Abstract
BACKGROUND:
Microsatellite instability (MSI) is the genetic pathway underlying 15% of sporadic colorectal carcinoma (CRC) and hereditary non-polyposis CRC. MSI-H CRC has a distinct clinicopathological characteristic including excess mucin and signet ring component, proximal colon, Crohn’s like reaction, lymphocytic infiltration, and better survival.
AIM:
This research aims to screen Egyptian CRC patients for MSI status by IHC testing of expression of the MMR proteins in correlation to its clinicopathological features.
MATERIAL AND METHODS:
Immunohistochemistry study for mismatch repair proteins (MMR) was done on 115 cases of CRC. Their expressions were assessed and correlated to clinicopathological parameters in an attempt to obtain the most significant predictors of MSI.
RESULTS:
MSI (low and high) represents 67% of the study cases. The most frequent expression pattern was combined loss of MLH, and PMS2 (38% of MSI) followed by a combined loss of MSH2, and MSH6 (29% of MSI). There was significant correlation of expression pattern of MMR proteins with the laterality, lymphovascular emboli, perineural invasion, grade, T stage, N stage, signet ring component, tumor infiltrating lymphocyte, and peritumoral lesion (0.014, 0.035, 0.012, 0.033, 0.013, 0.000, 0.041, 0.012, and 0.009 respectively). Proximal location (right sided) and lower grade, higher nodal stage, and marked TIL were selected as predictors of MS-H CRC (0.005, 0.031, 0.025, and 0.000 respectively).
CONCLUSION:
All clinicopathological and histological parameters should be assessed in CRC for the sake of predicting MSI. The optimal approach to MSI evaluation is (IHC) assessment of MMR proteins.
Keywords: Microsatellite instability, Colorectal carcinoma, Immunohistochemistry, Mismatch repair, Tumour-infiltrating lymphocyte
Introduction
Colorectal cancer (CRC) is the most commonly observed cancer worldwide [1]. It is one of the most common cause of cancer-related death worldwide [2]. Cancer colon, as any cancer is caused by environmental and genetic factors [3]. 75% of cancer colon is sporadic and the remaining 30% has a hereditary contribution [4]. Hereditary CRC is appeared in two forms. The first is preceded by familial adenomatosis polyposis (FAP). The second is Lynch syndrome (LS) that has a defect in mismatch repair (MMR) gene and often referred to as hereditary nonpolyposis colorectal cancer. However, there was another category that exhibit gathering of CRC and/or adenomas in families with an identifiable hereditary syndrome, and are known as familial CRC. The genetic basis of familial CRC remains unknown [5], [6].
There are two molecular genetic pathways that underlie colorectal carcinogenesis. The first pathway is chromosomal instability that involves the activation of proto-oncogenes such as K-ras, and inactivation of tumour-suppressor genes, such as APC, TP53, DCC, SMAD2, and SMAD4 [7], [8]. Chromosomal instability occurs in 85% of sporadic CRC and FAP [9]. The second pathway is the microsatellite instability (MSI) mutational pathway. MSI results from inactivation, mutational and/or epigenetic silencing of mismatch repair (MMR) [8], [10], [11]. MSI is not limited to hereditary non-polyposis cancer colon (HNPCC) but also present in sporadic CRC [9], [11].
The genetic basis for instability in MSI tumours is an inherited germline alteration in any one of the five human MMR genes: MLH, MSH2, MSH6, PMS2, and PMS1 [8], [12]. More specifically, germline mutations in MSH2 and MLH1 are responsible for most HNPCC families, while MSH6 is less common and PMS2 and PMS1 are rare [8]. MSI can be present in 10-15% of sporadic colorectal carcinoma. Acquired hypermethylation of MLH1 promotor and subsequent transcriptional silencing is the cause of high MSI in sporadic CRC [8], [9], [13].
Abnormal expression of MMR in CRC may be due to complete loss of expression, expression of an only truncated protein that does not bind to the antibody or weak /patchy cytoplasmic reaction if the mutation forms premature truncated but stable protein [14].
Hashmi et al., 2017 reported that MSI-H cancers often stimulate a host response that leads to migration of activated T cells into tumour cells. T cell cytotoxicity is activated. The T cells are CD8+, TCR+ cells. Therefore improved prognosis of MSI-H colonic cancers is related to the upregulated immune system that prevents the emergence of metastatic deposit [9].
There were distinct clinicopathological characteristics of CRC with MSI. These include poor differentiation, excess mucin and signet ring component, proximal colon, medullary feature, Crohn’s like reaction and lymphocytic infiltration [9]. It is noted that the survival rate of CRC with high MSI is better when it is compared with MSS tumour evidenced by in tumoral lymphocytosis of MSI tumours [9], [15]. However, it sometimes associated with metachronous cancer and resistant to traditional chemotherapeutic agent [16], [17].
Investigation for the presence of MSI in CRC is really important due to many factors. It decides the extent of surgical treatment, the prophylactic surgery of hysterectomy and oophorectomy, and screening of the family member for the presence of the same mutation [9], [18]. Recognition of MSI phenotype can be done by histopathology and IHC because of CRC with MSI shares morphological features such as young patient age, right-sided location, mucinous and signet ring histology and intratumoral lymphocytosis. This fact allows the pathologist to dispense on PCR which remains the gold standard for recognition of MSI phenotype as it is not practicable and expensive in routine pathology lab [9], [15], [19].
Our study aims to screen CRC patients for MSI status in a big histopathology lab in Egypt (Referral lab for a big sector of Egyptian population) by immunohistochemical testing of expression of the MMR proteins and its relation to the clinicopathological features in CRC patients.
Material and Methods
A group of 115 cases of CRC were conducted in the present study. They were retrieved consecutively from the archives of Professor Elia, Anis Ishak Laboratory Pathology Centre (Cairo, Egypt), from 2015 to 2018. One representative slide of all cases were retrieved and reviewed. Then, the allocated paraffin-fixed tissue blocks were selected that showed both tumour and adjacent non-tumor colonic epithelium. The clinical and pathological information as patient’s age, gender, tumour laterality (the right side from cecum to splenic flexure), lymphovascular invasion, perineural invasion, T stage, and N stage were obtained from the records. The cases were grouped according to the age of 2 groups (< 50, ≥ 50).
Histopathology
The histopathological features of each slide were reviewed by 3 pathologists as regard the variants (mucinous, cribriform, signet ring, medullary, and poorly differentiated,) according to WHO 2010 [20]. The presence or absence of necrosis (focal and diffuse) was recorded. As regards, intratumoral lymphocytic infiltration, the presence of small round lymphocytes within neoplastic epithelial cells was divided into mild to moderate (up to three intraepithelial lymphocytes (IEL)/HPF) and marked (> 3 IEL/HPF) according to the CAP guidelines [9]. The presence of peritumoral lymphocytic reaction requires 2 or more large lymphoid aggregate. The lab rules include written informed consents of all patients and approval of using their specimen for research purposes.
Immunohistochemistry
IHC examination was performed using a Ventana Benchmark Ultra machine automated staining system. A four-antibody panel of MMR proteins, including MLH1, MSH2, MSH6, and PMS2, are conducted. The primary antibodies used were: anti MLH-1 (M1) Mouse Monoclonal Primary Antibody 1.4 μg/ml) (Ventana, Tucson, AZ, USA), MSH2 (G219-1129), Mouse Monoclonal Primary Antibody 3.04 μg/ml) (Ventana, Tucson, AZ, USA), anti MSH6 (44) Mouse Monoclonal Primary Antibody 0.101 μg/ml) (Ventana, Tucson, AZ, USA), and PMS2 (EPR3947, Rabbit Monoclonal Primary Antibody 11.84 μg/ml) (Ventana, Tucson, AZ, USA). Adjacent normal colonic epithelium, lymphocytes, and stromal cells served as positive internal controls; we use it to check for accuracy. Positive external controls from CRC positive for MLH-1, MSH-2, MSH-6, and PMS2 are used. Negative controls were done by replacing the primary antibody with PBS.
According to the CAP protocol for immunohistochemistry interpretation, any nuclear staining even patchy is taken as “no loss of expression” (Figure 1: C, D, E, and F; Figure 2: C and F). Only absolute absence of nuclear staining was considered “loss of expression” (Figure 2: D and E) provided that internal controls are positive. Hence, carcinoma was considered MSI when nuclear staining was absent for at least one protein [21]. MSI-positive markers were reexamined to confirm the results.
Figure 1.
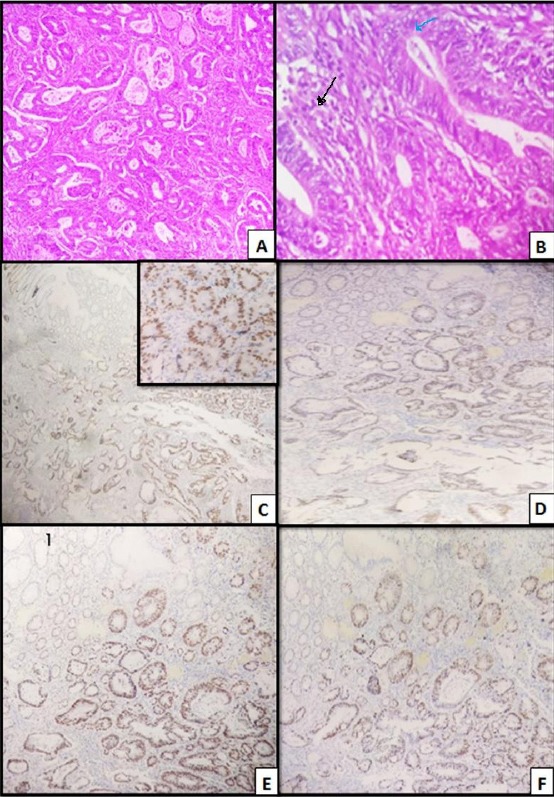
Colonic adenocarcinoma, moderately differentiated, grade II; A) H&E (x 40), Distorted acini of moderately differentiated malignant cells; B) H&E (x 400), showing TIL (blue arrow). PTL (black arrow); C) MLH1, moderately positive nuclear staining of tumor cells (x 40), Inset (x400); D) MSH2, moderately positive nuclear staining of tumor cells (x 100); E) MSH6 (x 100), moderately positive nuclear staining of tumor cells (x 100); F) PMS 2(x100), moderately positive nuclear staining of tumor cells (x 100)
Figure 2.
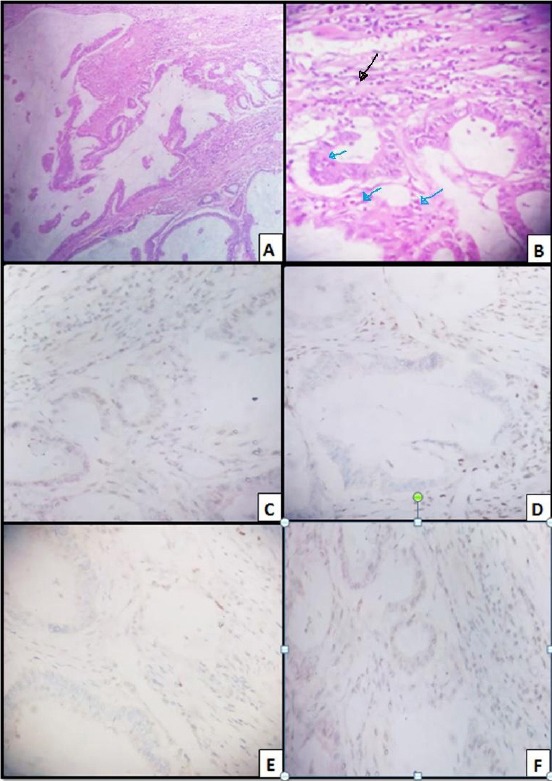
Colonic mucinous carcinoma; A) H&E (x 100), Pools of mucin entangling malignant glands; B) H&E (x 400), TIL (blue arrows); PTL (black arrow); C) MLH 1; Weakly positive nuclear staining of tumor cells (x 400), with positive lymphocytes, internal control; D) MSH2, Negative nuclear staining of tumor cells (x 400). with positive lymphocytes, internal control; E) MSH6, Negative nuclear staining of tumour cells (x 40); F) PMS 2, Weakly positive staining of tumour cells (x 400)
In the present study, expression of proteins was then grouped into 10 categories (A-J) Table (1 and 2): A) no loss of expression; B) loss of expression of all four proteins; C) combined loss of MLH1/PMS2; D) combined loss of MSH2/MSH6; E) combined loss of MSH6/PMS2; F) combined loss of MLH/MSH2 and isolated loss of any of the four proteins G: MLH1; H) MSH2; I) MSH6 and J) PMS2.
MSI-H is considered when two or more markers are demonstrated to be unstable (lost expression). MSI- low is considered when only one marker is unstable. MSS is the case when no markers are unstable. We follow Fujiyoshi et al., 2017 study definition of MSI that was approved for Bethesda [2], [22]. In Fujiyoshi et al., 2017 study, they include MSI-L as MSS. However, in our study, we constellate the results of expression of the 4 markers into 3 categories (MSS, MSI-L, and MSI-H).
Statistical Analysis
All statistical analysis is done with the SPSS version 20 software program. Categorical data obtained are statistically evaluated using the X2 test. Whereas the only continuous data in the study (age) is evaluated using Mean ± stander deviation. The tests are considered statistically significant when the P value less than 0 .05. All clinicopathological parameters of the studied cases are tested for association with the results of expression of the four markers and the status of MSI (Table 1, 2, 3, and 4). Then all candidate predictors with a P < 0.05 in univariate analysis are included in a multivariate logistic regression model in an attempt to discover the factors predicting CRC with MSI. In this study, we use the MSI-H model as a reference model (Table 5).
Table 1.
Expression pattern of MMR protein and clinicopathological characteristics of CRC
| MSS (38) |
MSI (77) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | P | |||
| n | 38 | 1 | 29 | 22 | 1 | 1 | 4 | 7 | 9 | 3 | |||
| Gender | M | n | 25 | 0 | 18 | 14 | 0 | 1 | 2 | 5 | 5 | 2 | 0.84 |
| % | 66 | 0 | 62 | 64 | 0 | 100 | 50 | 71 | 56 | 67 | |||
| F | n | 13 | 1 | 11 | 8 | 1 | 0 | 2 | 2 | 4 | 1 | ||
| % | 34 | 100 | 38 | 36 | 100 | .0 | 50 | 29 | 44 | 33 | |||
| Age | < 50 | n | 17 | 1 | 13 | 13 | 1 | 1 | 2 | 2 | 2 | 1 | 0.51 |
| % | 45 | 100 | 45 | 59 | 100 | 100 | 50 | 29 | 22 | 33 | |||
| < 50 | n | 21 | 0 | 16 | 9 | 0 | 0 | 2 | 5 | 7 | 2 | ||
| % | 55 | 0 | 55 | 41 | 0 | 0 | 50 | 71 | 78 | 67 | |||
| R | n | 16 | 1 | 22 | 18 | 1 | 0 | 3 | 3 | 8 | 1 | 0.014* | |
| % | 42 | 100 | 76 | 82 | 100 | 0 | 75 | 43 | 89 | 33 | |||
| L | L | n | 22 | 0 | 7 | 4 | 0 | 1 | 1 | 4 | 1 | 2 | |
| % | 57.9 | 0 | 24 | 18 | 0 | 100 | 25 | 57.1 | 11.1 | 66.7 | |||
| LVE | A | n | 30 | 1 | 19 | 10 | 1 | 0 | 1 | 4 | 9 | 2 | 0.035* |
| % | 79 | 100 | 66 | 46 | 100 | 0 | 25 | 57 | 100 | 67 | |||
| P | n | 8 | 0 | 10 | 12 | 0 | 1 | 3 | 3 | 0 | 1 | ||
| % | 21 | 0 | 34 | 54 | 0 | 100 | 75 | 43 | 0 | 33 | |||
| PNI | A | n | 34 | 1 | 29 | 17 | 1 | 0 | 4 | 7 | 9 | 3 | 0.012* |
| % | 89.5 | 100 | 100 | 77 | 100 | 0 | 100 | 100 | 100 | 100 | |||
| P | n | 4 | 0 | 0 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| % | 10.5 | 0 | 0 | 23 | 0 | 100 | 0 | 0 | 0 | 0 | |||
| G | G2 | n | 30 | 0 | 20 | 9 | 1 | 0 | 4 | 5 | 7 | 3 | 0.033* |
| % | 79 | 0 | 69 | 41 | 100 | 0 | 100 | 71 | 78 | 100 | |||
| G3 | n | 8 | 1 | 9 | 13 | 0 | 1 | 0 | 2 | 2 | 0 | ||
| % | 21 | 100 | 31 | 59 | 0 | 100 | 0 | 29 | 22 | 0 | |||
| T | T2 | n | 6 | 0 | 7 | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 0.013* |
| % | 16 | 0 | 24 | 14 | 100 | 0 | 0 | 43 | 0 | 0 | |||
| T3 | n | 31 | 1 | 20 | 17 | 0 | 0 | 4 | 4 | 9 | 3 | ||
| % | 82 | 100 | 69 | 77 | 0 | 0 | 100 | 57 | 100 | 100 | |||
| T4 | n | 1 | 0 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| % | 3 | 0 | 7 | 9 | 0 | 100 | 0 | 0 | 0 | 0 | |||
| N | N0 | n | 20 | 0 | 23 | 11 | 1 | 0 | 1 | 5 | 9 | 2 | 0.000* |
| % | 53 | 0 | 79 | 50 | 100 | 0 | 25 | 71 | 100 | 67 | |||
| N1 | n | 5 | 1 | 4 | 10 | 0 | 0 | 3 | 2 | 0 | 1 | ||
| % | 13 | 100 | 14 | 45 | 0 | 0 | 75 | 29 | 0 | 33 | |||
| N2 | n | 13 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | ||
| % | 34 | 0 | 7 | 4 | 0 | 100 | 0 | 0 | 0 | 0 | |||
| V | NOS | n | 31 | 1 | 21 | 10 | 1 | 0 | 4 | 5 | 7 | 3 | 0.3 |
| % | 81 | 100 | 72 | 45 | 100 | 0 | 100 | 72 | 78 | 100 | |||
| MUC | n | 6 | 0 | 8 | 10 | 0 | 1 | 0 | 1 | 2 | 0 | ||
| % | 16 | 0 | 28 | 46 | 0 | 100 | 0 | 14 | 22 | 0 | |||
| Sign. | n | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| % | 3 | 0 | 0 | 9 | 0 | 0 | 0 | 14 | 0 | 0 | |||
G: Gender; L: Laterality; LVE: Lymphovascular Emboli; PNI: perineural Invasion; T: T stage; N: Nodal stage; V: Histopathologic variant; NOS: Non otherwise specified; and MUC: Mucinous; sign: signet ring; A: no loss of expression of any marker; B: loss of expression of all markers; C: loss of expression of both MLH, and PMS2; D: loss of expression of both MSH2, and MSH6; E: loss of expression of both of MSH6, and PMS2; F: loss of expression of both of MLH, and MSH2; G: isolated loss of expression of MLH; H: isolated loss of expression of MSH2; I: isolated loss of expression of MSH6; J: isolated loss of expression of PMS2.
Table 2.
Expression pattern of MMR protein and histopathological characteristics of CRC
| A | B | C | D | E | F | G | H | I | J | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n. | 38 | 1 | 29 | 22 | 1 | 1 | 4 | 7 | 9 | 3 | |||
| Mucinous component | Absent | n | 26 | 1 | 13 | 7 | 0 | 0 | 2 | 5 | 4 | 2 | 0.353 |
| % | 68 | 100 | 45 | 32 | 0 | 0 | 50. | 72 | 44 | 67 | |||
| <10% | n | 3 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 2 | 0 | ||
| % | 8 | 0 | 10 | 5 | 0 | 0 | 25. | 0 | 22 | 0 | |||
| 10-50% | n | 3 | 0 | 6 | 4 | 1 | 0 | 1 | 1 | 1 | 1 | ||
| % | 8 | 0 | 21 | 18 | 100 | 0 | 25 | 14 | 11. | 33 | |||
| ≥50% | n | 6 | 0 | 7 | 10 | 0 | 1 | 0 | 1 | 2 | 0 | ||
| % | 16 | 0 | 24 | 50 | 0 | 100 | 0 | 14 | 22 | 0 | |||
| Signet ring component | A | n | 36 | 1 | 28 | 19 | 1 | 0 | 4 | 6 | 9 | 3 | 0.041* |
| % | 95 | 100 | 97 | 86 | 100 | 0 | 100 | 86 | 100 | 100 | |||
| P | n | 2 | 0 | 1 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | ||
| % | 5 | 0 | 3 | 14 | 0 | 100 | 0 | 14 | 0 | 0 | |||
| TIL | Absent | n | 27 | 0 | 15 | 8 | 1 | 1 | 0 | 3 | 2 | 1 | 0.012* |
| % | 71 | 0 | 52 | 36 | 100 | 100 | 0 | 43 | 22 | 33 | |||
| Mild | n | 11 | 1 | 14 | 14 | 0 | 0 | 4 | 3 | 7 | 2 | ||
| % | 29 | 100 | 48 | 64 | 0 | 0 | 100 | 43 | 78 | 67 | |||
| Marked | n | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| % | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | |||
| PTL | Absent | n | 38 | 0 | 26 | 18 | 1 | 1 | 4 | 5 | 9 | 2 | 0.009* |
| % | 100 | 0 | 90 | 82 | 100 | 100 | 100 | 71 | 100 | 67 | |||
| Present | n | 0 | 1 | 3 | 4 | 0 | 0 | 0 | 2 | 0 | 1 | ||
| % | 0 | 100 | 10 | 18 | 0 | 0 | 0 | 29 | 0 | 33 | |||
| Necrosis | Absent | n | 10 | 0 | 11 | 2 | 0 | 0 | 1 | 2 | 4 | 0 | 0.172 |
| % | 26 | 0 | 38 | 9 | 0 | 0 | 25 | 29 | 44 | 0 | |||
| Focal | n | 23 | 0 | 15 | 13 | 1 | 1 | 3 | 5 | 5 | 3 | ||
| % | 61 | 0 | 52 | 59 | 100 | 100 | 75 | 71 | 56 | 100 | |||
| Diffuse | n | 5 | 1 | 3 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| % | 13 | 100 | 10 | 32 | 0 | 0 | 0 | 0 | 0 | 0 | |||
TIL: tumour infiltrating lymphocyte; PTL: peritumoral lesion; A: no loss of expression of any marker; B: loss of expression of all markers; C: loss of expression of both MLH, and PMS2; D: loss of expression of both MSH2, and MSH6; E: loss of expression of both of MSH6, and PMS2; F: loss of expression of both of MLH, and MSH2; G: isolated loss of expression of MLH; H: isolated loss of expression of MSH2; I: isolated loss of expression of MSH6; J: isolated loss of expression of PMS2.
Table 3.
Clinicopathological characteristics about the MSI status of CRC
| MSS | MSI-L | MSI-H | P | |||
|---|---|---|---|---|---|---|
| n | 38 (33%) | 23 (20%) | 54 (47%) | 115 | ||
| Gender | M | n | 25 | 14 | 33 | 088 |
| % | 66 | 61 | 61 | |||
| F | n | 13 | 9 | 21 | ||
| % | 34 | 39 | 39 | |||
| Age | < 50 | n | 17 | 7 | 29 | 0.169 |
| % | 45 | 30 | 54 | |||
| < 50 | n | 21 | 16 | 25 | ||
| % | 55 | 70 | 46 | |||
| R | n | 16 | 15 | 42 | 0.002* | |
| % | 42 | 65 | 78 | |||
| L | L | n | 22 | 8 | 12 | |
| % | 58 | 35 | 22 | |||
| LVE | A | n | 30 | 16 | 31 | 0.092 |
| % | 79 | 70 | 57 | |||
| P | n | 8 | 7 | 23 | ||
| % | 21 | 30 | 43 | |||
| PNI | A | n | 34 | 23 | 48 | 0.253 |
| % | 90 | 100 | 99 | |||
| P | n | 4 | 0 | 6 | ||
| % | 10 | 0 | 11 | |||
| G | G2 | n | 30 | 19 | 30 | 0.016* |
| % | 79 | 83 | 56 | |||
| G3 | n | 8 | 4 | 24 | ||
| % | 21 | 17 | 44 | |||
| T | T2 | n | 6 | 3 | 11 | 0.324 |
| % | 16 | 13 | 21 | |||
| T3 | n | 31 | 20 | 38 | ||
| % | 81 | 87 | 70 | |||
| T4 | n | 1 | 0 | 5 | ||
| % | 3 | 0 | 9 | |||
| N | N0 | n | 20 | 17 | 35 | 0.001* |
| % | 53 | 74 | 65 | |||
| N1 | n | 5 | 6 | 15 | ||
| % | 13 | 26 | 28 | |||
| N2 | n | 13 | 0 | 4 | ||
| % | 34 | 0 | 7 | |||
| V | NOS | n | 31 | 19 | 33 | 0.146 |
| % | 81 | 83 | 61 | |||
| MUC | n | 6 | 3 | 19 | ||
| % | 16 | 13 | 35 | |||
| Signet | n | 1 | 1 | 2 | ||
| % | 3 | 4 | 4 | |||
G: Gender; L: Laterality; LVE: Lymphovascular Emboli; PNI: perineural Invasion; T: T stage; N: Nodal stage; V: Histopathologic variant; NOS: Non otherwise specified; and MUC: Mucinous.
Table 4.
Histopathological characteristics of the MSI status of CRC
| MSS | L MSI | H MSI | P | |||
|---|---|---|---|---|---|---|
| n. | 38 | 23 | 54 | 115 | ||
| Mucinous component | Absent | n | 26 | 13 | 21 | 0.089 |
| % | 68 | 57 | 40 | |||
| <10% | n | 3 | 3 | 4 | ||
| % | 8 | 13 | 7 | |||
| 10-50% | n | 3 | 4 | 11 | ||
| % | 8 | 17 | 20 | |||
| ≥50% | n | 6 | 3 | 18 | ||
| % | 16 | 13 | 33 | |||
| Signet ring component | A | n | 36 | 22 | 49 | 0.653 |
| % | 95 | 96 | 91 | |||
| P | n | 2 | 1 | 5 | ||
| % | 5 | 4 | 9 | |||
| TIL | Absent | n | 27 | 6 | 25 | 0.004* |
| % | 71 | 26 | 46 | |||
| Mild | n | 11 | 16 | 29 | ||
| % | 29 | 70 | 54 | |||
| Marked | n | 0 | 1 | 0 | ||
| % | 0 | 4 | 0 | |||
| PTL | Absent | n | 38 | 20 | 46 | 0.048* |
| % | 100 | 87 | 85 | |||
| Present | n | 0 | 3 | 8 | ||
| % | 0 | 13 | 15 | |||
| Necrosis | Absent | n | 10 | 7 | 13 | 0.23 |
| % | 26 | 30 | 24 | |||
| Focal | n | 23 | 16 | 30 | ||
| % | 61 | 70 | 56 | |||
| Diffuse | n | 5 | 0 | 11 | ||
| % | 13 | 0 | 20 | |||
TIL: tumour infiltrating lymphocyte; PTL: peritumoral lesion.
Table 5.
Multivariate analysis of factors predicting CRC with MSI-H Parameter Estimates
| MSIa | B | Std. Error | Sig. | 95% Confidence Interval for Exp (B) | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| MSS | Intercept | 1.541 | 390.22 | 0.997 | ||
| laterality | -1.466- | 0.526 | 0.005 | 0.082 | 0.646 | |
| grade | 1.279 | 0.594 | 0.031 | 1.121 | 11.516 | |
| N stage | -1.892- | 0.847 | 0.025 | 0.029 | 0.793 | |
| TIL | -13.860- | 0.527 | 0.000 | 3.404E-007 | 2.686E-006 | |
| MSI-L | Intercept | 3.910 | 2550.3 | 0.999 | ||
| grade | 1.551 | 0.701 | 0.027 | 1.194 | 18.625 | |
a: the reference category is MSI-H; TIL: tumour infiltrating lymphocyte; N: nodal stage.
Results
A group of 115 cases of CRC are included in this study: 72 male and 43 females. The mean age of the patients was 50.88 ± 14.14 years, with an age range of 15-82 years. 72.2% of the cases (n = 83) are adenocarcinoma (NOS) Figure 1A, 24.3% of the cases (n = 28) are mucinous carcinoma Figure 2A, and 3.5% of the cases (n= 4) are signet ring carcinoma. All the assessed clinicopathological parameters categories are illustrated and correlated with the pattern of expression of the 4 markers of MSI (A-J) (Table 1, and 2) and the status of MSI (MSS, MSI-L, and MSI-H) (Table 3, and 4). The significance of the clinicopathological parameters as predictors of MSI-H is illustrated in Table 5.
Expression pattern of MMR protein, clinicopathological and histopathological characteristics of CRC (Table 1, and 2)
A total of 72 males and 43 females were enlisted in the study. Age of the cases was subgrouped into < 50 (53 cases) and ≥ 50 years (62 cases). Eighty-three cases were of adenocarcinoma NOS, 28 cases were of the mucinous type, and 4 cases were of signet ring type. The clinicopathological characteristics of each expression pattern of MMR proteins are included in Table 1.
A (No loss of expression of any MMR proteins): (38 cases) (Figure 1: C, D, E, F)
Around 66% of the cases of this pattern are made with 55% of them ≥ 50 years old, and 58% left-sided. Most of the cases of this pattern reveal no lymphovascular emboli (LVE) (79%), no perineural invasion (PNI) (90%), grade 2 (79%), stage T3 (82%), stage N0 (53%), and of adenocarcinoma NOS (81%). Most of the cases of this pattern do not have a mucinous component (68%), or signet ring component (95%), or TIL (71%), or PTL (100%) and show focal necrosis in 61% of the cases.
B (loss of expression of all MMR proteins)
Only one female case, 30 years old, has right-sided cancer colon. It is of adenocarcinoma NOS G3, stage T3N1, and doesn’t have LVE, or PNI, mucinous or signet ring component. It shows mild TIL, PTL, and diffuse necrosis.
C (combined loss of expression of MLH and PMS2 proteins): (29 cases)
Around 62% of the cases of this pattern are male. 55% of them are ≥ 50 years old. 76% is the right side. Most of the cases of this pattern do not have LVE (66%), or PNI (100%). They are of grade 2 (69%), stage T3 (69%), and stage N0 (97%). Most of them are either of adenocarcinoma NOS or mucinous carcinoma (72%, 28% respectively). Some of the cases in this pattern are associated with absence of the mucinous component, or mucinous component ≥ 50% (45%, 21% respectively), absence of signet ring component (95%), TIL (absent, mild) (52%, 48% respectively), and absence of PTL (90%) and show focal necrosis in 52% of the cases.
D (combined loss of expression of MSH2 and MSH6 proteins): (22 cases) (Figure 2: D, and E)
Around 64% of the cases of this pattern are male. 59% of them are < 50 years old. 82% is the right side. Most of the cases of this pattern reveal the presence of LVE (54%) and absence of PNI (77%). Most of them are grade 3 (59%), stage T3 (77%), and stage N0 (50%). They are of adenocarcinoma NOS, mucinous carcinoma, and signet ring carcinoma (45%, 46%, and 9% respectively). This pattern in most of the cases is associated with the presence of mucinous component ≥ 50% in 50% of the cases in this pattern. There is no signet ring component in 86% of the cases of this pattern. TIL (absent, mild) is present in 36%, 64% of this pattern, respectively (Figure 1B, and 2B). Absence of PTL is evidenced in 82% of the cases in this pattern. It shows focal necrosis in 59% of the cases.
E (combined loss of expression of MSH6and PMS2 proteins)
Only one female case, 35-years-old, has right-sided CRC. It is of adenocarcinoma G2, stage T2N0, and has LVE, PNI, mucous component 20%, signet ring component, and focal necrosis. It doesn’t show TIL or PTL.
F (combined loss of expression of MLH and MSH2 proteins)
Only one male case, 24years old, has left-sided CRC. It is of mucinous carcinoma G3, stage T4N2b, and has LVE, PNI, mucous component 60%, signet ring component, and focal necrosis. It doesn’t show TIL or PTL.
G (Isolated loss of expression of MLH1): (4 cases)
In 4 cases, 2 of them are male, 2 of them are < 50 years old. 3/4 cases are left sided. Most of the cases of this pattern reveal LVE (75%), no PNI (100%), grade 2 (100%), stage T3 (100%), stage N1 (75%), and of adenocarcinoma NOS (100%). This pattern in most of the cases have mucinous component <50%, no mucinous component, (50%, 50% respectively), no signet ring component (100%), or TIL (mild) (100%), or no PTL (100%) and show focal necrosis in 75% of the cases.
H (Isolated loss of expression of MSH2): (7 cases)
In 7 cases, 5 of them are male, 5 of them are ≥ 50 years old. 4 cases are left sided. Most of the cases of this pattern reveal no LVE (57%), no PNI (100%). They are of grade 2 (71%), stage T3 (57%), and stage N0 (71%), and adenocarcinoma NOS (72%). Most of the cases in this pattern are associated with the absence of mucinous component (72%), absence of signet ring component (86%), TIL (absent, mild) (43% each), absence of PTL (71%) and show focal necrosis in 71% of the cases.
I (Isolated loss of expression of MSH6): (9 cases)
In 9 cases, 5 of them are male, 7 of them are ≥ 50 years old, 8 cases are right sided. Most of the cases of this pattern reveal no LVE (100%), no PNI (100%), grade 2 (78%), stage T3 (100%), stage N0 (100%), and of adenocarcinoma NOS (78%). This pattern in most of the cases is associated with no mucinous component, mucinous component ≥ 50%, mucinous component < 10%), (44%, 22%, 22% respectively), no signet ring component (100%), TIL (mild) (78%), and no PTL (100%). It shows focal necrosis in 56% of the cases.
J (Isolated loss of expression of PMS2): (3 cases)
In 3 cases, 2 of them are male. 2 of them are ≥ 50 years old. 2cases are left sided. Most of the cases of this pattern reveal no LVE (67%), no PNI (100%). They are of G2 adenocarcinoma (NOS) stage T3 (100%), and stage N0 (67%). This pattern in most of the cases is associated with the absence of mucinous component (67%), absence of signet ring component (100%), mild TIL (67%), and absence of PTL (67%). It shows focal necrosis in 100% of the cases. In the present study, there is significant association of expression pattern of MMR proteins with the laterality, the presence of lymphovascular emboli, the presence of perineural invasion, grade, T stage, N stage, the presence of signet ring component, and the extent of tumour-infiltrating lymphocyte, the presence of peritumoral lesion (0.014, 0.035, 0.012, 0.033, 0.013, 0.000, 0.041, 0.012, and 0.009 respectively). No significant association of expression pattern of MMR proteins with gender, age category, histopathological type, the presence and amount of mucinous component, or necrosis (0.84, 0.51, 0.3, 0.35, 0.17 respectively).
Clinicopathological and histopathologic characteristics about the MSI status of CRC (Table 3, and 4)
MSI status of the CRC in our study is subdivided into 3 groups (MSS (38 cases), MSI-L (23 cases), and MSI-H (54 cases). Table 3 and 4 illustrate the clinicopathological and histopathological characteristics of each category of MSI.
MSS (microsatellite stable CRC)
About 66% of the cases of this group are male. 55% of them are ≥ 50 years old, 58% are left sided. Most of the cases of this status are characterised by the absence of LVE (79%), and absence of PNI (90%). They are of grade 2 (79%), stage T3 (82%), and stage N0 (53%). 81% of the cases in this status are of adenocarcinoma NOS. Most of the cases in this status do not have either mucinous component (68%), or signet ring component (95%), nor TIL (71%), nor PTL (100%). It shows focal necrosis in 61% of the cases.
MSI-L (low microsatellite instability CRC)
About 61% of the cases of this group are male, 70% of them are ≥ 50 years old, 65% are right sided. Most of the cases of this status are characterised by the absence of LVE (70%), and absence of PNI (100%). They are of grade 2 (83%), stage T3 (87%), and stage N0 (74%). 83% of the cases in this status are of adenocarcinoma NOS. the mucinous component in this status ranges from 0, < 10%, 10-50%, and ≥ 50% in 57%, 13%, 17%, 13% of the cases respectively. It is characterised by the absence of signet ring component (96%), mild TIL (70%), and absence of PTL (87%). It shows focal necrosis in 70% of the cases.
MSI-H (high microsatellite instability CRC)
Around 61% of the cases of this group are male, 54% of them are < 50 years old, 78% are right sided. Most of the cases of this status are characterised by the absence of LVE (57%), and absence of PNI (89%). They are of grade 2, 3(56%, 44% respectively), stage T3 (70%), and stage N0 (65%). They are of adenocarcinoma NOS, mucinous, and signet ring carcinoma (61%, 35%, and 4% respectively). The mucinous component in this status ranges from 0, < 10%, 10-50%, and ≥ 50% in 40%, 7%, 33%, and 20% of the cases respectively). It is characterised by the absence of signet ring component (91%), mild TIL (54%), and absence of PTL (85%). It shows focal necrosis in 56% of the cases.
In the present study, there is a significant association of MSI status with the laterality, grade, N stage, the presence and grade of tumour-infiltrating lymphocyte, the presence of a peritumoral lesion (0.002, 0.016, 0.001, 0.004, and 0.048 respectively). No significant association of MSI status with gender, age category, the presence of lymphovascular emboli, the presence of perineural invasion, T stage, histopathological type, extent of mucinous component, the presence of signet ring component or necrosis (0.88, 0.16, 0.09, 0.25, 0.32, 0.14, 0.08, 0.65, and 0.23 respectively).
Multivariate analysis of factors predicting CRC with MSI-H (Table 5)
Multivariate logistic regression analysis was conducted including all of the above candidate predictors (laterality, grade, N stage, the extent of tumour-infiltrating lymphocyte, the presence of peritumoral lesion that is significant at univariate analysis). Proximal location (right sided) and lower grade, higher nodal stage, and marked TIL are selected as predictors of MS-H CRC when compared to the model of MSS based on a P < 0.05 (0.005, 0.031, 0.025, and 0.000 respectively). However, lower grade only is subsequently selected as predictors of MSI-H when compared to MSI-L based on a P < 0.05 (0.027). The final model of MSI-H predictors is shown in Table 5.
Discussion
The annual incidence of CRC worldwide raises a significant public health impact. Familial CRC represents 20% of all CRC. Lynch syndrome represents 3.5% of all CRC [23]. These figures, besides awareness of the role of genomic instability in initiation and progression in CRC, raise the need for molecular screening of all CRC for Lynch syndrome [14]. Hashmi et al., 2017 reported that PCR amplification of microsatellite repeats remain the gold standard for recognition of MSI phenotype, this approach is not feasible in routine pathology lab [9], [24]. Therefore Hampel 2018 study recommends that IHC is preferred a method to screen for LS as IHC is equally sensitive to PCR, inexpensive, more readily available, and predicts the nonworking gene, so it has a big role in limiting the number of the gene to be sequenced [24]. Hampel 2018 study recommendation confirms the previous conclusion of the national cancer institute workshop on microsatellite instability for cancer detection and Familial Predisposition [22], [24].
The presence of MSI defines a subset of colorectal carcinomas with special molecular aetiology and characteristic clinicopathological features inclusive of increased survival [9], [15]. Since MSI-H CRCs share some morphological features such as young patient age, right-sided location, mucinous and signet ring histology, and intratumoral lymphocytosis, careful observation of tumour histology help identifying these tumours [19]. Thomas, in his study, concluded that quantification of TILs might provide a simple, single criterion for choosing CRC patients as candidates for MSI testing [25].
Frequency of expression pattern of MMR proteins and MSI status of CRC (Table 1, 2, 3, and 4)
In our study, the frequency of MSI (low and high) is 67% of CRC cases of the study (Table 3). MSI-L account for 20% of CRC cases of the study. MSI-H account for 47% of CRC cases of the study. The frequency of loss of expression is found to be quite variable in different studies. Tumours with d MMR status accounted for 34% of the total study cases that was done on the Pakistani population [9]. 21% in Singapore population [26], 6.9% in Chinese population [27] and approximately 15% in western studies [28]. The higher frequency of MSI in cases of CRC of our study compared to the previous studies in the same issue is attributed to that the pathology lab that we retrieve the cases from it is the unique lab if not the only lab in investigation of the expression of MMR protein by immunohistochemistry at this time, so the cases, expected clinically to have MSI, are transferred to this lab for immunohistochemical investigation for MMR protein.
In our study, the expression pattern of the d MMR proteins has variable patterns. No loss of expression of MMR proteins (MSS) accounts for 33% of the study cases. However, MSI includes all other patterns of lost expression. Loss of expression of all MMR proteins (MLH, MSH2, MSH6, and PMS2) is present in one case of the study (0.12% of MSI). The combined loss of 2 MMR proteins includes 4 categories the most frequent in our study is combined loss of MLH, and PMS2 29/115 (38% of MSI) then combined loss of MSH2, and MSH6 22/115 (29% of MSI). The other 2 categories of the combined loss of 2 MMR proteins include only 2 cases (one for each category (MLH + MSH2); (MSH6 + PMS2)) each represents 0.12% of MSI cases of the study. These frequencies as regard combined loss of MMR proteins are in agreement to studies done on Pakistani and Shanghai population [9], [11]. Isolated loss of one MMR proteins (MSI-L) accounts for 23/115 (30% of MSI). Lost MLH expression is present in 4/115 (5% of MSI). Lost MSH2 expression is present in 7/115 (9% of MSI). Lost MSH6 expression is present in 9/115 (12% of MSI). Lost PMS2 expression is present in 3/115 (4% of MSI). This means that the most frequent isolated loss of one MMR proteins in our study is lost MSH6, followed by MSH2 then MLH1, and lastly PMS2.
These results are in agreement with Yuan et al., 2015 as regard MSH6 and PMS2 [11]. Yuan et al., 2015 reported and confirmed that MMR gene products existing in cells always stay as heterodimers complex. And MLH1 and MSH2 are obligatory partners, combined with their secondary partners PMS2 and MSH6 respectively. If the degradation of the former partners occurs, caused by the mutation of the respective MMR gene, the later partners will not exist anymore. But the reverse is not true [11]. This fact was applied by Hall et al., in his study and screened CRC using MSH6, PMS2 proteins only instead of 4 [29]. This means that it is unlogic to find the isolated loss of PMS2 alone without loss of MLH1. However, 4% of MSI in our study, and 9.6% of Kumar et al., 2018 study showed isolated loss of PMS2 without loss of the MLH1 [30]. Hashmi et al., 2017 study found an isolated loss of MLH1 in 5% of study cases which agree with our result as regard isolated loss of MLH1 (5% of MSI) [9]. This controversy between our results and Yuan et al., 2015 can be explained on the basis that some pathogenic MLH1 missense mutations or MLH1 promotor hypermethylation functionally inactivates MLH1 protein and preserve its antigenicity leading to the occurrence of isolated loss of PMS2 [31], [32]. Hashmi et al., 2017 study, like our study, showed isolated loss of MLH1 (without its secondary partner) in 5% of the studied cases of CRC [9]. The frequent failure of antigenic retrieval of MLH1 protein is the logic cause for the lower frequency of isolated loss of MLH1 compared to other studies the problem that similarly faces Yuan et al., 2015 study [11].
Correlation of Expression pattern of MMR protein/MSI status with clinicopathological and histopathological characteristics of CRC
The biological explanations for the characteristic morphology of MSI-H colorectal cancer are not known [33]. However, the increased lymphocytic reaction in MSI-H colorectal cancer may be explained by stimulated immunogenicity associated with the generation of abnormal proteins transcribed from mutant genes [15], [33]. In our study, there is significant correlation of expression pattern of MMR proteins with the laterality, lymphovascular emboli, perineural invasion, grade, T stage, N stage, signet ring component, tumor infiltrating lymphocyte, and peritumoral lesion (0.014, 0.035, 0.012, 0.033, 0.013, 0.000, 0.041, 0.012, and 0.009 respectively) Table (1, 2). These mean that lost expression of MMR proteins (MSI-L, MSI-H) in our study commonly occurs in the proximal colon up to the splenic flexure, and significantly characterized by the presence of lymphovascular emboli, absence of perineural invasion, lower grade, higher T stage, lower N stage, the presence of signet ring component, mild to marked tumor infiltrating lymphocyte, and the presence of peritumoral lesion. While no loss of expression of any MMR proteins (MSS) commonly occurs in the distal colon and rectum, associated with the absence of lymphovascular emboli, presence of perineural invasion, lower T stage, higher N stage, absence of signet ring component, absence of tumour infiltrating lymphocyte, and peritumoral lesion.
These results are approving nearly all studies dealt with this issue [9], [11], [19], [27], [34], [35], [36]. But we are different from most of the studies as to regard the extent of mucinous component and the presence of signet ring component that show no significant correlation with MSI status of the tumour in our study (Table 4) [27], [34], [36]. However Hashmi et al., 2017 and Yuan et al., 2015 studies agree with our result in failure of detection of significant correlation of MSI status of CRC and the extent of mucinous and signet ring component of the tumour [9], [11]. This limited detection of the mucinous and signet ring component can be explained by examination of one slide per case so the exact amount of both components cannot be detected at the research level but if assessed in routine examination of the slides during the initial diagnosis of the mother, the father will be more accurate in prediction of MSI status of CRC. But it puts a big burden on the pathologists.
Multivariate analysis of factors predicting CRC with MSI-H (Table 5)
The current study succeeds in confirmation of that proximal colon CRC, and the presence of marked TIL, lower grade and higher nodal stage have a significant predictive value of MSI-H as compared to MSS CRC. While only lower grade has a significant predictive value of MSI-H when compared to MSI-L CRC. This result when MSI-H model is taken as a reference model.
There is a failure of confirmation of some clinicopathological and histopathological parameters as significant predictors of MSI-H that previously reported in the previous models of MSI-H predictors such as age, mucinous component, and signet ring component [33], [37], [38]. The reasons behind these controversies with our study are in different cut off value used for any of the clinicopathological parameters investigated or inclusion of additional parameters in these studies. For instance, Greenspan et al., 2009 considered any mucinous differentiation, no matter what the overall percentage rather than having at least 50% mucinous differentiation. While in our study, we consider the percentage of the mucinous component. Also, their models included histologic heterogeneity and the type of growth pattern that they are not included in our study [37]. Jenkins et al., 2007 had much younger patients, which probably enriched the number of HNPCC cases in their study [33]. Revised Bethesda Guidelines for Microsatellite Instability included the Presence of synchronous, metachronous colorectal or other HNPCC-associated tumours regardless of age [38]. The big obstacle of our study is the inability to investigate germline mutations of MMR genes for this large number of cases. However, our study cases are not selected according to their ages, so the effectiveness of the clinicopathological and histological parameters can be noticed accurately.
In conclusion, the prevalence of MSI in our study is relatively high in comparison to international literature. Although they add a big burden on the pathologists, all clinicopathological and histological parameters should be assessed in all CRC for the sake of predicting MSI. Since it is not applicable to test all cases of CRC for MSI, selected cases only (according to clinicopathological predictors) will be proceeded to IHC for MLH1, MSH2 only. Consequently, the optimal approach to MSI evaluation is the immunohistochemical (IHC) analysis of tumours, followed by germline MSH2/MLH1 testing. IHC is easily available and inexpensive as part of the routine services inthe department of pathology.
Acknowledgement
The authors gratefully acknowledge all staff member of Prof. Elia Anis Ishak Lab.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Gkekas I, Novotny JAN, Pecen L, Strigård K, Palmqvist R, Gunnarsson ULF. Microsatellite Instability as a Prognostic Factor in Stage II Colon Cancer Patients, a Meta-Analysis of Published Literature. Anticancer Res. 2017;37(12):6563–74. doi: 10.21873/anticanres.12113. [DOI] [PubMed] [Google Scholar]
- 2.Fujiyoshi K, Yamaguchi T, Kakuta M, Takahashi A, Arai Y, Yamada M, et al. Predictive model for high-frequency microsatellite instability in colorectal cancer patients over 50 years of age. Cancer Med. 2017;6(6):1255–63. doi: 10.1002/cam4.1088. https://doi.org/10.1002/cam4.1088 PMid:28544821 PMCid:PMC5463087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayar I, Akbas EM, Isik A, Gokce A, Peker K, Demirtas L, et al. Relationship among mismatch repair deficiency, CDX2 loss, p53 and E-cadherin in colon carcinoma and suitability of using a double panel of mismatch repair proteins by immunohistochemistry. Polish J Pathol. 2015;3(3):246–53. doi: 10.5114/pjp.2015.54958. https://doi.org/10.5114/pjp.2015.54958. [DOI] [PubMed] [Google Scholar]
- 4.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044–58. doi: 10.1053/j.gastro.2010.01.054. https://doi.org/10.1053/j.gastro.2010.01.054 PMid:20420945 PMCid:PMC3057468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.PDQ Cancer Genetics Editorial Board PCGE. PDQ Cancer Information Summaries. National Cancer Institute (US); 2002. Genetics of Colorectal Cancer (PDQ®):Health Professional Version [Internet] [Google Scholar]
- 6.Kumar V, Abbas AK, Aster JC, Perkins JA. Vinay Kumar, Abul K Abbas, Jon C Aster, Jon C PJ., editors. Robbins basic pathology [Internet] 10th ed. 2018. [cited 2018Dec d19]. p. 935. Available from: https://smtebooks.eu/book/7756/robbins-basic-pathology-10th-edition-pdf .
- 7.Fearon ER. Molecular genetics of colorectal cancer. Ann N Y Acad Sci. 1995;768:101–10. doi: 10.1111/j.1749-6632.1995.tb12114.x. https://doi.org/10.1111/j.1749-6632.1995.tb12114.x PMid:8526339. [DOI] [PubMed] [Google Scholar]
- 8.Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis:MSI-H versus MSI-L. Disease markers. 2004;20(4, 5):199–206. doi: 10.1155/2004/368680. https://doi.org/10.1155/2004/368680 PMid:15528785 PMCid:PMC3839332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashmi AA, Ali R, Hussain ZF, Faridi N, Khan EY, Bakar SMA, et al. Mismatch repair deficiency screening in colorectal carcinoma by a four-antibody immunohistochemical panel in Pakistani population and its correlation with histopathological parameters. World J Surg Oncol. 2017;15(1):4–11. doi: 10.1186/s12957-017-1158-8. https://doi.org/10.1186/s12957-017-1158-8 PMid:28651545 PMCid:PMC5485685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–9. doi: 10.1126/science.8484122. https://doi.org/10.1126/science.8484122 PMid:8484122. [DOI] [PubMed] [Google Scholar]
- 11.Yuan L, Chi Y, Chen W, Chen X, Wei P, Sheng W, et al. Immunohistochemistry and microsatellite instability analysis in molecular subtyping of colorectal carcinoma based on mismatch repair competency. Int J Clin Exp Med. 2015;8(11):20988–1000. [PMC free article] [PubMed] [Google Scholar]
- 12.Peltomäki P. DNA mismatch repair and cancer. Mutat Res. 2001;488(1):77–85. doi: 10.1016/s1383-5742(00)00058-2. https://doi.org/10.1016/S1383-5742(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 13.Matsubara N. Diagnostic application of hMLH1 methylation in hereditary non-polyposis colorectal cancer. Disease markers. 2004;20(4-5):277–82. doi: 10.1155/2004/371941. https://doi.org/10.1155/2004/371941 PMid:15528793 PMCid:PMC3839271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulogiannis G, Frayling IM, Arends MJ. DNA mismatch repair deficiency in sporadic colorectal cancer and Lynch syndrome. Histopathology. 2010;56(2):167–79. doi: 10.1111/j.1365-2559.2009.03392.x. https://doi.org/10.1111/j.1365-2559.2009.03392.x PMid:20102395. [DOI] [PubMed] [Google Scholar]
- 15.Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-Infiltrating Lymphocytes Are a Marker for Microsatellite Instability in Colorectal Carcinoma BACKGROUND. Cells with deficient DNA mismatch repair develop microsatellite. 2001:2417–22. https://doi.org/10.1002/1097-0142(20010615)91:12<2417::AID-CNCR1276>3.3.CO;2-L. [PubMed] [Google Scholar]
- 16.Sengupta SB, Yiu C-Y, Boulos PB, De Silva M, Sams VR, Delhanty JDA. Genetic instability in patients with metachronous colorectal cancers. Br J Surg. 1997;84(7):996–1000. doi: 10.1002/bjs.1800840725. https://doi.org/10.1002/bjs.1800840725 PMid:9240146. [DOI] [PubMed] [Google Scholar]
- 17.De Angelis P, Kravik KL, De Angelis PM. DNA damage signaling in response to 5-fluorouracil in three colorectal cancer cell lines with different mismatch repair and TP53 status. Int J Oncol. 2011;39(3):673–82. doi: 10.3892/ijo.2011.1080. https://doi.org/10.3892/ijo.2011.1080 PMid:21674128. [DOI] [PubMed] [Google Scholar]
- 18.Stupart DA, Goldberg PA, Baigrie RJ, Algar U, Ramesar R. Surgery for colonic cancer in HNPCC:total vs segmental colectomy. Color Dis. 2011;13(12):1395–9. doi: 10.1111/j.1463-1318.2010.02467.x. https://doi.org/10.1111/j.1463-1318.2010.02467.x PMid:20969713. [DOI] [PubMed] [Google Scholar]
- 19.Jass JR, Do KA, Simms LA, Iino H, Wynter C, Pillay SP, et al. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42(5):673–9. doi: 10.1136/gut.42.5.673. https://doi.org/10.1136/gut.42.5.673 PMid:9659163 PMCid:PMC1727100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theise ND, Nakashima O, Park YN, Nakanuma Y. WHO Classification of Tumours of the Digestive System. Lyon: IARC Press;International Agency for Research on Cancer; 2010. pp. 225–227. [Google Scholar]
- 21.APMG Mismatch Repair Genes Resource. J Natl Compr Canc Netw. 2011:1311–20. [Google Scholar]
- 22.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for Cancer Detection and Familial Predisposition:Development of International Criteria for the Determination of Microsatellite Instability in Colorectal Cancer. 1998 [PubMed] [Google Scholar]
- 23.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. https://doi.org/10.3322/caac.20073 PMid:20610543. [DOI] [PubMed] [Google Scholar]
- 24.Hampel H. Population Screening for Hereditary Colorectal Cancer. Surg Oncol Clin N Am. 2018;27(2):319–25. doi: 10.1016/j.soc.2017.11.006. https://doi.org/10.1016/j.soc.2017.11.006 PMid:29496092. [DOI] [PubMed] [Google Scholar]
- 25.Smyrk TC, Watson P, Kaul K, Lynch HT. Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer. 2001;91(12):2417–22. https://doi.org/10.1002/1097-0142(20010615)91:12<2417::AID-CNCR1276>3.0.CO;2-U. [PubMed] [Google Scholar]
- 26.Chew M-H, Koh P-K, Tan M, Lim K-H, Carol L, Tang C-L. Mismatch Repair Deficiency Screening via Immunohistochemical Staining in Young Asians with Colorectal Cancers. World J Surg. 2013;37(10):2468–75. doi: 10.1007/s00268-013-2134-2. https://doi.org/10.1007/s00268-013-2134-2 PMid:23887594. [DOI] [PubMed] [Google Scholar]
- 27.Zhi W, Ying J, Zhang Y, Li W, Zhao H, Lu N, et al. DNA Mismatch Repair Deficiency in Colorectal Adenocarcinoma and its Association with Clinicopathological Features. 2015 [Google Scholar]
- 28.Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E. Microsatellite instability as a marker of prognosis and response to therapy:A meta-analysis of colorectal cancer survival data. Eur J Cancer. 2010;46(15):2788–98. doi: 10.1016/j.ejca.2010.05.009. https://doi.org/10.1016/j.ejca.2010.05.009 PMid:20627535. [DOI] [PubMed] [Google Scholar]
- 29.Hall G, Clarkson A, Shi A, Langford E, Leung H, Eckstein RP, et al. Immunohistochemistry for PMS2 and MSH6 alone can replace a four antibody panel for mismatch repair deficiency screening in colorectal adenocarcinoma. Pathology. 2010;42(5):409–13. doi: 10.3109/00313025.2010.493871. https://doi.org/10.3109/00313025.2010.493871 PMid:20632815. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Jain M, Yadav A, Kumari N, Krishnani N. Pattern of mismatch repair protein loss and its clinicopathological correlation in colorectal cancer in North India. S Afr J Surg. 2018;56(1):25–9. https://doi.org/10.17159/2078-5151/2018/v56n1a2285 PMid:29638089. [PubMed] [Google Scholar]
- 31.Kato A, Sato N, Sugawara T, Takahashi K, Kito M, Makino K, et al. Isolated Loss of PMS2 Immunohistochemical Expression is Frequently Caused by Heterogenous MLH1 Promoter Hypermethylation in Lynch Syndrome Screening for Endometrial Cancer Patients. Am J Surg Pathol. 2016;40(6):770–6. doi: 10.1097/PAS.0000000000000606. https://doi.org/10.1097/PAS.0000000000000606 PMid:26848797 PMCid:PMC4885527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salahshor S, Koelble K, Rubio C, Lindblom A. Microsatellite Instability and hMLH1 and hMSH2 Expression Analysis in Familial and Sporadic Colorectal Cancer. Lab Investig. 2001;81(4):535–41. doi: 10.1038/labinvest.3780262. https://doi.org/10.1038/labinvest.3780262 PMid:11304573. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins MA, Hayashi S, O'Shea A-M, Burgart LJ, Smyrk TC, Shimizu D, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability:a population-based study. Gastroenterology. 2007;133(1):48–56. doi: 10.1053/j.gastro.2007.04.044. https://doi.org/10.1053/j.gastro.2007.04.044 PMid:17631130 PMCid:PMC2933045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon YS, Yu CS, Kim TW, Kim JH, Jang SJ, Cho DH, et al. Mismatch repair status in sporadic colorectal cancer:Immunohistochemistry and microsatellite instability analyses. J Gastroenterol Hepatol. 2011;26(12):1733–9. doi: 10.1111/j.1440-1746.2011.06784.x. https://doi.org/10.1111/j.1440-1746.2011.06784.x PMid:21615788. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145(1):148–56. [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158(2):527–35. doi: 10.1016/S0002-9440(10)63994-6. https://doi.org/10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenson JK, Huang S-C, Herron C, Moreno V, Bonner JD, Tomsho LP, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol. 2009;33(1):126–33. doi: 10.1097/PAS.0b013e31817ec2b1. https://doi.org/10.1097/PAS.0b013e31817ec2b1 PMid:18830122 PMCid:PMC3500028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Rüschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–8. doi: 10.1093/jnci/djh034. https://doi.org/10.1093/jnci/djh034 PMid:14970275 PMCid:PMC2933058. [DOI] [PMC free article] [PubMed] [Google Scholar]


