Abstract
Lanthanide luminescence, while ideal for in vivo applications owing to sharp emission bands within the optical window, requires high intensity, short wavelength excitation of small organic “antenna” chromophores in vicinity of the lanthanide complex to access excited f-orbital states via intersystem crossing. Here, we explored Cherenkov radiation of the radioisotopes 18F and 89Zr as an in situ source of antenna excitation. The effective inter- and intramolecular excitation of the terbium(III) complexes of a macrocylic polyaminocarboxylate ligand (q = 0, ϕ = 47%) as well as its analogue functionalized to append an intramolecular Cherenkov excitation source (q = 0.07, ϕ = 63%) was achieved. Using conventional small animal fluorescence imaging equipment, we have determined a detection limit of 2.5 nmol [Tb(L1)]- and [Tb(L2)]- in presence of 10 μCi of 18F or 89Zr. Our system is the first demonstration of the optical imaging of discrete luminescent lanthanide complexes without external short-wave excitation.
Graphical Abstract
COMMUNICATION
Exciting isotopes: Cherenkov radiation, emitted by clinically utilized radioisotopes, was employed as an in situ, inter- and intramolecular excitation source for biocompatible, discrete terbium(III) complexes. The successful detection of the emitted lanthanide luminescence with conventional bioimaging equipment is demonstrated.
Lanthanide-based luminescence represents an attractive alternative to commonly employed, conventional organic chromophores.[1–3] The unique electronic configuration of trivalent lanthanides gives rise to sharp emission bands ranging from green for Tb(III) (λem= 490, 545 nm), to red for Eu(III) (λem= 613, 690 nm) and near-IR for Nd(III) (λem= 1080 nm). Due to the Laporte-forbidden nature of the f-f transitions, aromatic chromophores (‘antennae’) are required for efficient excitation of metal-based luminescence after intersystem crossing.[4–5] The antenna must possess triplet state energy comparable to lanthanide acceptor states and allows efficient energy transfer with excitation wavelengths of 250–350 nm. Because of the large difference in antenna excitation and lanthanide emission wavelength, the effective Stokes-shift is conveniently large and minimizes inner filter effects; f-orbital centered deexcitation is long lived and allows for time-resolved detection. The applicability of luminescent lanthanides for bioassays requires short wavelength excitation or time resolution of emitted luminescence. Additionally, the lack of tissue depth penetration of light below 500 nm wavelength negates in vivo optical imaging applications.[6] A potential solution to this problem is the use of an internal excitation source that obviates the need for high-energy, external excitation.
In this context, we propose the application of Cherenkov radiation (CR) of radionuclides for the in situ excitation of the antenna of discrete Eu(III) and Tb(III) complexes. As radioisotopes decay and emit charged particles, the electromagnetic waves generated by the propagating particle traveling in dielectric media results in phase interference, observed as CR.[7] CR in aqueous solutions is continuous and wavelength dependent, with maximum intensity below 400 nm. This identifies discrete luminescent lanthanide complexes with antennae as highly suitable acceptors for Cherenkov radiation energy transfer (CRET).[8]
The concept of CRET has been successfully explored to excite Cu, Er, Y and CdTeSe based quantum dots and fluorescein.[8–13] However, organic fluorophores with favorable emission properties for biological applications absorb at longer wavelengths (> 400 nm), which coincides with low intensity of CR. We hypothesized that an application of CRET to discrete luminescent lanthanide complexes would be feasible and ideal; excitation of the lanthanide antenna occurs at short wavelengths where CR exhibits maximum intensity, which produces efficient intersystem crossing to yield the desired lanthanide-based luminescence emission (Scheme 1).
Scheme 1.
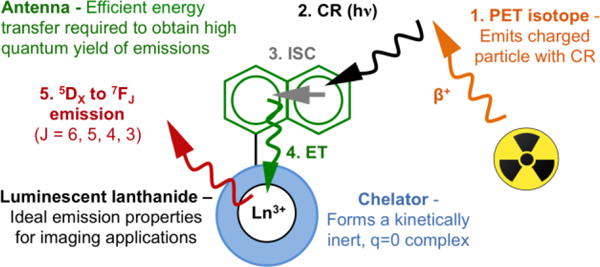
Schematic description of CR mediated excitation of a discrete, luminescent lanthanide complex.
In order to furnish a lanthanide complex with significant φ and comparable to organic fluorophores suitable for in vivo applications, several design criteria were considered as essential: 1. Incorporation of an antenna in vicinity of the metal center to allow for efficient excitation and intersystem crossing to the f-orbital centered excited state 2. Full coordinative saturation to limit access of H2O to the first coordination sphere, preventing vibrational, radiationless deexcitation, 3. Hydrophilicity and high kinetic inertness of the over-all complex to ascertain biocompatibility, 4. Structural handles for functionalization for covalent introduction of the CR source.
Here, we tested Tb/Eu/La(III) complexes with and without presence of an antenna to probe efficiency and detection limit for intermolecular excitation using 10–30 μCi 18F or 89Zr, which corresponds to typical quantities of activity employed for in vivo PET imaging studies.[14–15] Subsequently, we designed and synthesized a Tb(III) complex that allows for covalent attachment of the CR emitting isotope 89Zr, generating a Tb(III) complex with an intramolecular CR source (Scheme 2).
Scheme 2.
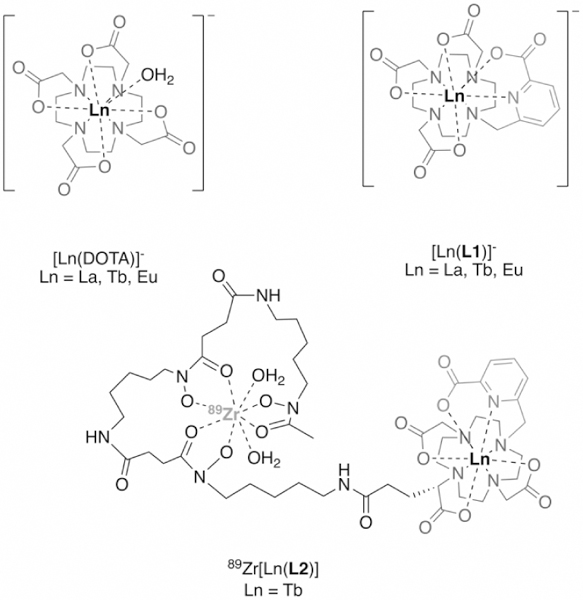
Structure of lanthanide complexes [Ln(DOTA)]-, [Ln(L1)]- and 89Zr[Ln(L2)]- investigated in this work.
Lanthanide complexes of DO3Apic (here referred to as L1)[16] exhibit high thermodynamic stability and efficiently exclude water from the inner hydration sphere. The picolinate arm provides convenient bidentate donation and serves as an efficient antenna to produce a quantum yield (ϕ) of 47% for [Tb(L1)]- (Table 1). The corresponding [Eu(L1)]- complex produced ϕ = 1.5% while [La(L1)]- is not emissive. The Tb(III), Eu(III) and La(III) complexes of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) were also synthesized and employed as controls without antenna.
Table 1.
Summary of hydration number, quantum yields and extinction coefficient for complexes evaluated.
| Complex | Hydration number q |
Quantum yield ϕ [a] |
Brightness B (ε × ϕ) [M–1cm–1] |
|---|---|---|---|
| [Tb(DOTA)]- | 0.96 | 0.1% | 20.0 |
| [Eu(DOTA)]- | 1.00 | 0.1% | 1.96 |
| [La(DOTA)]- | 1.00 | - | - |
| [Tb(L1)]- | 0.00 | 47% | 25345 |
| [Eu(L1)]- | 0.02 | 1.5% | 633 |
| [La(L1)]- | 0.00 | - | - |
| [Tb(L2)]- | 0.07 | 63% | 44151 |
Quantum yield calculations were carried out as reported previously relative to Ru(bipy)3 as a reference standard in H2O. The estimated relative error is 10−15%.20
Solutions of 0.025 – 600 nmol complex in 500 μL of phosphate buffered saline (PBS) were prepared; these quantities are comparable to a typical dose of fluorophore administered for targeted in vivo fluorescence imaging studies.[17–19] To assess feasibility and efficiency of CR mediated excitation, aqueous solutions of [Ln(DOTA)]- and [Ln(L1)]- complexes were doped with 10 μCi 18F or 89Zr and imaged using a commercial small animal fluorescence optical imaging scanner. Radiance emission was collected between 515 – 840 nm and quantified using ROI analysis. We observed increasing signal intensity proportional to increasing concentration of lanthanide for terbium(III) and europium(III) complexes; furthermore, the emission intensity of complexes [Eu(L1)]- and [Tb(L1)]- is enhanced by orders of magnitude when compared to the corresponding [Eu(DOTA)]- and [Tb(DOTA)]- complexes (Figure 1A, Figure S13, Supporting Information).
Figure 1.
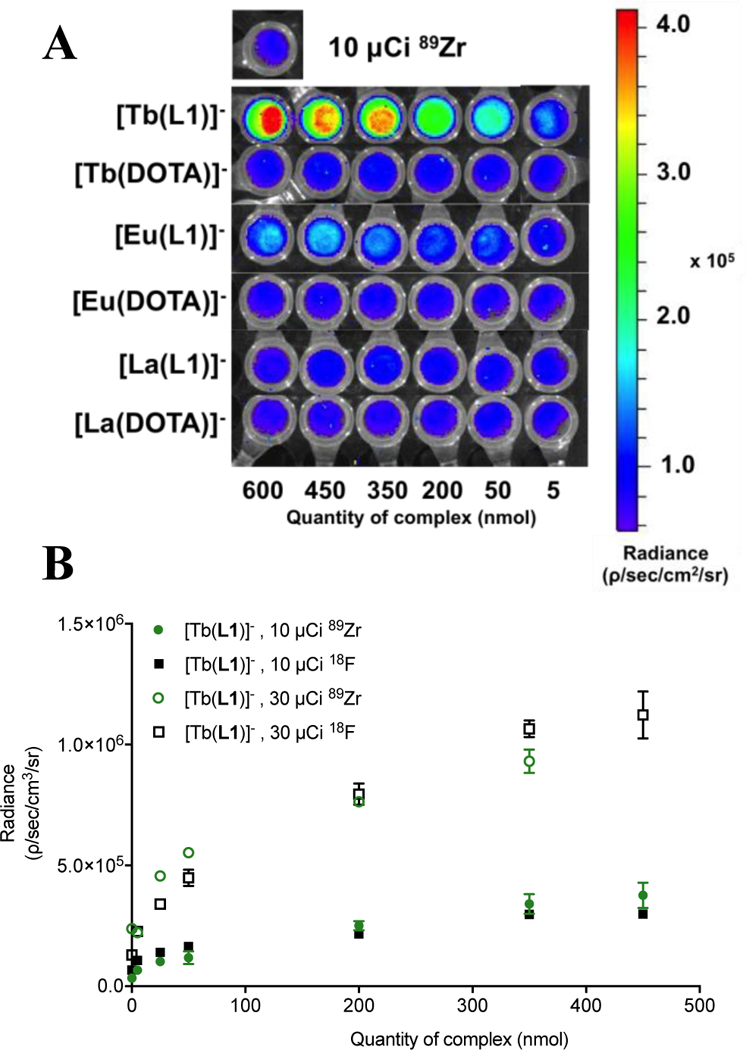
(A) Images of phantoms to quantify radiance emerging from 89Zr (10 μCi) doped samples containing increasing quantities of lanthanide complex. (B) Quantified radiance for complex [Tb(L1)]- in presence of 10 or 30 μCi of 89Zr or 18F.
This indicates that excitation of the antenna by CR is the dominant excitation pathway. As expected, complexes of lanthanum show no enhanced emission in presence of a CR emitting source (Figure 1A). The lanthanide-based emission is further confirmed by detection of the characteristic emission bands of [Tb(L1)]- using conventional fluorescence spectrophotometry (Figure S12, Supporting Information). The picolinate antenna of L1 is not suited to produce a strongly emissive Eu complex, which is reflected in the comparatively low radiance enhancement obtained for [Eu(L1)]- (Figure 1A) and the low quantum yield value. We note a non-linear increase of radiance especially at high concentrations of lanthanide, indicating that a finite amount of photons is released by a given amount of activity and thus a limit of excitation is reached.[8] Increase of activity to 30 μCi 18F or 89Zr in samples leads to proportionally enhanced emission intensity, followed by saturation of the maximum intensity (Figure 1B, Table S2, Supporting Information).
CR mediated excitation of the antenna to indirectly produce lanthanide luminescence emission opens possibilities for multimodality and sensing applications in vitro and in vivo. However, the absorption of short wavelength CR by proteins can strongly attenuate efficient intermolecular excitation (Figure S16, S17). To address this, we designed a Tb(III) complex with an appended desferrioxamine to incorporate 89Zr as an intramolecular CR source. The synthesis of the corresponding L2 ligand was achieved through step-wise functionalization of DO2AtBu with 1, followed by alkylation with 3. Debenzylation and subsequent amidation with desferrioxamine mesylate yields 5, which is followed by deprotection of the tBu protective groups and complexation with Tb(III) to afford [Tb(L2)]- (Scheme 3). [Tb(L2)]- exhibits a ϕ of 63% and a q = 0.07, thus representing a suitable functionalized analogue of [Tb(L1)]- (Table 1) and confirming complexation exclusively by the polyaza-macrocycle.
Scheme 3.
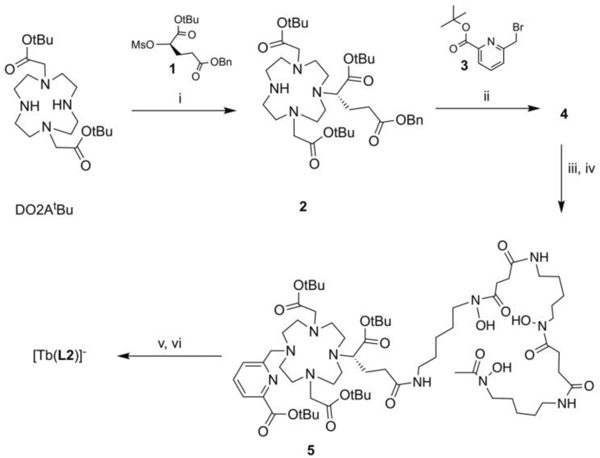
Synthesis of [Tb(L2)]-: (i) K2CO3, MeCN, 70 °C, 18 h, (ii) 3, K2CO3, MeCN, 70 °C, 18 h (iii) 10% w/w Pd/C, H2, MeOH, rt, 3 h (iv) DFO-mesylate, HBTU, DIPEA, DMF, 40 °C, 18 h (v) TFA/DCM 1:1, rt, 20 h (vi) Tb(OTf)3, H2O, pH 7.
To compare [Tb(L2)]- with an intramolecular CR source with [Tb(L1)]-, we first carried out the direct radiolabeling of [Tb(L2)]- using 89Zr-oxalate. Radiolabeling proceeds quantitatively within 10 minutes at room temperature, producing the radiolabeled, bimetallic complex 89Zr[Tb(L2)] (Figure 2). Subsequently, solutions with varying amounts of [Tb(L2)]- (0.1 – 10 nmol) were incubated with 10 μCi of 89Zr-oxalate followed by HPLC analysis to ascertain that 89Zr was fully complexed by [Tb(L2)]-. Radiance of the bimetallic 89Zr[Tb(L2)] construct in comparison with [Tb(L1)]- was collected and quantified. Phantom imaging revealed analogous behavior of [Tb(L1)]- in presence of 10 μCi of 89Zr-oxalate and 89Zr[Tb(L2)], with a detection limit as low as 2.5 nmol for 89Zr[Tb(L2)] in the range of 0.1 – 10 nmol of Tb(III) complex (Figure 3). This confirms that CR mediated excitation of discrete Tb(III) complexes in both inter- and intramolecular fashion. We note that under these conditions, the ratio of 89Zr[Tb(L2)] to [Tb(L2)]- is approximately 1:10 – 1:1000 in solution, which represents a typical ligand to complex ratio for radiometallated peptides and antibodies utilized for targeted in vivo imaging.[21] This raises the question of which portion of emitted luminescence arises from additional, intermolecular excitation processes. A plasma incubation experiment of both complexes (Figure S21, Table S3) confirms enhanced attenuation for [Tb(L1)]- (−37% for 100 nmol) whereas protein-mediated attenuation of emission was minimized for 89Zr[Tb(L2)] (−8% for 100 nmol).
Figure 2.
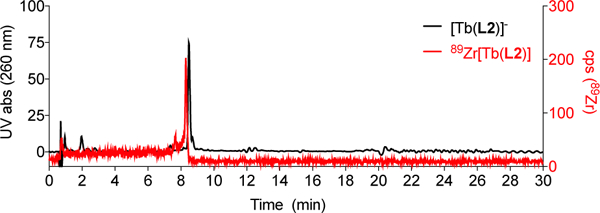
Overlay of HPLC traces of 89Zr[Tb(L2)] and [Tb(L2)]- using analytical method C.
Figure 3.
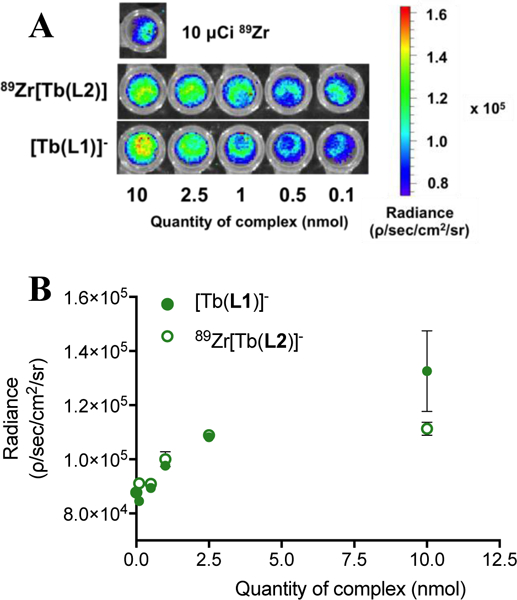
(A) Images of phantoms to quantify of radiance emerging from 89Zr doped samples containing lanthanide complexes [Tb(L1)]- and [Tb(L2)]- at Tb(III) concentrations ranging from 0.1–10 nmol. (B) Quantified radiance for complexes [Tb(L1)]- and 89Zr[Tb(L2)] with 10 μCi of 89Zr.
Studies on distance and concentration effects between CR and luminescent lanthanide are required as indicated by the detected difference in emission intensity for [Tb(L1)]- and 89Zr[Tb(L2)] observed at 10 nmol. required, specifically for intramolecular systems of the L2 type, but are beyond the scope of this communication.[10
In summary, we have successfully employed CR-emissive radioisotopes to excite the antenna of discrete luminescent lanthanide complexes in an inter- and intramolecular fashion. Solutions of ≥ 2.5 nmol 89Zr –doped [Tb(L1)]- and 89Zr[Tb(L2)] complex produce luminescence detectable by a conventional small animal fluorescence imager. The in situ excitation of discrete luminescent lanthanide complexes opens possibilities for in vivo applications of lanthanide luminescence in tandem with PET imaging or radiotherapy.
Supplementary Material
Acknowledgements
E.B. acknowledges funding sources, specifically the NIH for a Pathway to Independence Award (NHLBI R00HL125728–03), a REACH award (U01HL127522 – 18150031) and Stony Brook University for startup funds.
Footnotes
Prof. Peter Smith-Jones and Prof. Nashaat Turkman are kindly acknowledged for generous donation of 18F. We thank Jaclyn Ashcraft, Gregory Quevedo and Justin Devaraj for chemical synthesis of precursors.
Supporting information for this article is given via a link at the end of the document.
Experimental Section
Experimental details on chemical synthesis, sample preparation, analytical methods and raw data of photophysical measurements are provided in the Supporting Information.
References
- [1].Faulkner S, Pope SJ, Burton‐Pye BP, Appl. Spectr. Res. 2005, 40, 1–31. [Google Scholar]
- [2].Moore EG, Samuel APS, Raymond KN, Acc. Chem. Res. 2009, 42, 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Eliseeva SV, Bünzli J-CG, Chem. Soc. Rev. 2010, 39, 189–227. [DOI] [PubMed] [Google Scholar]
- [4].Heffern MC, Matosziuk LM, Meade TJ, Chem. Rev. 2013, 114, 4496–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thibon A, Pierre VC, Anal. Bioanal. Chem. 2009, 394, 107–120. [DOI] [PubMed] [Google Scholar]
- [6].Amoroso AJ, Pope SJ, Chem. Soc. Rev. 2015, 44, 4723–4742. [DOI] [PubMed] [Google Scholar]
- [7].Ruggiero A, Holland JP, Lewis JS, Grimm J, J. Nucl. Med. 2010, 51, 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dothager RS, Goiffon RJ, Jackson E, Harpstrite S, Piwnica-Worms D, PloS One 2010, 5, e13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bernhard Y, Collin B, Decréau RA, Chem. Commun. 2014, 50, 6711–6713. [DOI] [PubMed] [Google Scholar]
- [10].Kotagiri N, Niedzwiedzki DM, Ohara K, Achilefu S, Angew. Chem. Int. Ed. 2013, 52, 7756–7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao Y, Shaffer TM, Das S, Pérez-Medina C, Mulder WJ, Grimm J, Bioconj. Chem. 2017, 28, 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shaffer TM, Pratt EC, Grimm J, Nat. Nanotech. 2017, 12, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ma X, Kang F, Xu F, Feng A, Zhao Y, Lu T, Yang W, Wang Z, Lin M, Wang J, PLOs One 2013, 8, e77926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adams CJ, Wilson JJ, Boros E, Mol. Pharmaceutics 2017, 14, 2831–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meimetis LG, Boros E, Carlson JC, Ran C, Caravan P, Weissleder R, Bioconj. Chem. 2016, 27, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Regueiro-Figueroa M, Bensenane B, Ruscsák E, Esteban-Gómez D, Charbonnière LJ, Tircsó G, Tóth I, Blas A, Rodríguez-Blas T, Platas-Iglesias C, Inorg. Chem. 2011, 50, 4125–4141. [DOI] [PubMed] [Google Scholar]
- [17].Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, Wiedenmann B, Grötzinger C, Nat. Biotechnol. 2001, 19, 327–331. [DOI] [PubMed] [Google Scholar]
- [18].Falati S, Gross P, Merrill-Skoloff G, Furie BC, Furie B, Nat. Med. 2002, 8, 1175–1181. [DOI] [PubMed] [Google Scholar]
- [19].Frangioni JV, Curr. Opin. Chem. Biol. 2003, 7, 626–634. [DOI] [PubMed] [Google Scholar]
- [20].O’Malley WI, Abdelkader EH, Aulsebrook ML, Rubbiani R, Loh C-T, Grace MR, Spiccia L, Gasser G, Otting G, Tuck KL, Graham B, Inorg. Chem. 2016, 55, 1674–1682. [DOI] [PubMed] [Google Scholar]
- [21].Zeglis BM, Lewis JS, JoVE 2015, 96, e52521. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


