Finally, hierarchical levels of biological organization may differ in their propensity for convergence. For example, we may find more shared mechanisms at the level of neural circuits and brain regions than gene expression or protein abundance. These factors are not mutually exclusive nor exhaustive, but are variations on the theme of evolutionary relatedness between organisms, sex differences in behaviour, and the relationship between behavioural and mechanistic convergence.
The scope for evaluating alternative hypotheses concerning the evolution of behavioural mechanisms is currently limited due to the inadequate taxonomic breadth for which we understand mechanisms driving affiliative behaviour. Most of what we know about how genomes and neural circuits regulate parental care and pair bonding in vertebrates comes from a select few mammalian species. This research bias hinders our understanding of how genomes and neural circuits evolve to produce behavioural diversity, and studying mechanisms across a breadth of species is critical for two reasons. First, there are a range of unique, adaptive behaviours that cannot be easily studied in traditional laboratory organisms. Second, understanding how different organisms regulate similar behaviours can provide insight into whether the convergent evolution of complex behavioural phenotypes relies on core mechanisms that are generalizable across species or if there are many possible mechanistic solutions. With the development of technologies applicable across a broad array of organisms and the inclusion of more diverse species in research programmes, there is promise that our understanding of behaviour will move in an evolutionarily informed direction to provide insights for diverse fields, from evolutionary biology to human health.
3. Pair bonding
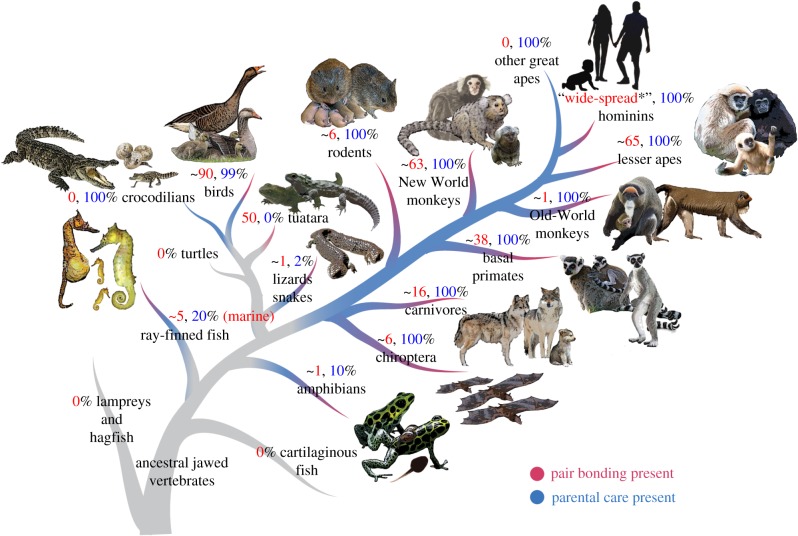
We define pair bonding here as a selective and enduring affiliation that is formed exclusively between two conspecifics and maintained outside of the immediate process of mating. In some species, heterosexuality, reproductive monogamy and biparental care are associated with pair bonding, although these phenomena are not required for pair bonding. Manifestations of partner affiliation are species-specific, ranging from maintaining close physical proximity (e.g. Tiliqua skinks [9]), coordinated motor displays (e.g. Hippocampus seahorses [18]), cooperative predator vigilance (e.g. Siganid rabbitfishes [19]) and allogrooming (e.g. common marmosets [20]). Affiliative behaviours are often reciprocated between partners and provide mutual fitness benefits, including mate and offspring guarding/provisioning and assisted resource defence [21]. The duration of partnerships likewise varies across species, ranging from one reproductive season to life-long. Pair bonding is phylogenetically widespread, occurring in every major vertebrate lineage (figure 2) as well as in invertebrates [22]. However, with a few exceptions (birds, lesser apes and New World monkeys), pair bonding is rare, occurring in only approximately 5% of marine teleosts, 1% of amphibians and reptiles and 9% of mammals [5].
Figure 2.
Distribution and estimated prevalence of pair bonding and parental care across select vertebrate lineages. Data sources: Pair bonding—mammals: [5–7]; birds: [8]; reptiles: [9–11]; amphibians: [12,13]; fishes: [14–16]. Parental care—mammals, birds, reptiles, amphibians, fishes: [17]. Representative pair bonding and parental species illustrated. All prevalence percentages are at the species level, except for parental care estimates of ray-finned fishes, which are at the family level. The absence of estimated prevalence percentage indicates it is unknown. *Among Westernized societies.
Variability of pair-bonding behaviour and phylogenetic prevalence questions the extent to which underlying mechanisms have converged and whether this depends on similar life-history background. For example, in female mammals, highly conserved mechanisms governing the mother–infant bond appear to have been repeatedly co-opted during independent transitions to pair bonding [23]. Hence, we may expect pair bonding mechanisms to be more converged among female mammals relative to males or non-mammals. However, it is unclear how these mechanisms relate to those mediating pair bonding in males and in species without an evolutionary history of maternal care, which is the case for most non-mammalian vertebrates. Likewise, in males, pre-existing territorial mechanisms are hypothesized to be co-opted for pair bonding [24], which may further favour divergence in pair-bonding mechanisms between sexes. Finally, convergence might further depend upon the level of mechanistic organization, from circuitry, to protein, to genetic levels. Whether and how these factors have shaped patterns of mechanistic convergence across vertebrates remains poorly understood.
(a). Mechanisms mediating pair bonding
Extensive research into the brain regions, neural circuits, signalling molecules and genes that regulate pair bonding is largely limited to a single mammalian species, Microtus ochrogaster (prairie voles). Recently, however, complementary studies have begun in at least fourteen additional vertebrate species among whom pair bonding has independently evolved. These species represent three phylogenetically distant lineages (mammals, birds, fishes) and diverse life-history backgrounds. Where data permit, we compare similarity in pair-bonding mechanisms in relation to the level of mechanistic organization, phylogenetic distance, life-history background (parental versus non-parental) and sex. We restrict our review to select brain regions of the highly conserved vertebrate social decision-making network [25], as well as the oxytocin-like, arginine vasopressin (AVP)-like and dopaminergic systems, as these are the neural components for which sufficient comparative data exist.
(i). Brain regions and circuits
In prairie voles, learned association of a partner with sexual reward promotes selective affiliation (82). Accordingly, pair bond formation relies on neural circuitry associated with sexual motivation, reward learning and social salience. The current prairie vole model [26] posits that mating induces ventral tegmental area dopaminergic and paraventricular oxytocin input to the nucleus accumbens, olfactory bulb and amygdala. Concurrently, social stimuli from the mating partner induce amygdala-glutamate input to the accumbens/ventral pallidum circuit and medial amygdala AVP input to the ventral pallidum and lateral septum (in males). Nucleus accumbens oxytocin interacts with dopamine to promote synaptic plasticity that allows mating partner stimuli to persistently activate the accumbens/ventral pallidum circuit, leading to an enduring social affiliation and pair bond maintenance. In males, pair bond maintenance further involves anterior hypothalamic AVP signalling, specifically to mediate mate-guarding [26]. Immediate early gene studies have implicated several other brain regions in prairie vole pair bonding, including the prefrontal cortex, medial preoptic area, ventral hypothalamus and bed nucleus of the stria terminalis [27,28].
Aside from prairie voles, immediate early gene expression and functional brain imaging studies are limited to humans [29,30], titi monkeys [31] and zebra finches [32]. Brain imaging studies suggest the nucleus accumbens, hypothalamus and amygdala are important for pair bonding in both humans and titi monkeys, similar to prairie voles. Interestingly, in prairie voles, humans and zebra finches, the involvement of these brain regions is implicated in both males and females (in titi monkeys this has only been tested in males). Only the amygdala is implicated in pair bonding across all aforementioned species. In prairie voles of both sexes, immediate early gene expression in the medial amygdala is heightened during mating-induced pair bond formation [33,34]. In male titi monkeys, positron emission tomography imaging shows that glucose uptake in the medial amygdala is reduced in males in long-term pair bonds relative to solitary counterparts [31]. Similarly, in male and female humans, the amygdala is differentially activated when viewing pictures of a beloved, compared to a friend or acquaintance [29,30]. Finally, in female zebra finches, medial amygdala neural activity is correlated with partner contact behaviour [32]. Thus, there appears to be convergence in the role of the amygdala, nucleus accumbens and hypothalamus in pair bonding across both male and female mammals; and of the amygdala across mammals and birds, at least in females. Additional studies of both sexes, especially among reptiles and anamniotes, are needed to resolve the extent to which brain regions have converged in their regulation of pair bonding.
(ii). Signalling molecules and genes
The oxytocin-like system (oxytocin in mammals; mesotocin in birds, reptiles and amphibians; and isotocin in fish) is the best-studied neuromodulator of pair bonding, and is presumed to modulate social recognition specifically [26]. Oxytocin administration promotes selective partner attraction in several mammalian species including humans [35], marmosets [36] and prairie voles [26], whereas oxytocin receptor antagonists attenuate this behaviour in marmosets [36], prairie voles [26] and zebra finches [37]. Consistently, a general nonapeptide antagonist (targeting both isotocin and arginine vasotocin receptors) reduces affiliation towards a partner and antagonism towards non-partners in convict cichlid fish [38]. Studies such as these, as well as those examining endogenous oxytocin levels [39,40], show that oxytocin signalling is involved in both male and female pair bonding in mammals and birds, negating early speculation that converged oxytocin signalling for the repeated evolution of pair bonding is unique to female mammals. Furthermore, a recent investigation found that genetic variants of the oxytocin receptor gene contribute to individual differences in romantic relationship status in humans and pair-bonding behaviour in prairie voles [41], suggesting a possible general role of oxytocin receptor polymorphisms in pair bonding. In summary, oxytocin signalling appears to promote pair bonding across phylogenetically diverse species and sexes.
AVP (arginine vasotocin—AVT—in non-mammalian species) and the V1a receptor have been extensively studied in the context of pair bonding, and are presumed to regulate territoriality and social recognition/memory [42]. AVP delivery induces partner preference in titi monkeys and prairie voles [24], whereas blocking the V1aR receptor reduces partner preference formation in prairie voles [24] and convict cichlid fish [38]. In humans, higher plasma AVP levels are related to more attachment security and social support, and fewer negative interactions between spouses [43]. Sufficient species data for AVP signalling within specific brain regions is limited to the lateral septum, where AVP-like signalling regulates pair bond formation in Microtus voles [44], a non-human primate (common marmoset [45]), other rodents (pine voles [46] and California mice [46]) and teleost fishes (Chaetodon butterflyfishes [47]). These studies indicate no sex differences in the role of AVP-V1aR signalling, neither peripherally nor within the lateral septum specifically (though it has only been studied in Chaetodon males). These preliminary studies refute the expectation that AVP's involvement in pair bonding is more common among males than females, which is based on the idea that the AVP system that mediates territoriality has been co-opted for pair bonding [24]. Rather, it appears that AVP involvement in general, and in the lateral septum in particular, has converged across vertebrates equally in both sexes. Functional studies, especially in females, are needed to confirm this idea. Finally, the V1aR gene (avpr1a) has also been the focus of investigations into genetic mechanisms of the evolution of pair bonding, as early work in prairie voles suggested a microsatellite in the 5′ regulatory region of avpr1a promoted the emergence of pair-bonding behaviour [24]. However, a more comparative analysis of the avpr1a microsatellite across 21 Microtus species and eight Peromyscus species found no role for microsatellite involvement in the evolution of pair bonding in these genera [24].
The involvement of dopamine and two of its receptor subtypes, D1R and D2R, in pair bonding has been studied in five phylogenetically divergent species: common marmosets, titi monkeys, prairie voles, zebra finches and butterflyfishes. In all species examined, the dopaminergic system appears to play an important role in governing pair bonding in both sexes (though data in titi monkeys are restricted to males). In prairie voles, partner preference is enhanced by a D2R agonist and inhibited by D1R activation within the nucleus accumbens [44]. After pair bonds have been established, D1R density in the nucleus accumbens increases, likely inhibiting the formation of new partnerships [44]. Similarly, in common marmosets, D2Rs regulate partner proximity behaviour in new pairs, whereas D1Rs do so in long-term pairs [20]. In zebra finches, dopamine levels in the brain increase with pair bond formation [48] and decrease with pair bond maintenance [49]. These results suggest that dopaminergic signalling pathways are different during pair bond formation and maintenance. Although dopamine signalling seems to be generally important for pair bonding, the limited data currently available suggest that the specific functional roles of D1R and D2R within the nucleus accumbens may vary among species [50].
While the pair-bonding studies have focused mostly on candidate genes, genomic technologies have progressed to allow untargeted gene expression measurements in many species. A recent study used RNA sequencing to determine if similar gene expression profiles could be identified across vertebrates that had independently evolved pair-bonding behaviour [51]. To test this hypothesis, the authors used RNA sequencing of whole brains from reproductive males of monogamous and non-monogamous species pairs including rodents, songbirds, dendrobatid frogs and cichlid fishes. This study did not find differential gene expression in the traditional gene candidates described above, but instead found other genes associated with the evolution of monogamy, like Dscam (Down syndrome cell adhesion molecule) and Grm6 (glutamate metabotropic receptor 6). The lack of significant expression differences in genes known to regulate monogamy is difficult to interpret, as the study used whole brain (rather than brain region-specific) sequencing, sampled males that were not necessarily pair bonded or with a mate, and had a sample size of only one per species. While future studies with more anatomically and behaviourally informed sampling and larger sample sizes are necessary, this study is valuable as the first to find similar gene expression patterns underlying monogamy across wide evolutionary distances.
(b). Future directions in pair-bonding research
Recent investigations into diverse species among whom pair bonding has independently evolved provide first clues of the extent of mechanistic convergence across vertebrates and how it has been shaped by key factors. Based on the few studies available, it tentatively appears that key signalling molecules (i.e. nonapeptides and dopamine) are ubiquitously involved in pair bonding across vertebrate species irrespective of phylogenetic distance, life-history background (parental versus non-parental) and sex. Additional research characterizing genetic and neurochemical signalling within brain regions across diverse taxa is needed to further elucidate genetic and neural network convergence of pair bonding.
4. Parental care
Next to pair bonding, the other affiliative behaviour that has arisen independently many times across all major vertebrate taxa is parental care. The extent, duration and intensity of care vary across taxa [52,53], but broadly defined, parental care encompasses any behaviour(s) on the part of parents that increases offspring survival [54], in particular under harsh environmental conditions [55,56] or in the case of altricial young [57,58]. Parental behaviours fall largely into the categories of (i) offspring defence and (ii) offspring provisioning with food products either physiologically produced by or caught/collected by parents. While parental care is phylogenetically widespread, the prevalence of parental care varies across clades (figure 2). Parental care is extremely common in mammals (100% of species) and birds (greater than 99% of species), but comparatively rare in reptiles (approx. 3% of species), amphibians (approx. 10% of species) and fishes (approx. 20% of species) [17]. Similarly, clades vary in their most common care strategy, i.e. in the frequency with which male uniparental, female uniparental or biparental care are observed.
Comparing across taxa, we can readily observe similar care behaviours across sexes and species on the one hand, and clear differences in the details of care behaviours and the sex performing these behaviours on the other. For example, provisioning of physiological products encompasses fascinating examples, including lactation in female mammals [59], crop-milk production by both males and females in doves and pigeons [60], provisioning with unfertilized trophic eggs by female frogs [61], and provisioning with skin in caecilians and fish [62,63]. The juxtaposition of broad-scale similarities and detailed differences across highly divergent taxa begs the question of the extent to which parental behaviours are mediated by shared versus distinct mechanisms across species and sexes. Despite the well-recognized ecological, evolutionary and societal importance of parental care, its underlying mechanisms remain poorly understood and such questions, therefore, remain largely unanswered.
(a). Mechanisms mediating parental care
Despite the diversity of parental care observed across species, most of what is known about the mechanisms mediating parental care comes from research with female laboratory rodents. Where studies have explored neural mechanisms mediating parental care outside of laboratory rodents, the evidence is accumulating for mechanistic overlap across sexes and within and across vertebrate lineages. Below, we discuss existing data with a focus on mechanisms that have been explored across various taxa. Since we focus on brain regions and molecules for which comparative data are available, the list of potential mechanisms is not exhaustive. Our aim is to provide a framework for discussion and emphasize valuable areas for future work. In particular, more comparative mechanistic studies are needed to leverage taxonomic and behavioural diversity to provide a holistic view of parental care and its evolution.
(i). Brain regions and circuits
Parental care is best conceptualized as a complex set of inter-related behaviours controlled by multiple brain regions involved in the sensory, social, motivational and cognitive aspects of care [64]. The high density of neuromodulators and widespread connections with multiple brain regions makes the hypothalamic preoptic area well-positioned to modulate parental care [64–66]. In rodents, preoptic area immediate early gene induction increases during parental care [67,68] and lesions inhibit care in both sexes [69,70]. Similarly, in birds, increased immediate early gene activity in the preoptic area is associated with parental behaviour [71] and preoptic area lesions disrupt parental care [72]. In fish, increased preoptic area activity is associated with male parental care [73] and direct simulation of the preoptic area increases nesting [74]. Although studies in reptiles and amphibians are lacking, existing data suggest widespread convergence in the role of the preoptic area in parental care.
Beyond the preoptic area, data outside of mammals are sparse. The bed nucleus of the stria terminalis appears to regulate motivational aspects of parental care via interactions with the mesolimbic dopamine system, including the ventral tegmental area. Female retrieval of pups is disrupted when the bed nucleus of the stria terminalis is lesioned in rats and when ventral tegmental area activity is inhibited in mice [75]. Increased immediate early gene abundance in the bed nucleus of the stria terminalis is also associated with pup care in males [76]. Similarly, increased immediate early gene activation in the bed nucleus of the stria terminalis is observed following brooding behaviour in quail [71] and interactions with chicks in ring doves [77]. Along with the medial amygdala, the bed nucleus of the stria terminalis is also important in processing offspring cues in mammals, in particular, odour cues coming from the main olfactory bulb and vomeronasal organ (reviewed in [26]). Whether and how the extended amygdala is involved in parental care in other species is currently unknown, but may be particularly interesting given that birds, some reptiles and fishes lack a vomeronasal system. In summary, while there is some evidence for shared functionality of core brain regions in parental care across vertebrates, comparative studies are sorely lacking. Moving forward, such studies will be critical for understanding region-specific functional variation associated with the diverse life histories and ecologies associated with parental care across lineages.
(ii). Signalling molecules and genes
In addition to its role in pair bonding, oxytocin is well known for its role in various aspects of maternal care, including lactation, mother-infant bonding and maternal aggression [59]. Studies on the non-mammalian homologs of oxytocin are less common, but evidence exists for a convergent role of mesotocin in maternal care in birds [78] and isotocin in paternal care in fish [73]. Oxytocin cells are concentrated in the preoptic area, well-positioned to influence the tuning of parental care circuits described above [26]. Indeed, changes in oxytocin signalling within the preoptic area and connections to mesolimbic reward pathways are known to be critical for maternal behaviours [26]. Changes in oxytocin signalling in brain regions important for perception and cognition have also been shown to facilitate care behaviours and responsiveness to offspring. For example, increased expression of oxytocin receptors in auditory cortex increases the salience of pup vocalizations for mothers [79], and oxytocin knockout mice exhibit impairments in social cognition and the initiation of maternal care [80,81]. Though oxytocin's effects have been described primarily in the context of maternal care, there is some evidence for increased oxytocin/isotocin signalling associated with paternal care in rodents [82], humans [83] and fish [73].
Though there is some functional overlap in the roles of oxytocin and AVP, there appears to be greater sex and species specificity in the relationship between AVP and parental behaviour than between oxytocin and parental behaviour [82]. AVP has been associated with paternal involvement in biparental versus female uniparental species of Peromyscus mice through decades of research by Catherine Marler and colleagues [84], and a recent study has linked this work to regulation at the genomic level [85]. By contrast, parental care studies specifically focusing on AVP in non-mammalian species are nearly non-existent. A single study in frogs found no effect of AVT on parental care [86], and a study in stickleback fish found increased AVT associated with egg care, although parental care is confounded with aggressive behaviour in this case [87]. Thus, while a wealth of evidence indicates that AVP is involved in sociality, sex and species specificity suggest that molecular convergence, in this case, represents variation on a theme of social behaviour evolution generally rather than parental care specifically [88]. Given these variations, here too comparative studies will be valuable for linking variation in underlying mechanisms to behavioural, ecological and life-history variation that reflects the unique selective factors acting among species and lineages.
Finally, long known to be important in feeding behaviour, recent work has uncovered a role of galanin signalling in mediating parental care [89]. Like the nonapeptides, populations of galanin neurons are located in the preoptic area and are heavily interconnected both within and outside of this brain region. Except for differences in projections to periventricular vasopressin (males) versus oxytocin (females) neurons, galanin neuron projection and connectivity patterns are conserved across sexes and mediate overlapping parental behaviours [75]. Although detailed circuit level analysis in other organisms is still technologically challenging, some RNA sequencing experiments in fishes suggest that galanin may also be important in parental care in non-mammalian animals. In both the bluegill sunfish and the midshipman fish, galanin mRNA abundance is increased in courting, parental males compared to non-courting, non-parental males [90,91]. While galanin was initially described as regulating feeding behaviour, recent recognition of the importance of this and other feeding-related signalling molecules in parental care has led to the hypothesis that feeding-related neural and molecular substrates are repeatedly co-opted in the evolution of parental care and other social behaviours [92].
(b). Future directions in parental care research
A fundamental question left open by heavily female-biased research is how sex differences in parental care behaviour arise and evolve. Despite marked sex differences in behaviour, the neural circuits underlying parental care appear largely conserved between sexes, as is the expression of some key molecules, such as galanin [75]. Some behavioural variation can be linked to the expression of key signalling molecules such as AVP and dopamine, and to receptor distributions. However, the existence of flexible parenting (e.g. induction of parental care in the typically non-parenting sex or in adoptive parents) indicates that circuitry must be largely conserved and that there are multiple routes of access to the activation of parental care circuits [64,93]. Whether and how changes in circuit tuning that mediate behavioural flexibility within species may be co-opted for the evolution of alternative care strategies among species is one of the many interesting research areas that will profit from additional comparative data.
5. Conclusion
Our brief review of the preliminary comparative data available indicates that parallel, independent transitions to parental care and pair bonding both rely on at least partially overlapping brain regions and molecules. Additional comparative data across non-mammalian taxa (especially amphibians and reptiles) is needed to determine the extent of this overlap across vertebrates. Central to this effort will be global -omic assessments of neural components (e.g. epigenomes, genomes, transcriptomes) on a brain region-specific level, using technologies transferable across traditional and non-traditional model organisms that allow characterization of broad-scale patterns and identification of novel molecular mechanisms. Bridging the gap between patterns of broad-scale regulation (e.g. circulating hormones, expression of thousands of genes), highly specific circuit-level modulation (e.g. subpopulations of specific neurons), and patterns of genetic variation is a central challenge in building a holistic picture of the mechanisms regulating affiliation [75]. Moreover, integrating comparative work with approaches from the expanding neurogenetic toolkit will be important for functionally verifying these observations. These studies will shed light on why shared neural substrates are repeatedly involved in the independent evolution of affiliation, and how mechanisms are evolutionarily tuned to give rise to species-specific variations in care strategies and behaviour.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to this review article by developing content, drafting the article and approving the final version.
Competing interests
We declare we have no competing interests.
Funding
L.A.O. is supported by funds from Stanford University and National Science Foundation grant nos IOS-1822025 and IOS-1827333.
References
- 1.Yi X, et al. 2010. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329, 75–78. ( 10.1126/science.1190371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manceau M, Domingues VS, Linnen CR, Rosenblum EB, Hoekstra HE. 2010. Convergence in pigmentation at multiple levels: mutations, genes and function. Phil. Trans. R. Soc. B 365, 2439–2450. ( 10.1098/rstb.2010.0104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapheim KM. 2016. Genomic sources of phenotypic novelty in the evolution of eusociality in insects. Curr. Opin. Insect Sci. 13, 24–32. ( 10.1016/j.cois.2015.10.009) [DOI] [PubMed] [Google Scholar]
- 4.Steiger S, Stökl J. 2017. Pheromones involved in insect parental care and family life. Curr. Opin. Insect Sci. 24, 89–95. ( 10.1016/j.cois.2017.09.006) [DOI] [PubMed] [Google Scholar]
- 5.Lukas D, Clutton-Brock TH. 2013. The evolution of social monogamy in mammals. Science 341, 526–530. ( 10.1126/science.1238677) [DOI] [PubMed] [Google Scholar]
- 6.Opie C, Atkinson QD, Dunbar RIM, Shultz S. 2013. Male infanticide leads to social monogamy in primates. Proc. Natl Acad. Sci. USA 110, 13 328–13 332. ( 10.1073/pnas.1307903110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henrich J, Boyd R, Richerson PJ. 2012. The puzzle of monogamous marriage. Phil. Trans. R. Soc. B 367, 657–669. ( 10.1098/rstb.2011.0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockburn A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383. ( 10.1098/rspb.2005.3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull CM. 2000. Monogamy in lizards. Behav. Processes. 51, 7–20. ( 10.1016/S0376-6357(00)00115-7) [DOI] [PubMed] [Google Scholar]
- 10.Chapple DG. 2003. Ecology, life-history, and behavior in the Australian scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetological Monogr. 17, 145–180. ( 10.1655/0733-1347(2003)017[0145:ELABIT]2.0.CO;2) [DOI] [Google Scholar]
- 11.Moore JA, Daugherty CH, Godfrey SS, Nelson NJ. 2009. Seasonal monogamy and multiple paternity in a wild population of a territorial reptile (tuatara). Biol. J. Linnean Soc. 98, 161–170. ( 10.1111/j.1095-8312.2009.01271.x) [DOI] [Google Scholar]
- 12.Summers K, Tumulty J. 2013. Parental care, sexual selection, and mating systems in neotropical poison frogs. In Sexual selection: perspectives and models from the Neotropics (eds RH Macedo, G Machado), pp. 289–320. New York, NY: Elsevier Academic Press. [Google Scholar]
- 13.Gillette JR, Jaeger RG, Peterson MG. 2000. Social monogamy in a territorial salamander. Anim. Behav. 59, 1241–1250. ( 10.1006/anbe.2000.1437) [DOI] [PubMed] [Google Scholar]
- 14.Whiteman EA, Cote IM. 2004. Monogamy in marine fishes. Biol. Rev. 79, 351–375. ( 10.1017/S1464793103006304) [DOI] [PubMed] [Google Scholar]
- 15.Froese R, Pauly D. 2012. FishBase 2012. See www.fishbase.org.
- 16.Brandl SJ, Bellwood DR. 2014. Pair-formation in coral reef fishes: an ecological perspective. Oceanogr. Mar. Biol. Annu. Rev. 52, 1–79. [Google Scholar]
- 17.Roland AB, O'Connell LA. 2015. Poison frogs as a model system for studying the neurobiology of parental care. Curr. Opin. Behav. Sci. 6, 76–81. ( 10.1016/j.cobeha.2015.10.002) [DOI] [Google Scholar]
- 18.Vincent AC, Sadler LM. 1995. Faithful pair bonds in wild seahorses, Hippocampus whitei. Anim. Behav. 50, 1557–1569. ( 10.1016/0003-3472(95)80011-5) [DOI] [Google Scholar]
- 19.Brandl SJ, Bellwood DR. 2015. Coordinated vigilance provides evidence for direct reciprocity in coral reef fishes. Sci. Rep. 5, 14556 ( 10.1038/srep14556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carp S, Taylor J, Womack S, French J. 2018. Dopamine modulation of reunion behavior in short and long term marmoset pairs. Front. Ecol. Evol. 6, 46 (doi:103389/fevo.2018) [Google Scholar]
- 21.Reichard UH, Boesch C. 2003. Monogamy: mating strategies and partnerships in birds, humans and other mammals. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Mathews LM. 2003. Tests of the mate-guarding hypothesis for social monogamy: male snapping shrimp prefer to associate with high-value females. Behav. Ecol. 14, 63–67. ( 10.1093/beheco/14.1.63) [DOI] [Google Scholar]
- 23.Curtis JT, Wang Z. 2003. The neurochemistry of pair bonding. Curr. Dir. Psychol. Sci. 12, 49–53. ( 10.1111/1467-8721.01224) [DOI] [Google Scholar]
- 24.Freeman SM, Young L. 2013. Oxytocin, vasopressin, and the evolution of mating systems in mammals. In Oxytocin, vasopressin, and related peptides in the regulation of behavior, vol. 11 (eds E Choleris, DW Pfaff, M Kavaliers), pp. 128–144. Cambridge, UK: Cambridge University Press.
- 25.O'Connell LA, Hofmann HA. 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639. ( 10.1002/cne.22735) [DOI] [PubMed] [Google Scholar]
- 26.Numan M, Young LJ. 2016. Neural mechanisms of mother–infant bonding and pair bonding: similarities, differences, and broader implications. Horm. Behav. 77, 98–112. ( 10.1016/j.yhbeh.2015.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cushing BS, Mogekwu N, Le W-W, Hoffman GE, Carter CS. 2003. Cohabitation induced Fos immunoreactivity in the monogamous prairie vole. Brain Res. 965, 203–211. ( 10.1016/S0006-8993(02)04199-9) [DOI] [PubMed] [Google Scholar]
- 28.Dominguez JM, Hull EM. 2005. Dopamine, the medial preoptic area, and male sexual behavior. Physiol. Behav. 86, 356–368. ( 10.1016/j.physbeh.2005.08.006) [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Aron A, Brown L, Cao G, Feng T, Weng X. 2011. Reward and motivation systems: a brain mapping study of early-stage intense romantic love in Chinese participants. Hum. Brain Mapp. 32, 249–257. ( 10.1002/hbm.21017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acevedo BP, Aron A, Fisher HE, Brown LL. 2012. Neural correlates of long-term intense romantic love. Social Cogn. Affect. Neurosci. 7, 145–159. ( 10.1093/scan/nsq092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. 2007. Neural correlates of pair-bonding in a monogamous primate. Brain Res. 1184, 245–253. ( 10.1016/j.brainres.2007.09.087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svec L, Licht K, Wade J. 2009. Pair bonding in the female zebra finch: a potential role for the nucleus taeniae. Neuroscience 160, 275–283. ( 10.1016/j.neuroscience.2009.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim MM, Young LJ. 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125, 35–45. ( 10.1016/j.neuroscience.2003.12.008) [DOI] [PubMed] [Google Scholar]
- 34.Curtis JT, Wang Z. 2003. Forebrain c-fos expression under conditions conducive to pair bonding in female prairie voles (Microtus ochrogaster). Physiol. Behav. 80, 95–101. ( 10.1016/S0031-9384(03)00226-9) [DOI] [PubMed] [Google Scholar]
- 35.Scheele D, Wille A, Kendrick KM, Stoffel-Wagner B, Becker B, Güntürkün O, Maier W, Hurlemann R. 2013. Oxytocin enhances brain reward system responses in men viewing the face of their female partner. Proc. Natl Acad. Sci. USA 110, 20 308–20 313. ( 10.1073/pnas.1314190110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith AS, Agmo A, Birnie AK, French JA. 2010. Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 57, 255–262. ( 10.1016/j.yhbeh.2009.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen A, Tomaszycki M. 2012. Oxytocin antagonist treatments alter the formation of pair relationships in zebra finches of both sexes. Horm. Behav. 62, 113–119. ( 10.1016/j.yhbeh.2012.05.009) [DOI] [PubMed] [Google Scholar]
- 38.Oldfield RG, Hofmann HA. 2011. Neuropeptide regulation of social behavior in a monogamous cichlid fish. Physiol. Behav. 102, 296–303. ( 10.1016/j.physbeh.2010.11.022) [DOI] [PubMed] [Google Scholar]
- 39.Gouin J-P, et al. 2010. Marital behavior, oxytocin, vasopressin, and wound healing. Psychoneuroendocrinology 35, 1082–1090. ( 10.1016/j.psyneuen.2010.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snowdon CT, Pieper BA, Boe CY, Cronin KA, Kurian AV, Ziegler TE. 2010. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pair bonded tamarins. Horm. Behav. 58, 614–618. ( 10.1016/j.yhbeh.2010.06.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King LB, Walum H, Inoue K, Eyrich NW, Young LJ. 2016. Variation in the oxytocin receptor gene predicts brain region-specific expression and social attachment. Biol. Psychiatry 80, 160–169. ( 10.1016/j.biopsych.2015.12.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young LJ, Wang ZX. 2004. The neurobiology of pair bonding. Nat. Neurosci. 7, 1048–1054. ( 10.1038/nn1327) [DOI] [PubMed] [Google Scholar]
- 43.Gouin J-P, Carter CS, Pournajafi-Nazarloo H, Malarkey WB, Loving TJ, Stowell J, Kiecolt-Glaser JK. 2012. Plasma vasopressin and interpersonal functioning. Biol. Psychol. 91, 270–274. ( 10.1016/j.biopsycho.2012.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young KA, Gobrogge KL, Liu Y, Wang ZX. 2011. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol. 32, 53–69. ( 10.1016/j.yfrne.2010.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young LJ. 1999. Oxytocin and vasopressin receptors and species-typical social behaviors. Horm. Behav. 36, 212–221. [DOI] [PubMed] [Google Scholar]
- 46.Insel TR, Wang ZX, Ferris CF. 1994. Patterns of brain vasopressin receptor distribution associated with social-organization in microtine rodents. J. Neurosci. 14, 5381–5392. ( 10.1523/JNEUROSCI.14-09-05381.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dewan AK, Ramey ML, Tricas TC. 2011. Arginine vasotocin neuronal phenotypes, telencephalic fiber varicosities, and social behavior in butterflyfishes (Chaetodontidae): potential similarities to birds and mammals. Horm. Behav. 59, 56–66. ( 10.1016/j.yhbeh.2010.10.002) [DOI] [PubMed] [Google Scholar]
- 48.Banerjee SB, Dias BG, Crews D, Adkins-Regan E. 2013. Newly paired zebra finches have higher dopamine levels and immediate early gene Fos expression in dopaminergic neurons. Eur. J. Neurosci. 38, 3731–3739. ( 10.1111/ejn.12378) [DOI] [PubMed] [Google Scholar]
- 49.Alger SJ, Juang C, Riters LV. 2011. Social affiliation relates to tyrosine hydroxylase immunolabeling in male and female zebra finches (Taeniopygia guttata). J. Chem. Neuroanat. 42, 45–55. ( 10.1016/j.jchemneu.2011.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hostetler CM, et al. 2017. Effects of pair bonding on dopamine D1 receptors in monogamous male titi monkeys (Callicebus cupreus). Am. J. Primatol. 79, e22612 ( 10.1002/ajp.22612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young RL, et al. 2019. Conserved transcriptomic profiles underpin monogamy across vertebrates. Proc. Natl Acad. Sci. USA 116, 1331–1336. ( 10.1073/pnas.1813775116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Royle NJ, Alonzo SH, Moore AJ. 2016. Co-evolution, conflict and complexity: what have we learned about the evolution of parental care behaviours? Curr. Opin. Behav. Sci. 12, 30–36. ( 10.1016/j.cobeha.2016.08.004) [DOI] [Google Scholar]
- 53.Bales KL. 2017. Parenting in animals. Curr. Opin. Psychol. 15, 93–98. ( 10.1016/j.copsyc.2017.02.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clutton-Brock TH. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- 55.Clutton-Brock T, Harvey PH. 1978. Mammals, resources and reproductive strategies. Nature 273, 191–195. ( 10.1038/273191a0) [DOI] [PubMed] [Google Scholar]
- 56.Royle NJ, Russell AF, Wilson AJ. 2014. The evolution of flexible parenting. Science 345, 776–781. ( 10.1126/science.1253294) [DOI] [PubMed] [Google Scholar]
- 57.Gittleman JL. 1994. Female brain size and parental care in carnivores. Proc. Natl Acad. Sci. USA 91, 5495–5497. ( 10.1073/pnas.91.12.5495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shultz S, Dunbar RI. 2010. Social bonds in birds are associated with brain size and contingent on the correlated evolution of life-history and increased parental investment. Biol. J. Linnean Soc. 100, 111–123. ( 10.1111/j.1095-8312.2010.01427.x) [DOI] [Google Scholar]
- 59.Numan M, Insel TR. 2006. The neurobiology of parental behavior. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 60.Johnston RF, Janiga M. 1995. Feral pigeons. Oxford, UK: Oxford University Press. [Google Scholar]
- 61.Weygoldt P. 1987. Evolution of parental care in dart poison frogs (Amphibia: Anura: Dendrobatidae). J. Zool. Syst. Evol. Res. 25, 51–67. ( 10.1111/j.1439-0469.1987.tb00913.x) [DOI] [Google Scholar]
- 62.Buckley J, Maunder RJ, Foey A, Pearce J, Val AL, Sloman KA. 2010. Biparental mucus feeding: a unique example of parental care in an Amazonian cichlid. J. Exp. Biol. 213, 3787–3795. ( 10.1242/jeb.042929) [DOI] [PubMed] [Google Scholar]
- 63.Kupfer A, Müller H, Antoniazzi MM, Jared C, Greven H, Nussbaum RA, Wilkinson M. 2006. Parental investment by skin feeding in a caecilian amphibian. Nature 440, 926–929. ( 10.1038/nature04403) [DOI] [PubMed] [Google Scholar]
- 64.Pereira M, Ferreira A. 2016. Neuroanatomical and neurochemical basis of parenting: dynamic coordination of motivational, affective and cognitive processes. Horm. Behav. 77, 72–85. ( 10.1016/j.yhbeh.2015.08.005) [DOI] [PubMed] [Google Scholar]
- 65.Dulac C, Kimchi T. 2007. Neural mechanisms underlying sex-specific behaviors in vertebrates. Curr. Opin. Neurobiol. 17, 675–683. ( 10.1016/j.conb.2008.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fischer EK, O'Connell LA. 2018. Circuit architecture underlying distinct components of parental care. Trends Neurosci. 41, 334–336. ( 10.1016/j.tins.2018.04.003) [DOI] [PubMed] [Google Scholar]
- 67.Fleming AS, Suh EJ, Korsmit M, Rusak B. 1994. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures of maternal and social interactions in rats. Behav. Neurosci. 108, 724–734. ( 10.1037/0735-7044.108.4.724) [DOI] [PubMed] [Google Scholar]
- 68.Numan M, Numan M. 1996. A lesion and neuroanatomical tract-tracing analysis of the role of the bed nucleus of the stria terminalis in retrieval behavior and other aspects of maternal responsiveness in rats. Dev. Psychobiol. 29, 23–51. () [DOI] [PubMed] [Google Scholar]
- 69.Jacobson CD, Terkel J, Gorski RA, Sawyer CH. 1980. Effects of small medial preoptic area lesions on maternal behavior: retrieving and nest building in the rat. Brain Res. 194, 471–478. ( 10.1016/0006-8993(80)91226-3) [DOI] [PubMed] [Google Scholar]
- 70.Lee AW, Brown RE. 2007. Comparison of medial preoptic, amygdala, and nucleus accumbens lesions on parental behavior in California mice (Peromyscus californicus). Physiol. Behav. 92, 617–628. ( 10.1016/j.physbeh.2007.05.008) [DOI] [PubMed] [Google Scholar]
- 71.Ruscio MG, Adkins-Regan E. 2004. Immediate early gene expression associated with induction of brooding behavior in Japanese quail. Horm. Behav. 46, 19–29. ( 10.1016/j.yhbeh.2004.02.002) [DOI] [PubMed] [Google Scholar]
- 72.Slawski BA, Buntin JD. 1995. Preoptic area lesions disrupt prolactin-induced parental feeding behavior in ring doves. Horm. Behav. 29, 248–266. ( 10.1006/hbeh.1995.1018) [DOI] [PubMed] [Google Scholar]
- 73.O'Connell LA, Matthews BJ, Hofmann HA. 2012. Isotocin regulates paternal care in a monogamous cichlid fish. Horm. Behav. 61, 725–733. ( 10.1016/j.yhbeh.2012.03.009) [DOI] [PubMed] [Google Scholar]
- 74.Demski LS, Knigge KM. 1971. The telencephalon and hypothalamus of the bluegill (Lepomis macrochirus): evoked feeding, aggressive and reproductive behavior with representative frontal sections. J. Comp. Neurol. 143, 1–16. ( 10.1002/cne.901430102) [DOI] [PubMed] [Google Scholar]
- 75.Kohl J, Dulac C. 2018. Neural control of parental behaviors. Curr. Opin. Neurobiol. 49, 116–122. ( 10.1016/j.conb.2018.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Jong TR, Chauke M, Harris BN, Saltzman W. 2009. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus). Horm. Behav. 56, 220–231. ( 10.1016/j.yhbeh.2009.05.001) [DOI] [PubMed] [Google Scholar]
- 77.Buntin L, Berghman LR, Buntin JD. 2006. Patterns of Fos-like immunoreactivity in the brains of parent ring doves (Streptopelia risoria) given tactile and nontactile exposure to their young. Behav. Neurosci. 120, 651 ( 10.1037/0735-7044.120.3.651) [DOI] [PubMed] [Google Scholar]
- 78.Chokchaloemwong D, Prakobsaeng N, Sartsoongnoen N, Kosonsiriluk S, El Halawani M, Chaiseha Y. 2013. Mesotocin and maternal care of chicks in native Thai hens (Gallus domesticus). Horm. Behav. 64, 53–69. ( 10.1016/j.yhbeh.2013.04.010) [DOI] [PubMed] [Google Scholar]
- 79.Marlin BJ, Mitre M, D'amour JA, Chao MV, Froemke RC. 2015. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504. ( 10.1038/nature14402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sala M, et al. 2011. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol. Psychiatry 69, 875–882. ( 10.1016/j.biopsych.2010.12.022) [DOI] [PubMed] [Google Scholar]
- 81.Rich ME, Lee H-J, Caldwell HK. 2014. Impairments in the initiation of maternal behavior in oxytocin receptor knockout mice. PLoS ONE 9, e98839 ( 10.1371/journal.pone.0098839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rilling JK, Mascaro JS. 2017. The neurobiology of fatherhood. Curr. Opin. Psychol. 15, 26–32. ( 10.1016/j.copsyc.2017.02.013) [DOI] [PubMed] [Google Scholar]
- 83.Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. 2010. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology 35, 1133–1141. ( 10.1016/j.psyneuen.2010.01.013) [DOI] [PubMed] [Google Scholar]
- 84.Bester-Meredith JK, Marler CA. 2003. Vasopressin and the transmission of paternal behavior across generations in mated, cross-fostered Peromyscus mice. Behav. Neurosci. 117, 455–463. ( 10.1037/0735-7044.117.3.455) [DOI] [PubMed] [Google Scholar]
- 85.Bendesky A, Kwon Y-M, Lassance J-M, Lewarch CL, Yao S, Peterson BK, He MX, Dulac C, Hoekstra HE. 2017. The genetic basis of parental care evolution in monogamous mice. Nature 544, 434–439. ( 10.1038/nature22074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schulte LM, Summers K. 2017. Searching for hormonal facilitators: are vasotocin and mesotocin involved in parental care behaviors in poison frogs? Physiol. Behav. 174, 74–82. ( 10.1016/j.physbeh.2017.03.005) [DOI] [PubMed] [Google Scholar]
- 87.Kleszczyńska A, Sokołowska E, Kulczykowska E. 2012. Variation in brain arginine vasotocin (AVT) and isotocin (IT) levels with reproductive stage and social status in males of three-spined stickleback (Gasterosteus aculeatus). Gen. Comp. Endocrinol. 175, 290–296. ( 10.1016/j.ygcen.2011.11.022) [DOI] [PubMed] [Google Scholar]
- 88.Goodson JL, Thompson RR. 2010. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr. Opin. Neurobiol. 20, 784–794. ( 10.1016/j.conb.2010.08.020) [DOI] [PubMed] [Google Scholar]
- 89.Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. 2014. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509, 325–330 ( 10.1038/nature13307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Partridge CG, MacManes MD, Knapp R, Neff BD. 2016. Brain transcriptional profiles of male alternative reproductive tactics and females in bluegill sunfish. PLoS ONE 11, e0167509 ( 10.1371/journal.pone.0167509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tripp JA, Feng NY, Bass AH. 2018. Behavioural tactic predicts preoptic-hypothalamic gene expression more strongly than developmental morph in fish with alternative reproductive tactics. Proc. R. Soc. B. 285, 20172742 ( 10.1098/rspb.2017.2742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fischer EK, O'Connell LA. 2017. Modification of feeding circuits in the evolution of social behavior. J. Exp. Biol. 220, 92–102. ( 10.1242/jeb.143859) [DOI] [PubMed] [Google Scholar]
- 93.Rosenblatt JS. 1967. Nonhormonal basis of maternal behavior in the rat. Science 156, 1512–1513. ( 10.1126/science.156.3781.1512) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.