Abstract
BACKGROUND:
Chronic hepatitis C virus infection represents a more frequent cause of liver cirrhosis and hepatocellular carcinoma. Statins, inhibit HCV replication in vitro, enhance the antiviral effect of the already known antiviral drugs and reduce their resistance.
AIM:
To determine the impact of additional therapy (treatment with Atorvastatin 20 mg) to the standard antiviral therapy (pegylated interferon alpha-peg-IFN α and ribavirin) on achieving sustained virological response (SVR).
MATERIAL AND METHODS:
In the study which is comparative, open-label, prospective-retrospective, 70 patients diagnosed with chronic hepatitis C virus infection who met criteria for treatment with standard antiviral therapy combined with anti-lipemic therapy (Atorvastatin 20 mg) were included. Patients in the study were divided into two groups: one group of 35 patients receiving combination therapy (Atorvastatin + peg-IFN α + Ribavirin) and another group of 35 patients received only standard antiviral therapy. Those parameters were followed in all patients: genotyping, quantification of the virus, histological assessment of liver inflammation and fibrosis degree (before starting treatment), the presence of steatosis, laboratory analysis: hematology, liver, lipid and carbohydrate status, insulin blood level (the calculation of HOMA-IR) and body mass index (BMI) calculation. The overall treatment of the patients depends from the virus genotype, thus, patients with genotype 1 and 4 received 48 weeks standard antiviral therapy, but patients with genotypes 2 and 3 received 24 weeks of antiviral therapy. SVR was considered an undetectable level of HCV RNA levels 24 weeks after completion of antiviral therapy. The results were statistically analysed, and all results for p < 0.05 were considered statistically significant.
RESULTS:
Combination therapy leads to a slightly higher percentage of SVR (85.71%) in patients with chronic hepatitis C versus standard therapy (74.29%), but in a group of patients with genotype 3 this rate of SVR amounting to 95.83%. Combination therapy leads to significant improvement of lipid and glucose status after treatment, and in terms of side effects, there was no appearance of serious adverse events that would be a reason for discontinuation of the therapy.
CONCLUSION:
Combination therapy Atorvastatin + pegylated interferon alpha + Ribavirin leads to high rate of SVR of 95.83% in patients with chronic hepatitis C, genotype 3. Statins can be used safely in patients with chronic hepatitis C.
Keywords: Hepatitis C virus infection, Statins, Pegylated interferon alpha, Ribavirin, Sustained virological response
Introduction
Hepatitis C viral (HCV) infection is one of the main causes of liver diseases worldwide. The severity of the diseases is quite different, in the spectrum from acute hepatitis, through liver cirrhosis to hepatocellular carcinoma [1]. The end stage of liver disease has a definite need for liver transplantation as the only curative method [2]. The hepatitis C virus uses the host’s lipoprotein metabolism for its life cycle [3]. HCV circulates into the bloodstream as lipoviroparticle composed of triglyceride-rich lipoproteins containing both, apolipoproteins B and E, viral RNA, and structural nuclear viral protein [4], [5].
During the HCV life cycle, from the viral entry into the cell, until the liberation of the virions, the virus modulates the cellular fat metabolism [6]. To facilitate its replication, the hepatitis C virus increases lipogenesis, resulting in accumulation of the triglycerides and the cholesterol in hepatocytes [7], [8].
Cholesterol is synthesised in hepatocytes through a mevalonate pathway, which is promoted by several enzymes, including HMG-CoA (3-hydroxy-3-methyl-glutaryl-coenzyme A) reductase (HMGR). Normally, HMGR expression is consistent with the intracellular cholesterol level, but despite significant cholesterol accumulation, HMGR expression is substantially enhanced in the HCV-infected liver. The Mevalonate pathway for de novo synthesis of cholesterol is also responsible for the synthesis of farnesyl pyrophosphate and geranylgeranyl pyrophosphate (GGPP), which are essential for viral replication [9]. The hepatitis C virus induces a paradoxical condition in which cholesterogenesis is stimulated, but the current cholesterol synthesis is interrupted, or disturbed, by diverting to the synthesis of the intermediate GGPP needed for viral replication [10]. Due to the suppression of cholesterol synthesis, cholesterol levels and its fractions in HCV infected patients are lower.
Statins are a class of competitive inhibitors of HMG-CoA reductase, which are used for controlling cholesterol levels in patients with hyperlipidemia, but have a strong pleiotropic effect, modulating the inflammation, the angiogenesis, the apoptosis and the cell growth [11], [12], [13]. It is of importance to emphasise the effect of statins on the inhibition of HCV replication, which determined their significant anti-HCV effect [14]. Statins block HCV replication by inhibiting the de novo synthesis of cholesterol and inhibition of geranylgeranyl pyrophosphate [15], [16]. Thus, statin therapy can reduce the level of HCV RNA in the blood despite the fact that cholesterol synthesis is suppressed, then it leads to a rise in cholesterol levels after SVR is reached, and it has also been observed that patients with a higher cholesterol baseline are more likely to respond positively to antiviral therapy [17], [18].
Several studies have shown that statins inhibit HCV replication in vitro, that they improve the antiviral activity of already known antiviral drugs and that they reduce their resistance [19], [20]. In vivo studies have shown that statin monotherapy in conventional doses does not inhibit HCV replication [21]. In contrast, statins increase the rate of sustained virologic response when combined with pegylated interferon and ribavirin [22], [23], [24].
In addition to the positive effect of statins, along with antiviral therapy with pegylated interferon and ribavirin over the virologic response, it should be noted the influence of statins on the process of fibrosis, thus preventing the development of a more advanced form of liver disease [25]. However, the possible protective role of the occurrence of HCC, although it has not been proven in randomised studies, should not be neglected.
The purpose of the study is to determine the impact of additional therapy (atorvastatin 20 mg treatment) to standard antiviral therapy (Pegylated interferon alpha-peg-IFN α + Ribavirin) on achieving a sustained virologic response
Material and Methods
The study is comparative, open, prospectively-retrospective, which was conducted at the University Clinic of Gastroenterohepatology in Skopje in the period from 2015 to 2017. A total of 70 patients were included with diagnosed chronic hepatitis C virus infection that met criteria for treatment with antiviral therapy in combination with antilipemic therapy. Patients were divided into two study groups:
A group of 35 patients who, besides antiviral therapy with peg-IFN α and Ribavirin, received additional antilipemic therapy Atorvastatin 20 mg, orally once daily for the entire duration of the study.
A group of 35 patients who received standard antiviral therapy with peg-IFN α subcutaneously administered once a week (180 μg for peg-IFN α2a and 1.5 μg/kg for peg-IFN α2b), for a duration of 24 or 48 weeks depending on the genotype (48 weeks for genotype 1 and 4, and 24 weeks for genotype 2 and 3) and Ribavirin administered orally at a daily dose of 800 to 1200 mg depending on the genotype and body weight.
Inclusion criteria
Patients aged over 18 years, regardless of gender and race, verified C hepatitis virus infection (seropositive HCV patients, and PCR-confirmed HCV RNA positivity), naive or pre-treated patients with standard antiviral therapy, respondents with ability to follow the instructions given by the expert and meet the requirements of the provided study treatment, signed informed consent.
Exclusion criteria
Active intravenous drug addicts, the presence of other types of viruses (HBV or HIV), dialysis patients, patients with other etiology of liver disease (autoimmune hepatitis, Wilson’s disease, haemochromatosis, primary biliary cirrhosis, primary sclerosing cholangitis, α1 antitrypsin deficiency), hepatic decompensation, previous liver transplantation, alcohol abuse (> 20 g/day), presence of HCC and allergy data on the above-mentioned drugs.
The following examinations were made in all patients: - genotyping (determination the genotype of the virus); - quantification of HCV RNA (viral load or number of copies); - liver biopsy under ultrasound control for histological assessment of the degree of inflammation and fibrosis, using the Knodel scale and HAI (histological activity index). According to the presence of fibrotic changes in the liver, patients were divided into three groups: Group 1 - the absence of fibrosis; Group 2 - the presence of fibrosis; and Group 3 - verified liver cirrhosis; - ultrasound examination of the abdomen for assessment of hepatic steatosis. Estimation of steatosis is done before starting with antiviral therapy and after completing the therapy. In addition, steatosis was graded from 0-2 degrees: 0 - absence of steatosis; degree 1- mild steatosis, with quite easily increased echogenicity of the liver parenchyma and clear visualization of the diaphragm and walls of the intrahepatic vessels; and degree 2 - severe steatosis with impaired or no visualization of the diaphragm and blood vessel walls; - laboratory analysis (haematological, hepatological, lipid, glucid status, insulinemia with HOMA-IR calculation); - autoantibodies to exclude autoimmune liver disease; - thyroid status (TSH, fT4); - Body Mass Index (BMI) calculation, according to the formula: weight in kg/(height in meters)2.
All patients were evaluated for the virological response achieved (12 weeks after initiation of treatment, at the end of treatment and 6 months after the treatment), and for a sustained virologic response was considered an undetectable level of HCV RNA in the blood, 24 weeks after completion of therapy.
Before the start of the study, the protocol, informed consent and other accompanying documents were reviewed and approved by the Ethics Committee for Research over Humans at the UKIM Medical Faculty Skopje. Patients signed written informed consent for participation in the study, which was conducted in accordance with the Declaration of Helsinki.
Statistical analysis
The obtained data were processed with statistical computer program SPSS 17 for Windows. Various statistical tests were used, such as:
- descriptive statistics (arithmetic mean, standard deviation, standard error, median and interquartile interval) for a description of quantitative features,
- frequencies and percentages for a description of categorical features,
- independent parametric and nonparametric tests (Student t-test for independent samples, Chi-square test, Fisher exact test, Mann-Whitney test) were used to compare the analysis groups,
- for comparison of the analysed parameters in the analysed period, dependent parametric and nonparametric tests were used (Student t-test for dependent samples, Wilcoxon Matched Pairs test, ANOVA Repeated-measurements, Friedman ANOVA test),
- for all analyses, the value of < 0.05 was considered statistically significant, and p < 0.01 for highly significant.
Results
The results of our study showed a non-significant difference between the groups before the treatment regarding the gender, age, drug abuse, the genotypic distribution, viral load, histology activity index, presence of fibrosis and steatosis, transaminase activity, lipid and carbohydrate status. Table 1 shows all the baseline characteristics of the whole group, of both analysed patient groups, and the comparison of the two groups.
Table 1.
Baseline characteristics and comparison between two groups of patients with CHC treated with or without atorvastatin + antiviral therapy
| Variable | N (%) | Group I n (%) | Group II n (%) | P value |
|---|---|---|---|---|
| Gender, n% | ||||
| Male | 50 (71.43) | 27 (77.14) | 23 (65.71) | A0.29 |
| Female | 20 (28.57) | 8 (22.86) | 12 (34.29) | |
| Age, years, mean ± SD | ||||
| 70 | 36.37 ± 7.7 | 36.23 ± 9.1 | B0.94 | |
| Drug abuse, n (%) | ||||
| Yes | 40 (57.14) | 23 (65.71) | 17 (48.57) | A0.15 |
| No | 30 (42.86) | 12 (34.29) | 18 (51.43) | |
| Genotype n (%) | ||||
| Subtype 1 | 22 (31.43) | 10 (28.57) | 12 (34.29) | Fisher exact, 0.7 |
| Subtype 2 | 1 (1.43) | 0 | 1 (2.86) | |
| Subtype 3 | 46 (65.71) | 24 (68.57) | 22 (62.86) | |
| Subtype 4 | 1 (1.43) | 1 (2.86) | 0 | |
| Viral load, n% | ||||
| Low | 37(52.86) | 20 (57.14) | 17 (48.57) | A0.47 |
| High | 33 (47.14) | 15 (42.86) | 18 (51.43) | |
| Liver biopsy Knodell HAI, n % | ||||
| 1 | 12 (17.91) | 6 (17.65) | 6 (18.18) | C0.24 |
| 2 | 22 (32.84) | 13 (38.24) | 9 (27.27) | |
| 3 | 13 (19.4) | 8 (23.53) | 5 (15.15) | |
| 4 | 11 (16.42) | 5 (14.71) | 6 (18.18) | |
| 5 | 4 (5.97) | 1 (2.94) | 3 (9.09) | |
| 6 | 1 (1.49) | 0 | 1 (3.03) | |
| 7 | 1 (1.49) | 0 | 1 (3.03) | |
| 9 | 1 (1.49) | 1 (2.94) | 0 | |
| 11 | 1 (1.49) | 0 | 1 (3.03) | |
| 18 | 1 (1.49) | 0 | 1 (3.03) | |
| Presence of fibrosis, n% | ||||
| No fibrosis | 50 (71.43) | 28 (80) | 22 (62.86) | Fisher exact, 0.165 |
| Fibrosis present | 17 (24.29) | 7 (20) | 10 (28.57) | |
| Cirrhosis | 3 (4.29) | 0 | 3 (8.57) | |
| Steatosis, n% | ||||
| No steatosis | 32 (45.71) | 14 (40) | 18 (51.43) | Fisher exact, 0.6 |
| Mild | 34 (48.57) | 19 (54.29) | 15 (42.86) | |
| Severe | 4 (5.71) | 2 (5.71) | 2 (5.71) | |
| BMI, mean ± SD | ||||
| 25.52±4.4 | 25.77 ± 4.2 | 25.27 ± 4.6 | B0.64 | |
| AST (10-34 U/L), mean ± SD | ||||
| 72.53±75.2 | 63.6 ± 45.3 | 81.46 ± 96.3 | C0.44 | |
| ALT (10-45 U/L), mean ± SD | ||||
| 108.86±89.7 | 99.97 ± 68.9 | 117.7 ± 106.8 | C0.73 | |
| Trigliceride (0.0-2.0 mmol/L), mean ± SD | ||||
| 1.15 ± 0.6 | 1.17 ± 0.6 | 1.14 ± 0.6 | C0.9 | |
| Total Cholesterol (0.0-5.5 mmol/L), mean ± SD | ||||
| 4.27 ± 1.1 | 4.16 ± 1.3 | 4.38 ± 0.9 | C0.41 | |
| HDL-C (0.9-2.0 mmol/L), mean ± SD | ||||
| 1.15 ± 0.3 | 1.12 ± 0.3 | 1.17 ± 0.3 | C0.5 | |
| LDL-C (2.2-3.7 mmol/L), mean ± SD | ||||
| 2.54 ± 0.96 | 2.47 ± 1.1 | 2.61 ± 0.8 | C0.53 | |
| Fasting glucose (3.6-6.5 mmol/L), mean ± SD | ||||
| 5.38 ± 0.7 | 5.35 ± 0.7 | 5.41 ± 0.7 | C0.7 | |
| Fasting insulin (2-17 µiu/ml), mean ± SD | ||||
| 12.45 ± 10.8 | 12.95 ± 11.1 | 11.98 ± 10.7 | C0.72 | |
| HOMA-IR, mean ± SD | ||||
| 2.96 ± 2.7 | 3.12 ± 3.04 | 2.81 ± 2.5 | C0.67 | |
Group I (with Atorvastatin); group II (without Atorvastatin).
The virological response was determined after 12 weeks of treatment (early virologic response, EVR), at the end of treatment (end of treatment response, ETR), and 6 months after treatment (sustained virologic response, SVR) (Table 2).
Table 2.
Virologic response
| Variable | N (%) | Group I N (%) | Group II N (%) | P value |
|---|---|---|---|---|
| Virologic response ЕVR | ||||
| Yes | 45(93.75) | 22 (95.65) | 23 (92) | Fisher exact, p = 1.0 |
| No | (36.25) | 1 (4.35) | 2 (8) | |
| Virologic response ЕTR | ||||
| Yes | 53(85.48) | 25 (89.29) | 28 (82.35) | Fisher exact, p = 0.49 |
| No | 9(14.52) | 3 (10.71) | 6 (17.65) | |
| Virologic response SVR | ||||
| Yes | 56(80) | 30 (85.71) | 26 (74.29) | P = 0.23 |
| No | 14(20) | 5 (14.29) | 9 (25.71) | |
Group I (with Atorvastatin), group II (without Atorvastatin); p (Chi-square test): EVR (early virologic response-12 week of the beginning of therapy); ETR (end of virologic treatment response); SVR (sustained virologic response – 6 months after the end of treatment).
The early virologic response resulted in 45 (93.75%) patients, 22 of those (95.65%) were in the Atorvastatin group, and 23 (92%) were in the group without anti-lipemic therapy. End of treatment response resulted in 53 (85.48%) patients, 25 of those (89.29%) were in the Atorvastatin group, and 28 (82.35%) were in the atorvastatin-free group; and sustained virological response, respectively, was achieved in 56 (80%) patients, 30 of those (85.71%) were in the anti-lipemic therapy group, and 26 (74.29%) were in the group without additional anti-lipemic therapy. The statistical analysis showed the non-significant difference between the group taking anti-lipemic therapy and the group not taking anti-lipemic therapy, all in co-relation to the frequency of early virological response (p = 1.0), end of treatment virological response (p = 0.49) and 6 months after the treatment (p = 0.23).
In the group of participants receiving Atorvastatin, a sustained virologic response was achieved significantly more frequently in patients with genotype 3 compared to those with genotype 1 (95.83% vs 60%, p = 0.019), Table 3.
Table 3.
Virologic response 6 months after the treatment
| HCV genotype in pts with Atorvastatin | SVR | NVR | P value |
|---|---|---|---|
| n (%) | n (%) | ||
| 1 | 6 (60) | 4 (40) | Fisher exact, P = 0.019* |
| 3 | 23 (95.83) | 1 (4.17) |
SVR (sustained virologic response); NVR (no virologic response);
p < 0.05.
Figure 1 shows the difference between the rate of achieved SVR, depending on the type of therapy, as well genotype 1 and 3, whereas although the statistical analysis did not confirm a significant difference (p = 0.7, p = 0.34) between the groups, the rate of achieved SVR (95.83%) in the group of patients with genotype 3 who received Atorvastatin is remarkable.
Figure 1.
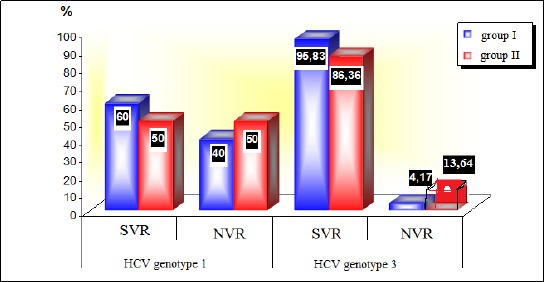
SVR (sustained virologic response) NVR (no virologic response) group I (with Atorvastatin), group II (without Atorvastatin)
Table 4 shows the values of cholesterol, HDL, LDL, TG in three points (before treatment, at the end of treatment and 6 months after treatment) in both groups of participants.
Table 4.
Cholesterol, HDL, LDL, TG in three points (before treatment, at the end of treatment and 6 months after treatment) in both groups of participants
| Group II | |||||
|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | P value | |
| Cholesterol (mmol/l) | |||||
| BT | 35 | 4.16 ± 1.3 | 35 | 4.38 ± 0.9 | A0.41 |
| ET | 35 | 3.83 ± 1.3 | 35 | 4.43 ± 0.7 | B0.0008** |
| AT | 35 | 4.47 ± 1.4 | 35 | 4.89 ± 1.1 | B0.038* |
| HDL (mmol/l) | |||||
| BT | 35 | 1.12 ± 0.3 | 35 | 1.17 ± 0.3 | A0.5 |
| ET | 35 | 0.97± 0.3 | 35 | 1.1 ± 0.3 | B0.02* |
| AT | 35 | 2.0 ± 0.35 | 35 | 1.2 ± 0.2 | B0.36 |
| LDL (mmol/l) | |||||
| BT | 35 | 2.47 ± 1.1 | 35 | 2.61 ± 0.8 | A0.53 |
| ET | 35 | 2.16 ± 1.1 | 35 | 2.56 ± 0.6 | A0.06 |
| AT | 35 | 2.77 ± 1.2 | 35 | 3.04 ± 1.1 | A0.33 |
| TG (mmol/l) | |||||
| BT | 35 | 1.17 ± 0.6 | 35 | 1.14 ± 0.6 | B0.9 |
| ET | 35 | 1.39 ± 0.6 | 35 | 1.71 ± 2.3 | B0.8 |
| AT | 35 | 1.32 ± 0.6 | 35 | 1.32 ± 0.7 | B0.9 |
Group I (with Atorvastatin), group II (without Atorvastatin); a(Student t test); b(Mann-Whitney test);
p < 0.05;
p < 0.01; BT - before treatment; ET-end of treatment; AT-after treatment (6 months).
Before the start of the treatment, the two groups of subjects had non-significantly different cholesterol level values (p = 0.41). At the end of the treatment, in the group of patients receiving additional antilipemic therapy, significantly lower cholesterol values were measured compared to the standard therapy treatment group. (p = 0.0008). Even after 6 months of completed treatment, patients who received Atorvastatin in addition to antiviral therapy had significantly lower cholesterol values than patients receiving only antiviral therapy (p = 0.038).
Before the start of the treatment, participants from the two groups had non-significantly different values of HDL (p = 0.5). For a value of p = 0.02, a significant difference in HDL level values at the end of the treatment between the groups was confirmed. In 6 months after completed treatment, differences in HDL level values between the two groups remain statistically non-significant (p = 0.36).
In the whole period of analysis, statistically non-significant differences were shown in the mean values of LDL level between the group of patients treated with and without Atorvastatin (p > 0.05).
The differences between the two groups in terms of baseline (pre-therapy) and TG control values (at the end and 6 months after therapy) were statistically non-significant (p = 0.9, p = 0.8, p = 0.9 respectively).
Table 5 presents the values of fasting glucose, fasting insulin and HOMA IR in three points (before treatment, at the end of treatment and 6 months after treatment), in both groups of participants
Table 5.
Fasting glucose, fasting insulin and HOMA IR in three points (before treatment, at the end of treatment and 6 months after treatment), in both groups of participants
| Group II | P value | ||||
|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | ||
| Fasting glucose (mmol/L) | |||||
| BT | 35 | 5.35 ± 0.7 | 35 | 5.41 ± 0.7 | A0.7 |
| ET | 35 | 4.92 ± 0.7 | 35 | 5.28 ± 0.8 | A0.049* |
| AT | 35 | 5.19 ± 0.7 | 35 | 5.34 ± 0.6 | A0.415 |
| Fasting insulin (mmol/L) | |||||
| BT | 31 | 12.95 ± 11.1 | 35 | 11.98 ± 10.7 | B0.72 |
| ET | 26 | 9.53 ± 7.6 | 35 | 15.15 ± 10.9 | A0.032* |
| AT | 25 | 13.55 ± 10.6 | 35 | 10.86 ± 7.4 | A0.33 |
| HOMA IR | |||||
| BT | 31 | 3.12 ± 3.04 | 35 | 2.81 ± 2.5 | B0.67 |
| ET | 26 | 2.15 ± 2.1 | 35 | 3.51 ± 3.4 | B0.01* |
| AT | 25 | 3.19 ± 2.7 | 35 | 2.38 ± 1.7 | B0.45 |
Group I (with Atorvastatin), group II (without Atorvastatin); a (Student t test); b(Mann-Whitney test);
p < 0.05; BT-before treatment; ET-end of treatment; AT-after treatment (6 months).
Before the start of the therapy, there was no significant difference in the average values of fasting glucose between group I and group II (p = 0.7). At the end of the treatment, the average fasting glucose values were significantly lower in the group of patients who, in addition to standard antiviral therapy, received antilipemic therapy (4.92 ± 0.7 vs 5.28 ± 0.8; p = 0.049), while control values after 6 months of completed treatment were non-significantly lower (5.19 ± 0.7 Sun 5.34 ± 0.6; p = 0.415).
Values of fasting insulin before treatment start were non-significantly different between the two groups of patients (p = 0.72). At the end of the treatment, in the group with Atorvastatin, mean fasting insulin values were of 9.53 ± 7.6, which were significantly lower compared to the group without Atorvastatin, where mean values were of 10.86 ± 7.4 (p = 0.032).
Six months after the completed treatment program, non-significantly higher mean fasting insulin values were evaluated in the Atorvastatin group compared to the group without antilipemic therapy (p = 0.33).
Both groups of participants had non-significantly different values of the HOMA-IR index before the start of the treatment and 6 months after the treatment (p = 0.67, p = 0.45 consequently), and significantly different at the end of the treatment (p = 0.01). In a group, I at the end of the treatment, significantly lower values for HOMA IR was obtained, in contrary to group II.
Table 6 shows the distribution of the frequency of adverse events that occurred in patients during the treatment.
Table 6.
Distribution of the frequency of adverse events that occurred in patients during the treatment
| Side effects | Group I | Group II | P value |
|---|---|---|---|
| N (%) | N (%) | ||
| Thrombocytopenia | 14 (40) | 16 (45.7) | A0.63 |
| Leukopenia | 17 (48.6) | 18 (51.4) | A0.81 |
| Anemia | 4 (11.4) | 2 (5.7) | B0.34 |
| Flu like symptoms | 12 (34.3) | 4 (11,.4) | A0.023* |
| Loss of appetite and weight loss | 18 (51.4) | 13 (37.1) | A0.23 |
| Hair loss | 7 (20) | 1 (2.9) | B0.027* |
| Hypothyreosis | 2 (5.7) | 1 (2.9) | B0.5 |
| Hyperthyreosis | 1 (2.9) | 1 (2.9) | B0.75 |
| Nausea, vomitus | 1 (2.9) | 1 (2.9) | B0.75 |
| Fatigue, malaise | 6 (17.1) | 4 (11.4) | B0.49 |
| Cutaneous allergic reaction to the drug | 1 (2.9) | 0 | B0.5 |
| Dry skin | 2 (5.7) | 0 | B0.25 |
| Vertigo | 1 (2.9) | 0 | B0.5 |
| Infection | 1 (2.9) | 0 | B0.5 |
| Anxiety | 10 (28.6) | 7 (20) | A0.4 |
| Depression | 1 (2.9) | 0 | B0.5 |
| Skin changes | 3 (8.6) | 1 (2.9) | B0.31 |
| Headache | 1 (2.9) | 1 (2.9) | B0.75 |
| Insomnia | 1 (2.9) | 2 (5.7) | B0.5 |
Group I (with Atorvastatin); group II (without Atorvastatin); ap (Chi-square test); bp (Fisher exact test);
p < 0.05.
The most common adverse reaction in the group which was receiving Atorvastatin was reduced appetite and decreased body weight (51.4%), followed by leucopenia (48.6%) and thrombocytopenia (40%). Haematological alterations were the most common adverse reactions in the group treated with standard antiviral therapy only-leukopenia and thrombocytopenia (51.4%, 45.7% respectively). A statistically significant difference between the two groups of patients was confirmed only about the frequency of occurrence of flu-like symptoms (p = 0.023) and hair loss (p = 0.027). Patients in the first group significantly more frequently had the appearance of flu-like symptoms and hair loss (34.3%, 20% consequently), compared with the second group of participants (11.4%, 2.9% respectively).
Discussion
Statins that inhibit the enzyme on the mevalonate pathway, HMG CoA reductase, have shown to play an important action not only in fat metabolism but also in the modulation of hepatic steatosis and fibrosis, and are presumed to have an important anti-proliferative, anti-angiogenic and antioxidant effect, with a potentially protective action against the development of HCC. In vitro studies, such as studies of Ikeda et al., [26]; and Aiziki et al., [27], have clearly shown that the statins inhibit the replication of viral RNA, more likely by inhibiting geranylgeranylation of cellular proteins, rather than by inhibiting cholesterol synthesis, showing almost the same efficacy as the most potent clinical treatment. However, studies have shown that not all statins have the same impact. According to them, atorvastatin, fluvastatin and simvastatin have stronger anti-HCV activity, lovastatin moderate activity, and pravastatin does not possess such activity, although it inhibits HMG-CoA reductase.
In vitro study of Ikeda et al., have shown that statins can be used as adjuvant therapy for interferon as well, just as ribavirin together with interferon shows synergistic antiviral activity [28].
In vivo studies, however, showed different results. Statin monotherapy did not lead to an improvement in the virologic response, probably due to the synergistic effect of statins with interferon, as demonstrated in the study of O’Leary et al., [29] and Forde KA et al., [30].
Most studies have analysed the effect of Fluvastatin on the virologic response, and the number of studies that analysed other statins is lower. There are studies that are potentiating the positive impact of Fluvastatin as an additional therapy of standard antiviral therapy over virologic response, especially for genotype 1, such as the study of Selic Kurincic et al., [31]. The study of Atsukawa et al., [32], showed the reduction of viral relapse in patients with genotype 1b, as well as the studies of Georgescu et al., [33], Sesaki et al., [34] and Kondo et al., [35]. In contrary to those, Shavakhi et al., published results where additional therapy with Atorvastatin in patients with genotype 1 over 12 weeks does not lead to a better SVR [36]. The study of Malaguarnera et al., showed that the use of Rosuvastatin has a significant influence over lipid metabolism, inflammatory status and fibrosis, but also no significantly improves the effect of standard therapy on the virologic response [37]. The author Zhu et al., in his meta-analysis, concluded that additional statin therapy to previous standard IFN-α and ribavirin therapy improves SVR, RVR and EVR without additional adverse events and thus can be considered as an adjuvant treatment to IFN-α and ribavirin [38].
Our results showed a higher percentage of the achieved sustained virologic response as a marker for the success of treatment in the statin group (85.71%) versus 74.29% of the SVR achieved in the antiviral therapy group only. But when we additionally made a comparison between Genotype 1 and 3 as the most common genotypes in our study, we obtained an even higher percentage of SVR achieved in genotype 3 and combined treatment, of a high 95.83% versus 83.36% achieved in genotype 3 and standard antiviral therapy. Although no significant difference has been obtained (p = 0.34), this is a remarkable result of a sustained virological response achieved in patients with genotype 3 (practically only one patient out of 23 did not achieve SVR). This high percentage of virological response is achieving with the new direct antiviral drugs (DAA), mainly for other genotypes but not for genotype 3 [39], 40]. In the study of Sette H Jr et al., the rate of sustained virological response is lowest in patients with genotype 3, which is 90.7%, while in patients with genotype 1 it is 95.8%, in genotype 2 100%, and in genotype 4 also 100% [41]. Our results in the group of patients with genotype 1 have also shown the difference between the two groups in terms of therapy, but in this case, we are talking about a much lower SVR rate (60% versus 50%, p = 0.7). In the Atorvastatin group, patients with genotype 3 who have achieved sustained virological response are significantly higher, compared to patients with genotype 1, p = 0.019, which coincides with the conclusion of Zhu et al., in their meta-analysis, that additional statin therapy should be used in other genotypes other than genotype 1, as in our case, in genotype 3. The early virological response (EVR) and end-of-treatment virologic response (ETR) analysis also showed that they were evident in a higher percentage in patients belonging to the statin group, 95.65% and 89.29%, compared to the patients put into the standard antiviral therapy group (92% and 82.35%), but the comparison between the two groups of patients was statistically non-significant (p = 1.0 and p = 0.49, respectively).
Several parameters were analyzed in our two investigated groups (one group with antilipemic therapy, the other without it), and then compared to each other, such as: gender, age, genotype, the presence of steatosis, fibrosis and liver inflammation, body mass index, transaminase activity, but also more laboratory parameters that reflect the fat and sugar metabolism during antiviral therapy, actually before and after therapy. Differences between the two groups were observed about lipid and glucose status. Specifically, patients receiving additional anti-lipemic therapy had significantly lower total cholesterol levels at the end of treatment and 6 months after, compared to the other group (p = 0.0008 and p = 0.038). Significantly lower results were detected for HDL but only at the end of the treatment (p = 0.02), but higher mean value 2.0 ± 0.35 and p = 0.36 was observed 6 months later, and the results for LDL were non-significantly lower (p = 0.06 and p = 0.33). Considering this lipid profile, we can conclude that antiviral therapy supplemented with Atorvastatin, leads to a decrease in total cholesterol and LDL 6 months after treatment, and an increase in HDL levels. According to these results, patients who received statins with antiviral therapy will have fewer chances to suffer from cardiovascular disease.
About the glucose status, statistically significantly lower values of fasting glucose, fasting insulin, and HOMA IR index at the end of therapy in the Atorvastatin group were obtained, p = 0.049, p = 0.032 and p = 0.01 respectively. This result suggests that additional statin therapy does not lead to a worsening of fasting glucose values and insulin resistance, as written in some studies [42], but rather to their improvement. This improvement in the glucose profile may also be due to the achieved sustained virological response in 85.71% of treated patients with Atorvastatin and antiviral therapy, thereby reversing the diabetogenic effect of the virus, as confirmed in other studies [43], [44]. Improving glucose status means reducing the chance of developing fibrosis and more advanced form of liver disease. Other parameters (gender, age, genotype, presence of steatosis, fibrosis, degree of inflammatory activity, and transaminase activity) compared between the two groups did not show significant differences.
The safety profile of drugs is also of particular importance. Adverse events commonly occurring in both groups were: decreased appetite and decreased body weight, leucopenia, thrombocytopenia, flu-like symptoms, anxiety, fatigue and malaise, anaemia, hair loss, thyroid disorders, and others. In one patient due to a skin allergic reaction, therapy with peg-IFN α2a was discontinued and started with peg-IFN α2b after which there were no side effects, and the treatment was completed according to the genotype 3 protocol. In the remaining patients, adverse events were not life-threatening and were not a cause for discontinuation of therapy. In our study, a statistically significant difference between the group of patients with Atorvastatin and without was confirmed only about the frequency of occurrence of flu-like symptoms (p = 0.023) and hair loss (p = 0.027). These symptoms lasted for short period of time and were reversible, indicating that statins can be safely used in patients with chronic hepatitis C. Verpaalen et al., in their review writes about the positive impact of statins in the treatment of HCV infection, but also their excellent safety profile and low cost [45], which coincides with our conclusions.
In our study, the influence of statins on the progression of fibrosis, cirrhosis and the occurrence of HCC was not analysed. But since the goal of antiviral therapy is precisely preventing the onset of cirrhosis and HCC in this group of patients, we should not underestimate the significance of the statins in this field. Simon TG et al., in their review, indicates the association of atorvastatin and fluvastatin with a dose-dependent reduction in the incidence of cirrhosis and HCC in patients with HCV infection [46]. We should consider all those facts when planning a strategy for the treatment of patients with Chronic Hepatitis C.
In conclusion: 1) Combined therapy of Atorvastatin + Pegylated interferon alfa + Ribavirin leads to a high rate of sustained virological response of 95.83%, in patients with chronic hepatitis C, genotype 3; 2) Combined therapy leads to an improvement in lipid and glucose status after the treatment; and 3) Adverse events do not differ between the Atorvastatin group and the standard antiviral therapy group and do not lead to discontinuation of therapy. Therefore, statins can be safely used in patients with chronic hepatitis C.
Abbreviations
- CHC:
chronic hepatitis C
- NS:
not statistically significant
- S:
statistically significant
- BMI:
body mass index
- AST:
aspartate transaminase
- ALT:
alanine aminotransferase
- HDL-C:
high-density lipoprotein cholesterol
- LDL-C:
low-density lipoprotein cholesterol
- HOMA-IR:
Homeostasis Model Assessment of Insulin Resistance
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Civan J, Hann HW. Hepatitis C Virus Mediated Hepatocellular Carcinoma:A Focused Review for a Time of Changing Therapeutic Options. N A J Med Sci. 2014;7(1):8–16. https://doi.org/10.7156/najms.2014.0701008. [Google Scholar]
- 2.Tsoulfas G, Goulis I, Giakoustidis D, et al. Hepatitis C and liver transplantation. Hippokratia. 2009;13(4):211–215. [PMC free article] [PubMed] [Google Scholar]
- 3.Aizawa Y, Seki N, Nagano T, Abe H. Chronic hepatitis C virus infection and lipoprotein metabolism. World Journal of Gastroenterology. 2015;21(36):10299–1031. doi: 10.3748/wjg.v21.i36.10299. https://doi.org/10.3748/wjg.v21.i36.10299 PMid:26420957 PMCid:PMC4579877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim CW, Chang KM. HCV virology and life cycle. Clin Mol Hepatol. 2013;19:17–25. doi: 10.3350/cmh.2013.19.1.17. https://doi.org/10.3350/cmh.2013.19.1.17 PMid:23593605 PMCid:PMC3622851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLauchlan J. Lipid droplets and hepatitis C virus infection. Biochim Biophys Acta. 2009;1791:552–559. doi: 10.1016/j.bbalip.2008.12.012. https://doi.org/10.1016/j.bbalip.2008.12.012 PMid:19167518. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y-Z, Qian X-J, Zhao P, Qi Z-T. How hepatitis C virus invades hepatocytes:The mystery of viral entry. World Journal of Gastroenterology. 2014;20(13):3457–3467. doi: 10.3748/wjg.v20.i13.3457. https://doi.org/10.3748/wjg.v20.i13.3457 PMid:24707128 PMCid:PMC3974512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felmlee DJ, Hafirassou ML, Lefevre M, Baumert TF, Schuster C. Hepatitis C Virus, Cholesterol and Lipoproteins - Impact for the Viral Life Cycle and Pathogenesis of Liver Disease. Viruses. 2013;5(5):1292–1324. doi: 10.3390/v5051292. https://doi.org/10.3390/v5051292 PMid:23698400 PMCid:PMC3712309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enjoji M, Kohjima M, Kotoh K, Nakamuta M. Metabolic disorders and steatosis in patients with chronic hepatitis C:Metabolic strategies for antiviral treatments. International journal of hepatology. 2012;2012 doi: 10.1155/2012/264017. https://doi.org/10.1155/2012/264017 PMid:22701799 PMCid:PMC3373124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(7):2561–2566. doi: 10.1073/pnas.0409834102. https://doi.org/10.1073/pnas.0409834102 PMid:15699349 PMCid:PMC549027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert J, Bain V, et al. Elevated lipogenesis and diminished cholesterol synthesis in patients with hepatitis C viral infection compared to haelthy humans. Hepatology. 2013;57:1697–1704. doi: 10.1002/hep.25990. https://doi.org/10.1002/hep.25990 PMid:23417775. [DOI] [PubMed] [Google Scholar]
- 11.Davies JT, Delfino SF, Feinberg CE, et al. Current and Emerging Uses of Statins in Clinical Therapeutics:A Review. Lipid insights. 2016;9:13–29. doi: 10.4137/LPI.S37450. https://doi.org/10.4137/LPI.S37450 PMid:27867302 PMCid:PMC5110224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. https://doi.org/10.1146/annurev.pharmtox.45.120403.095748 PMid:15822172 PMCid:PMC2694580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janicko M, Drazilova S, Pella D, Fedacko J, Jarcuska P. Pleiotropic effects of statins in the diseases of the liver. World Journal of Gastroenterology. 2016;22(27):6201–6213. doi: 10.3748/wjg.v22.i27.6201. https://doi.org/10.3748/wjg.v22.i27.6201 PMid:27468210 PMCid:PMC4945979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon TG, Butt AA. Lipid dysregulation in hepatitis C virus, and impact of statin therapy upon clinical outcomes. World Journal of Gastroenterology. 2015;21(27):8293–8303. doi: 10.3748/wjg.v21.i27.8293. https://doi.org/10.3748/wjg.v21.i27.8293 PMid:26217081 PMCid:PMC4507099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Gale M, Keller BC, Huang H, Brown MS, Goldstein JL, Ye J. Identification of FBL2 as a geranylgeranylated cellular protein required for hepatitis C virus RNA replication. Mol Cell. 2005;18:425–434. doi: 10.1016/j.molcel.2005.04.004. https://doi.org/10.1016/j.molcel.2005.04.004 PMid:15893726. [DOI] [PubMed] [Google Scholar]
- 16.Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, et al. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383–1389. doi: 10.1111/j.1572-0241.2008.01876.x. https://doi.org/10.1111/j.1572-0241.2008.01876.x PMid:18410471. [DOI] [PubMed] [Google Scholar]
- 17.Shavakhi A, Minakari M, Bighamian A, Sadeghian S, et al. Statin efficiacy in the treatment of hepatitis C genotip I. J Res Med Sci. 2014;19(Suppl 1):S1–S4. [PMC free article] [PubMed] [Google Scholar]
- 18.Vere CC, Streba CT, Streba L, Rogoveanu I. Statins in the Treatment of Hepatitis C. Hepatitis Monthly. 2012;12:369–71. doi: 10.5812/hepatmon.5998. https://doi.org/10.5812/hepatmon.5998 PMid:22879825 PMCid:PMC3412552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delang L, Paeshuyse J, et al. Statins potentiate the in vitro anti-hepatitis C virus activity of selective hepatitis C virus inhibitors and delay or prevent resistance development. Hepatology. 2009;50:6–16. doi: 10.1002/hep.22916. https://doi.org/10.1002/hep.22916 PMid:19437494. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117–125. doi: 10.1002/hep.21232. https://doi.org/10.1002/hep.21232 PMid:16799963. [DOI] [PubMed] [Google Scholar]
- 21.O'Leary JG, Chan JL, McMahon CM, Chung RT. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses:A pilot clinical trial. Hepatology. 2007;45:895–8. doi: 10.1002/hep.21554. https://doi.org/10.1002/hep.21554 PMid:17393518. [DOI] [PubMed] [Google Scholar]
- 22.Butt AA, Yan P, Bonilla H, Abou-Samra AB, Shaikh OS, Simon TG, et al. Effect of addition of statins to antiviral therapy in hepatitis C virus-infected persons:Results from ERCHIVES. Hepatology. 2015;62(2):365–74. doi: 10.1002/hep.27835. https://doi.org/10.1002/hep.27835 PMid:25847403. [DOI] [PubMed] [Google Scholar]
- 23.Mihaila RG. Statins in Chronic Hepatitis C:Stage result. Biomed Res. 2014;25(4):463–469. [Google Scholar]
- 24.Rao GA, Pandya PK. Statin therapy improves sustained virologic response among diabetic patients with chronic hepatitis C. Gastroenterology. 2011;140:144–152. doi: 10.1053/j.gastro.2010.08.055. https://doi.org/10.1053/j.gastro.2010.08.055 PMid:20833169. [DOI] [PubMed] [Google Scholar]
- 25.Simon TG, King LY, Zheng H, Chung RT. Statin Use is Associated with a Reduced Risk of Fibrosis Progression in Chronic Hepatitis C. Journal of hepatology. 2015;62(1):18–23. doi: 10.1016/j.jhep.2014.08.013. https://doi.org/10.1016/j.jhep.2014.08.013 PMid:25135867 PMCid:PMC4272642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117–125. doi: 10.1002/hep.21232. https://doi.org/10.1002/hep.21232 PMid:16799963. [DOI] [PubMed] [Google Scholar]
- 27.Aizaki H, Lee KJ, Sung VM, Ishiko H, Lai MM. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology. 2004;324(2):450–61. doi: 10.1016/j.virol.2004.03.034. https://doi.org/10.1016/j.virol.2004.03.034 PMid:15207630. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda M, Kato N. Life style-related diseases of the digestive system:cell culture system for the screening of anti-hepatitis C virus (HCV) reagents:suppression of HCV replication by statins and synergistic action with interferon. J Pharmacol Sci. 2007;105(2):145–50. doi: 10.1254/jphs.fm0070050. https://doi.org/10.1254/jphs.FM0070050 PMid:17928739. [DOI] [PubMed] [Google Scholar]
- 29.O'Leary JG, Chan JL, McMahon CM, Chung RT. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses:A pilot clinical trial. Hepatology. 2007;45:895–8. doi: 10.1002/hep.21554. https://doi.org/10.1002/hep.21554 PMid:17393518. [DOI] [PubMed] [Google Scholar]
- 30.Forde KA, Law C, O'Flynn R, Kaplan DE. Do statins reduce hepatitis C RNA titers during routine clinical use? World J Gastroenterol. 2009;15(40):5020–7. doi: 10.3748/wjg.15.5020. https://doi.org/10.3748/wjg.15.5020 PMid:19859994 PMCid:PMC2768880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selic Kurincic T, Lesnicar G, Poljak M, Meglic Volkar J, Rajter M, Prah J, et al. Impact of added fluvastatin to standard-of-care treatment on sustained virological response in naïve chronic hepatitis C Patients infected with genotypes 1 and 3. Intervirology. 2014;57(1):23–3. doi: 10.1159/000354541. https://doi.org/10.1159/000354541 PMid:24080608. [DOI] [PubMed] [Google Scholar]
- 32.Atsukawa M, Tsubota A, Kondo C, Itokawa N, Narahara Y, Nakatsuka K, et al. Combination of fluvastatin with pegylated interferon/ribavirin therapy reduces viral relapse in chronic hepatitis C infected with HCV genotype 1b. J Gastroenterol Hepatol. 2013;28(1):51–6. doi: 10.1111/j.1440-1746.2012.07267.x. https://doi.org/10.1111/j.1440-1746.2012.07267.x PMid:22989264. [DOI] [PubMed] [Google Scholar]
- 33.Georgescu EF, Streba L, Teodorescu R, Mateescu G, Abagiu MT. 10 potential enhancement of both early (evr) and sustained (svr) virological response by fluvastatin in chronic hepatitis c treated with standard pegifn-ribavirin therapy. A pilot study. Journal of Hepatology. 2011;54:S5. https://doi.org/10.1016/S0168-8278(11)60012-3. [Google Scholar]
- 34.Sezaki H, Suzuki F, Akuta N, Yatsuji H, et al. An open pilot study exploring the efficacy of fluvastatin, pegylated interferon and ribavirin in patients with hepatitis C virus genotype 1b in high viral loads. Intervirology. 2009;52(1):43–8. doi: 10.1159/000213504. https://doi.org/10.1159/000213504 PMid:19372703. [DOI] [PubMed] [Google Scholar]
- 35.Kondo C, Atsukawa M, Tsubota A, Itokawa N, Fukuda T, Matsushita Y, et al. An open-label randomized controlled study of pegylated interferon/ribavirin combination therapy for chronic hepatitis C with versus without fluvastatin. J Viral Hepat. 2012;19:615–622. doi: 10.1111/j.1365-2893.2011.01584.x. https://doi.org/10.1111/j.1365-2893.2011.01584.x PMid:22863265. [DOI] [PubMed] [Google Scholar]
- 36.Shavakhi A, Minakari M, Bighamian A, et al. Statin efficacy in the treatment of hepatitis C genotype I. Journal of Research in Medical Sciences:The Official Journal of Isfahan University of Medical Sciences. 2014;19(Suppl 1):S1–S4. [PMC free article] [PubMed] [Google Scholar]
- 37.Malaguarnera M, Vacante M, Russo C, et al. Rosuvastatin reduces nonalcoholic fatty liver disease in patients with chronic hepatitis C treated with α-interferon and ribavirin:Rosuvastatin reduces NAFLD in HCV patients. Hepatitis Monthly. 2011;11(2):92–98. [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Q, Li N, Han Q, Zhang P, Yang C, Zeng X, et al. Statin therapy improves response to interferon alfa and ribavirin in chronic hepatitis C:A systematic review and meta-analysis. Antiviral Res. 2013;98:373–9. doi: 10.1016/j.antiviral.2013.04.009. https://doi.org/10.1016/j.antiviral.2013.04.009 PMid:23603497. [DOI] [PubMed] [Google Scholar]
- 39.Lai Wei, Seng Gee Lim, Qing Xie, Kính Nguyen Văn, Teerha Piratvisuth, Yan Huang, et al. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia:a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;4(2):127–134. doi: 10.1016/S2468-1253(18)30343-1. https://doi.org/10.1016/S2468-1253(18)30343-1. [DOI] [PubMed] [Google Scholar]
- 40.Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med. 2015;373:2608–2617. doi: 10.1056/NEJMoa1512612. https://doi.org/10.1056/NEJMoa1512612 PMid:26575258. [DOI] [PubMed] [Google Scholar]
- 41.Cheinquer H, Sette H, Jr, Wolff FH, de Araujo A, Coelho-Borges S, Soares SRP, et al. Treatment of Chronic HCV Infection with the New Direct Acting Antivirals (DAA):First Report of a Real World Experience in Southern Brazil. Annals of hepatology. 2017;16(5):727–33. doi: 10.5604/01.3001.0010.2717. https://doi.org/10.5604/01.3001.0010.2717 PMid:28809742. [DOI] [PubMed] [Google Scholar]
- 42.Ganda OP. Statin-induced diabetes:incidence, mechanisms, and implications. F1000Research. 2016;5 doi: 10.12688/f1000research.8629.1. https://doi.org/10.12688/f1000research.8629.1 PMid:27408693 PMCid:PMC4926726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgado-Borrego A, Jordan SH, Negre B, et al. Reduction of Insulin Resistance with Effective Clearance of Hepatitis C Infection:Results from The Halt-C Trial. Clin Gastroenterol Hepatol. 2010;8(5):458–462. doi: 10.1016/j.cgh.2010.01.022. https://doi.org/10.1016/j.cgh.2010.01.022 PMid:20156586 PMCid:PMC2856733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero-Gómez M, Fernández-Rodríguez CM, Andrade RJ, et al. Effect of sustained virological response to treatment on the incidence of abnormal glucose values in chronic hepatitis C. J Hepatol. 2008;48(5):721–727. doi: 10.1016/j.jhep.2007.11.022. https://doi.org/10.1016/j.jhep.2007.11.022 PMid:18308416. [DOI] [PubMed] [Google Scholar]
- 45.Verpaalen B, Neyts J, Delang L. Are statins a viable option for the treatment of infections with the hepatitis C virus? Antiviral Res. 2014;105:92–9. doi: 10.1016/j.antiviral.2014.02.020. https://doi.org/10.1016/j.antiviral.2014.02.020 PMid:24613180. [DOI] [PubMed] [Google Scholar]
- 46.Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus:Results from ERCHIVES. Hepatology. 2016;64(1):47–57. doi: 10.1002/hep.28506. https://doi.org/10.1002/hep.28506 PMid:26891205 PMCid:PMC4917438. [DOI] [PMC free article] [PubMed] [Google Scholar]


