Abstract
OBJECTIVE.
The purpose of this study was to evaluate the incidence of clinically significant diaphragmatic injuries and local tumor progression after microwave ablation of hepatic tumors abutting the diaphragm.
MATERIALS AND METHODS.
This retrospective study included 55 peripheral hepatic tumors abutting the diaphragm treated by microwave ablation versus a control group of 15 centrally located tumors. Treated tumors were further subdivided according to the use of artificial ascites (fluid vs no fluid) and whether instilled fluid achieved displacement of the liver surface away from the diaphragm (displaced vs nondisplaced). Measurements of tumor size, distance to the diaphragm, ablation zone size, displacement distance, length of the ablation zone along the liver capsule, diaphragm thickness, diaphragmatic hernia, and local tumor progression were made on pre- and postablation CT and MRI. The electronic medical record was reviewed for patient self-reported pain scores and other symptoms. Data were analyzed by use of the Kruskal-Wallis and Fisher exact tests.
RESULTS.
There were no cases of diaphragmatic hernia in peripheral or central tumors. Postablation diaphragm thickness was higher in peripheral hepatic tumors than in control tumors. Peripheral tumors had an overall higher incidence of postprocedure shoulder pain (18% vs 0%) and local tumor progression (5.5% vs 0%) compared with control tumors, but these differences did not achieve statistical significance (p = 0.2 and p = 1, respectively).
CONCLUSION.
Our study shows that microwave ablation of peridiaphragmatic hepatic tumors is safe, without incidence of diaphragmatic hernia, and can be performed with a low rate of local tumor progression.
Keywords: ablation, diaphragm, microwave, peripheral
Percutaneous image-guided thermal ablation has become an accepted treatment method for many tumors in the liver, kidney, lung, and bone [1—4]. In the liver, thermal ablation is considered a first-line therapy for small (< 3 cm) hepatocellular carcinoma in the setting of cirrhosis and a second-line therapy for the treatment of medically or surgically inoperable oligometastatic colorectal metastases [5]. Percutaneous ablation has also been used successfully to treat benign hepatic tumors, such as giant cavernous hemangiomas and hepatocellular adenomas [6, 7].
Radiofrequency has been the most common energy source used for percutaneous thermal ablation of hepatic tumors to date. However, physical limitations that constrain ablation zone size have resulted in radiofrequency ablation being most effective for treating tumors smaller than 2 cm [8–12]. High-powered microwave ablation systems have shown the potential to create larger ablation zones than radiofrequency ablation devices, with similar applicator size and shape [13–15]. Although larger ablations may enhance treatment efficacy by improving ablative margins, the risk of collateral damage may also be increased.
Radiofrequency ablation of peripheral liver lesions abutting the diaphragm has been associated with increased postprocedural pain, rare diaphragmatic hernias, and an increased rate of local tumor progression [1618]. To date, reports on the safety and efficacy of hepatic microwave ablation have focused on the overall safety profile [19, 20], without specific attention to the sequela of diaphragmatic injury or local tumor progression rates when treating peridiaphragmatic tumors. Recently, a single-center retrospective study of liver lesions treated by thermal ablation reported two cases of diaphragmatic hernia in the microwave group (2/654 [0.3%]), compared with zero of 376 in the radiofrequency group. However, this particular study was not controlled for tumor location or ablation zone size [21].
The purpose of this single-center retrospective case series was to determine the incidence of clinically significant diaphragmatic injuries associated with hepatic microwave ablation.
Materials and Methods
Patient Selection
This study was approved by the institutional review board and complied with HIPAA. A retrospective review was performed of all cases of percutaneous microwave ablation of liver tumors performed at our institution between 2010 and 2013 with at least 1 month of imaging follow-up. From this dataset, a study group was identified according to a study by Kang et al. [22], who defined patients at risk for diaphragmatic injury as those with ablation zones that directly contacted or were within 5 mm of the liver capsule and diaphragm. The study group included 42 patients (28 men and 14 women; mean age, 59.4 years) with 55 hepatic tumors (30 hepatocellular carcinomas, 23 metastases, and two hepatocellular adenomas). A control cohort consisted of 13 patients (11 men and two women; mean age, 62.7 years) with 15 consecutively treated centrally located (> 1.5 cm from the diaphragm) hepatic tumors (12 hepatocellular carcinomas, two metastatic tumors, and one hepatocellular adenoma) also treated by percutaneous microwave ablation.
Microwave Ablation Procedure
Percutaneous microwave ablation was performed under general anesthesia using a high-powered microwave ablation system (Certus 140, NeuWave Medical). Imaging guidance for antenna placement was provided by either ultrasound (E9, GE Healthcare) or CT fluoroscopy (Light-Speed Advantage, GE Healthcare). One of five board-certified radiologists experienced in percutaneous ablation performed all procedures. Ablation time and power were recorded during all procedures. In some cases, intraperitoneal fluid was administered for thermal protection at the discretion of the performing radiologist. All patients underwent an immediate postablation contrast-enhanced CT scan. Patients were monitored for immediate postprocedural complications during an overnight hospital admission.
Measurements
Preprocedure imaging was reviewed for tumor size, distance of the tumor to the diaphragm (Fig. 1A), and preablation diaphragm thickness. Immediate postablation imaging was done according to a standard biphasic protocol with 5-mm contiguous axial slices. These images were reviewed to measure the displacement distance provided by any intraperitoneal fluid between the hepatic capsule and diaphragm, ablation zone size, length of the ablation zone contacting the liver capsule, and postablation diaphragm thickness (Fig. 1B). Ablation zones were evaluated on the portal venous phase. Tumor size and ablation zone size were defined as the mean of the longest and orthogonal diameters. Diaphragm thickness and distance to the diaphragm were measured in the coronal plane in which the liver surface, ablation zone, and diaphragm were visible. Distance to the diaphragm and displacement distance were measured as the shortest distance to the diaphragm on coronal images.
Fig. 1—
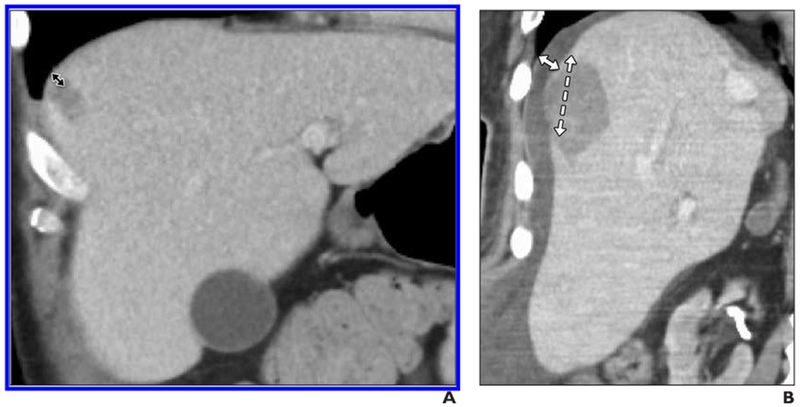
59-year-old man with metastatic colorectal cancer and 1.6-cm metastasis in segment 8 of liver.
A, Preprocedure coronal CT scan shows close proximity of tumor to diaphragm (double-headed arrow).
B, Immediate postprocedure coronal CT scan. Tumor has been ablated with two microwave antennas.
Approximately 1200 mL of intraperitoneal 5% dextrose in water was used to separate liver capsule from diaphragm.
Solid arrow represents displacement distance, and dashed arrow is length of ablation zone abutting capsule.
Patient Symptoms
The electronic medical record was reviewed for nursing documentation during the patient’s postprocedure overnight hospital admission. The number of patients reporting any shoulder pain during the admission was recorded. Pain scores were obtained from the nursing record of patients’ self-reported pain on the standard Visual Analog Scale 10-point pain scale.
Patient Follow-Up
Patients were followed serially with either CT or MRI at target dates of 1, 3, 6, and 12 months after ablation. Imaging and radiologic reports were reviewed for diaphragmatic hernia and local tumor.
Statistical Analysis
Because the instillation of intraperitoneal fluid does not guarantee that the diaphragm will be displaced away from the hepatic surface at the ablation zone, data were analyzed in several different ways. First, an overall analysis of peripheral versus central liver tumors was performed. Second, to take into account whether fluid actually separated the liver surface from the diaphragm, the study group was separated into the following groups for subanalysis: displaced (visible fluid separating hepatic capsule and diaphragm) and nondisplaced (no visible fluid separating hepatic capsule and diaphragm). Third, to account for the possibility that artificial ascites may lessen thermal damage, even if it does not visibly separate the hepatic capsule and diaphragm, the study group was again divided into fluid and no-fluid groups for reanalysis. All study subgroups were compared with the control group.
Group-specific summary descriptive measures were obtained for continuous variables, and differences were assessed with Kruskal-Wallis tests. Categoric variables were tabulated by group, and Fisher exact test was used to assess differences. Statistical significance was defined as p < 0.05 (two-sided). There was no adjustment of p values for multiplicity. Exploratory plots were used to assess possible violations in test assumptions. Ablations were used as the unit of analysis; multiple ablations within the same subject were assumed to be independent. All statistical graphics and computations were obtained in R software (version 3.0.I, R Foundation for Statistical Computing) and were based on n – 1.
Results
Patient Population
Of the total 55 ablation zones identified for the study groups, 30 were in the nondisplaced group (Fig. 2), and 25 were in the displaced group (Fig. 3). When subdivided by fluid, 37 cases were in the fluid group, and 18 were in the no-fluid group. There were 15 ablation zones in the central control group (Fig. 4). There was no statistically significant difference in the mean patient age, tumor size, and imaging follow-up between groups.
Fig. 2—
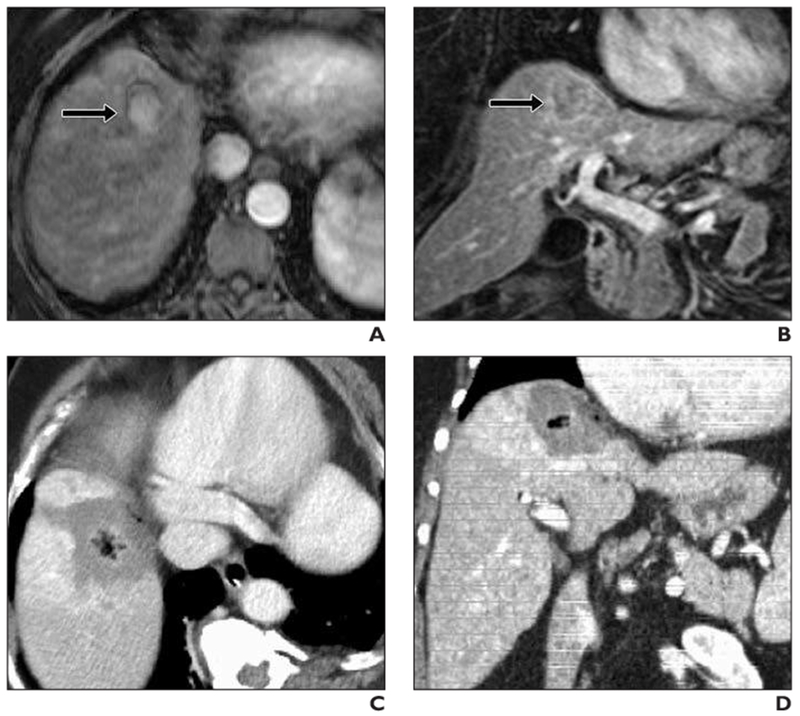
61-year-old woman with cirrhosis and 3-cm hepatocellular carcinoma at hepatic dome treated with microwave ablation (3 microwave antennas, 7 minutes, 65 Watts).
A, Preprocedure axial T1-weighted contrast-enhanced MRI shows 3-cm hypervascular lesion (arrow) at hepatic dome.
B, Preprocedure coronal T1-weighted contrast-enhanced MRI shows close proximity of tumor (arrow) to undersurface of diaphragm and heart.
C, Immediate postprocedure IV contrast-enhanced CT. Note large ablation zone in contact with medial diaphragm with no apparent diaphragm injury.
D, Immediate postprocedure coronal IV contrast-enhanced CT shows that ablation zone abuts diaphragm over long segment. Patient experienced only intermittent sharp abdominal pain (rated 5/10) immediately after procedure.
Fig. 3—
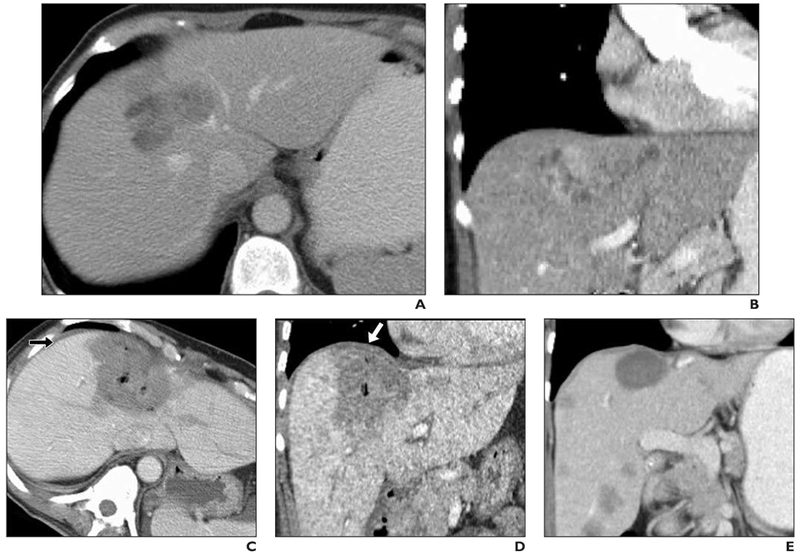
59-year-old woman with 6-cm metastatic uterine sarcoma in segment 8 of liver.
A, Preprocedure IV contrast-enhanced CT scan.
B, Preprocedure coronal IV contrast-enhanced CT scan.
C, Immediate postprocedure IV contrast-enhanced CT. Large ablation zone contacts diaphragm anteriorly. Note thin layer of fluid (arrow) as result of hydrodissection (450 mL of 5% dextrose in water) that separates liver capsule from diaphragm.
D, Immediate postprocedure coronal IV contrast-enhanced CT. Large ablation zone extends to hepatic dome. Note minimally increased thickness of diaphragm adjacent to ablation zone (arrow). Patient experienced mild shoulder pain (3/10 maximum) after procedure.
E, Follow-up CT 5 months later shows marked decrease in size of ablation zone, no evidence of diaphragm hernia or residual thickening, no local tumor progression, but new hepatic metastases.
Fig. 4—
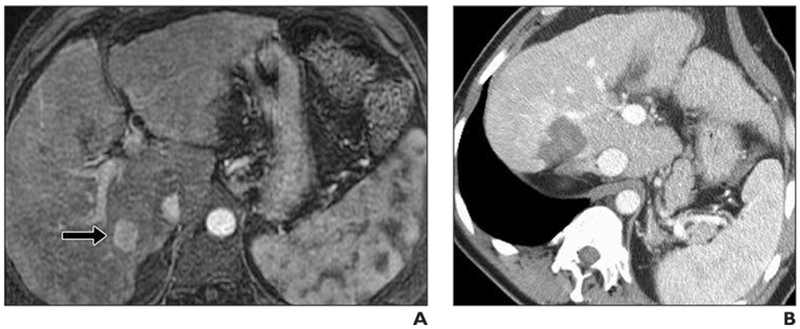
62-year-old man with cirrhosis and hepatocellular carcinoma.
A, Tumor (2 cm) (arrow) was identified in segment 6 of liver. This was ablated with single microwave antenna (3.5 minutes, 95 Watts).
B, Immediate postprocedure IV contrast-enhanced CT scan. Patient experienced mild (2/10) dull achy abdominal pain after procedure.
Ablation Power and Time
Ablation power and time was not significantly different when comparing peripheral liver lesions to central liver lesions (Table 1). In the subanalysis, power was statistically significantly lower in the nondisplaced group (70.8W ± 19.1) compared with the displaced (80.8W ± 23.7) and control (79.3W ± 19.6) groups (p = 0.02; Tables 1 and 2). There was no statistically significant difference in power between the no fluid (73.9W ± 23.2), fluid (76.1W ± 21.2), and control (79.3W ± 19.6) groups (Tables 2 and 3). No statistically significant difference in ablation times was detected across groups. The slightly larger ablation zone sizes in the treatment of peripheral tumors as compared with central tumors were marginally statistically significant (p = 0.05).
TABLE 1:
Peripheral Versus Central Tumors
| Characteristic | Peripheral (n = 55) | Central (n = 15) | p |
|---|---|---|---|
| Tumor size (cm) | 2.5 ± 1.4 | 2.5 ± 1.0 | 0.7 |
| Distance from tumor to diaphragm (mm) | 4.6 ± 6.2 | 41.5 ± 20.4 | < 0.001 |
| Displacement distance (mm) | 2.5 ± 4.3 | 0 | 0.002 |
| Ablation zone size (cm) | 4.3 ± 1.2 | 3.6 ± 0.8 | 0.05 |
| Length of ablation zone butting capsule (cm) | 3.6 ± 1.4 | 0 | < 0.001 |
| Ablation time (min) | 6.5 ± 3 | 5.5 ± 2.5 | 0.2 |
| Ablation power (W) | 75.4 ± 21.7 | 79.3 ± 19.6 | 0.1 |
| No. of diaphragmatic hernias | 0 | 0 | |
| Diaphragm thickness before ablation (mm) | 3.1 ± 1.2 | 3.0 ± 0.5 | 0.6 |
| Diaphragm thickness immediately after ablation (mm) | 4.0 ± 1.9 | 3.1 ± 0.5 | 0.2 |
| Patients with shoulder pain, no. (%) | 10 (18) | 0 (0) | 0.2 |
| Average pain score | 2.8 ± 2.1 | 2.4 ± 1.2 | 0.4 |
| Initial pain score | 3.5 ± 3.0 | 3.1 ± 2.2 | 0.6 |
| Local tumor progression, no. (%) | 3 (5.5) | 0 (0) | 1 |
| Imaging follow-up (mo) | 11.1 ± 7.9 | 14.1 ± 8.5 | 0.1 |
Note—Except where noted otherwise, data are mean ± SD. Peripheral tumors are those with ablation zone < 5 mm from diaphragm. Peripheral liver tumors include all lesions ablated regardless of artificial ascites. Central tumors are those with ablation zone > 1.5 cm from diaphragm.
TABLE 2:
Effect of Fluid Displacement
| Ablation Abutting Diaphragm | ||||
|---|---|---|---|---|
| Characteristic | Nondisplaced (n = 30) | Displaced (n = 25) | Control (n = 15) | p |
| Tumor size (cm) | 2.5 ± 1.5 | 2.6 ± 1.2 | 2.5 ± 1.0 | 0.6 |
| Distance from tumor to diaphragm (mm) | 4.9 ± 6.6 | 4.2 ± 6.0 | 41.5 ± 20.4 | < 0.001 |
| Displacement distance (mm) | 0 | 5.6 ± 5.0 | 0 | < 0.001 |
| Ablation zone size (cm) | 4.5 ± 1.3 | 4.1 ± 1.2 | 3.6 ± 0.8 | 0.1 |
| Length of ablation zone abutting capsule (cm) | 3.7 ± 1.6 | 3.4 ± 1.2 | 0 | < 0.001 |
| Ablation time (min) | 6.0 ± 2.7 | 7.2 ± 3.3 | 5.5 ± 2.5 | 0.2 |
| Ablation power (W) | 70.8 ± 19.1 | 80.8 ± 23.7 | 79.3 ± 19.6 | 0.02 |
| No. of diaphragmatic hernias | 0 | 0 | 0 | |
| Diaphragm thickness preablation (mm) | 3.2 ± 1.2 | 3.0 ± 1.2 | 3.0 ± 0.5 | 0.5 |
| Diaphragm thickness immediately postablation (mm) | 3.6 ± 1.3 | 4.4 ± 2.4 | 3.1 ± 0.5 | 0.3 |
| Patients with shoulder pain, no. (%) | 5 (16.7) | 5 (20) | 0 (0) | 0.2 |
| Average pain score | 3.1 ± 2.2 | 2.5 ± 1.8 | 2.4 ± 1.2 | 0.4 |
| Initial pain score | 4.2 ± 3.0 | 2.7 ± 2.8 | 3.1 ± 2.2 | 0.1 |
| Local tumor progression, no. (%) | 2 (6.7) | 1 (4) | 0 (0) | 1 |
| Imaging follow-up (mo) | 10.6 ± 6.6 | 11.6 ± 9.4 | 14.1 ± 8.5 | 0.2 |
Note—Except where noted otherwise, data are mean ± SD. Peripheral liver lesions are subdivided according to whether peritoneal fluid displaced the diaphragm away from the liver capsule (displaced vs nondisplaced).
TABLE 3:
Effect of Artificial Ascites
| Ablation Abutting Diaphragm | ||||
|---|---|---|---|---|
| Characteristic | No Fluid (n = 18) | Fluid (n = 37) | Control (n= 15) | p |
| Tumor size (cm) | 2.5 ± 1.7 | 2.6 ± 1.3 | 2.5 ± 1.0 | 0.7 |
| Distance from tumor to diaphragm (mm) | 6.3 ± 7.5 | 3.7 ± 5.5 | 41.5 ± 20.4 | < 0.001 |
| Displacement distance (mm) | 0 | 3.8 ± 4.9 | 0 | < 0.001 |
| Ablation zone size (cm) | 4.4 ± 1.4 | 4.3 ± 1.2 | 3.6 ± 0.8 | 0.1 |
| Length of ablation zone abutting capsule (cm) | 3.5 ± 1.4 | 3.6 ± 1.4 | 0 | < 0.001 |
| Ablation time (min) | 5.5 ± 1.9 | 7.0 ± 3.3 | 5.5 ± 2.5 | 0.2 |
| Ablation power (W) | 73.9 ± 23.2 | 76.1 ± 21.2 | 79.3 ± 19.6 | 0.2 |
| No. of diaphragmatic hernias | 0 | 0 | 0 | |
| Diaphragm thickness before ablation (mm) | 3.3 ± 1.1 | 3.0 ± 1.2 | 3.0 ± 0.5 | 0.4 |
| Diaphragm thickness immediately after ablation (mm) | 3.7 ± 1.3 | 4.1 ± 2.1 | 3.1 ± 0.5 | 0.5 |
| Patients with shoulder pain, no. (%) | 3 (16.7) | 7 (18.9) | 0 (0) | 0.3 |
| Average pain score | 3.0 ± 2.3 | 2.8 ± 2.0 | 2.4 ± 1.2 | 0.7 |
| Initial pain score | 3.9 ± 2.7 | 3.3 ± 3.1 | 3.1 ± 2.2 | 0.7 |
| Local tumor progression, no. (%) | 1 (5.6) | 2 (5.4) | 0 (0) | 1 |
| Imaging follow-up (mo) | 11.8 ± 7.4 | 10.8 ± 8.3 | 14.1 ± 8.5 | 0.2 |
Note—Except where noted otherwise, data are mean ± SD. Peripheral liver lesions are subdivided according to the use of artificial ascites.
Diaphragm Injury
There were no cases of diaphragmatic hernia in any patient in our study, including both the study and control groups (Tables 2 and 3). Diaphragm thickness on the immediate postablation CT was higher in the peridiaphragmatic subjects, but was not statistically significant (p = 0.2; Table 1). This remained true when controlling for artificial ascites and diaphragm displacement (Tables 2 and 3). There were a total of 10 cases of shoulder pain in the entire study group (control and peridiaphragmatic tumors). There were no cases of postprocedure shoulder pain in the control group versus five of 30 (16.7%) in the nondisplaced group, five of 25 (20%) in the displaced group, three of 18 (16.7%) in the no-fluid group, and seven of 37 (18.9%) in the fluid group (Tables 2 and 3). These differences in reported shoulder pain were not statistically significant between all groups.
Pain
Pain scores were higher in patients with peripheral liver tumors than in patients with centrally located tumors, but this difference was not statistically significant (Table 1). The initial self-reported pain score in the nondisplaced group (4.2 ± 3.0) was higher than those for the displaced (2.7 ± 2.8) and control (3.1 ± 2.2) groups but was not statistically significant (Table 2). There were no statistically significant differences in the initial patient self-reported pain scores among the no-fluid (3.9 ± 2.7), fluid (3.3 ± 3.1), and control (3.1 ± 2.2) groups (Table 3). The mean pain scores across the patient hospital admissions were also not statistically significantly different between groups (Tables 2 and 3).
Local Tumor Progression
The overall rate of local tumor progression in our study was low (3/69 [4.3%]). The frequency of local tumor progression was higher for peridiaphragmatic tumors than for central tumors (3/55 [5.5%] vs 0/15 [0.0%]) but this difference was not statistically significant (p = 1). Median follow-up of central tumors was 16 months (1–27 months), and median follow-up of peripheral tumors was 10 months (1–33 months).
Discussion
This retrospective study was undertaken to determine whether microwave ablation of subdiaphragmatic liver tumors is associated with a substantial risk of clinically significant diaphragm injuries and local tumor progression. We found that there was no significant difference in postprocedure pain and diaphragm hernias when comparing peripheral and central ablations. In fact, there were no cases of a significant complication in our entire study population. We also gathered data on surrogate measures of diaphragm injury, as described by prior authors [18, 22, 23]. Although postablation diaphragm thickness and shoulder pain were higher after microwave of peridiaphragmatic tumors compared with control tumors, these differences were not statistically significant. Therefore, our results suggest that collateral heating of the diaphragm may result in transient postprocedure symptoms but is not associated with a high rate of major complications, such as diaphragmatic perforation or hernia.
Many cases of hepatocellular carcinoma and hepatic metastases are peripherally located in close proximity to the diaphragm. As microwave devices become more commonly used in the treatment of peripheral and larger tumors, it is expected that more ablation zones will come into contact with the diaphragm. Therefore, an understanding of the risks associated with treating peridiaphragmatic tumors is needed to elucidate whether protective or alternative strategies are necessary. Indeed, case reports and retrospective studies of radiofrequency ablation of subdiaphragmatic liver lesions have suggested an increased risk of both diaphragm injury and local tumor progression [16–18, 22, 23]. However, many of these cases involved deployable radiofrequency electrodes and multiple treatments. Deployable electrodes may increase the likelihood of diaphragm injury due to both a diaphragm burn and protrusion of a tine through the diaphragm muscle. One recent study, although not controlled for ablation location, found an overall increased (but still low) incidence of diaphragm injury in microwave cases compared with radiofrequency ablation [21]. Although we report no cases of diaphragmatic hernia in this study, our sample size was smaller and therefore possibly underpowered to detect a low incidence complication. It is likely that, in the future with longer follow-up and more patients, diaphragm hernia will be encountered, but the data in this study suggest that the incidence will be low.
Local tumor progression was slightly higher for peridiaphragmatic tumors versus central control tumors (5.5% vs 0%), but this difference was not statistically significant. One explanation for the higher local tumor progression rate with peripheral tumors may be that these lesions were ablated more cautiously because of concern for collateral damage to the diaphragm and body wall. Indeed, in our study, physicians chose overall lower power for peridiaphragmatic tumors compared with central control tumors (75.4 vs 79.3 Watts), a difference that does not meet statistical significance but suggests a trend (p = 0.1). Another explanation for an increased rate of local tumor progression in peridiaphragmatic locations is the difficulty in imaging and positioning antennas in subdiaphragmatic tumors at the hepatic dome compared with centrally located hepatic lesions. This difficulty in imaging dome tumors increases the complexity of both needle placement and monitoring of the ablation zone. Both of these factors could ultimately increase the incidence of local tumor progression. Regardless, our overall rate of local tumor progression is comparable to [24, 25] or lower than [26, 27] that of historical microwave ablation controls and substantially lower compared with most large radiofrequency series [12, 18, 28, 29].
Because of the known risk to surrounding structures of peripheral hepatic ablations, hydrodissection has been studied for its thermoprotective effect. Both animal and human studies have found that the use of peritoneal fluid decreases both collateral injuries and postprocedure pain for radiofrequency ablation of peripheral liver tumors [30, 31]. Additionally, artificial ascites has been shown to improve ultrasound visibility of the targeted tumor, particularly at the hepatic dome [32, 33]. Our data show no significant difference in measures of diaphragm injury regardless of hydrodissection or fluid placement. It is important to note that the protective effects of fluid found with radiofrequency ablation may not translate to microwave given the physical differences in the mechanism of tissue heating. Specifically, fluid displacement has been shown to be beneficial in radiofrequency ablation by protecting nontargeted tissues from electrical current, which is not present in microwave ablations [30, 34]. However, pain scores and the incidence of shoulder pain were higher in the no-fluid group, but did not achieve statistical significance, likely because of high standard deviations, the subjective nature of pain scores, and relatively small sample sizes.
Our study was limited by its retrospective nature and small sample size. Additionally, we did not account for subjects contributing more than one ablation. A multivariable analysis was not done because of the lack of any statistical significance being detected among the measured parameters and the paucity of observed adverse events. Another limitation is the relatively short duration of follow-up, although we think that the mean imaging follow-up time in this study (≈ 1 year) was sufficient to detect most significant diaphragmatic injuries [21, 35, 36]. The range of follow-up in this study (1–33 months) is comparable to that reported in a comprehensive analysis of the literature (1–34 months) [35]. In the interest of completeness, we gathered all reported imaging data associated with diaphragmatic injury in previous studies, including postprocedure diaphragm thickness. Any differences between peripheral and central hepatic tumors were minimal, not statistically significant, and of dubious clinical significance. Our other measure of diaphragm injury was pain (both overall and shoulder pain), which by nature is subjective. Furthermore, there is inherent variability in the collection of pain data because of documentation and medication administration. The measurement of fluid displacement may have been underestimated in this study because it could be measured only retrospectively on the immediate postablation scan. By this time, the infused fluid may have diffused away from the diaphragm. Despite these limitations, the lack of any major complications from treatment of over 50 peridiaphragmatic tumors suggests that microwave ablation in this location is safe and well tolerated, and the true incidence of serious complications is likely to be low.
In summary, our study suggests that microwave ablation of hepatic tumors immediately adjacent to the diaphragm is safe and without significant risk of diaphragm injury, regardless of the use of artificial ascites. The rate of local tumor progression is minimally higher (but not statistically significant) in hepatic dome tumors, likely because of the more difficult approach for needle placement, decreased ability to image the tumor and ablation zone with ultrasound and CT, and resultant lower treatment power.
References
- 1.Livraghi T , Solbiati L , Meloni MF , Gazelle GS , Halpern EF , Goldberg SN . Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study . Radiology 2003; 226:441–451 [DOI] [PubMed] [Google Scholar]
- 2.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Renal cell carcinoma: clinical experience and technical success with radiofrequency ablation of 42 tumors. Radiology 2003; 226:417–424 [DOI] [PubMed] [Google Scholar]
- 3.Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H. Percutaneous radiofrequency ablation of malignancies in the lung. AJR 2000; 174:57–59 [DOI] [PubMed] [Google Scholar]
- 4.Dupuy DE, Liu D, Hartfeil D, et al. Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer 2010; 116:989–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol 2009; 20(suppl 7):S342–S347 [DOI] [PubMed] [Google Scholar]
- 6.van der Sluis FJ, Bosch JL, Terkivatan T, de Man RA, Ijzermans JN, Hunink MG. Hepatocellular adenoma: cost-effectiveness of different treatment strategies. Radiology 2009; 252:737–746 [DOI] [PubMed] [Google Scholar]
- 7.Sharpe EE 3rd, Dodd GD 3rd. Percutaneous radiofrequency ablation of symptomatic giant hepatic cavernous hemangiomas: report of two cases and review of literature. J Vasc Interv Radiol 2012;23:971–975 [DOI] [PubMed] [Google Scholar]
- 8.Tungjitkusolmun S, Staelin ST, Haemmerich D, et al. Three-dimensional finite-element analyses for radio-frequency hepatic tumor ablation. IEEE Trans Biomed Eng 2002; 49:3–9 [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol 2006; 59:432–441 [DOI] [PubMed] [Google Scholar]
- 10.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol 1996; 3:636–644 [DOI] [PubMed] [Google Scholar]
- 11.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2008; 47:82–89 [DOI] [PubMed] [Google Scholar]
- 12.Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005; 234:961–967 [DOI] [PubMed] [Google Scholar]
- 13.Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng 2010; 38:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Liang P, Yu X, Liu F, Chen L, Wang Y. A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol 2011; 79:124–130 [DOI] [PubMed] [Google Scholar]
- 15.Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007; 72(suppl 1): 124–131 [DOI] [PubMed] [Google Scholar]
- 16.Koda M, Ueki M, Maeda N, Murawaki Y. Diaphragmatic perforation and hernia after hepatic radiofrequency ablation. AJR 2003; 180:1561–1562 [DOI] [PubMed] [Google Scholar]
- 17.Shibuya A, Nakazawa T, Saigenji K, Furuta K, Matsunaga K. Diaphragmatic hernia after radiofrequency ablation therapy for hepatocellular carcinoma. AJR 2006; 186(suppl 5):S241–S243 [DOI] [PubMed] [Google Scholar]
- 18.Head HW, Dodd GD 3rd, Dalrymple NC, et al. Percutaneous radiofrequency ablation of hepatic tumors against the diaphragm: frequency of diaphragmatic injury. Radiology 2007; 243:877–884 [DOI] [PubMed] [Google Scholar]
- 19.Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: treatment with percutaneous microwave ablation—complications among cohort of 1136 patients. Radiology 2009; 251:933–940 [DOI] [PubMed] [Google Scholar]
- 20.Livraghi T, Meloni F, Solbiati L, Zanus G; Collaborative Italian Group Using AMICA System. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 2012; 35:868–874 [DOI] [PubMed] [Google Scholar]
- 21.Ding J, Jing X, Liu J, Wang Y, Wang F, Du Z. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol 2013; 68:608–615 [DOI] [PubMed] [Google Scholar]
- 22.Kang TW, Rhim H, Kim EY, et al. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol 2009; 10:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang TW, Rhim H, Lee MW, et al. Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: comparison of effects of thermal protection and therapeutic efficacy. AJR 2011; 196:907–913 [DOI] [PubMed] [Google Scholar]
- 24.Iannitti DA, Martin RC, Simon CJ, et al. Hepatic tumor ablation with clustered microwave antennae: the US Phase II trial. HPB (Oxford) 2007; 9:120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhardwaj N, Strickland AD, Ahmad F, et al. Microwave ablation for unresectable hepatic tumours: clinical results using a novel microwave probe and generator. Eur J Surg Oncol 2010; 36:264–268 [DOI] [PubMed] [Google Scholar]
- 26.Groeschl RT, Wong RK, Quebbeman EJ, et al. Recurrence after microwave ablation of liver malignancies: a single institution experience. HPB (Oxford) 2013; 15:365–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seki S, Sakaguchi H, Iwai S, et al. Five-year survival of patients with hepatocellular carcinoma treated with laparoscopic microwave coagulation therapy. Endoscopy 2005; 37:1220–1225 [DOI] [PubMed] [Google Scholar]
- 28.Kei SK, Rhim H, Choi D, Lee WJ, Lim HK, Kim YS. Local tumor progression after radiofrequency ablation of liver tumors: analysis of morphologic pattern and site of recurrence. AJR 2008; 190:1544–1551 [DOI] [PubMed] [Google Scholar]
- 29.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR 2007; 188:480–488 [DOI] [PubMed] [Google Scholar]
- 30.Laeseke PF, Sampson LA, Brace CL, Winter TC 3rd, Fine JP, Lee FT Jr. Unintended thermal injuries from radiofrequency ablation: protection with 5% dextrose in water. AJR 2006; 186(suppl 5):S249–S254 [DOI] [PubMed] [Google Scholar]
- 31.Hinshaw JL, Laeseke PF, Winter TC 3rd, Kliewer MA, Fine JP, Lee FT Jr. Radiofrequency ablation of peripheral liver tumors: intraperitoneal 5% dextrose in water decreases postprocedural pain. AJR 2006; 186(suppl 5):S306–S310 [DOI] [PubMed] [Google Scholar]
- 32.Ohmoto K, Tsuzuki M, Yamamoto S. Percutaneous microwave coagulation therapy with intraperitoneal saline infusion for hepatocellular carcinoma in the hepatic dome. AJR 1999; 172:65–66 [DOI] [PubMed] [Google Scholar]
- 33.Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation with artificial ascites for hepatocellular carcinoma in the hepatic dome: initial experience. AJR 2008; 190:91–98 [DOI] [PubMed] [Google Scholar]
- 34.Brace CL, Laeseke PF, Prasad V, Lee FT. Electrical isolation during radiofrequency ablation: 5% dextrose in water provides better protection than saline. Conf Proc IEEE Eng Med Biol Soc 2006; 1:5021–5024 [DOI] [PubMed] [Google Scholar]
- 35.Zhou M, He H, Cai H, et al. Diaphragmatic perforation with colonic herniation due to hepatic radiofrequency ablation: a case report and review of the literature. Oncol Lett 2013; 6:1719–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JS, Kim HS, Myung DS, et al. A case of diaphragmatic hernia induced by radiofrequency ablation for hepatocellular carcinoma. Korean J Gastroenterol 2013; 62:174–178 [DOI] [PubMed] [Google Scholar]


