Abstract
BACKGROUND:
Minimizing the number of therapy failures and decreasing the diabetic complications can be achieved by the application of personalising diabetes therapy, based on patient`s genetics, however, currently, personalised Medicine (PM) in diabetes mellitus management is not extensively applied.
AIM:
To assess the knowledge, attitudes, and willingness of physicians in practising of PM in diabetes management.
METHODS:
A cross-sectional analytical study was implemented among 126 physicians from six different governmental hospitals and 12 primary care centres selected by the stratified random sampling technique in the Tabuk region of Saudi Arabia. A structured self-administered questionnaire was utilised for data collection. A simple scoring system (scale of 5 points) was utilised to assess knowledge and willingness. Likert scale was applied to evaluate the attitudes towards practising PM in DM management by the fixed choice response formats.
RESULTS:
The majority of the participants (97.62%) claimed not receiving any PM and/or genomic medicine training. Most of them (82.54%) expressed unsatisfactory knowledge concerning personalised DM, whereas the medium level of attitudes was reported among 57.14% of them and a good level of willingness had been observed among 76.98% of the physicians.
CONCLUSION:
Emphasizing on essential personalised DM management knowledge aspects should be given a considerable priority. Fortunately, positive attitudes and goodwill of physicians towards PM are encouraging and should be supported by policymakers.
Keywords: Personalized Medicine, Precision Medicine, Diabetes Miletus, Genomics, KAP Studies
Introduction
Diabetes mellitus (DM) is considered one of the biggest worldwide health problems of the 21st century as well as it is a considerable contributor to the morbidity and mortality all over the world caused by the non-communicable diseases. Currently, there are 415 million adults living with diabetes mellitus in different areas of the world, and this is expected to increase by more than 50% over the coming 20 years. In addition to that number, there are 318 million adults with impaired glucose tolerance, which at higher risk of developing diabetes in the future [1], [2]. Saudi Arabia has one of the highest diabetic prevalence (14.4%) country in the world [3]. Also, Saudi Arabia has one of the highest annual incidence rates of type 1 diabetes in children aged 0-14 years, with 31.4 new cases per 100,000 population [4]. Despite the availability of many effective oral therapies to treat diabetes, a considerable percentage of patients do not achieve the required glucose-control rate or may be subjected to adverse effects, from these drugs [5]. Therefore, it is suggested that the response of patients to anti-diabetic therapies is subjected to inter-individual variability, possibly due to genetic factors regarding drug absorption, distribution, and metabolism. Therefore, exploring genetic markers associated with drug reaction can assist physicians with the decisions of drug selection, dose modification, therapy duration, and escaping adverse drug effects [6].
Most of type 2 diabetic patients have polygenetic forms of the disease in which each gene locus shares only a little risk [7]. For example, the association of the E23K variant in KCNJ11 with an increased risk for secondary failure of sulfonylurea in type 2 DM patients explains how a gene can influence the response to a drug [8]. Also, patients with OCT1 polymorphisms (organic cation transporter 1), which is the main port of entry for metformin into hepatocytes and enterocytes have a decreased response to metformin [9]. Fortunately, nowadays, there are many treatment options for diabetes patients that can improve outcomes for many of them. However, there is still a need to define the appropriate treatment for each, since a good number of monotherapy treatments fail within three years and diabetes related-complication and death continue [10], [11]. Therefore, personalising diabetes therapy based on the genetics of patients can reduce the number of treatment failures and decreasing the diabetes-associated complications but, there is a limited implementation of personalised medicine (PM) in treatment of DM [12].
Personalised medicine (PM) involves identifying specific information about a specific patient and then prescribing a drug that is particular for that patient [13]. The National Academy of Sciences (NAS) defined PM as “the use of genomic, epigenomic, exposure and other data to define individual patterns of disease, potentially leading to better individual treatment” [14]. Its goal is to utilise specific information about the patient to specify the appropriate intervention [15]. The intervention could range from a common drug given to several patients to a drug specific for the patient’s genetic profile [16]. Evidence-based support exists for benefits of applying PM for diabetes management in patients with certain monogenic forms of diabetes, and it is expected that applying this strategy for the more common forms of DM will occur with expanding knowledge of polygenic determinants of diabetes [17].
The application of PM in the clinical practice is expected to occur. However, physicians need to be aware of the correct test at the correct time and interpret the test result in the right way and alter their prescription behaviour accordingly. However, we are at an early stage of its application, and a dramatic shift will be needed in the main fields of medical research for this new area of medicine to be fully implemented [18]. Also, numerous challenges in health research exits as there is a need for more understand of the diseases ’mechanisms and translate research results into practice [19].
The study aimed to assess knowledge, attitudes, and willingness of physicians to practice PM in diabetes mellitus management in Tabuk, Saudi Arabia.
Material and Methods
A cross-sectional analytical design has been adopted in this study, which has been conducted in six different governmental hospitals and 12 primary care centres in Tabuk province. The province has an area of 108,000 km² and a population of 791,535 in 2015 [20]; its capital is Tabuk city and composed of six sub-directorates (Tabuk city, Taimaa, Haqel, Duba, Alwajh and Umlujj).
All physicians specialised in internal medicine and/or its diabetic related sub-specialities, namely, endocrinology, paediatrics, community medicine and geriatrics, were included. Physicians who are not providing direct patient care, e.g. administrators and not practising clinical medicine or diabetic related sub-specialities were excluded.
The researchers have followed 3 steps to develop a standardised self-administered questionnaire: The first step was a literature survey of relevant PM studies to identify the pertinent variables and formulating a preliminary questionnaire by the researchers. The second step was taken the opinion of 4 family medicine, and 2 research methodology professors and consultants on the proposed questionnaire in which the nominal group technique has been utilised and then modifications and adjustments have been done. The third step was testing the validity and reliability of the approved questions by the studied experts. Therefore, the studied physicians were asked to respond to a structured 35-items self-administrated questionnaire, designed to evaluate the diabetes management practice variables as related to PM. The independent variables including age, gender, health facility, highest qualification, source of PM awareness, duration of work experience in years and training in PM and/or genomic medicine and dependent variables (knowledge, attitudes and willingness) were selected and revised by five medical research experts. Approximately 20 minutes were needed by the participants to complete it. Ten Knowledge questions with correct or incorrect responses were applied. Assessment of attitude has been based on the Likert scaling method [21] using 10 statements scored as follows: 3 for agreement, 2 for undecided and 1 for disagreement. The total score has been computed by summing the individual scores. Forced-choice response scale (yes or no) was applied to assess willingness degree.
Multi-stage random sampling technique was applied to allocate the physicians. At the first stage, three sub-directorates from Tabuk province (Tabuk city, Taimaa, and Haqel) have been randomly selected by simple random sampling technique. At the second stage, two governmental hospitals and four primary health care centres were selected by the stratified random sampling technique from each sub-directorate. At the third stage, physicians have been selected by systematic random sampling technique. Only, the eligible subjects have been recruited (126 physicians).
Pre-test study has been conducted throughout the first two months in the preparatory phase of the study to: formulate the research problem for more precise investigation, refine the study variables, and test the validity and reliability of the study tools and instruments.
Ethical approval for carrying out the study was obtained from the regional research committee of Tabuk region. Furthermore, all of the study participants were briefly informed that all their personal information would be kept confidential. Refusal to answer any question or withdraw from the study at any time was granted to all participants. They were informed that there was no “correct” or “incorrect” answer, and they were requested to express their opinions and beliefs freely. Their informed consents were taken.
Data entry and analysis were done using the Statistical Package for Social Sciences version 22 (SPSS Inc., Chicago, IL). Arithmetic means and standard deviations were applied to describe continuous data, whereas frequency and percentages were applied for categorical data. Chi-squared tests were utilised to assess the associations between categorical data and unpaired t-test to test for the significant difference between two continuous variables. A p-value of less than 0.05 was considered for statistical significance.
Results
The study included 148 physicians, with a response rate of 85.06%. Physicians who were not in direct contact with patients (n = 6) and/or not practising diabetic management (n = 16) were excluded. Thus, a total of 126 physicians participated in the study. Of the participants, 53.17% and 46.83% were males and females, respectively, as shown in Table (1) with average age and experience years of 37 ± 3.68 and 13 ± 6.42, respectively. Only 9.52% of them have obtained a doctorate or Saudi Board degrees, and surprisingly, 2.38% of them received PM and/or Genomic training. The main source of their PM knowledge (57.14%) was the internet.
Table 1.
General Characteristics of the Studied Physicians
| Character | Health Facility | Total (N = 126) | ||||
|---|---|---|---|---|---|---|
| Hospitals (N = 68) | Primary Health Care Centers (N = 58) | |||||
| No. | % | No. | % | No. | % | |
| Age (years): Mean± SD | 39.46 ± 4.54 | 34.12 ± 2.68 | 37.00 ± 3.68 | |||
| t = 8.17, P< 0.05 | ||||||
| Sex: | ||||||
| Males | 41 | 60.29 | 26 | 44.83 | 67 | 53.17 |
| Females | 27 | 39.71 | 32 | 55.17 | 59 | 46.83 |
| χ2 = 0.34, P>0.05 | ||||||
| Highest Qualification: | ||||||
| MBBS | 15 | 22.06 | 38 | 65.52 | 53 | 42.06 |
| Diploma | 14 | 20.59 | 5 | 8.62 | 19 | 15.08 |
| Master | 28 | 41.17 | 14 | 24.14 | 42 | 33.34 |
| Doctorate or Saudi Board | 11 | 16.18 | 1 | 1.72 | 12 | 9.52 |
| χ2= 26.62, P< 0.05 | ||||||
| Experience years: Mean ± SD | 16.16 ± 8.84 | 9.27 ± 3.57 | 13 ± 6.42 | |||
| t = 5.89, P > 0.05 | ||||||
| PM and/or Genomic Training: | ||||||
| Received | 3 | 4.41 | - | 0.00 | 3 | 2.38 |
| Did not receive | 65 | 95.59 | 58 | 100.00 | 123 | 97.62 |
| Source of PM Knowledge: | ||||||
| Internet | 39 | 57.35 | 33 | 56.90 | 72 | 57.14 |
| Text books | 10 | 14.71 | 12 | 20.69 | 22 | 17.46 |
| Medical journals | 11 | 16.18 | 4 | 6.89 | 15 | 11.91 |
| In-service training | 5 | 7.35 | 7 | 12.07 | 12 | 9.52 |
| Others | 3 | 4.41 | 2 | 3.45 | 5 | 3.97 |
| χ2= 0.45, P > 0.05 | ||||||
It’s obvious from Table 2 and Figure 1 that only 11.1% of participants have a good level of knowledge regarding important PM elements in DM management. Also, the fair and poor levels of knowledge among them were 6.4% and 82.5%, respectively.
Table 2.
Personalized DM Medical Knowledge Assessment of the Studied Physicians
| Personalised DM Medical Aspect | Studied Physicians’ Knowledge (N. 126) | |||
|---|---|---|---|---|
| Correct Answer | Incorrect Answer | |||
| No. | % | No. | % | |
| Definition | 7 | 5.56 | 119 | 94.44 |
| Rationale | 10 | 7.94 | 116 | 92.06 |
| Vision | 8 | 6.35 | 118 | 93.65 |
| Objectives | 9 | 7.14 | 117 | 92.86 |
| Components | 9 | 7.14 | 117 | 92.86 |
| Clinical Diagnostic Methods | 26 | 20.63 | 100 | 79.37 |
| Required laboratory investigations | 22 | 17.46 | 104 | 82.54 |
| Recent PM drugs | 16 | 12.70 | 110 | 87.30 |
| Most important defective genes | 7 | 5.56 | 119 | 94.44 |
| Recent Management Strategies | 12 | 9.52 | 114 | 90.48 |
| Overall Assessment: | No. | % | ||
| Good | 14 | 11.1 | ||
| Fair | 8 | 6.4 | ||
| Poor | 104 | 82.5 | ||
Figure 1.
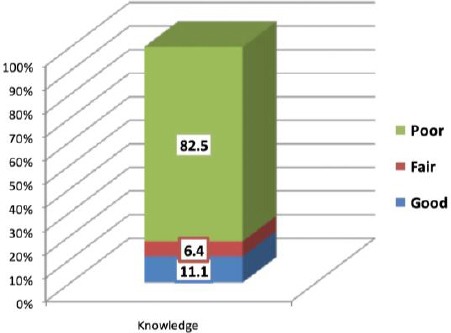
Overall Assessment of knowledge of Physicians Regarding Personalized Medicine in Diabetes Miletus Management
It’s clear from Table 3 and Figure 2 that nearly more than half (57.1%) of participants have positive attitudes towards important PM aspects in DM management.
Table 3.
Attitudes of the Studied Physicians towards Important Personalized DM Medical Aspects
| Personalised DM Medical Aspect | Studied Physicians’ Attitudes (N. 126) | |||||
|---|---|---|---|---|---|---|
| Favourable | Undecided | Unfavourable | ||||
| No. | % | No. | % | No. | % | |
| Importance in Medical Progress | 79 | 62.70 | 41 | 32.54 | 6 | 4.76 |
| Physicians’ Acceptance | 102 | 80.95 | 20 | 15.87 | 4 | 3.18 |
| Easy Patients’ Accessibility | 42 | 33.33 | 49 | 38.89 | 35 | 27.78 |
| Current Achievements | 48 | 38.10 | 50 | 39.68 | 28 | 22.22 |
| Ethical Considerations | 82 | 65.08 | 27 | 21.43 | 17 | 13.49 |
| Wide Spread Applicability | 38 | 30.15 | 51 | 40.48 | 37 | 29.37 |
| Medical Curricula Sufficiency | 9 | 7.14 | 36 | 28.57 | 81 | 64.29 |
| Needed Research | 100 | 79.37 | 15 | 11.90 | 11 | 8.73 |
| Current High Costs | 73 | 57.93 | 29 | 23.02 | 24 | 19.05 |
| Future Mapping of Medicine | 105 | 83.33 | 19 | 15.08 | 2 | 1.59 |
| Overall Attitudes Assessment: | No. | % | ||||
| Favourable | 72 | 57.1 | ||||
| Un-decided | 34 | 27.0 | ||||
| Un-favorable | 20 | 15.9 | ||||
Figure 2.
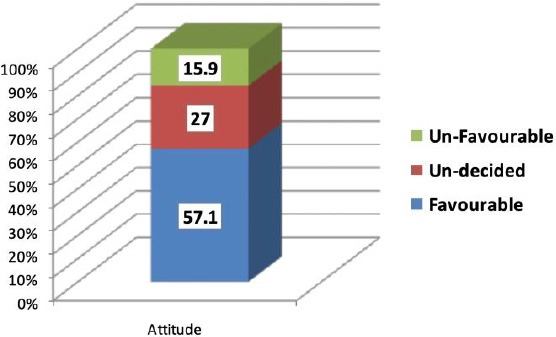
Overall Assessment of Attitudes of Physicians towards Personalized Medicine in Diabetes Miletus Management
Also, one may notice that 64.29%, 29.37%, 27.78%, 22.22%, and 19.05% of them have an unfavourable attitude towards medical curricula sufficiency, widespread applicability, easy patients’ accessibility, current achievements and current high costs of practising PM in DM management, respectively.
Finally, the results indicate that 77% of participants have a good willingness degree regarding PM practising in DM management, with 90.48%, 82.54%, and 80.95% of them reporting that they agree to practice PM in DM management, looking for training on personalized diabetic management, and intersected in personalized diabetic management practicing, respectively, Table 4 and Figure 3.
Table 4.
Willingness Assessment of the Studied Physicians Regarding Personalized DM Medical Practice
| Personalised DM Medical Aspect | Studied Physicians’ Willingness (N. 126) | |||
|---|---|---|---|---|
| YES | NO | |||
| No. | % | No. | % | |
| Intersected in PM Practicing | 102 | 80.95 | 24 | 19.05 |
| Agree to Practice PM | 114 | 90.48 | 12 | 9.52 |
| Ready to Learn PM Practicing Principles | 92 | 73.02 | 34 | 26.98 |
| Keen to have PM Practicing Degree | 90 | 71.43 | 36 | 28.57 |
| Looking for training on PM | 104 | 82.54 | 22 | 17.46 |
| Will search for recent PM topics-as needed | 87 | 69.05 | 39 | 30.95 |
| Disseminate PM materials on colleagues | 71 | 56.35 | 55 | 43.65 |
| Try to organize PM workshop(s) | 48 | 38.10 | 78 | 61.90 |
| Try to attend PM conference(s) | 62 | 49.21 | 64 | 50.79 |
| Eager to Practice PM | 97 | 76.98 | 29 | 23.02 |
| Overall Enthusiasms’ Assessment: | No. | % | ||
| Good | 97 | 77.0 | ||
| Fair | 22 | 17.4 | ||
| Poor | 7 | 5.6 | ||
Figure 3.
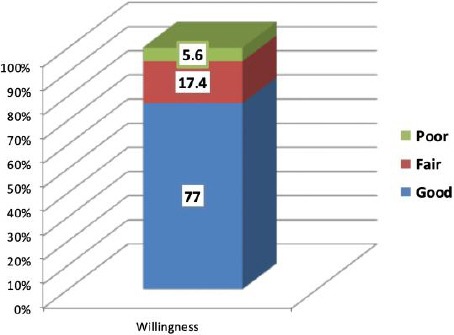
Overall Assessment of Physicians’ Willingness to Practice Personalized Medicine in Diabetes Miletus Management
Discussion
Because of the multifactorial and complex nature of diabetes mellitus, variability has been reported in the response of patients to most currently available oral hypoglycemics. Therefore, different guidelines recommended using a patient-specific approach and choosing management strategies that provide the patient with the most benefit and the least harm [22].
Despite the existence of several studies investigating diabetes, there is a lack of evidence regarding knowledge, attitudes and practices of physicians and their influence on diabetes and its risk in the community [23].
The World Health Organization (WHO) in 2016 recommended that countries with high diabetic prevalence, as the case in Saudi Arabia have to fight the rise in diabetes prevalence, to minimise diabetes-related premature deaths and to improve access to main diabetes therapies and basic technologies. Such commitment should address the key gaps in the diabetes knowledge base and conduct evaluation studies of innovative programs intended to change attitudes and behaviour [24]. Therefore this study was conducted to explore the knowledge, the extent of positive attitudes, and willingness of physicians to practice PM in diabetes management to provide sound and practical data for diabetic decision makers’ program development.
Personalised treatment of diabetes based on patient genomic can reduce the number of therapy failures and reducing the rate of diabetic-related complications and consequently improve the patients` care [12]. However, in the present study, only 2.38% of surveyed physicians received PM and/or Genomic training.
With many advances in PM on the horizon, the researcher has expected that the PM knowledge of the studied physicians would be satisfactory and increased exponentially. However, the current results showed a considerable very low gap in physicians’ knowledge regarding the basic principles of personalised medicine as only 11.1% of physicians had a good knowledge regarding important personalised diabetic management elements. Also, insufficient knowledge was observed in another study carried among oncologists [25]. However, a better level of knowledge was reported in another study conducted by Chow-White P et al. among oncologists [26].
The practice of PM for diabetes (PMFD) includes 4 processes [27]. First, is the specification of genes and biomarkers for diabetes; Second, is the allocation of resources to early detect or prevent diabetes; Third, is the selection of personalised treatments for affected patients; Fourth, is the measurement of circulating biomarkers of diabetes to evaluate the response to prevention and/or therapy. It is important to notice that the detection of efficiency in anti-diabetic drug development is possible if genetically [28] or nutritionally [29] drug determinants are identified in patients with diabetes. Also, patients who are at high risk for diabetes, as evidenced by genetic testing can be subjected to preventive measures, such as alteration in lifestyle or therapies, to prevent or at least delay the diabetes development [30]. Thus, PM allows prescribing personalised drugs with less time lost with an inappropriate response or with side effects [31]. Unfortunately, in the present study, definition, rationale, vision, objectives, components, recent drugs, most important defective genes and recent management strategies were identified correctly by a minority of physicians.
Despite the lack of knowledge about personalised diabetic management reported among physicians in the present study, they expressed a favourable attitude towards important personalised diabetic management aspects as most of them had a favourable attitude towards physician`s acceptance. However, a considerable proportion of them had a favourable concern regarding high cost and ethical consideration. The results also indicated good willingness degree among physicians regarding personalised diabetic management practising, with the majority of them reported that they agree to practice personalised diabetic management, looking for training on personalised diabetic management, and intersected in personalised diabetic management practising. Recent studies also reported that physicians in the USA perceived their lack the knowledge and skills to incorporate genomic medicine in their practices; despite their positive attitude towards it [32], [33].
The present study has some important limitations. The cross-sectional design was merely descriptive without investigating the factors that could be associated with physicians` knowledge and willingness. Due to the limitations of international studies in that area, we could not sufficiently compare our findings with others. Also, the results were based on a self-administered structured questionnaire (quick, easy, participants answer at their convenience, and cost-efficient but may lack conscientious responses, differences in understanding and interpretation by participants and lack of personalisation). Therefore, carrying out the study only in Tabuk city could impact the generalizability of results. However, the transferability model of generalizability [34] can be adopted and applied in the Tabuk region and the northern parts of Saudi Arabia, given the proximal similarity of such geographical and demographic parts. Finally, we have to realise that both individual- and system-level factors likely contribute to differences by race and ethnicity in use and responses to PM practice as stressed by Kaphingst, and Goodman (2016) [35]. Thus, given the demographic and predominant consanguineous marriage pattern in KSA, it is of great importance to conduct national genomic studies and investigations to determine the needed PM areas for the Saudi population properly.
In conclusion, a high priority should be given to continuing medical education for physicians regarding essential personalised diabetic management knowledge. Additionally, discovering the positive attitudes and favourable willingness of physicians towards the application of PM in patient’s care are promising and should be encouraged by decision makers.
Acknowledgement
The authors would like to thank Dr Tahani Khalil Family Medicine Residency Joint Program director, King Salman Military hospital, Tabuk for her tremendous support in making this project a success.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.International Diabetes Federation (IDF), IDF DIABETES ATLAS Seventh edition, 2015. Available from: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/13-diabetes-atlas-seventh-edition.html .
- 2.Murray CJL, Lopez AD. Measuring the Global Burden of Disease. New England Journal of Medicine. 2013;369(5):448–457. doi: 10.1056/NEJMra1201534. https://doi.org/10.1056/NEJMra1201534 PMid:23902484. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, Diabetes Country Profiles, Saudi Arabia, 2016. Available from: https://www.who.int/diabetes/country-profiles/sau_en.pdf .
- 4.Diabetes UK, List of countries by incidence of Type 1 diabetes ages 0 to 14. Available from: https://www.diabetes.org.uk/About_us/News_Landing_Page/UK-has-worlds-5th-highest-rate-of-Type-1-diabetes-in-children/List-of-countries-by-incidence-of-Type-1-diabetes-ages-0-to-14/
- 5.Pacanowski MA, Hopley CW, Aquilante CL. Interindividual variability in oral antidiabetic drug disposition and response:the role of drug transporter polymorphisms. Expert Opinion on Drug Metabolism &Toxicology. 2008;4(5):529–544. doi: 10.1517/17425255.4.5.529. https://doi.org/10.1517/17425255.4.5.529 PMid:18484913. [DOI] [PubMed] [Google Scholar]
- 6.Hu C. Pharmacogenomics in type 2 diabetes management:towards personalized medicine. J Transl Med. 2012;10(Suppl 2):A19. https://doi.org/10.1186/1479-5876-10-S2-A19 PMCid:PMC3479974. [Google Scholar]
- 7.O'Rahilly S, Barroso I, Wareham NJ. Genetic Factors in Type 2 Diabetes:The End of the Beginning? Science. 2005;307(5708):370–373. doi: 10.1126/science.1104346. https://doi.org/10.1126/science.1104346 PMid:15662000. [DOI] [PubMed] [Google Scholar]
- 8.Sesti G, Laratta E, Cardellini M, Andreozzi F, Del Guerra S, Irace C, Gnasso A, Grupillo M, Lauro R, Hribal ML, Perticone F, Marchetti P. The E23K Variant of KCNJ11 Encoding the Pancreatic β-Cell Adenosine 5′-Triphosphate-Sensitive Potassium Channel Subunit Kir6.2 Is Associated with an Increased Risk of Secondary Failure to Sulfonylurea in Patients with Type 2 Diabetes. J Clin Endocrinol Metab. 2006;91(6):2334–9. doi: 10.1210/jc.2005-2323. https://doi.org/10.1210/jc.2005-2323 PMid:16595597. [DOI] [PubMed] [Google Scholar]
- 9.Shu Y, Sheardown SA, Brown C, Owen RP, Zhang S, Castro RA, Ianculescu AG, Yue L, Lo JC, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007;117(5):1422–1431. doi: 10.1172/JCI30558. https://doi.org/10.1172/JCI30558 PMid:17476361 PMCid:PMC1857259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, Sulfonylurea, Metformin, or Insulin in patients with type 2 Diabetes Mellitus progressive pequirement for multiple therapies (UKPDS 49) JAMA. 1999;281(21):2005–2012. doi: 10.1001/jama.281.21.2005. https://doi.org/10.1001/jama.281.21.2005 PMid:10359389. [DOI] [PubMed] [Google Scholar]
- 11.Riedel AA, Heien H, Wogen J, Plauschinat CA. Loss of glycemic control in patients with type 2 Diabetes Mellitus who were receiving initial Metformin, Sulfonylurea, or Thiazolidinedione monotherapy. Pharmacotherapy. 2007;27(8):1102–10. doi: 10.1592/phco.27.8.1102. https://doi.org/10.1592/phco.27.8.1102 PMid:17655510. [DOI] [PubMed] [Google Scholar]
- 12.Kleinberger JW, Pollin TI. Personalized medicine in diabetes mellitus:current opportunities and future prospects. Ann N Y Acad Sci. 2015;1346(1):45–56. doi: 10.1111/nyas.12757. https://doi.org/10.1111/nyas.12757 PMid:25907167 PMCid:PMC4480162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodcock J. The prospects for “Personalized Medicine”in drug development and drug therapy. Clinical Pharmacology &Therapeutics. 2007;81(2):164–169. doi: 10.1038/sj.clpt.6100063. https://doi.org/10.1038/sj.clpt.6100063 PMid:17259943. [DOI] [PubMed] [Google Scholar]
- 14.Abrahams E, Silver M. The History of Personalized Medicine. In: E Gordon, S Koslow., editors. Integrative Neuroscience and Personalized Medicine. New York, NY: Oxford University Press; 2010. [Accessed April 30, 2018]. pp. 3–16. Available from: https://www.oxfordscholarship.com/view/10.1093/acprof:oso/9780195393804.001.0001/acprof-9780195393804-chapter-001 . https://doi.org/10.1093/acprof:oso/9780195393804.003.0001. [Google Scholar]
- 15.Brown PM. Personalized medicine and comparative effectiveness research in an era of fixed budgets. EPMA J. 2010;1(4):633–640. doi: 10.1007/s13167-010-0058-6. https://doi.org/10.1007/s13167-010-0058-6 PMid:23199118 PMCid:PMC3405343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McWilliam A, Lutter R, Nardinelli C. Healthcare impact of personalized medicine using genetic testing:an exploratory analysis for warfarin. Personalized Medicine. 2008;5(3):279–284. doi: 10.2217/17410541.5.3.279. https://doi.org/10.2217/17410541.5.3.279 PMid:29783488. [DOI] [PubMed] [Google Scholar]
- 17.Malandrino N, Smith RJ. Personalized Medicine in Diabetes. Clinical Chemistry. 2011;57(2):231–240. doi: 10.1373/clinchem.2010.156901. https://doi.org/10.1373/clinchem.2010.156901 PMid:21127150. [DOI] [PubMed] [Google Scholar]
- 18.European Commission, Health Research Directorate. Personalised Medicine-Opportunities and Challenges for European HealthCare. Workshop report. 2010. Available from: https://ec.europa.eu/research/health/pdf/13th-european-health-forum-workshop-report_en.pdf .
- 19.Zhang XD. Precision Medicine, Personalized Medicine, Omics and Big Data:Concepts and Relationships. J Pharmacogenomics Pharmacoproteomics. 2015;6(1):1000e144. https://doi.org/10.4172/2153-0645.1000e144. [Google Scholar]
- 20.General Statistics Authority, Kingdom of Saudi Arabia. Population Characteristics Survey, Saudi Arabia (in Arabic) 2018. [Accessed March 22, 2018]. Available from: https://www.stats.gov.sa/sites/default/files/population_characteristics_surveysar.pdf .
- 21.Wuensch KL. What is a likert scale? and how do you pronounce'likert? 'East Carolina University. 2005;4 [Google Scholar]
- 22.Elk N, Iwuchukwu OF. Using Personalized Medicine in the Management of Diabetes Mellitus. Pharmacotherapy:The Journal of Human Pharmacology and Drug Therapy. 2017;37(9):1131–1149. doi: 10.1002/phar.1976. https://doi.org/10.1002/phar.1976 PMid:28654165. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti R, Finger RP, Lamoureux E, Islam MT, Dirani MD, Bhuiyan A, Islam SZ, Wahab MA, Islam FMA. Rationale and methodology for a population-based study of diabetes and common eye diseases in a rural area in Bangladesh:Bangladesh Population based Diabetes and Eye Study (BPDES) Bangladesh Journal of Medical Science. 2015;14(4):367–375. https://doi.org/10.3329/bjms.v14i4.25767. [Google Scholar]
- 24.World Health Organization. Global report on diabetes. 2016:8–11. [Google Scholar]
- 25.Ciardiello F, Adams R, Tabernero J, Seufferlein T, Taieb J, Moiseyenko V, Ma B, Lopez G, Vansteenkiste JF, Esser R, Tejpar S. Awareness, understanding, and adoption of precision medicine to deliver personalized treatment for patients with cancer:A multinational survey comparison of physicians and patients. Oncologist. 2016;21(3):292–300. doi: 10.1634/theoncologist.2015-0279. https://doi.org/10.1634/theoncologist.2015-0279 PMid:26888693;PMCid:PMC4786350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow-White P, Ha D, Laskin J. Knowledge, attitudes, and values among physicians working with clinical genomics:a survey of medical oncologists. Hum Resour Health. 2017;27(15(1)):42. doi: 10.1186/s12960-017-0218-z. https://doi.org/10.1186/s12960-017-0218-z PMid:28655303 PMCid:PMC5488429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klonoff DC. Personalized medicine for diabetes. Journal of Diabetes Science and Technology. 2008;2(3):335–341. doi: 10.1177/193229680800200301. https://doi.org/10.1177/193229680800200301 PMid:19885196 PMCid:PMC2769744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman EP. Skipping toward Personalized molecular medicine. New England Journal of Medicine. 2007;357(26):2719–2722. doi: 10.1056/NEJMe0707795. https://doi.org/10.1056/NEJMe0707795 PMid:18160693. [DOI] [PubMed] [Google Scholar]
- 29.Sho-ichi Y, Seiji U, Seiya O. Food-derived advanced glycation end products (AGEs):A novel therapeutic target for various disorders. Current Pharmaceutical Design. 2007;13(27):2832–2836. doi: 10.2174/138161207781757051. https://doi.org/10.2174/138161207781757051. [DOI] [PubMed] [Google Scholar]
- 30.Collins CD, Purohit S, Podolsky RH, Zhao HS, Schatz D, Eckenrode SE, Yang P, Hopkins D, Muir A, Hoffman M, McIndoe RA, Rewers M, She JX. The application of genomic and proteomic technologies in predictive, preventive and personalized medicine. Vascular Pharmacology. 2006;45(5):258–267. doi: 10.1016/j.vph.2006.08.003. https://doi.org/10.1016/j.vph.2006.08.003 PMid:17030152. [DOI] [PubMed] [Google Scholar]
- 31.Ruano G, Goethe JW, Caley C, et al. Physiogenomic comparison of weight profiles of olanzapine- and risperidone-treated patients. Mol Psychiatry. 2007;12(5):474–482. doi: 10.1038/sj.mp.4001944. https://doi.org/10.1038/sj.mp.4001944 PMid:17199131. [DOI] [PubMed] [Google Scholar]
- 32.Denus Sd, Letarte N, Hurlimann T, Lambert JP, Lavoie A, Robb L, Sheehan NL, Turgeon J, Vadnais B. An evaluation of pharmacists'expectations towards pharmacogenomics. Pharmacogenomics. 2013;14(2):165–175. doi: 10.2217/pgs.12.197. https://doi.org/10.2217/pgs.12.197 PMid:23327577. [DOI] [PubMed] [Google Scholar]
- 33.Owusu Obeng A, Fei K, Levy KD, Elsey AR, Pollin TI, Ramirez AH, Weitzel KW, Horowitz CR. Physician-Reported Benefits and Barriers to Clinical Implementation of Genomic Medicine:A Multi-Site IGNITE-Network Survey. Journal of personalized medicine. 2018;8(3):E24. doi: 10.3390/jpm8030024. https://doi.org/10.3390/jpm8030024 PMid:30042363 PMCid:PMC6163471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Polit DF, Beck CT. Generalization in quantitative and qualitative research:Myths andstrategies. International Journal of Nursing Studies. 2010;47:1451–1458. doi: 10.1016/j.ijnurstu.2010.06.004. https://doi.org/10.1016/j.ijnurstu.2010.06.004 PMid:20598692. [DOI] [PubMed] [Google Scholar]
- 35.Kimberly AK. Importance of race and ethnicity in individuals'use of and responses to genomic information. Per Med. 2016;13(1):1–4. doi: 10.2217/pme.15.39. https://doi.org/10.2217/pme.15.39 PMid:29749865. [DOI] [PubMed] [Google Scholar]


