Abstract
Background
Diabetes mellitus is one of the most widespread diseases in the world, high glucose can damage islet cells, it is important to discover new natural products to inhibit high glucose damage. The protective effects and mechanisms of a novel Lentinus edodes mycelia polysaccharide (LMP) against damage induced by high glucose in MIN6 cells were explored.
Methods
Cell viability, malondialdehyde (MDA) inhibition, lactate dehydrogenase (LDH) release and the activity of superoxide dismutase (SOD) were evaluated under 40 mM glucose with or without LMP for 48 h. Cell signaling pathway analysis was performed to investigate the possible mechanisms of the protective effects of LMP in MIN6 cells.
Results
The results showed that LMP could increase cell viability and the activity of SOD, decrease the reactive oxygen species ( ROS) production, and reduce the MDA content and LDH release in high glucose-induced MIN6 cells. Moreover, LMP prevented high glucose-induced apoptosis by decreasing the expression of Bax and the activation of caspase-1 and caspase-3. Cell signaling pathway analysis showed that p38 mitogen-activated protein kinase (MAPK) and JNK pathways were inhibited and the Nrf2 pathway was activated after treated with LMP.
Conclusion
The protective effects of LMP against MIN6 cells damage induced by high glucose might rely on the regulation of the MAPK and Nrf2 pathways. These results indicated that LMP had great potential as a therapeutic agent for the treatment of diabetes mellitus.
Keywords: LMP, MIN6 cells, ROS, Oxidative stress, MAPK, Nrf2
Popular scientific summary
Polysaccharide from Lentinus edodes mycelia inhibits oxidative stress induced by high glucose in MIN6 cells.
Polysaccharide from Lentinus edodes mycelia inhibits high glucose-induced apoptosis of MIN6 cells.
Polysaccharide from Lentinus edodes mycelia protecting MIN6 cells against high glucose-induced damage may rely on mediating MAPK and Nrf2 pathway.
Type 2 diabetes mellitus (T2DM) is a metabolic disease, which leads to a high risk of complications and high morbidity (1). In 2017, there were 451 million people with diabetes worldwide, and it is expected that this figure will increase to 693 million by 2045 (2). Diabetes refers to a group of lifelong metabolic diseases characterized by chronic hyperglycemia due to insufficient insulin secretion and/or biological dysfunction (3). For T2DM, insulin resistance and pancreatic cell failure are the main factors, with a complex inter-relationship responsible for initiating its pathogenesis (4).
At present, the main clinical drugs for T2DM include sulfonylureas, α-glucosidase inhibitors and biguanides, but these drugs are associated with side effects (5). Active substances derived from natural products are generally considered to be less toxic than chemically synthesized drugs (6). Some natural products, especially the polysaccharides, extracted from medicinal plants and mushrooms have been used in the treatment of diabetes (7, 8). Polysaccharides extracted from mulberry were able to decrease blood glucose in rats (9). The natural biomacromolecule Dendrobium officinale polysaccharide had a remarkable hypoglycemic effect in mice with STZ-induced T2DM (10). In fact, several polysaccharide products have been used to treat DM in China, such as konjac glucomannan and pumpkin polysaccharide (11).
Lentinus edodes is one of the most popular edible fungi in the world. It is rich in various nutrients and biologically active substances, such as proteins, vitamins, minerals, and polysaccharides (12, 13). The production of fruiting bodies needs a long cultivation in a plastic bag, and its product quality is difficult to control; therefore, mycelia are the alternative or substitute products of mature fruiting bodies, for use in the formulation of nutraceuticals and medicine (14). At present, polysaccharides from mycelia have become a research hotspot in the field of polysaccharides. A new polysaccharide extracted from the Lentinus edodes mycelium (LMP) has been prepared in our laboratory, which exhibits antioxidant activity in islet cells (15). However, the inhibitory effects of these polysaccharides against glucose cytotoxicity are still undetermined. In this study, the protective effects of LMP on glucose-induced cell damage in MIN6 cells and the associated mechanism were explored, which might provide a basis for developing natural drugs for diabetes treatment.
Materials and methods
Chemicals and reagents
Hochest 33258 staining kit, LDH, MDA and SOD kits were purchased from Jiancheng Biologic Project Company (Nanjing, China); 4% paraformaldehyde and Bax (cat. no. sc-23959), Bcl-2 (cat. no. sc-509), nuclear factor erythroid 2-related factor 2 (Nrf2; cat. no. sc-518033), PCNA (cat. no. sc-25280), Caspase-1 (cat. no. sc-56036), and Caspase-3 (cat.no. sc-271028) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). β-actin (cat. no. 4970S), anti-p38 (cat. no. 9212), anti-phospho-p38 (cat. no. 9215), JNK (cat. no. 9252), and p-JNK (cat. no. 9251) antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-rabbit IgG (cat. no. SE134) and anti-mouse IgG secondary antibodies (cat. no. SE131) were obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China).
Cell culture
MIN6 cells were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). MIN6 cells were grown in DMEM supplemented with 10% fetal bovine serum, 100 U/mL of penicillin and 100 μg/mL of streptomycin at 37˚C in an incubator with 5% CO2.
MTT assay
MIN6 cells were seeded into 96-well plates at a density of 3 × 103 cells/well. Then, cells were treated with 40 mM glucose and various concentrations (0.0125, 0.025, and 0.05 mM) of LMP for 48 h at 37˚C; 20 μL of 5 mg/mL MTT was added to each well, followed by incubation for 4 h. The medium was removed and 150 μL dimethyl sulfoxide (DMSO) was added to each well to dissolve the thiazolyl blue tetrazolium bromide (MTT) formazan crystals. The optical density value was measured at 490 nm with a microplate reader.
Hoechst 33258 fluorescence staining
MIN6 cells were seeded in 12-well plates (8 × 104 cells/well) and treated with 40 mM glucose and different concentrations (0.0125, 0.025, and 0.05 mM) of LMP. Cells were washed twice with Buffer A for 5 min. After incubation with 4% paraformaldehyde solution for 30 min, cells were stained with 100 μL Hoechst 33258 for 10 min at room temperature, washed with Buffer A and mounted using 50% glycerol. Cells were observed with fluorescence microscope (20× magnification) (16).
ROS and LDH determination
The ROS were determinate by 2', 7'-dichlorofluorescein (DCFH-DA) (17). The cells were seeded in 12-well plates at a density of 8 × 104 cells per well and cultured for 48 h. The supernatant was collected from the plates after treatment. Then, cells were incubated with 500 μL dichlorodihydro-fluorescein diacetate (DCFH-DA) for 45 min at 37˚C in dark. Cells were washed twice with phosphate buffered saline (PBS) for 5 min. After incubation with 4% paraformaldehyde solution for 30 min, cells were observed with a fluorescence microscope (10× magnification). The supernatant was collected and the LDH release was determined with LDH assay kit, according to the manufacturer’s instructions. The absorbance was recorded at 450 nm.
MDA and SOD assay
Cells were treated as described in 2.4. After treatment, the MIN6 cells were washed twice with PBS, repeated freezing, and thawing to obtain homogenate. The homogenate was centrifuged at 4,000 rpm for 15 min, and the supernatant was collected for the MDA and SOD assay using commercial kits. The level of lipid peroxidation was indicated by the amount of MDA in the cells. The MDA and SOD content were determined by using MDA and SOD assay kits, according to the manufacturer’s instructions. The absorbance was recorded at 532 and 550 nm, respectively.
Western blot assay
The cells were cultured in flasks. When the growth density reached 60%, glucose and different concentrations (0.0125, 0.025, and 0.05 mM) of LMP were added for 48 h. Cells were harvested and lysed, and total protein extracts, cytoplasmic extracts, and nuclear extracts were prepared. The protein concentration of the extracts was estimated with bicinchoninic acid protein assay according to the manufacturer’s instruction. Equal amounts of total cell proteins (20 μg) were loaded and separated by 12% SDS poly-acrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes by electroblotting. After blocking with 5% non-fat milk for 1 h, the blots were incubated with primary antibodies overnight at 4˚C. Primary antibodies were used at a dilution of 1:2,000. The membranes were washed with TBST and incubated with the secondary antibodies at a dilution of 1:5,000 for 1 h. Finally, chemiluminescent detection was performed by using ECL reagents. β-actin and PCNA were used as loading controls. Densitometry analysis was performed by using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation of at least three independently performed experiments. Statistical analysis was conducted by one-way analysis of variance (ANOVA) by using SPSS software version 20.0 (SPSS, Inc., Chicago, IL, USA). P < 0.05 and P < 0.01 were considered statistically significant.
Results
Effect of LMP on cell viability
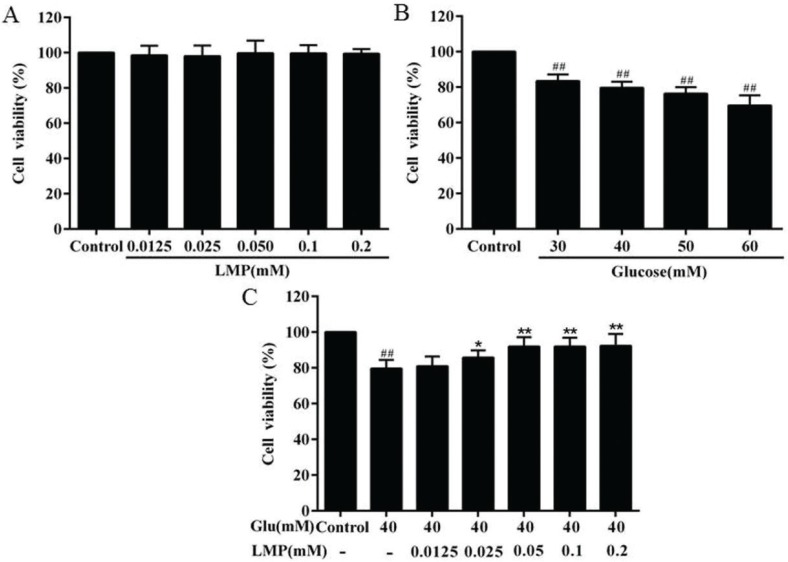
Cell viability was quantified by MTT assay. The result revealed that LMP had no significant (P > 0.05) effect on cell viability at concentrations between 0.0125 and 0.2 mM (Fig. 1a). The cell viability of MIN6 cells was determined after treatment with different concentrations of glucose. As shown in Fig. 1b, the cell viability of MIN6 cells significantly (P < 0.01) decreased in a concentration-dependent manner after treatment with glucose for 48 h, and 40 mM glucose was chosen for the subsequent experiments. As shown in Fig. 1c, after treatment with 0.0125, 0.025, and 0.05 mM LMP for 48 h, the cell viability was restored compared with the glucose-treated group in a concentration-dependent manner.
Fig. 1.
Protective effects of LMP on cell viability losses in MIN6 cells induced by glucose. (A) MIN6 cells were incubated with 0.0125, 0.025, 0.050, 0.1, 0.2 mM LMP for 48 h. Then the cell viability was evaluated by MTT assay. (B) The MIN6 cells were treated with glucose at different concentrations (30, 40, 50 and 60 mM) for 48 h. The cell viability was evaluated by MTT assay. (C) Protective effects of LMP on cell viability losses in MIN6 cells induced by 40 mM glucose. The untreated normal cells (control group) were assigned values of 100% and data are expressed as the mean ± standard deviation. ##P < 0.01 vs. control group, *P < 0.05, **P < 0.01 vs. group treated with glucose.
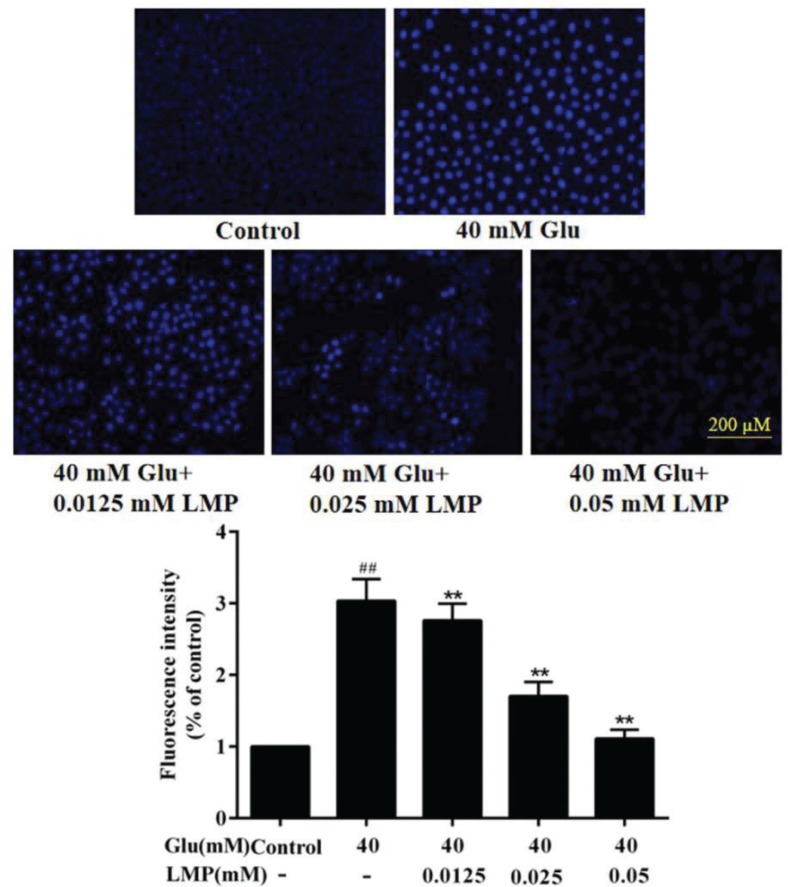
Hoechst 33258 fluorescent staining analysis
Hoechst 33258 can permeate the cells and make the nuclei appear blue, which can indicate condensation of chromatin and fragmentation (18). As shown in Fig. 2, after treatment with 40 mM glucose for 48 h, the fluorescence of cells treated with glucose was brighter than that of control cells. When MIN6 cells were incubated with 40 mM glucose and different concentrations of LMP simultaneously, the blue fluorescence gradually decreased with the increasing concentration of LMP. The results showed that LMP could inhibit chromatin fragmentation and condensation induced by glucose in MIN6 cells.
Fig. 2.
The apoptosis detection of the MIN6 cells. Cells were stained with Hoechst33258 and observed under a fluorescence microscope. Data are expressed as the mean ± standard deviation. ##P < 0.01 vs. control group, *P < 0.05, **P < 0.01 vs. group treated with glucose.
Effects of LMP on ROS and LDH production
As shown in Fig. 3a and b, treatment with 40 mM glucose led to an increase of ROS level in MIN6 cells compared with the control group, and the intracellular ROS levels in MIN6 cells were significantly (P < 0.01) reduced when the cells were incubated with 0.025 or 0.05 mM LMP for 48 h. To further investigate the protective effects of LMP in MIN6 cells, the release of LDH was measured. After 40 mM glucose treatment, the release of LDH from MIN6 cells was increased (P < 0.01), and LMP reversed this effect significantly (P < 0.05). This result indicated that the protective effect of LMP on MIN6 might be related to the inhibition of the release of LDH. Therefore, LMP not only decreased the LDH release, but also reduced ROS production, and thus alleviated glucose-induced cell toxicity in MIN6 cells.
Fig. 3.
Effects of LMP on ROS release and LDH overproduction in MIN6 cells after exposure to 40 mM glucose. (A) Results of MIN6 cells stained with DCFH-DA. (B) Quantitative analysis of ROS level in MIN6 cells. (C) The LDH release in MIN6 cells. Data are expressed as the mean ± standard deviation. ##P < 0.01 vs. control group, *P < 0.05, **P < 0.01 vs. group treated with glucose.
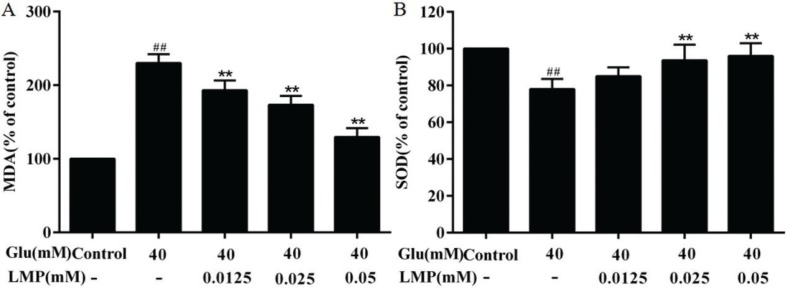
Effects of LMP on MDA and SOD production
As shown in Fig. 4a, after 40 mM glucose treatment, the level of MDA in MIN6 cells increased significantly (P < 0.01), indicating that the lipid peroxidation was enhanced in MIN6 cells. After treated with 0.0125, 0.025, and 0.05 mM LMP and 40 mM glucose together for 48 h, the MDA level in MIN6 cells decreased significantly (P < 0.01) with the increasing in LMP concentration, compared with the group treated only with 40 mM glucose. SOD is a scavenger of superoxide anion radicals, as shown in Fig. 4b, there was a significant (P < 0.01) reduction of the activity of SOD in the glucose-treated group compared with control group. After LMP was added at the same time, the activity of SOD in MIN6 cells increased gradually with the increase of LMP concentration.
Fig. 4.
Effects of LMP on the activities of MDA and SOD in MIN6 cells induced by 40 mM glucose. (A) Inhibition of LMP on MDA content in MIN6 cells induced by 40 mM glucose. (B) Promotion of SOD activity in MIN6 cells by LMP after exposure to 40 mM glucose. Data are expressed as the mean ± standard deviation. ##P < 0.01 vs. control group, *P < 0.05, **P < 0.01 vs. group treated with glucose.
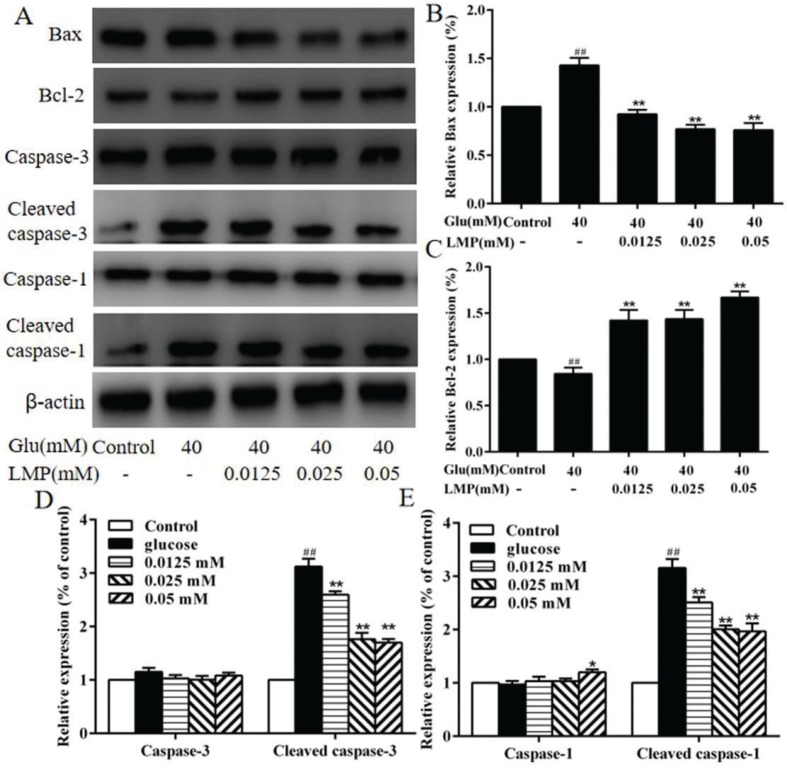
The expression of apoptosis-related proteins
The expression of Bax, Bcl-2, caspase-3, cleaved caspase-3, caspase-1, and cleaved caspase-1 in MIN6 cells was detected by western blot. As shown in Fig. 5, the protein expression of Bcl-2 was decreased significantly (P < 0.01) with 40 mM glucose, and the expression of Bax, cleaved caspase-3, and cleaved caspase-1 was increased (P < 0.01) after treated with 40 mM glucose compared with control cells, and the addition of LMP could reverse these effects significantly. Meanwhile, glucose and different concentrations of LMP had no obvious (P > 0.05) effect on the expression of caspase-3 and caspase-1, respectively (Fig. 5d and e).
Fig. 5.
The effects of LMP on the expression of apoptosis-related proteins in MIN6 cells (A) The expression levels of Bax, Bcl-2, Caspase-3, Cleaved caspase-3, Caspase-1 and Cleaved caspase-1 were detected by western blot assay. The levels of β-actin were used as an internal control. (B, C, D and E) Quantitative analysis of the protein expression of Bax, Bcl-2, Caspase-3, Cleaved caspase-3, Caspase-1, Cleaved caspase-1. Data are expressed as the mean ± standard deviation. ##P < 0.01 vs. control group, *P < 0.05, **P < 0.01 vs. group treated with glucose.
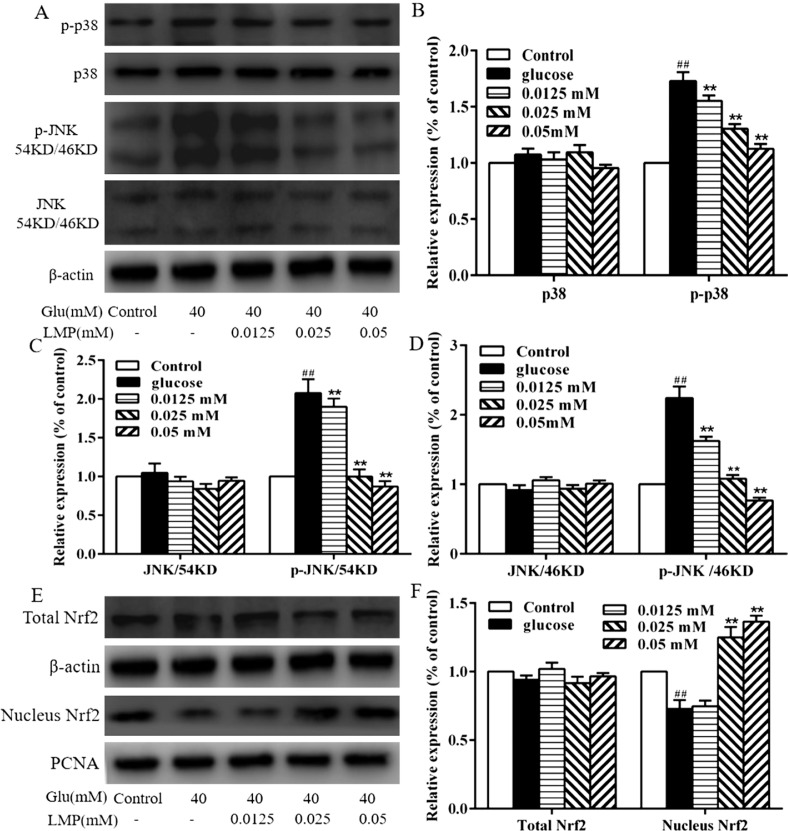
Effect of LMP on the MAPK and Nrf2 pathways
Western blot analysis was used to investigate the effect of LMP on p38 MAPK and JNK expression. As shown in Fig. 6, the expression of total p38 MAPK and JNK did not differ from each group. p-p38 and p-JNK were significantly (P < 0.01) increased in 40 mM glucose-treated group compared with the control group. Treatment with LMP led to a decrease in p-p38 and p-JNK in MIN6 cells to basal level (Fig. 6b, c and d). A concentration of 40 mM glucose significantly (P < 0.01) decreased the protein expression of Nrf2 in the nucleus, whereas 0.025 and 0.05 mM LMP significantly (P < 0.01) increased the translocation of Nrf2 in the nucleus of MIN6 cells. These results indicated that the protective effects of LMP in MIN6 cells damaged by 40 mM glucose might rely on the MAPK and Nrf2 cell-signaling pathways.
Fig. 6.
The effect of LMP on MAPK and Nrf2 pathways. (A, B, C and D) The protein expression of p-p38, p38, p-JNK and JNK by western blot analysis using specific antibodies, The levels of β-actin were used as an internal control. (E and F) The expressions of total Nrf2 and Nucleus Nrf2 by western blot analysis using specific antibodies, the levels of β-actin and PCNA were used as an internal control. Data are expressed as the mean ± standard deviation. ##P < 0.01 vs. control group, *P < 0.05, **P < 0.01 vs. group treated with glucose.
Discussion
Under normal physiological conditions, the production and elimination of ROS in the body is in a dynamic equilibrium (19). Oxidative stress refers to elevated intracellular levels of ROS that cause lipids, protein, and DNA damage, which have been shown to have direct and deleterious consequences in diabetes (20, 21). The study showed that hyperglycemia could generate ROS, which in turn causes oxidative stress on the beta cell (22, 23). Our results showed that high glucose treatment alone significantly increased ROS levels in MIN6 cells, and the level of ROS reduced after treatment with LMP.
Lipid peroxides are the product of the reaction of ROS with polyunsaturated fatty acids, which can reflect the degree of damage to the body caused by oxidative stress (24). MDA, one of the lipid peroxidation products, is regarded as biomarkers of oxidative stress in cells (25). Several previous studies have shown that the level of MDA in subjects with T2DM is increased (26, 27). In this study, we found that MDA level in the group treated with 40 mM glucose was significantly higher than that in the control group, and LMP could decrease the MDA levels compared to the 40 mM glucose-treated group. It has been previously reported that diabetic patients exhibited high ROS production accompanied by decreased expression of antioxidant enzymes (28). SOD, one of the most important antioxidant enzymes, catalyzes the dismutation of O2 − to H2O2 and O2 and inhibits the formation of free radical (29). It has been reported that pumpkin polysaccharides protected islets cells from STZ injury in vitro via increasing the levels of SOD and reducing the level of MDA (30). The results of the present study showed that the 40 mM glucose significantly reduced the expression of SOD in MIN6 cells; however, SOD activity in MIN6 cells was recovered after treatment with LMP.
Apoptosis, a process of programmed cell death characterized by cell shrinkage, chromatin condensation and DNA cleavage (31). Islet β-cell apoptosis plays an crucial role in the development of T2DM (32, 33). β-cell apoptosis can occur through many pathways (34). Mitochondrial apoptotic pathway can be regulated by Bcl-2 family and caspase family (32). It has been reported that polysaccharide could inhibit pancreatic β-cells apoptosis through regulating the expression of Bcl-2 family and caspase family proteins (35, 36). Our study showed that LMP could inhibit MIN6 cells apoptosis by regulating mitochondrial apoptotic pathway.
MAPK is an important signaling pathway and plays a key role in regulating cellular function (37). MAPK cascade is the divergent combination of at least three protein kinases such as MAPKKK (MKKK/MEKK, MAP3K), MAPKK (MEK/MKK), and MAPK (MPK), stimulating each other by phosphorylation (38, 39). ROS induces phosphorylation of MAPK-signaling proteins such as RTK and MAP3K, resulting in the activation of MAPK pathways (40, 41). In eukaryotes, the MAPK-signaling pathways include p38, JNK, and ERK. p38 MAPK- and JNK-signaling pathways are associated with the response of cells to stresses, such as inflammation and ROS (42). Therefore, the inhibition of MAPK-signaling pathway has protective effects against islet cell dysfunction. In our study, glucose activated the p38 MAPK and JNK pathways in MIN6 cells, and LMP decreased the phosphorylation of p38 and JNK in a concentration-dependent manner, indicating that the protective effects of LMP on damage in MIN6 cells induced by glucose might partially be mediated by regulating the MAPK pathway.
The transcription factor Nrf2 is a key protein in the regulation of the endogenous antioxidant response and is considered as a promising therapeutic target for diseases caused by oxidative stress (43). By activating the Nrf2 antioxidant-signaling pathway, the ability of the body to resist oxidative stress damage can be significantly enhanced (44, 45). Therefore, the Nrf2 pathway is a potential target for the treatment of diabetes (46). Nrf2 is a key transcription factor that determines redox status by regulating numerous antioxidant enzymes (47). It has been reported that antrodia cinnamomea polysaccharide (48) and Lycium barbarum polysaccharides could activate Nrf2 (49). It has also been reported that curcumin resulted in enhanced nuclear translocation of Nrf2, which plays a role in the cellular protection against oxidative stress (50). Nrf2 is involved in the expression of various antioxidant proteins (such as detoxifying enzymes) via antioxidant response element binding site, hyperglycemia leads to oxidative stress and results in changes in levels of Nrf2, persistent hyperglycemia decreases its expression of Nrf2, and evidence has also indicated decreased levels of Nrf2 in diabetes (51). In this study, LMP was able to promote nuclear translocation of Nrf2, and the expression of SOD, a downstream enzyme of Nrf2, increased after treatment with LMP.
Conclusions
The present study showed that LMP could recover the glucose-induced redox changes. These results indicated that LMP might become a potential therapeutic agent for diabetes, due to its inhibition of glucose toxicity.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No.31770017), Cultivation Plan for Youth Agricultural Science and Technology Innovative Talents of Liaoning Province (No.2015013), Project Supported by Scientific Research Fund of Liaoning Provincial Education Department (No. LQN201714), Startup Foundation for Doctors of Liaoning Province (No.20170520258). Project Supported for Youth and Middle-aged Science and Technology Innovative Talents of Shenyang City (No.RC180240).
Conflict of interests
There are no conflicts of interest to declare.
References
- 1.Pang YL, Zhu HH, Xu JQ, Yang LH, Liu LJ, Li J. β-arrestin-2 is involved in irisin induced glucose metabolism in type 2 diabetes via p38 MAPK signaling. Exp Cell Res 2017; 360: 199–204. doi: 10.1016/j.yexcr.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Cho NH, Shaw JE, Karuranga S, Huang Y, Fernandes JDDR, Ohlrogge AW, et al. . IDF diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pr 2018; 138: 271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Skyler JS, Oddo C. Diabetes trends in the USA. Diabetes Metab Res 2002; 18: 21–6. doi: 10.1002/dmrr.289. [DOI] [PubMed] [Google Scholar]
- 4.Kenneth S, Polonsky MD. The past 200 years in diabetes. New Engl J Med 2015; 367: 1332–40. doi: 10.1056/NEJMra1110560. [DOI] [PubMed] [Google Scholar]
- 5.Wang PC, Zhao S, Yang BY, Wang QH, Kuang HX. Anti-diabetic polysaccharides from natural sources: a review. Carbohtd Polym 2016; 148: 86–97. doi: 10.1016/j.carbpol.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Zhong HY, Zeng JH, Ge J. The pharmacological effect of polysaccharides from Lentinus edodes on the oxidative status and expression of VCAM-1mRNA of thoracic aorta endothelial cell in high-fat-diet rats. Carbohyd Polym 2008; 74: 445–50. doi: 10.1016/j.carbpol.2008.03.018. [DOI] [Google Scholar]
- 7.Huang M, Wang FQ, Zhou XH, Yang HX, Wang Y. Hypoglycemic andhypolipidemic properties of polysaccharides from Enterobacter cloacae Z0206in KKAy mice. Carbohyd Polym 2015; 117: 91–8. doi: 10.1016/j.carbpol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Tang TT, Duan XY, Ke Y, Zhang L, Shen YB, Hu B, et al. . Antidiabetic activities of polysaccharides from Anoectochilus roxburghii and Anoectochilus formosanus in STZ-induced diabetic mice. Int J Biol Macromol 2018; 112: 882–8. doi: 10.1016/j.ijbiomac.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Ren CJ, Zhang Y, Cui WZ, Lua GB, Wang YW, Gao HJ, et al. . A polysaccharide extract of mulberry leaf ameliorates hepatic glucose metabolism and insulin signaling in rats with type 2 diabetes induced by high fat-diet and streptozotocin. Int J Biol Macromol 2015; 7: 951–9. doi: 10.1016/j.ijbiomac.2014.09.060. [DOI] [PubMed] [Google Scholar]
- 10.Wang KP, Wang HX, Liu YG, Shui WZ, Wang JF, Cao P, et al. . Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. J Funct Foods 2018; 40: 261–71. doi: 10.1016/j.jff.2017.11.004. [DOI] [Google Scholar]
- 11.Diao YL, Jiang W, Zhu T, Meng DL, Shan JJ. Antidiabetic activities of natural plant polysaccharides and their advances. J Int Pharm Res 2011; 38: 275–279. doi: 10.13220/j.cnki.jipr.2011.04.002. [DOI] [Google Scholar]
- 12.Chang R, MD FACP. Functional properties of edible mushrooms. Nutr Rev 1996; 54: S91–3. doi: 10.1111/j.1753-4887.1996.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen SY, Yuan B, Xua JJ, Chen GT, Hua QH, Zhao LY. Simultaneous separation and determination of six arsenic species in Shiitake (Lentinus edodes) mushrooms: method development and applications. Food Chem 2018; 262: 134–41. doi: 10.1016/j.foodchem.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 14.Cao XY, Liu RH, Liu JL, Huo YP, Yang W, Zeng M, et al. . A novel polysaccharide from Lentinus edodes mycelia exhibits potential antitumor activity on laryngeal squamous cancer cell line Hep-2. Appl Biochem Biotech 2013; 171: 1444–53. doi: 10.1007/s12010-013-0441-6 [DOI] [PubMed] [Google Scholar]
- 15.Liu JL, Wang WY, Yu H, Cao XY, Wang Y, Liu MJ, et al. . Antioxidant activity of polysaccharides extracted from lentinus edodes mycelia and their protective effects on INS-1 cells. J Biosciences 2016; 32: 1679–88. doi: 10.14393/BJ-v32n1a2016-33809 [DOI] [Google Scholar]
- 16.Wang ZH, Su GY, Zhang ZG, Dong H, Wang YH, Zhao HY, et al. . 25-Hydroxyl-protopanaxatriol protects against H2O2 -induced H9c2 cardiomyocytes injury via PI3K/Akt pathway and apoptotic protein down-regulation. Biomed Pharmacother 2018; 99: 33–42. doi: 10.1016/j.biopha.2018.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Aceitunoa VC, Ahna S, Simub SY, Singh P, Mathiyalagan R, Lee HA, et al. . Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed Pharmacother 2016; 84: 158–65. doi: 10.1016/j.biopha.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Li YL, Gan GP, Zhang HZ, Wu HZ, Li CL, Huang YP, et al. . A flavonoid glycoside isolated from Smilax china L. rhizome in vitro anticancer effects on human cancer cell lines. J Ethnopharmacol 2007; 113: 0–124. doi: 10.1016/j.jep.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Li JJ, Wang F, Xia YJ, Dai WQ, Chen K, Li SN, et al. . Astaxanthin pretreatment attenuates hepatic ischemia reperfusion-induced apoptosis and autophagy via the ROS/MAPK pathway in mice. Mar Drugs 2015; 13: 3368–87. doi: 10.3390/md13063368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24: R453–62. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy V.P, Zhu XW, Perry G, Smith MA. Oxidative stress in diabetes and Alzheimer’s disease. J Alzheimers Dis 2009; 16: 763–74. doi: 10.3233/JAD-2009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson RP, Harmon JS. Diabetes, glucose toxicity, and oxidative stress: a case of double jeopardy for the pancreatic islet β cell. Free Radical Bio Med 2006; 41: 177–84. [DOI] [PubMed] [Google Scholar]
- 23.Robertson R, Zhou H, Zhang T, Harmon JS. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys 2007; 48: 139–46. doi: 10.1080/10641960701361601. [DOI] [PubMed] [Google Scholar]
- 24.Xia LL, Tang YB, Shao K. Effect of alprostadil on hemorheology, immune function, MDA, SOD and ROS in patients with diabetic nephropathy. J Hainan Med Univ 2016; 22: 59–62. doi: 10.13210/j.cnki.jhmu.20160330.011. [DOI] [Google Scholar]
- 25.Bastos AS, Graves DT, Loureiro AP, Rossa Júnior C, Abdalla DS, Faulin TE. Lipid peroxidation is associated with the severity of periodontal disease and local inflammatory markers in patients with type 2 diabetes. J Clin Endocrinol Metab 2012; 97: 1353–62. doi: 10.1210/jc.2011-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bandeira SDM, Guedes GDS, Fonseca LJSD, Pires AS, Gelain DP, Moreira JCF, et al. . Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid per-oxidation and SOD activity. Oxid Med cell Longev 2012; 2012: 1–13. doi: 10.1155/2012/819310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davì G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Sign 2005; 7: 256–268. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 28.Matzinger M, Fischhuber K, Heiss EH. Activation of Nrf2 sig-naling by natural products-can it alleviate diabetes? Biotechnol Adv 2018; 36: 1738–67. doi: 10.1016/j.biotechadv.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culotta VC. Superoxide dismutase, oxidative stress, and cell metabolism. Curr Top Cell Regul 2000; 36: 117. doi: 10.1016/S0070-21372137(01)80005-4. [DOI] [PubMed] [Google Scholar]
- 30.Zhu HY, Chen GT, Meng GL, Xu JL. Characterization of pumpkin polysaccharides and protective effects on streptozotocin-damaged islet cells. Chin J Nat Medicines 2015; 13: 199–207. doi: 10.1016/S1875-53645364(15)30005-4. [DOI] [PubMed] [Google Scholar]
- 31.Shu BS, Zhang JJ, Jiang ZY, Cui GF, Veeranac S, Zhong GH. Harmine induced apoptosis in Spodoptera frugiperda Sf9 cells by activating the endogenous apoptotic pathways and inhibiting DNA topoisomerase I activity. Pestic Biochem Phys 2019; 155: 26–35. doi: 10.1016/j.pestbp.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Tomita T. Apoptosis in pancreatic β-islet cells in type 2 diabetes. Bosnian J Basic Med 2016; 16: 162–79. doi: 10.17305/bjbms.2016.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonardi O, Mints G, Hussain M. Beta-cell apoptosis in the pathogenesis of human type 2 diabetes mellitus. Eur J Endocrinol 2003; 149: 99–102. doi: 10.1530/eje.0.1490099. [DOI] [PubMed] [Google Scholar]
- 34.Lupi R, Prato SD. Beta-cell apoptosis in type 2 diabetes: quantitative and functional consequences. Diabetes Metab 2008; 34: S56–64. doi: 10.1016/s1262-36363636(08)73396-2. [DOI] [PubMed] [Google Scholar]
- 35.Guo J, Wang JL, Song S, Liu Q, Huang YL, Xu YF, et al. . Sphallerocarpus gracilis polysaccharide protects pancreatic β-cells via regulation of the bax/bcl-2, caspase-3, pdx-1 and insulin signalling pathways. Int J Biol Macromol 2016; 93: 829–36. doi: 10.1016/j.ijbiomac.2016.08.083. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, He ZH, Liu XC, Chen ZH, Sun JL, Wu ZJ, et al. . Oral administration of Angelica sinensis polysaccharide protects against pancreatic islets failure in type 2 diabetic mice: pancreatic β-cell apoptosis inhibition. J Funct Foods 2019; 54: 361–70. doi: 10.1016/j.jff.2019.01.037. [DOI] [Google Scholar]
- 37.Wada TJ, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004; 23: 2838–49. [DOI] [PubMed] [Google Scholar]
- 38.Raja V, Majeed U, Kang H, Andrabi KI, Johna R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ Exp Bot 2017; 137: 142–57. doi: 10.1016/j.envexpbot.2017.02.010. [DOI] [Google Scholar]
- 39.Huang GH, Shi LZ, Chi HB. Regulation of JNK and p38 MAPK in the immune system: signal integration, propagation and termination. Cytokine 2009; 48: 0–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Sig Transd 2011; 2011: 792–639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, et al. . NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. Embo J 2003; 22, 3898–3909. doi: 10.1093/emboj/cdg379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trempolec N, Dave-Coll N, Nebreda AR. SnapShot: p38 MAPK signaling. Cell 2013; 152: 656–656. doi: 10.1016/j.cell.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 43.Chu XY, Liu YM, Zhang HY. Activating or Inhibiting Nrf2? Trends Pharmacol Sci 2017; 38: 953–55. doi: 10.1016/j.tips.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, León R. Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther 2016; 157: 84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Lu MC, Ji JA, Jiang ZY, You QD. The Keap1-Nrf2-ARE pathway as a potential preventive and therapeutic target: an update. Med Res Rev 2016; 36: 924–63. doi: 10.1002/med.21396 [DOI] [PubMed] [Google Scholar]
- 46.Fu JQ, Hou YY, Xue P, Wang HH, Xu YY, Qu WD, et al. . Nrf2 in type 2 diabetes and diabetic complications: Yin and Yang. Curr Opin Toxicol 2016; 1: 9–19. doi: 10.1016/j.cotox.2016.08.001. [DOI] [Google Scholar]
- 47.Cao SM, Du JL, Hei QH. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp Ther Med 2017; 14: 4919–27. doi: 10.3892/etm.2017.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu YG, Yang AH, Qu YD, Wang ZQ, Zhang YQ, Liu Y, et al. . Ameliorative effects of Antrodia cinnamomea polysaccharides against cyclophosphamide-induced immunosuppression related to Nrf2/HO-1 signaling in BALB/c mice. Int J Biol Macromol 2018; 116: 8–15. doi: 10.1016/j.ijbiomac.2018.04.178. [DOI] [PubMed] [Google Scholar]
- 49.Yang DM, Zhang JQ, Fei YF. Lycium barbarum polysaccharide attenuates chemotherapy-induced ovarian injury by reducing oxidative stress: LBP attenuates ovarian injury. J Obstet Gynaecol Res 2017; 43: 1621–28. doi: 10.1111/jog.13416 [DOI] [PubMed] [Google Scholar]
- 50.Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol 2008; 46: 1279–87. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 51.Kumar A, Mittal R. Nrf2: a potential therapeutic target for diabetic neuropathy. Inflammopharmacology 2017; 25: 393–402. doi: 10.1007/s10787-017-0339-y. [DOI] [PubMed] [Google Scholar]