Introduction
Multiple myeloma is a hematologic malignancy characterized by significant bone disease – lytic lesions are present in over 60% of patients at diagnosis, as are pathologic fractures, osteoporosis, and hypercalcemia1. Due to the frequency and morbidity of bone disease in these patients, guidelines from the National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), International Myeloma Working Group and European Myeloma Network all recommend use of the bisphosphonates zoledronic acid and pamidronate in patients receiving primary myeloma therapy2,3. In 2018, NCCN and ASCO updated their recommendations to include the osteoclast inhibitor denosumab as an alternative anti-resorptive agent, after evidence that it is noninferior to zoledronic acid in delaying time to first skeletal-related event (SRE)4,5.
There is considerable evidence to support the use of bisphosphonates from randomized controlled trials. Pamidronate and zoledronic acid have been shown to significantly reduce SREs (defined as pathologic fracture, radiation or surgery on bone and spinal cord compression) and bone pain, with a relative risk reduction of 16–17% in some studies6,7 and absolute risk reduction of 0.9 SRE per year in another8. Whether bisphosphonates improve survival is controversial and may vary by the specific agent used9. Pamidronate was associated with a survival benefit in one study for relapsed or refractory myeloma patients (median 21 months survival in users versus 14 months in those receiving placebo; p =0.04)8. The MRC Myeloma IX trial of 1,960 newly diagnosed patients found that zoledronic acid improved survival compared to clodronate, but only in patients with bone disease or SRE at baseline (hazard ratio (HR) 0.82; 95%CI 0.70–0.95)10,11.
The compelling evidence regarding the benefit of bisphosphonates resulted in a change of NCCN guidelines in 2011 to recommend bisphosphonate treatment for all newly diagnosed myeloma patients receiving primary myeloma therapy, without contraindication. We performed a population-based analysis to investigate whether newly diagnosed myeloma patients received bisphosphonates in accordance with guidelines. We examined predictors for bisphosphonate use, and also whether use is associated with a change in survival on a population level.
Methods
Data Source
This retrospective cohort study used data from the Surveillance, Epidemiology and End Results (SEER)-Medicare Database12. SEER is a population-based tumor registry developed by the National Cancer Institute (NCI) that captures time of diagnosis, tumor details, and sociodemographic characteristics for individuals with cancer over 18 geographic areas of the United States (U.S.), representing approximately 28% of the population13. Medicare captures billed claims submitted through inpatient (Part A), outpatient (Part B), and prescription drug (Part D) coverages. The SEER and Medicare files are linked to provide data about individual patients from their time of cancer diagnosis onward14.
Cohort Selection
Individuals 65 years or older diagnosed with multiple myeloma (SEER Site Recode 34000) between 2012 to 2013 were included in this analysis. Patients were required to have received antineoplastic therapy appropriate for myeloma (Supplemental Data S1) within 6 months after diagnosis, and to have complete Medicare Parts A and B coverage from 12 months before diagnosis onward. We excluded patients who had received bisphosphonates prior to myeloma diagnosis, who had less than 6 months of follow-up after diagnosis, and individuals diagnosed by autopsy.
Patient Characteristics
Age at diagnosis was stratified into 65–74, 75–84, and ≥85 years. Race was recorded as white, black and other. Marital status was recorded as married and unmarried. Socioeconomic status (SES) was calculated from education, poverty level and income data from the 2000 census using reported methods15.
A patient was considered to have a SRE at diagnosis if he/she had a claim for one within 90 days before myeloma diagnosis and 60 days after. SRE was defined as pathologic fracture, spinal cord compression, radiation, and surgery to bone7,8,16. Codes used to identify SREs are given in Supplemental Data S216–19.
The presence of several comorbid conditions at any time before the diagnosis of myeloma was recorded – acute kidney injury, chronic kidney disease, use of hemodialysis, osteoporosis, osteopenia, and hypercalcemia. Comorbidity score was assessed using the Klabunde adaptation of the Charlson comorbidity index20. The initial antineoplastic regimen used after diagnosis, defined as all antineoplastic drugs a patient received within the first 90 days of treatment, was recorded and categorized as proteasome inhibitor-based (proteasome inhibitor with or without steroids), immunomodulatory imide agent-based, proteasome inhibitor + immunomodulatory agent, proteasome inhibitor + other cytotoxic agent, and other. The specific drugs in each class and their identifying codes are listed in Supplemental Data S1. Of note, steroids come in many formulations and accompanying drug codes. We examined their use, but do not believe our methodology fully captured use as we found that only 58% of patients received steroids as part of their antineoplastic regimen – significantly lower than what we expected since steroids are nearly always given.
Measures
The primary outcome of this analysis was the use of bisphosphonates, defined as whether a patient received a bisphosphonate within 6 months after start of his/her myeloma therapy. All bisphosphonates that have been evaluated for use in myeloma were included, with a full list given in Supplemental Data S3. Duration of bisphosphonate use, defined as the time interval between the first and last doses, was determined. We calculated the percentage of time patients received bisphosphonates while on anti-neoplastic therapy, whereby a patient treated according to guidelines would receive bisphosphonates 100% of the time that he/she is on anti-neoplastic therapy.
We assessed overall survival from diagnosis as a function of bisphosphonate use. Patients were followed from myeloma diagnosis to death, end of enrollment from SEER-Medicare, or 12/3½014, whichever was earliest.
Statistical Analyses
We conducted univariate and multivariable logistic regression analyses to evaluate which variables were associated with the use of bisphosphonates. Proportions were compared using the χ2 test. Kaplan-Meier analysis was used to evaluate survival, with hypothesis testing conducted using the log-rank test. Cox proportional hazards models were used to adjust for the effects of covariates on both outcomes, with the proportional hazards assumption evaluated by the Wald test21. For all models, we used α <0.05 and two-sided tests of statistical significance. All analyses were conducted using SAS 9.3 (SAS Institute, 2010).
This study used deidentified data and was deemed exempt by our Institutional Review Board.
Results
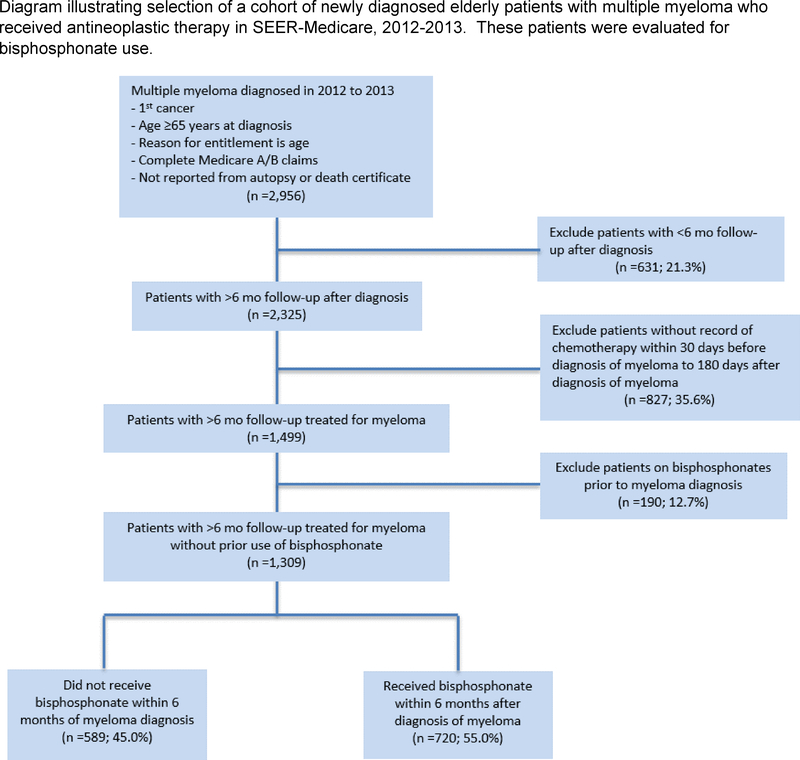
We identified 1,309 patients between January 1, 2012, and December 31, 2013 with a diagnosis of multiple myeloma who received chemotherapy within 6 months of diagnosis and who had >6 months of follow-up after diagnosis (Figure 1). Among them, 720 (55%) received at least one dose of a bisphosphonate within 6 months after the start of antineoplastic therapy, while 589 (45%) did not.
Figure 1.
Diagram illustrating selection of a cohort of newly diagnosed elderly patients with multiple myeloma who received antineoplastic therapy in SEER-Medicare, 2012–2013. These patients were evaluated for bisphosphonate use.
Zoledronic acid was the most commonly used bisphosphonate, in 640 of the 720 (89%) patients. Pamidronate was used in 139 (19%) patients. Some patients – 84 (12%) received more than one bisphosphonate agent over the course of their treatment, with the most common scenario being use of both zoledronic acid and pamidronate, in 66 patients. Median time to initiation of bisphosphonates from myeloma diagnosis was 52 days (IQR 26–152).
Among users, median number of doses given was 8 (max 33, interquartile range (IQR) 4–13), and median duration of bisphosphonate use was 294 days (IQR 89–569). On average, patients received bisphosphonates covering 66% (IQR 43–93%) of the time over which they received chemotherapy. By comparison, a patient receiving bisphosphonate every month he/she is on chemo would have a coverage of 100%.
Descriptive statistics for users of bisphosphonates, and factors associated with use, are summarized in Table 1. In multivariable analysis, factors associated with decreased use of bisphosphonates were chronic kidney disease (adjusted odds ratio [OR] 0.48; 95%CI 0.35–0.66), hemodialysis (AOR 0.42; 95%CI 0.24–0.75), and residence in a Southern region (AOR 0.66; 95%CI 0.45–0.99). Factors associated with increased use of bisphosphonates were having a SRE at time of diagnosis (AOR 2.60; 95%CI 1.98–3.40), hypercalcemia (AOR 1.74; 95%CI 1.26–2.41), and use of proteasome inhibitor + immunomodulatory drug regimens (AOR 1.70; 95%CI 1.36–2.85 compared to proteasome inhibitor regimens without immunomodulator).
Table 1.
Factors Associated with Use of Bisphosphonates in Myeloma Patients Who Received Chemotherapy.
| Total | Univariate |
Multivariable |
|||
|---|---|---|---|---|---|
| # User (%) | P-value | Point estimate | 95% Wald CI | ||
| Overall | 1,309 | 720 (55.0%) | N/A | N/A | N/A |
| Age group | |||||
| 65 – 74 | 735 | 409 (55.7%) | 0.016 | REF | REF |
| 75 – 84 | 485 | 275 (56.7%) | 1.22 | 0.94–1.58 | |
| ≥85 | 89 | 36 (40.5%) | 0.66 | 0.40–1.08 | |
| Gender | |||||
| Male | 753 | 417 (55.4%) | 0.75 | REF | REF |
| Female | 556 | 303 (54.5%) | 0.92 | 0.70–1.22 | |
| Race | |||||
| White | 1,027 | 583 (56.8%) | 0.031 | REF | REF |
| Black | 199 | 93 (46.7%) | 0.85 | 0.59–1.21 | |
| Othera | 83 | 44 (53.0%) | 0.75 | 0.46–1.25 | |
| Marital status | |||||
| Unmarried | 543 | 293 (54.0%) | 0.52 | REF | REF |
| Married | 766 | 427 (55.7%) | 0.92 | 0.71–1.19 | |
| Region | |||||
| North East | 219 | 125 (57.1%) | REF | REF | |
| North Central | 169 | 100 (59.2%) | 0.003 | 1.00 | 0.63–1.60 |
| South | 385 | 181 (47.0%) | 0.66 | 0.45–0.99 | |
| West | 536 | 314 (58.6%) | 1.13 | 0.78–1.63 | |
| SES rank | |||||
| Lowest quintile | 293 | 157 (53.6%) | REF | REF | |
| Second quintile | 368 | 184 (50.0%) | 0.007 | 0.79 | 0.56–1.11 |
| Third quintile | 275 | 173 (62.9%) | 1.25 | 0.86–1.82 | |
| Fourth quintile | 198 | 118 (59.6%) | 1.07 | 0.70–1.61 | |
| Highest quintile | 175 | 88 (50.3%) | 0.73 | 0.47–1.14 | |
| Acute kidney injury | |||||
| No | 830 | 503 (60.6%) | <0.001 | REF | REF |
| Yes | 479 | 217 (45.3%) | 0.85 | 0.62–1.17 | |
| Chronic kidney disease | |||||
| No | 771 | 498 (64.6%) | <0.001 | REF | REF |
| Yes | 538 | 222 (41.3%) | 0.48 | 0.35–0.66 | |
| Osteoporosis | |||||
| No | 934 | 518 (55.5%) | 0.60 | REF | REF |
| Yes | 375 | 202 (53.9%) | 0.76 | 0.57–1.02 | |
| Osteopenia | |||||
| No | 621 | 303 (48.8%) | <0.001 | REF | REF |
| Yes | 688 | 417 (60.6%) | 1.24 | 0.96–1.60 | |
| Hypercalcemia | |||||
| No | 1,038 | 553 (53.3%) | 0.014 | REF | REF |
| Yes | 271 | 167 (61.6%) | 1.74 | 1.26–2.41 | |
| Hemodialysis | |||||
| No | 1,229 | 699 (56.9%) | <0.001 | REF | REF |
| Yes | 80 | 21 (26.3%) | 0.42 | 0.24–0.75 | |
| Comorbidity score | |||||
| 0 | 549 | 341 (62.1%) | REF | REF | |
| 1 | 282 | 156 (55.3%) | <0.001 | 0.79 | 0.57–1.08 |
| ≥2 Missing | 46216 | 217 (47.0%) | 1.05 | 0.77–1.42 | |
| Antineoplastic regimen | |||||
| PI ± steroid | 605 | 319 (52.73%) | REF | REF | |
| IMID ± steroid | 192 | 98 (51.04%) | 0.78 | 0.54–1.12 | |
| PI + IMID ± steroid | 252 | 165 (65.48%) | 0.007 | 1.70 | 1.21–2.39 |
| PI + other cytotoxic ± steroid | 190 | 100 (52.63%) | 0.99 | 0.69–1.42 | |
| Otherb | 70 | 38 (54.29%) | 1.02 | 0.59–1.75 | |
| SRE at Diagnosis | |||||
| No | 818 | 368 (45.0%) | <0.001 | REF | REF |
| Yes | 491 | 352 (71.7%) | 2.60 | 1.98–3.40 | |
Note: Statistically significant results in bold.
Abbreviations: IMID = immunomodulatory imide agent; PI = proteasome inhibitor; SES = socioeconomic status; SRE = skeletal related events.
Other race includes: Asian, Hispanic, North American Native, and Unknown.
Other antineoplastic regimens include: other cytotoxic agents ± steroids (34 patients), IMID + other cytotoxic agents ± steroids (10 patients) and PI + IMID + other cytotoxic agents ± steroids (26 patients).
We examined the effects of bisphosphonate use on survival using multivariable proportional hazards analysis (Table 2). Users of bisphosphonates had a lower hazard ratio for death (HR 0.70; 95%CI 0.56–0.88) compared to non-users after adjusting for covariates, including comorbidities and antineoplastic therapy use. Other factors associated with decreased risk of death include female gender (HR 0.76; 95%CI 0.59–0.97) and proteasome inhibitor + immunomodulatory imide agent use (HR 0.67; 95%CI 0.48–0.92 compared to proteasome inhibitor use without immunomodulatory agent). Factors associated with increased risk were age ≥85 (HR 1.98; 95%CI 1.37–2.87 compared to ages 65–74), hemodialysis (1.50; 95%CI 1.01–2.22), hypercalcemia (HR 1.37; 95%CI 1.06–1.78), and higher comorbidity (HR 1.32 for comorbidity score of ≥2 compared to 0; 95%CI 1.01–1.72).
Table 2.
Multivariable Cox Proportional Hazards Analysis of Overall Survival in Myeloma Patients Who Received Chemotherapy.
| Parameter | HR (for death) | 95% CI |
|---|---|---|
| Bisphosphonate use | ||
| Users vs non-users | 0.70 | 0.56–0.88 |
| Age group | ||
| 75 – 84 vs 65 – 74 | 1.25 | 1.00–1.58 |
| ≥85 vs 65 – 74 | 1.98 | 1.37–2.87 |
| Gender | ||
| Female vs male | 0.76 | 0.59–0.97 |
| Race | ||
| Black vs white | 0.99 | 0.72–1.35 |
| Other vs white | 1.07 | 0.69–1.65 |
| Marital status | ||
| Married vs unmarried | 1.09 | 0.86–1.37 |
| Region | ||
| North Central vs North East | 1.03 | 0.68–1.56 |
| South vs North East | 1.23 | 0.86–1.76 |
| West vs North East | 1.05 | 0.76–1.47 |
| SES rank | ||
| Second quintile vs lowest quintile | 0.98 | 0.72–1.32 |
| Third quintile vs lowest quintile | 0.97 | 0.70–1.34 |
| Fourth quintile vs lowest quintile | 0.95 | 0.66–1.37 |
| Highest quintile vs lowest quintile | 0.85 | 0.58–1.26 |
| Acute kidney injury | ||
| Yes vs no | 1.16 | 0.88–1.53 |
| Chronic kidney disease | ||
| Yes vs no | 1.01 | 0.77–1.33 |
| Osteoporosis | ||
| Yes vs no | 1.21 | 0.94–1.54 |
| Osteopenia | ||
| Yes vs no | 1.02 | 0.82–1.28 |
| Hypercalcemia | ||
| Yes vs no | 1.37 | 1.06–1.78 |
| Hemodialysis | ||
| Yes vs no | 1.50 | 1.01–2.22 |
| Comorbidity score | ||
| 1 vs 0 | 0.85 | 0.63–1.16 |
| ≥2 vs 0 | 1.32 | 1.01–1.72 |
| Antineoplastic regimen | ||
| IMID ± steroid vs PI ± steroid | 0.77 | 0.56–1.07 |
| PI + IMID ± steroid vs PI ± steroid | 0.67 | 0.48–0.92 |
| PI + other cytotoxic ± steroid vs PI ± steroid | 0.97 | 0.71–1.31 |
| Other vs PI ± steroid | 0.79 | 0.49–1.29 |
| SRE at diagnosis | ||
| Yes vs no | 1.23 | 0.97–1.57 |
Note: Statistically significant results in bold.
Abbreviations: HR = hazard ratio; IMID = immunomodulatory imide agent; PI = proteasome inhibitor; SES = socioeconomic status; SRE = skeletal related events
Discussion
In this retrospective cohort study, we found that 55% of myeloma patients diagnosed between 2012–2013 were started on a bisphosphonate within 6 months of starting antineoplastic therapy. Zoledronic acid was much more frequently used than pamidronate. Though patients should be receiving bisphosphonates every month by guidelines, on average those in our cohort received 2 doses of bisphosphonates every 3 months (66% coverage). Having a SRE at the time of diagnosis was a strong predictor of bisphosphonate use, as were hypercalcemia and use of proteasome inhibitor + immunomodulatory imide combination regimens. Chronic kidney disease and hemodialysis were associated with decreased use. The use of bisphosphonates was associated with improved survival even after adjusting for demographic characteristics, comorbidities, and initial myeloma therapy.
Though the use of bisphosphonates in myeloma is well-supported by evidence and has been recommended by professional society guidelines for two decades22,23, the real-world adoption of this practice has not been examined until recently. Investigators at Amgen examined bisphosphonate use using electronic health records from Flatiron Health, consisting of data drawn from over 250 cancer clinics and covering 1.5 million U.S. cancer patients treated mostly at community practices. They found that of 11,112 myeloma patients treated between 2009 and 2016, 63% received at least one dose of pamidronate or zoledronic acid24. Median time to bisphosphonate initiation was 29 days. Patients with chronic kidney disease were less likely to receive bisphosphonates (72% of patients with stage 1 compared to 24% with stage 5; HR 0.22; 95%CI 0.18–0.28).
Two other population-based analyses of bisphosphonate use in myeloma have been published. One utilized the United States Veterans cancer registry to compare the use of zoledronic acid to pamidronate in 1,018 newly diagnosed patients between 2002–2009. Patients receiving zoledronic acid had significantly improved survival (HR 0.78; 95%CI 0.67–0.92) and fewer SREs (HR 0.75; 95%CI 0.60–0.94) compared to those who received pamidronate16. In comparison, our analysis found that users of bisphosphonates collectively had improved survival compared to non-users. We did not examine the impact of bisphosphonates on SRE development due to a few concerns. Administrative claims for SREs may not accurately reflect the time of occurrence, duration and nature of SREs, making it difficult to distinguish new SREs from existing ones19. Additionally, our dataset does not contain certain variables which reflect the degree of bone disease at diagnosis, such as results of skeletal imaging and presence of bone pain. These variables may have a significant effect on the development of SREs separate from bisphosphonate use and we could not control for them.
A second population-based study used 2 large managed-care databases comprised of commercially insured patients to compare users of zoledronic acid to those who did not receive any bisphosphonates. Over 1,655 myeloma patients (newly diagnosed and relapsed/refractory) were identified between 2001–2006. Those receiving zoledronic acid had improved survival (incidence rate ratio 0.58; p <0.001) and lower rates of SREs (incidence rate ratio 0.63; p <0.001). These benefits were most prolonged in those who used zoledronic acid the longest25. In comparison to this study, we examined a more uniform population (newly diagnosed, treated patients) in which everyone should receive a bisphosphonate, and incorporated more demographic and clinical variables.
The median time to initiation of bisphosphonates in our cohort was 52 days after the start of antineoplastic therapy. One potential explanation for the delay is the time associated with obtaining a baseline dental exam, which is recommended prior to bisphosphonate use5,26. Given that we only had data about bisphosphonate use over 2012–2013, we were not able to meaningfully examine duration of use. Most guidelines recommend use for at least 2 years2–4, though the optimal duration has not been clearly established27. We did find that on average, patients received 2 doses of bisphosphonates every 3 months, which is lower than the monthly dosing that is currently recommended by guidelines for patients with active myeloma on antineoplastic therapy2–4. However, there is now evidence that one dose of zoledronic acid every 3 months is noninferior to monthly dosing for the prevention of SREs28, suggesting that some patients may have received adequate therapy for bone disease.
Our findings that bisphosphonate use decreased with chronic kidney disease and hemodialysis, and increased with SRE at diagnosis and hypercalcemia conform to clinical practice and thus are not surprising. We also found that patients who receive proteasome inhibitor + immunomodulatory imide ± steroid (“triplet”) regimens had increased use of bisphosphonates, which supports the notion that those who receive the most up-to-date antineoplastic therapies are also more likely to receive other guideline-based care. Interestingly, these patients also had improved survival compared to those receiving proteasome inhibitors without immunomodulatory drugs, but we cannot tell if this effect is from increased use of up-to-date and guideline-based care, or differences in disease or patient characteristics. The finding that bisphosphonate use impacted survival is biologically plausible, and is in accordance with some prospective studies8,10. Bisphosphonates induce osteoclast apoptosis by inhibiting the mevalonate pathway required for the prenylation of proteins such as Ras29, helping to restore bone health. They have antiproliferative effects against myeloma cells, and also anti-angiogenic and immunomodulatory properties30,31.
Strengths of our study include the use of a nationally representative database and examination of users of bisphosphonates compared to non-users. Our study also has important limitations. Our population only included those aged 65 or older and reflects <10% of newly diagnosed myeloma patients in the U.S. during the time period included. We lacked data about dental health, an important mediator of bisphosphonate use. We also lacked other important covariates that could predict outcomes such as disease stage, cytogenetics, or response to therapy, although none of these variables should affect the decision to treat patients with bisphosphonates. The use of billing codes to capture data can lead to under-capture of interventions. Our data covered only the years 2012–2013 as more recent data had not yet been released at the time we initiated this study; nonetheless, this limits our ability to examine bisphosphonate use over a longer time interval.
In conclusion, we found that 55% of newly diagnosed myeloma patients who receive antineoplastic therapy were given a bisphosphonate in accordance with national guidelines. Given the clinical evidence supporting the effectiveness of bisphosphonates in myeloma, we advocate for efforts to increase bisphosphonate use in these patients.
Supplementary Material
Acknowledgement of research support
This work was supported by the National Cancer Institute under Grants R25 CA094061 (Dr. Leng), R01 CA166084 (Dr. Hershman), and R01 CA169121 (Dr. Wright).
References
- 1.Kyle RA, Gertz MA, Witzig TE, et al. : Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78:21–33, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Terpos E, Morgan G, Dimopoulos MA, et al. : International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol 31:2347–57, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terpos E, Kleber M, Engelhardt M, et al. : European Myeloma Network guidelines for the management of multiple myeloma-related complications. Haematologica 100:1254–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson K, Ismaila N, Flynn PJ, et al. : Role of Bone-Modifying Agents in Multiple Myeloma: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 36:812–818, 2018 [DOI] [PubMed] [Google Scholar]
- 5.NCCN: NCCN Clinical Practice Guidelines in Oncology: Multiple Myeloma, NCCN, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Berenson JR, Lichtenstein A, Porter L, et al. : Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med 334:488–93, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Rosen LS, Gordon D, Kaminski M, et al. : Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 98:1735–44, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Berenson JR, Lichtenstein A, Porter L, et al. : Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol 16:593–602, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Mhaskar R, Redzepovic J, Wheatley K, et al. : Bisphosphonates in multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev:Cd003188, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Morgan GJ, Davies FE, Gregory WM, et al. : First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 376:1989–99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan GJ, Davies FE, Gregory WM, et al. : Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: the Medical Research Council Myeloma IX Trial. Blood 119:5374–83, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Wright JD, Burke WM, Tergas AI, et al. : Comparative Effectiveness of Minimally Invasive Hysterectomy for Endometrial Cancer. J Clin Oncol 34:1087–96, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SEER: About the SEER Registries. https://seer.cancer.gov/registries/, 2018
- 14.Warren JL, Klabunde CN, Schrag D, et al. : Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 40:Iv-3–18, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Du XL, Fang S, Vernon SW, et al. : Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer 110:660–9, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Sanfilippo KM, Gage B, Luo S, et al. : Comparative effectiveness on survival of zoledronic acid versus pamidronate in multiple myeloma. Leuk Lymphoma 56:615–21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDougall JA, Bansal A, Goulart BH, et al. : The Clinical and Economic Impacts of Skeletal-Related Events Among Medicare Enrollees With Prostate Cancer Metastatic to Bone. Oncologist 21:320–6, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain A, Aly A, Daniel Mullins C, et al. : Risk of skeletal related events among elderly prostate cancer patients by site of metastasis at diagnosis. Cancer Med 5:3300–3309, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aly A, Onukwugha E, Woods C, et al. : Measurement of skeletal related events in SEER-Medicare: a comparison of claims-based methods. BMC Med Res Methodol 15:65, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, et al. : Development of a comorbidity index using physician claims data. J Clin Epidemiol 53:1258–67, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Cox DR: Regression Models and Life-Tables. J Royal Stat Soc 34:187–220, 1972 [Google Scholar]
- 22.Bloomfield DJ: Should bisphosphonates be part of the standard therapy of patients with multiple myeloma or bone metastases from other cancers? An evidence-based review. J Clin Oncol 16:1218–25, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Berenson JR, Hillner BE, Kyle RA, et al. : American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol 20:3719–36, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kim C, Hernandez RK, Cyprien L, et al. : Patterns of bisphosphonate treatment among patients with multiple myeloma treated at oncology clinics across the USA: observations from real-world data. Support Care Cancer, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henk HJ, Teitelbaum A, Perez JR, et al. : Persistency with zoledronic acid is associated with clinical benefit in patients with multiple myeloma. Am J Hematol 87:490–5, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Terpos E, Roodman GD, Dimopoulos MA: Optimal use of bisphosphonates in patients with multiple myeloma. Blood 121:3325–8, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Raje N, Vescio R, Montgomery CW, et al. : Bone Marker–Directed Dosing of Zoledronic Acid for the Prevention of Skeletal Complications in Patients with Multiple Myeloma: Results of the Z-MARK Study. Clinical Cancer Research 22:1378–1384, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Himelstein AL, Foster JC, Khatcheressian JL, et al. : Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: A randomized clinical trial. JAMA 317:48–58, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luckman SP, Hughes DE, Coxon FP, et al. : Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 13:581–9, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Corso A, Ferretti E, Lazzarino M: Zoledronic acid exerts its antitumor effect in multiple myeloma interfering with the bone marrow microenvironment. Hematology 10:215–24, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Modi ND, Lentzsch S: Bisphosphonates as antimyeloma drugs. Leukemia 26:589, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.