Abstract
Objectives
To assess if ubiquitinated proteins potentially present in neutrophil extracellular traps (NETs) can modify cellular responses and induce inflammatory mechanisms in systemic lupus erythematosus (SLE) patients and healthy subjects.
Materials and Methods
We studied 74 subjects with SLE and 77 healthy controls. Neutrophils and low-density granulocytes were isolated, and NETs were induced. Ubiquitin content was quantified in NETs by Western Blot, ELISA and immunofluorescence microscopy, while ubiquitination of NET proteins was assessed by immunoprecipitation. Monocyte-derived macrophages from SLE and controls were isolated and stimulated with NETs or ubiquitin. Calcium flux and cytokine synthesis were measured following these stimuli.
Results
NETs contain ubiquitinated proteins, with a lower expression of polyubiquitinated proteins in SLE subjects than in controls. Myeloperoxidase (MPO) is present in ubiquitinated form in NETs. SLE patients develop anti-ubiquitinated MPO antibodies, and titers positively correlate with SLEDAI score (p<0.01), and negatively correlate with complement components (p<0.01). Stimulation of monocyte-derived macrophages with NETs or with ubiquitin led to enhanced calcium flux. In addition, stimulation with NETs led to enhanced cytokine (TNF-α and IL-10) production in macrophages from SLE patients when compared to controls, which was hampered by inhibition of NET internalization by macrophages.
Conclusion
This is the first study to find ubiquitinated proteins in NETs, and evidence for adaptive immune responses directed towards ubiquitinated NET proteins in SLE. The distinct differences in ubiquitin species profile in NETs compared to healthy controls may contribute to dampened anti-inflammatory responses observed in SLE. These results also support a role for extracellular ubiquitin in inflammation in SLE.
Keywords: neutrophil extracellular traps, ubiquitin, macrophages, systemic lupus erythematosus
INTRODUCTION
Neutrophil extracellular traps (NETs) are extracellular fibers primarily composed of nucleic acids bound to neutrophil granule–derived proteins1. The process of NET formation (NETosis) has recently been associated with the pathogenesis of systemic lupus erythematosus (SLE), since it causes the exposure of modified (oxidized) extracellular DNA2,3. Increased NET formation occurs in SLE, in particular mediated by low-density granulocytes (LDGs), a proinflammatory neutrophil subset increased in lupus patients4. In addition, an impaired NET degradation has been described in SLE, promoting an imbalance that prolongs the half-life of NET components5,6. Macrophages, which have been found to be defective in phagocytosing autoantigens in SLE patients7, are involved in NET clearance8. While NET degradation by macrophages has not been found to be pro-inflammatory in healthy subjects8, the process has not been studied in SLE patients.
Post translational modifications (PTMs) can alter protein structure and function. Different NET components, such as histones, are susceptible to undergo PTMs (i.e. acetylation, citrullination)9,10. Ubiquitination is a PTM that has typically been associated with protein degradation, but it can also impact many cellular processes by modifying protein function and gene transcription11–13. In SLE patients, defects in ubiquitination have been proposed to promote loss of peripheral tolerance14. Furthermore, extracellular ubiquitin has been recently described as an immune system regulator in diverse conditions15–18. When extracellular ubiquitin binds its receptor, CXCR419, a signaling cascade promotes numerous intracellular processes, including increases in calcium flux19,20.
Since NET proteins may be the target of different PTMs and ubiquitination is associated with immune tolerance, it is relevant to address whether NETs from SLE patients are differentially ubiquitinated, in order to analyze the implications of this process in the immune dysregulation characteristic of this disease. Therefore, the aim of this study was to evaluate the potential role of ubiquitination in inflammatory responses triggered by NETs in SLE patients and healthy controls.
METHODS
For full Methods, see online supplementary text.
Patients and controls
We included 74 SLE subjects, who fulfilled ≥4 ACR21 classification criteria and had different levels of disease activity according to the SLEDAI score22, as well as 77 healthy controls.. All subjects signed informed consent; the protocol was approved by the local ethics committee at INCMNSZ (Ref. 1243) and at the NIH (94-AR-0066).
RESULTS
Most subjects were women (89%), with a mean age of 32 ± 11 years. Fifty-nine SLE subjects (78.6%) had a SLEDAI score ≥6. Most patients (64%) did not have any immunosuppressive treatment at the time of the blood draw. The most common disease activity at the time of inclusion was renal (68%). SLE-associated variables are shown in online supplementary table S1. Numbers and main characteristics of patients included for each experiment are shown in online supplementary table S2. Confirming previous results, when measured by Sytox green, lupus neutrophils had an increased NET synthesis compared with controls (p<0.001).
NETs contain ubiquitinated proteins and SLE NETs display lower K63-mediated polyubiquitination.
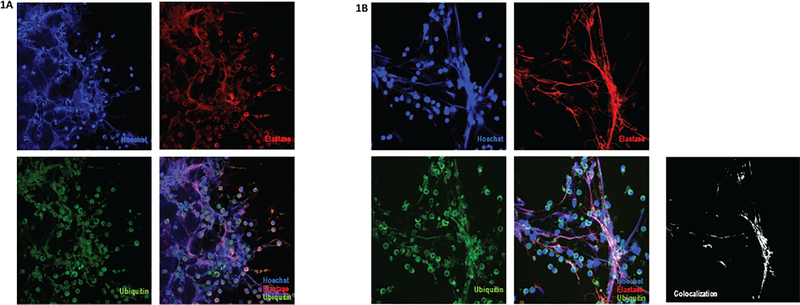
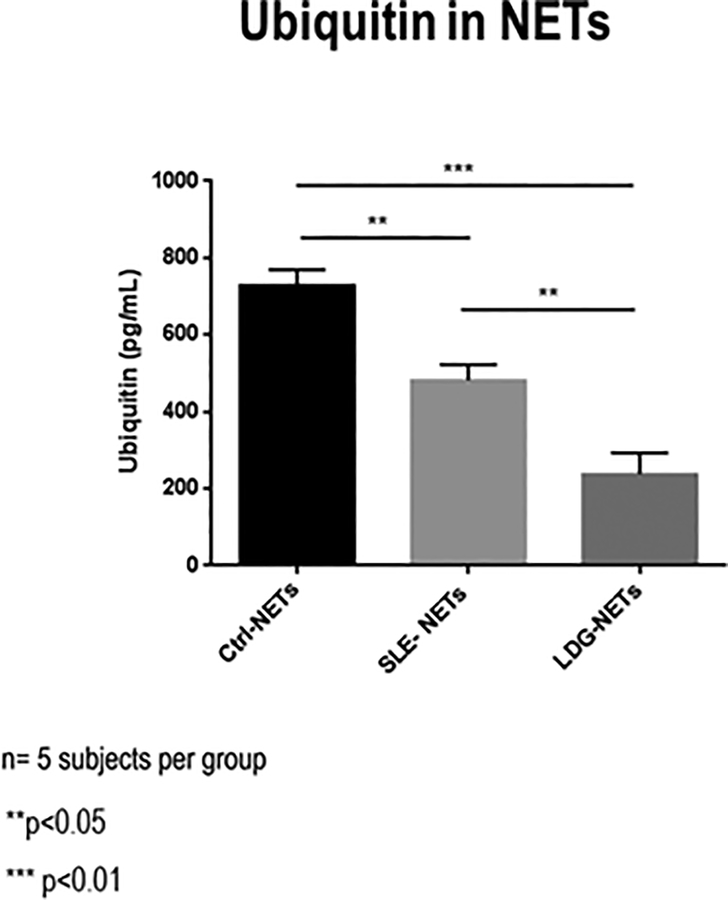
Ubiquitin was detected in NETs of both SLE patients and controls by immunofluorescence microscopy (Fig. 1). In order to confirm these results, an ELISA was performed to detect ubiquitin present in NETs. After analyzing NETs from control normal-density granulocytes (NDGs) and lupus NDGs and LDGs, we found a lower ubiquitin concentration in NETs derived from SLE neutrophils compared with healthy controls (p<0.05). NETs from lupus LDGs had the lowest amounts of ubiquitin (p<0.01 vs healthy controls, p<0.05 vs SLE NDGs; Fig. 2), suggesting that NETs produced by cells with a higher proinflammatory capacity3 have less ubiquitinated protein cargo.
Figure 1. Ubiquitin is expressed in NETs from SLE patients and healthy controls.
Immunofluorescence of NETs from a representative SLE patient (A) and a representative healthy control (B), analyzed with confocal microscopy; red represents neutrophil elastase, blue represents DNA and green represents ubiquitin. Images positive for neutrophil elastase and nuclear stain, with a characteristic morphology, were classified as NETs. A representative image is shown, demonstrating colocalization of ubiquitin with the other NET components (B); NIH Image J2 software analysis with the colocalization RGB plugin was performed to find colocalized pixels in red, green and blue channels. Results are displayed in black and white; white represents the merged image of the three channels.
Figure 2. Differential ubiquitin concentration in NETs from control and lupus NDGs and lupus LDGs.
ELISA for total ubiquitin in NETs from healthy control neutrophils (Ctrl-NETs), lupus NDGs (SLE NETs) and lupus LDGs (LDG-NETs) (n=5 subjects per group). Bars represent mean ± SEM of ubiquitin concentration in NETs from each group of subjects. ** p<0.01, ***p<0.001
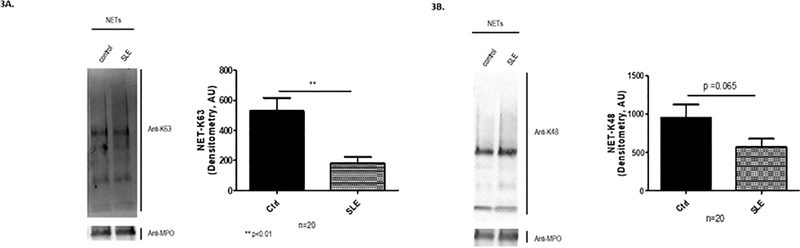
Furthermore, ubiquitinated proteins, specifically polyubiquitinated through lysine 48 and lysine 63, were detected by Western Blot in both NETs from SLE subjects and healthy controls (Fig. 3). A differential ubiquitination pattern was found between SLE subjects and healthy controls by Western Blot analysis, with a lower expression of K63 polyubiquitinated proteins in SLE subjects (p<0.05), as shown in Fig. 3A. There was also a trend for significant differences in diminished expression of K48 polyubiquitinated proteins in lupus NETs compared with controls (p=0.065, Fig. 3B). We did not find a significant difference in the ubiquitin content in NETs (p=0.124) in patients under immunosuppressive treatment in comparison to those untreated. Overall, these results indicate that ubiquitinated proteins are detected in NETs and that lupus and control NETs display different ubiquitination patterns.
Figure 3. NETs from SLE patients have decrease in K63 polyubiquitin species.
Western Blot analysis of NETs from SLE patients and controls was performed, and quantification was done by densitometry (n=20 subjects per group). Representative blots are included for both types of ubiquitin conjugation profile. Results are expressed as mean ± SEM. There was less expression of K63-dependent (A) and K48-dependent (B) polyubiquitinated proteins in SLE NETs than in healthy control NETs. ** p<0.01
Antibody responses to ubiquitinated NET proteins develop in SLE patients.
After demonstrating the presence of ubiquitinated proteins in NETs, we searched for specific NET components that could be ubiquitin targets. We specifically focused on MPO because it is a major component of NETs23, and because it has an antigenic role in certain autoimmune diseases. Using immunoprecipitation assays, we detected ubiquitinated MPO in NETs from SLE subjects and controls (online supplementary Figure S2).
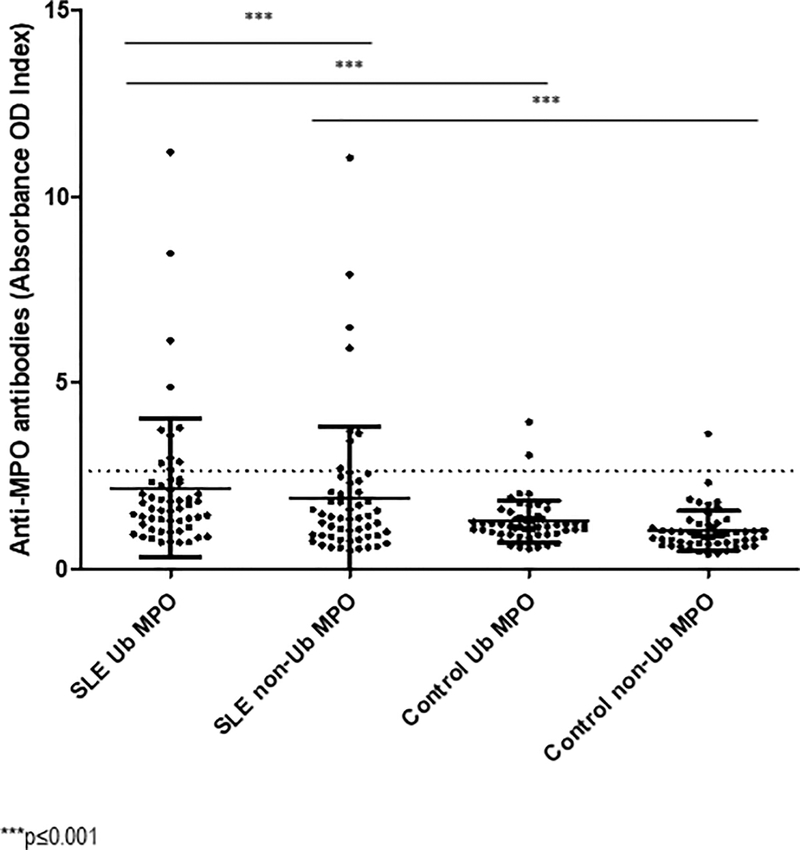
In order to determine if adaptive immune responses develop to ubiquitinated MPO (Ub-MPO), sera from SLE patients and healthy controls was analyzed by ELISA to detect antibodies against Ub-MPO and non-Ub-MPO. For this assay, we included sera from 57 SLE subjects, 55 healthy controls. Considering the established cutoff value described in Methods, 21% of SLE patients had antibodies against Ub-MPO, and 14% had antibodies against non-Ub-MPO, whereas only 3.6% and 1.8% of healthy controls had antibodies against both ubiquitinated and native MPO molecules, respectively. When compared to controls, SLE patients displayed significantly increased titers of anti-Ub-MPO by a normalized OD index (2.17 vs 1.26, p=0.0008), as well as anti-native MPO antibodies (1.89 vs 1, p=0.001). Furthermore, when comparing antibodies against ubiquitinated and native MPO proteins, there was a higher concentration against Ub-MPO in SLE subjects (2.17 vs 1.89, p<0.0001; fig 4).
Figure 4. Serum samples from SLE patients contain anti-Ub MPO antibodies.
An in-house ELISA was performed to quantify antibodies against Ub-MPO (purified from human neutrophils) and native (non-ubiquitinated) MPO (recombinant protein) in serum from 57 SLE patients and 55 healthy controls. Dots represent normalized values from individual subjects. The dotted line represents the established cutoff value. *** p≤0.001
Anti-Ub-MPO antibody levels positively correlated with SLEDAI (r=0.44, p<0.01), and negatively with complement components (C3: r=−0.474, p<0.01; C4: r=−0.426, p<0.01), while no correlation with anti-dsDNA antibody levels was found (p=0.43). Moreover, patients with positive anti-Ub-MPO titers had a higher mean SLEDAI score (17.8 vs 9.58, p=0.022) and a higher frequency of vasculitis (p=0.03) compared to those with negative anti-Ub-MPO antibodies. Overall, these results suggest that lupus patients, especially those with high disease activity, mount antibody responses to ubiquitinated NET components.
The activation of CXCR4, the receptor for extracellular ubiquitin, causes an increase in calcium flux.
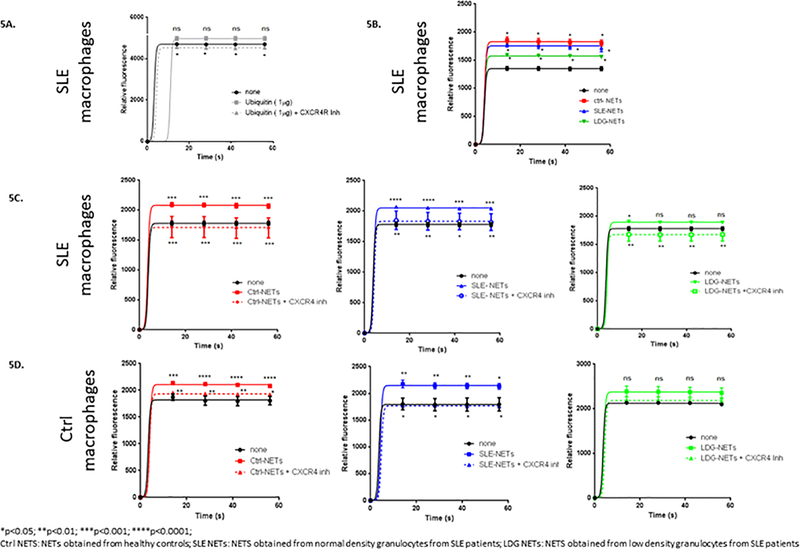
Extracellular ubiquitin is a natural CXCR4 agonist, and this axis is involved in various CXCR4-mediated cellular processes19. As NETs externalize ubiquitin, and SLE subjects and controls display different ubiquitination patterns in NETs, we assessed the role of ubiquitin and NETs on macrophage responses in the presence and absence of a CXCR4 inhibitor. When macrophages were stimulated with recombinant extracellular ubiquitin, there was a trend for an increase in basal calcium flux, which significantly diminished in the presence of a CXCR4 inhibitor (AMD 3100) (Fig. 5A). When SLE and control macrophages were stimulated with NETs from control or SLE NDGs or from SLE LDGs, increased calcium flux was also triggered, and it similarly decreased in the presence of a CXCR4 inhibitor (Fig. 5C and 5D). These results suggest that there is activation of CXCR4 by ubiquitin in NETs. When the response from SLE macrophages was evaluated, the largest increase in calcium flux was observed with control NETs, while the lowest increase occurred with LDG NETs (Fig. 5B). These findings correlated with the ubiquitin concentration gradient found by ELISA in NETs. We did not find significant differences in calcium flux when macrophages were stimulated with NETs derived from neutrophils exposed to PYR-41 (E1 inhibitor). This finding could be related to inability to completely remove ubiquitin in NETs (online supplementary Fig 3). Overall, these results suggest that ubiquitinated proteins are one of the NET components able to modify calcium flux in macrophages.
Figure 5. Extracellular ubiquitin and NETs increase calcium flux in macrophages.
Monocyte-derived macrophages from SLE subjects and healthy controls were obtained. They were stimulated with recombinant ubiquitin (1 μg) (A) or with 50 μg of NETs from healthy controls, lupus normal density granulocytes and lupus LDGs (B-D). In addition, a CXCR4 (extracellular ubiquitin receptor) inhibitor was used (A,C,D). Calcium flux was measured by Fluo-4 NW Calcium Assay Kit in all cases (5 independent experiments). Calcium flux was enhanced by recombinant ubiquitin (A) and by NETs (B-D), and significantly decreased when a CXCR4 inhibitor was added. There was also a differential calcium influx in response to NETs from healthy controls, SLE patients and LDGs. *p<0.05; ** p<0.01; ***p<0.001; ****p<0.0001; n.s.=non significant.
Macrophages internalize NETs and this process is proinflammatory in SLE.
Monocyte-derived macrophages from SLE subjects and controls were cultured in the presence of NETs, and internalized NET components after 6 h incubation (i.e. as assessed by neutrophil elastase, which is not expressed by macrophages (Fig 6A)). In order to address whether the internalization of lupus NET components is a proinflammatory process in macrophages8, macrophage supernatants were examined for cytokine release (TNF-α, IL-6 and IL-10) after exposure to NETs from SLE subjects (N=18). In SLE macrophages, TNF-α and IL-10 release was enhanced following stimulation with LPS-induced NETs, when compared with the effect observed in control macrophages (p<0.05; Fig. 6B–D). There was also a trend toward an increased IL-6 synthesis by SLE macrophages in response to NETs. This differential cytokine profile between SLE patients and controls was not found after stimulation with spontaneous NETs or LPS alone (data not shown). Finally, we also used chloroquine, mainly as a TLR antagonist24,25, as well as a NET internalization inhibitor26. We found that SLE macrophages that were pretreated with chloroquine showed a decrease in IL-6 and TNF-α synthesis, but not IL-10, after NET stimulation (p<0.01 and <0.05, respectively; Fig. 6B–D). Besides, upon CXCR4 inhibition, macrophages from controls had a decrease in TNF-α (p=0.027) and IL-10 (p=0.012) production (Fig. 6B–D). We found no significant effect on cytokine production from lupus macrophages, which could be related to the dysregulation and higher expression of CXCR4 in SLE patients27. These findings suggest that NET internalization by macrophages is not a silent process, at least in lupus macrophages, and that extracellular ubiquitin-CXCR4 pathway may be partially implicated in cytokine induction triggered by NET internalization. These results also suggest that chloroquine interferes with NET internalization by lupus macrophages.
Figure 6. SLE macrophages internalize NETs and synthesize cytokines in response to NET internalization.
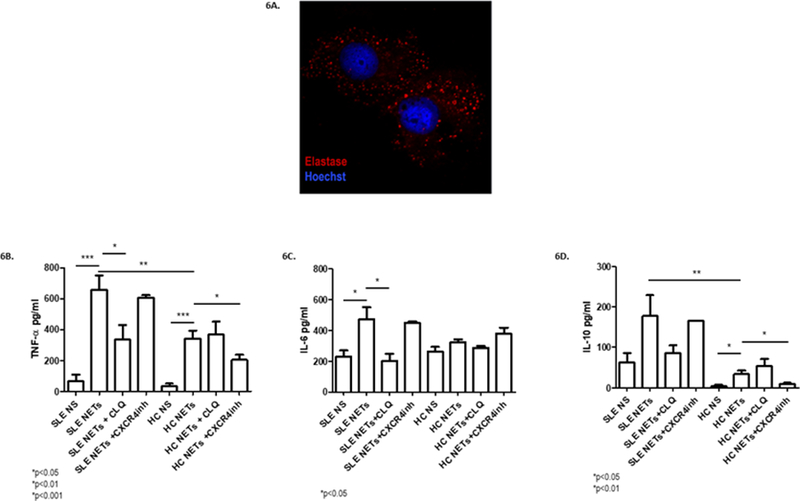
Monocyte-derived macrophages from SLE and healthy subjects were incubated with 50 μg of NDG NETs from SLE subjects for 6 hours (N=18). A) Immunofluorescence was performed and analyzed by confocal microscopy. Red represents neutrophil elastase and blue represents DNA. A representative image is shown, demonstrating internalization of neutrophil elastase from NETs by macrophages. B-D) Supernatants were obtained, and cytokine levels (TNF-α, IL-6 and IL-10) were measured by ELISA. Boxes show the pooled data (mean and SEM) of each cytokine assessed in non-stimulated (NS) SLE and control macrophages, macrophages stimulated with NETs, macrophages stimulated with NETs after chloroquine pretreatment, and macrophages stimulated with NETs after treatment with a CXCR4 inhibitor (AMD3100). There was a higher TNF-α and IL-10 synthesis in SLE macrophages compared with controls when they were stimulated with NETs. When SLE macrophages were pretreated with chloroquine, IL-6 and TNF-α release significantly decreased. After CXCR4 inhibition, there was a decrease in TNF-α and IL-10 synthesis in control macrophages; *p<0.05; ** p<0.01; ***p<0.001; n.s.=non significant.
DISCUSSION
Several groups have proposed that NETs play pathogenic roles in SLE28,29. Therefore, characterizing the role of specific NET modifications and their potential immunogenicity is fundamental in understanding how NETs can promote immune dysregulation and tissue damage. To our knowledge, we now describe for the first time that there are ubiquitinated proteins in NETs, as well as the ubiquitination of myeloperoxidase, one of the most abundant NET proteins.
We found polyubiquitinated proteins in NETs, which could have diverse implications in the immune system. Specifically, K63 ubiquitination is involved in DNA repair, signaling through NF-κB, and endosomal traffic regulation, all of which are related to the modulation of immune responses30. Also, we found decreased ubiquitination in NETs from SLE subjects compared with controls. Indeed, LDG NETs had the lowest expression of ubiquitinated proteins. This could be related to the lower expression of ubiquitin ligases in SLE, as demonstrated with Cbl-b in lymphocytes14. Regarding the effect that this differential ubiquitin expression in NETs may have, extracellular ubiquitin has been associated with a predominantly anti-inflammatory and counterregulatory response in sepsis and other diseases15,31. Therefore, its higher levels in healthy control NETs could constitute a regulatory mechanism to dampen the proinflammatory environment that initially leads to NETosis. This mechanism could be absent or deficient in SLE patients. Furthermore, K63 ubiquitination has been found to play an important role in cellular resistance to oxidative stress32. The fact that NETs from SLE patients have less K63 polyubiquitination could lead to a higher oxidative damage in the context of NETosis. This, in turn, could contribute to endothelial damage and the proatherogenic phenotype in SLE patients33.
We also found that NETs contain ubiquitinated MPO. This enzyme seems to play a dual role in inflammation and autoimmunity. Particularly in SLE, it has been associated with enhanced atherosclerosis33, as well as with nephritis5,29, through the role it plays within NETs. However, a decreased renal inflammation has also been described in MPO deficient mice34. We found MPO might induce a humoral response in SLE subjects, characterized by the production of more autoantibodies against both native and Ub-MPO molecules than healthy controls. The frequency of anti-native MPO antibodies in our study agrees with previous reports in SLE35,36. This is the first description of anti-Ub-MPO antibodies. Furthermore, SLE patients had a higher concentration of anti-Ub-MPO antibodies in comparison to non-Ub-MPO. Since ubiquitin is a highly conserved and very abundant protein, it has been hypothesized that it is unlikely to elicit an immune response and could even protect its substrates from immune recognition37. However, by masking certain epitopes, it could expose other, more immunogenic, peptides. It has been reported in a murine model that T cells specific for the PTM variant of a self-antigen are able to escape central tolerance and could provide help to B cells and promote pathogenic autoimmune responses38. Also, specifically regarding MPO, ubiquitination could be involved in its dual function in immunity, since this PTM may influence its function, its enzymatic action and its oxidative capacity, both intracellularly and as a NET component. MPO ubiquitination could not only have a direct impact on tissue injury due to oxidative stress, but also on the effect MPO has in other immune cells. Interestingly, MPO has been found to suppress dendritic cells, whereas in its enzymatically inactive form, it can activate lung macrophages34,39. Whether these interactions could be affected by ubiquitination remains to be defined.
We found a trend towards a higher macrophage calcium flux when we stimulated these cells with ubiquitin. This effect seems to be mediated by CXCR4, since its stimulation has been described to enhance calcium flux19 in other biologic systems, and after its inhibition, calcium flux decreased significantly. When macrophages were stimulated with NETs, the highest calcium flux was observed with NETs derived from controls, which had the higher ubiquitin concentration, suggesting that this differential effect may be driven by ubiquitin. To dissect the precise role of ubiquitin from NETs in the enhanced calcium flux, we used NETs from PYR-41 exposed neutrophils, which is a E1 activating enzyme inhibitor40. We found no significant differences when comparing to non PYR-41 exposed neutrophils. This could be secondary to the inability to attain complete removal of ubiquitin in NETs. Current available techniques are directed mainly at blocking the elongation of the ubiquitin chain, rather than removing the first ubiquitin molecule conjugated to the protein substrate. Most of these techniques were developed for polyubiquitin chain removal in a single protein substrate and are not suitable for a mixture of proteins prone to ubiquitination, such as those present in NETs41. Therefore, we consider that the lack of significant differences in the calcium flux are related to the limitation of the current technology available for the assessment of ubiquitination in NETs and to the lack of knowledge of the ubiquitin code (E1, E2, E3 ligases, deubiquitination enzymes42, ubiquitin like proteins43, and non-conventional ubiquitin linkages44) that guides ubiquitination during NETosis, a limitation of the present work. Overall, our findings partly support the role of ubiquitin in NETs as a trigger for calcium flux in macrophages, but do not allow us to conclude that it is the only NET component involved in this response. It will be relevant to determine if the concentration of ubiquitin in NETs makes them more or less immunogenic, which could be related to the specific ubiquitinated protein cargo and the lysine residue to which ubiquitin is anchored.
When SLE macrophages were stimulated with NETs, they were internalized and cytokine synthesis was documented. There was a higher synthesis of TNF-α and IL-10 by SLE macrophages compared with control macrophages after they were stimulated with LPS-induced NETs. This partially agrees with the findings by Farrera et al, who described a higher cytokine synthesis after stimulating with both LPS and NETs than with NETs alone8. These results with LPS may be secondary to a synergistic mechanism, which could be mediated by TLR4, a receptor that has been involved in SLE physiopathology25,45,46. Of note, pretreatment with chloroquine decreased TNF-α and IL-6 production in SLE macrophages. Chloroquine has been described to specifically alter macrophage IL-6 and TNF-α synthesis through TLR inhibition24. Macfarlane et al found that antimalarials inhibit IL-6 synthesis after TLR-9 stimulation, but did not have an effect on LPS-induced responses24, which was confirmed by Cepika et al47. Furthermore, we recently found that in rheumatoid arthritis synovial fibroblasts can internalize NETs and increase cytokine synthesis, and that NET internalization is inhibited by antimalarials26. This effect was not replicated in the case of IL-10, which could be partly explained by the other pathways involved in the synthesis of this cytokine48, and could also be related to its specific role in SLE. Although it is typically regarded as an anti-inflammatory cytokine, high levels of IL-10 have been described in lupus49,50 and it has even been considered as a disease activity biomarker51. Finally, control macrophages displayed decreased TNF-α and IL-10 synthesis after CXCR4 inhibition, which could implicate the ubiquitin- CXCR4 pathway in this process, since ubiquitin has been found to regulate TNF-α and IL-1015, but not IL-652 synthesis. We did not find similar changes in lupus macrophages, but this could be related to the abnormally enhanced expression and dysregulation of CXCR4 that has been reported in SLE patients27,53,54, which could have led to an incomplete inhibition of this pathway. Also, CXCR4 has a complex recycling process55, which could influence the effect of its inhibition in cytokine synthesis, without altering its effect on the initial calcium flux. Our data suggest that NET internalization by lupus macrophages is not a silent process and may contribute to inflammation in this disease.
In summary, this is the first study that demonstrates the presence of polyubiquitinated proteins in NETs, with a differential profile between SLE patients and healthy controls. MPO was found to be ubiquitinated in NETs and our data suggest it is a target of humoral responses in SLE. Furthermore, ubiquitin present in NETs is one of the components that regulates calcium flux in macrophages through CXCR4 signaling. Lupus macrophages synthesize inflammatory cytokines in response to NET internalization, a process that could be partly mediated by TLR4 and 9. Overall, abnormalities in mechanisms involved in NET internalization and the extracellular ubiquitination pathway could play important roles in the development of inflammatory responses in SLE.
Supplementary Material
Acknowledgements
We would like to thank the technical support provided by Dr. José Luis Maravillas, Dr. Guillermo Juárez Vega, Dr. Carlos Núñez Álvarez, and the Red de Apoyo a la Investigación CIC-UNAM.
Funding
This study was supported by the Intramural Research Program at NIAMS/NIH (ZIA AR041199) and a grant from the Consejo Nacional de Ciencia y Tecnología (CONACYT, 255941, SEP-Ciencia Básica 2015).
Footnotes
Competing interests
None
Contributor Information
Ana Barrera-Vargas, Department of Immunology and Rheumatology, Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán. Mexico City, Mexico..
Diana Gómez-Martín, Department of Immunology and Rheumatology and Red de Apoyo a la Investigación. CIC-UNAM. Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán. Mexico City, Mexico..
Carmelo Carmona-Rivera, Systemic Autoimmunity Branch. Intramural Research Program. National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health. Bethesda, MD, United States..
Javier Merayo-Chalico, Department of Immunology and Rheumatology. Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán. Mexico City. Mexico..
Jiram Torres-Ruiz, Department of Immunology and Rheumatology. Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán. Mexico City. Mexico..
Zerai Manna, Lupus Clinical Research Unit. Intramural Research Program. National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health. Bethesda, MD, United States..
Sarfaraz Hasni, Lupus Clinical Research Unit. Intramural Research Program. National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health. Bethesda, MD, United States..
Jorge Alcocer-Varela, Department of Immunology and Rheumatology, Instituto Nacional de Ciencias Médicas y Nutrición, Salvador Zubirán. Mexico City, Mexico..
Mariana J. Kaplan, Systemic Autoimmunity Branch. Intramural Research Program. National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health. Bethesda, MD, United States.
REFERENCES
- 1.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–5. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 2012;189:2689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lood C, Blanco LP, Purmalek MM, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 2016;22:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denny MF, Yalavarthi S, Zhao W, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol 2010;184:3284–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakkim A, Furnrohr BG, Amann K, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 2010;107:9813–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leffler J, Martin M, Gullstrand B, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol 2012;188:3522–31. [DOI] [PubMed] [Google Scholar]
- 7.Munoz LE, Gaipl US, Franz S, et al. SLE--a disease of clearance deficiency? Rheumatology (Oxford) 2005;44:1101–7. [DOI] [PubMed] [Google Scholar]
- 8.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol 2013;191:2647–56. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009;184:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu CL, Tangsombatvisit S, Rosenberg JM, et al. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther 2012;14:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YC, Penninger J, Karin M. Immunity by ubiquitylation: a reversible process of modification. Nat Rev Immunol 2005;5:941–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Martin D, Diaz-Zamudio M, Alcocer-Varela J. Ubiquitination system and autoimmunity: the bridge towards the modulation of the immune response. Autoimmun Rev 2008;7:284–90. [DOI] [PubMed] [Google Scholar]
- 13.Hicke L Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol 2001;2:195–201. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Martin D, Ibarra-Sanchez M, Romo-Tena J, et al. Casitas B lineage lymphoma b is a key regulator of peripheral tolerance in systemic lupus erythematosus. Arthritis Rheum 2013;65:1032–42. [DOI] [PubMed] [Google Scholar]
- 15.Majetschak M Extracellular ubiquitin: immune modulator and endogenous opponent of damage-associated molecular pattern molecules. J Leukoc Biol 2011;89:205–19. [DOI] [PubMed] [Google Scholar]
- 16.Daino H, Matsumura I, Takada K, et al. Induction of apoptosis by extracellular ubiquitin in human hematopoietic cells: possible involvement of STAT3 degradation by proteasome pathway in interleukin 6-dependent hematopoietic cells. Blood 2000;95:2577–85. [PubMed] [Google Scholar]
- 17.Majetschak M, Ponelies N, Hirsch T. Targeting the monocytic ubiquitin system with extracellular ubiquitin. Immunol Cell Biol 2006;84:59–65. [DOI] [PubMed] [Google Scholar]
- 18.Alonso S, Pethe K, Russell DG, et al. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci U S A 2007;104:6031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem 2010;285:15566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saini V, Staren DM, Ziarek JJ, et al. The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1alpha function through distinct receptor interactions. J Biol Chem 2011;286:33466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 22.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 23.Grayson PC, Carmona-Rivera C, Xu L, et al. Neutrophil-Related Gene Expression and Low-Density Granulocytes Associated With Disease Activity and Response to Treatment in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol 2015;67:1922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macfarlane DE, Manzel L. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol 1998;160:1122–31. [PubMed] [Google Scholar]
- 25.Zhang H, Fu R, Guo C, et al. Anti-dsDNA antibodies bind to TLR4 and activate NLRP3 inflammasome in lupus monocytes/macrophages. J Transl Med 2016;14:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmona-Rivera C, Carlucci PM, Moore E, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao LD, Liang D, Wu XN, et al. Contribution and underlying mechanisms of CXCR4 overexpression in patients with systemic lupus erythematosus. Cell Mol Immunol 2017;14:842–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmona-Rivera C, Zhao W, Yalavarthi S, et al. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 2015;74:1417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011;187:538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husnjak K, Dikic I. Ubiquitin-binding proteins: decoders of ubiquitin-mediated cellular functions. Annu Rev Biochem 2012;81:291–322. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Covarrubias L, Manning EW 3rd, Sorell LT, et al. Ubiquitin enhances the Th2 cytokine response and attenuates ischemia-reperfusion injury in the lung. Crit Care Med 2008;36:979–82. [DOI] [PubMed] [Google Scholar]
- 32.Silva GM, Finley D, Vogel C. K63 polyubiquitination is a new modulator of the oxidative stress response. Nat Struct Mol Biol 2015;22:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith CK, Vivekanandan-Giri A, Tang C, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol 2014;66:2532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Odobasic D, Muljadi RC, O’Sullivan KM, et al. Suppression of Autoimmunity and Renal Disease in Pristane-Induced Lupus by Myeloperoxidase. Arthritis Rheumatol 2015;67:1868–80. [DOI] [PubMed] [Google Scholar]
- 35.Faure-Fontenla MA, Rodriguez-Suarez RS, Arias-Velasquez R, et al. Antineutrophil cytoplasmic antibodies in systemic lupus erythematosus in childhood. J Rheumatol 1999;26:2480–1. [PubMed] [Google Scholar]
- 36.Spronk PE, Bootsma H, Horst G, et al. Antineutrophil cytoplasmic antibodies in systemic lupus erythematosus. Br J Rheumatol 1996;35:625–31. [DOI] [PubMed] [Google Scholar]
- 37.Weil R Does antigen masking by ubiquitin chains protect from the development of autoimmune diseases? Front Immunol 2014;5:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raposo B, Merky P, Lundqvist C, et al. T cells specific for post-translational modifications escape intrathymic tolerance induction. Nat Commun 2018;9:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grattendick K, Stuart R, Roberts E, et al. Alveolar macrophage activation by myeloperoxidase: a model for exacerbation of lung inflammation. Am J Respir Cell Mol Biol 2002;26:716–22. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Kitagaki J, Dai RM, et al. Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res 2007;67:9472–81. [DOI] [PubMed] [Google Scholar]
- 41.Huang X, Dixit VM. Drugging the undruggables: exploring the ubiquitin system for drug development. Cell Res 2016;26:484–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem 2012;81:203–29. [DOI] [PubMed] [Google Scholar]
- 43.Cappadocia L, Lima CD. Ubiquitin-like Protein Conjugation: Structures, Chemistry, and Mechanism. Chem Rev 2018;118:889–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 2005;309:127–30. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Yin H, Zhao M, et al. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol 2014;47:136–47. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Ferro M, Serrano Del Castillo C, Sanchez-Pernaute O. Cell Membrane-bound TLR2 and TLR4: Potential Predictors of Active Systemic Lupus Erythematosus and Lupus Nephritis. J Rheumatol 2016;43:1444–5. [DOI] [PubMed] [Google Scholar]
- 47.Cepika AM, Bendelja K, Vergles JM, et al. Monocyte response to LPS after exposure to corticosteroids and chloroquine with implications for systemic lupus erythematosus. Scand J Immunol 2010;72:434–43. [DOI] [PubMed] [Google Scholar]
- 48.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol 2010;10:170–81. [DOI] [PubMed] [Google Scholar]
- 49.Chun HY, Chung JW, Kim HA, et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol 2007;27:461–6. [DOI] [PubMed] [Google Scholar]
- 50.Houssiau FA, Lefebvre C, Vanden Berghe M, et al. Serum interleukin 10 titers in systemic lupus erythematosus reflect disease activity. Lupus 1995;4:393–5. [DOI] [PubMed] [Google Scholar]
- 51.Godsell J, Rudloff I, Kandane-Rathnayake R, et al. Clinical associations of IL-10 and IL-37 in systemic lupus erythematosus. Sci Rep 2016;6:34604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel MB, Proctor KG, Majetschak M. Extracellular ubiquitin increases in packed red blood cell units during storage. J Surg Res 2006;135:226–32. [DOI] [PubMed] [Google Scholar]
- 53.Wang A, Fairhurst AM, Tus K, et al. CXCR4/CXCL12 hyperexpression plays a pivotal role in the pathogenesis of lupus. J Immunol 2009;182:4448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang A, Guilpain P, Chong BF, et al. Dysregulated expression of CXCR4/CXCL12 in subsets of patients with systemic lupus erythematosus. Arthritis Rheum 2010;62:3436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarasova NI, Stauber RH, Michejda CJ. Spontaneous and ligand-induced trafficking of CXC-chemokine receptor 4. J Biol Chem 1998;273:15883–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.