Abstract
Purpose:
Pancreatic neuroendocrine tumors (PanNET) are a heterogeneous group of neoplasms with increasing incidence and unpredictable behavior. Whole-exome sequencing has identified recurrent mutations in the genes DAXX and ATRX, which correlate with loss of protein expression and alternative lengthening of telomeres (ALT). Both ALT and DAXX/ATRX loss were initially reported to be associated with a favorable prognosis; however, recent studies suggest the contrary. Our aims were to assess the prevalence and prognostic significance of ALT and DAXX/ATRX in both primary and metastatic PanNETs.
Experimental Design:
Telomere-specific FISH and DAXX/ATRX IHC was performed on a multi-institutional cohort of 321 patients with resected PanNET and 191 distant metastases from 52 patients. These results were correlated with clinicopathologic features, including disease-free survival (DFS) and disease-specific survival (DSS).
Results:
The prevalence of ALT and DAXX/ATRX loss in resected PanNETs was 31% and 26%, respectively, and associated with larger tumor size, higher WHO grade, lymph node metastasis, and distant metastasis (P < 0.001). The 5-year DFS and 10-year DSS of patients with ALT-positive and DAXX/ATRX-negative PanNETs were 40% and 50%, respectively, as compared with 96% and 89%, respectively, for wild-type PanNETs. Among distant metastases, ALT and DAXX/ATRX loss was 67% and 52%, respectively, and only occurred in the setting of an ALT-positive and DAXX/ATRX-negative primary PanNET. By multivariate analysis, both ALT and DAXX/ATRX loss were negative, independent prognostic factors for DFS.
Conclusions:
ALT and DAXX/ATRX loss in PanNETs was associated with shorter DFS and DSS and likely plays a significant role in driving metastatic disease. Clin Cancer Res; 23(2); 600–9. 2016 AACR.
Introduction
Pancreatic neuroendocrine tumors (PanNETs) are the second most common neoplasms of the pancreas (1). Within the United States, the annual incidence is approximately 1 per 100,000 individuals per year, but autopsy studies have shown a much higher prevalence that ranges from 0.8% to 10% (2–5). Moreover, given the increased accessibility and sensitivity of abdominal imaging techniques, the incidence of PanNETs has steadily increased over the last 30 years (1, 3). The 5-year survival following resection of a PanNET is 65% and the 10-year survival is 45%(5). In addition, >50% of patients will develop metastases. However, although many patients develop infiltrative, widely metastatic disease, others may present with slowly progressive, indolent tumors (1, 5). Therefore, a significant challenge in prognostic stratification and management of patients with Pan-NETs is predicting their biological behavior.
Recent advances in sequencing technologies have uncovered the molecular basis of numerous cancers that has led to new prognostic classification systems and actionable targets. Whole-exome sequencing of PanNETs has identified recurrent mutations in the death domain-associated protein (DAXX) and a-thalassemia/mental retardation X-linked (ATRX) genes. Jiao and colleagues found 43% of PanNETs harbored mutations in either DAXX or ATRX (6). Both DAXX and ATRX encode nuclear proteins that regulate the deposition of histone variant H3.3 during the assembly of pericentromeric and telomeric chromatin (7). Mutations in these genes are associated with loss of nuclear expression of their respective proteins by IHC and correlate with alternative lengthening of telomeres (ALT), a telomerase-independent telomere maintenance mechanism, which can be assayed using telomere specific FISH (8). Interestingly, Jiao and colleagues reported patients with PanNETs containing DAXX/ATRX alterations had an improved overall survival as compared with patients with wild-type tumors. However, the authors did note their patient cohort size was small and required further validation on a larger series. In contrast, Marinoni and colleagues found loss of DAXX/ATRX nuclear expression in PanNETs was associated with metastasis, shorter disease-free survival (DFS), and shorter disease-specific survival (DFS; ref. 9). This discrepancy may be attributed to differences in the patient populations investigated. All of the patients evaluated by Jiao and colleagues had metastatic disease, as opposed to 18% of patients reported by Marinoni and colleagues. But once again, the number of patients with adequate follow-up within the study by Marinoni and colleagues was small and divided in two separate cohorts. In addition, correlative telomere-specific FISH to assess for ALT was performed only on a subset of PanNETs. Moreover, the status of ALT and DAXX/ATRX in metastatic foci in relationship to their corresponding primary PanNET is unknown.
The aims of this study were to (i) identify the prevalence of ALT by telomere-specific FISH and loss of DAXX/ATRX expression by IHC in PanNETs using a large, multi-institutional cohort; (ii) determine the prognostic significance of ALT and DAXX/ATRX loss in PanNETs; and (iii) assess the status of ALT and DAXX/ATRX within paired primary PanNETs and their corresponding distant metastases.
Materials and Methods
Study population
Study approval was obtained from the University of Pittsburgh (IRB# PRO13020493) and Washington University (St. Louis, MO; 201404143) Institutional Review Boards. The surgical pathology archives from the Departments of Pathology at the University of Pittsburgh Medical Center (Pittsburgh, PA) and Barnes-Jewish Hospital (St. Louis, MO) were queried for neuroendocrine neoplasms of the pancreas between 1995 and 2012 that underwent enucleation, central pancreatectomy, pancreati coduodenectomy, or distal pancreatectomy. Cases were cross-referenced with clinical and follow-up data obtained from patient paper and/or electronic medical records. The study inclusion criteria consisted of the following: a solitary, well-differentiated neuroendocrine neoplasm [confirmed with positive immunolabeling for neuroendocrine markers (e.g., synaptophysin and chromogranin A)] centered within the pancreas, surveillance and survival data of >2 years, absence of a genetic syndrome associated with pancreatic neuroendocrine neoplasms (e.g., multiple endocrine neoplasia type 1 syndrome, von Hippel–Lindau syndrome, neurofibromatosis type 1 syndrome, and tuberous sclerosis complex syndrome), and cases with sufficient material for ancillary studies. In total, 321 patients with a resected PanNET fulfilled the aforementioned criteria. In addition, the surgical pathology archives from the respective institutions were cross-referenced to identify corresponding distant metastases with sufficient pathologic material for ancillary studies. Among 93 patients with distant metastases, 52 patients had pathologic material available for telomere FISH and DAXX/ATRX IHC. In total, 191 distant metastases were identified from these 52 patients.
Clinical and demographic data were reviewed for each case. Corresponding pathology gross reports and hematoxylin and eosin–stained slides were also reviewed for the following pathologic features: tumor size, location, lymphovascular invasion, perineural invasion, extension outside of the pancreas, and regional lymph node metastasis. Each PanNET was graded using the 2010 World Health Organization (WHO) classification system for pancreatic neuroendocrine neoplasms (10). Briefly, on the basis of mitotic rate and Ki67 IHC, the following criteria were used: grade 1 (G1), <2 mitoses/10 high-power fields (hpf) and Ki-67 of <3%; grade 2 (G2), 2 to 20 mitoses/10 hpf or Ki67 of 3% to 20%; and grade 3 (G3), >20 mitoses/10 hpf or Ki67 of >20%. The mitotic rate was derived from evaluation of multiple sections in 50 hpf (400, field diameter 0.55 mm2) and expressed as mitoses/10 hpf. For Ki67, at least 500 neoplastic nuclei were counted in the highest staining region for each case with careful exclusion of nonneoplastic cells (11). A labeling index was calculated and expressed as a percentage. For cases with discordant mitotic rate and Ki67 measurements, the highest grade was assigned. Pathologic primary tumor classification was determined according to the American Joint Committee on Cancer (AJCC) Staging Manual, seventh edition (12). Follow-up information was extracted from the patient’s paper and electronic medical records to include data on surveillance, disease recurrence/distant metastasis, and survival.
IHC
Immunohistochemical labeling was performed on 4-mm unstained whole slide sections from formalin-fixed, paraffin-embedded (FFPE) tissue blocks for each PanNET and distant metastases. Slides were deparaffinized with serial xylene treatments and subjected to antigen retrieval using heated citrate solution (pH 9.0) at 100 C for 10 minutes. Immunolabeling for Ki67 (mouse monoclonal, prediluted, Ventana Medical Systems), synaptophysin (rabbit polyclonal, prediluted, Cell Marque), chromogranin A (mouse monoclonal, prediluted, Ventana Medical Systems), DAXX (HPA008736 rabbit polyclonal, dilution 1:50, Sigma Aldrich), and ATRX (HPA001906 rabbit polyclonal, dilution 1:100, Sigma Aldrich) were performed on the automated Ventana Benchmark XT system using the biotin-free Ventana OptiView DAB IHC Detection Kit (Ventana Medical Systems).
Assessment of DAXX and ATRX was done blinded to any patient data, including outcome. Preserved or “positive” expression of DAXX and ATRX was defined as nuclear staining within tumor cells, using stromal cells as a positive internal control (Fig. 1). Loss or “negative” staining was scored in cases where the tumor lacked nuclear immunolabeling, but preserved expression within stromal cells was still identified. Intratu-moral heterogeneity or heterogeneous staining was defined as the clear presence of two distinct populations of tumor cells demonstrating preserved and loss of nuclear staining (Fig. 2). Each component (positive and negative nuclear staining) should comprise at least 10% of the neoplastic tissue. For subsequent statistical analysis, these cases were scored as loss or negative staining.
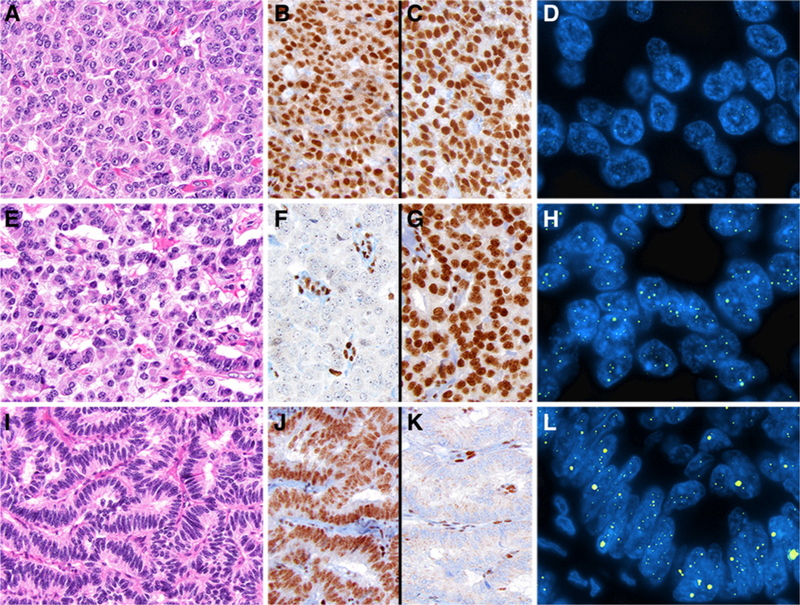
Figure 1.
Representative examples of PanNETs assessed by DAXX and ATRX IHC and telomere-specific FISH. A, PanNET with preserved nuclear expression for both DAXX (B) and ATRX (C) and absence of the ALT phenotype (D). E, PanNET with DAXX loss (F), but preserved expression for ATRX (G). The loss of DAXX expression correlated with the presence of large, ultrabright intranuclear foci by telomere-specific FISH, consistent with ALT (H). I, PanNET with preserved expression for DAXX (J), but ATRX loss (K) and ALT positive (L) by telomere-specific FISH.
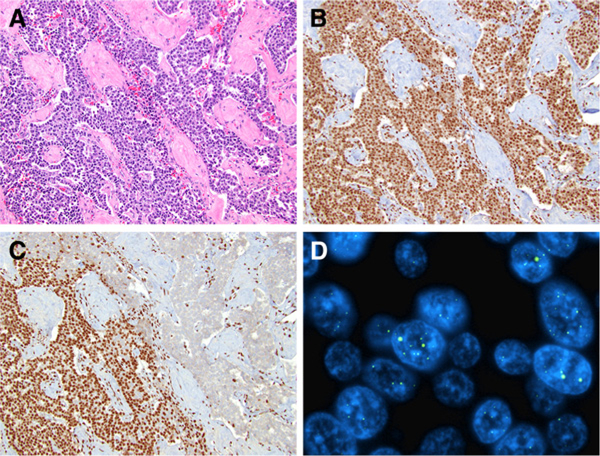
Figure 2.
Intratumoral heterogeneity for DAXX/ATRX protein expression and ALT in PanNETs. A, PanNET with preserved nuclear expression for DAXX (B), but ATRX loss (C, top right) within a subpopulation of neoplastic cells. Within areas of ATRX loss, telomere-specific FISH revealed large, bright signals consistent with ALT (D).
Tissue microarray construction and FISH
For telomere-specific FISH, high-density tissue microarrays (TMA) were constructed using archival FFPE tissue blocks from both resected PanNETs and distant metastases. Three 1.0 mm- sized cores were punched from representative areas of each tumor and harvested into recipient blocks. TMAs were cut at 4-mm sections. Sections were incubated for 30 minutes at 55 C, washed three times for 5 minutes in xylene, rinsed in successive 100%, 95%, and 70% ethanol baths, and washed in ddH2O and 1% Tween before being placed in antigen unmasking solution in a boiling steamer for 30 minutes. Next, slides were rinsed in ddH2O and dehydrated in successive ethanol washes of 70%, 95%, and 100%. Slides were incubated at 72 C for 10 minutes with an Alexa-488 telomeric-C PNA probe and hybridized overnight in a dark humidity chamber. Slides were washed with PNA wash buffer and PBST and incubated for 10 minutes in DAPI solution. After washing in ddH2O, slides were mounted with prolong anti-fade mounting medium. Images were taken on a Leica fluorescent light microscope (13).
Scoring for ALT was performed by assessing at least 250 nuclei from all three tissue cores for each case (at least 750 tumor nuclei). Using previously described criteria, ALT-positive cases were defined by the presence of large, ultrabright intranuclear foci consistent with telomere FISH signals in at least 1% of tumor nuclei and the total signal intensity for individual foci >10 fold than telomere signals from stromal cells (8, 14–16). Among ALT-positive PanNETs, although a 1% inclusion criterion was used, the percentage of tumor cells that were ALT-positive (percentage of tumor nuclei with large, ultrabright signals) ranged from 5.2% to24.3% (mean, 10.3%; median, 10%). Of note, areas of necrosis were excluded from evaluation. Among ALT-negative PanNETs, no large, ultrabright, intranuclear signals were found in over 750 tumor nuclei that were screened.
Statistical analysis
χ2 analysis or Fisher exact tests were used to compare categorical data, and ANOVA was used to compare continuous variables. Survival curves were constructed using the Kaplan–Meier method, and differences between groups were evaluated by the log-rank test. DFS was calculated from the date of surgery to the date of first distant metastasis/recurrence after surgery or to the date of last follow-up (in patients without distant metastasis/recurrence) for cases without synchronous distant metastasis. DSS was calculated from the date of surgery to the date of death due to disease or date of last follow-up (if death did not occur). The prognostic significance of clinical and pathologic characteristics was determined using univariate Cox regression analysis. Multivariate analyses of significant risk factors by univariate analysis were performed using Cox proportional hazard regression to identify independent risk factors for both DFS and DSS. All statistical analyses were performed using the SPSS Statistical software, version 22 (IBM), and statistical significance was defined as a P value of <0.05.
Results
Pancreatic neuroendocrine tumor study cohort
The study cohort consisted of 321 patients with a solitary PanNET treated by enucleation (n = 18), central pancreatectomy (n = 15), pancreaticoduodenectomy (n = 109), or distal pancreatectomy (n = 179) to include resection of identifiable metastases with curative intent. Patients ranged in age from 29 to 83 years (mean, 59.1 years) with a slight predominance in male gender (171 of 321, 53%). Thirty-six of 321 (11%) patients had a functional PanNET. The tumors were predominantly located within the pancreatic body and tail (n = 194, 60%) and ranged in size from 0.6 to 18 cm (mean, 3.4 cm). Although all PanNETs were morphologically well differentiated, on the basis of mitotic rate and Ki-67 proliferation index, PanNETs were classified into the following WHO grades: 185 (58%) grade 1 (G1), 132 (41%) grade 2 (G2), and 4 (1%) grade 3 (G3). Lymphovascular and perineural invasions were identified in 136 (42%) and 95 (30%) tumors, respectively. Using the AJCC prognostic staging system (seventh edition), the PanNETs were classified into the following pathologic tumor (pT) stages: 116 (36%) pT1, 99 (31%) pT2, and 106 (33%) pT3. Regional lymph nodes were submitted for histologic evaluation in 268 (83%) cases with involvement of 100 (of 268, 37%) cases. At the time of surgery, 51 (16%) patients were found to have synchronous distant metastases that were resected. Of the remaining 270 patients, metachronous distant metastases were identified in 42 (of 270, 16%) cases. The DFS rates for these 270 patients were 91% at 3 years and 86% at 5 years. For all 321 patients, the DSS rates were 91% at 5 years and 87% at 10 years.
Telomere-specific FISH and DAXX/ATRX IHC
The results of telomere-specific FISH for ALT and IHC for DAXX and ATRX are summarized in Table 1. Among 321 resected PanNETs, ALT was detected in 98 (31%) cases. Loss of nuclear expression for DAXX, ATRX, or both was identified in 39 (12%), 30 (9%), and 15 (5%) PanNETs, respectively (Fig. 1). Heterogeneous loss of expression was seen in 1 DAXX-negative and 3 ATRX-negative tumors (Fig. 2). While ALT correlated with DAXX/ATRX loss, 14 (6%) ALT-positive PanNETs had preserved expression for DAXX/ATRX. ALT-positive PanNETs were associated with a predilection for male patients (P 0.011), larger mean tumor size (P < 0.001), lack of functionality (P = 0.002), higher WHO grade (P < 0.001), lymphovascular invasion (P < 0.001), peri-neural invasion (P < 0.001), higher pathologic tumor (pT) stage (P < 0.001), regional lymph node (pN) metastasis (P < 0.001), synchronous distant metastasis (P < 0.001), and postoperative metachronous distant metastasis (P < 0.001). There was no statistically significant difference between ALT status and mean patient age (P = 0.195) or tumor location (P = 0.300). Theclinicopathologic characteristics of DAXX/ATRX-negative Pan-NETs were nearly identical to PanNETs with ALT.
Table 1.
Clinical and pathologic comparison of ALT and DAXX/ATRX status in PanNETs
| Patient or tumor characteristics | ALT-positive, n = 98 (31%) | ALT-negative, n = 223 (69%) | P | DAXX/ATRX-negative, n = 84 (26%) | DAXX/ATRX-positive, n = 237 (74%) | P |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 35 (35%) | 108 (51%) | 0.011a | 29 (35%) | 121 (51%) | 0.011a |
| Male | 63 (65%) | 115 (49%) | 55 (65%) | 116 (49%) | ||
| Mean age (range), years | 60.5 (31–85) | 58.6 (29–83) | 0.195 | 61.4 (31–85) | 58.4 (29–83) | 0.050 |
| Mean tumor size (range), cm | 5.0 (1.0–15.0) | 2.8 (0.6–18.0) | <0.001a | 5.0 (1.0–15.0) | 2.8 (0.6–18.0) | <0.001a |
| Functional | ||||||
| No | 95 (97%) | 190 (85%) | 0.002a | 81 (96%) | 204 (86%) | 0.008a |
| Yes | 3 (3%) | 33 (15%) | 3 (4%) | 33 (14%) | ||
| Location | ||||||
| Head and uncinate | 37 (38%) | 90 (40%) | 0.300 | 29 (35%) | 98 (41%) | 0.300 |
| Body and tail | 61 (62%) | 133 (60%) | 55 (65%) | 139 (59%) | ||
| WHO grade | ||||||
| Low (G1) | 28 (29%) | 157 (70%) | <0.001a | 25 (30%) | 160 (68%) | <0.001a |
| Intermediate (G2) | 66 (67%) | 66 (30%) | 56 (66%) | 76 (32%) | ||
| High (G3) | 4 (4%) | 0 (0%) | 3 (4%) | 1 | ||
| Lymphovascular invasion | ||||||
| Absent | 22 (22%) | 163 (73%) | <0.001a | 20 (24%) | 165 (70%) | <0.001a |
| Present | 76 (78%) | 60 (27%) | 64 (76%) | 72 (30%) | ||
| Perineural invasion | ||||||
| Absent | 55 (56%) | 185 (83%) | <0.001a | 35 (42%) | 191 (81%) | <0.001a |
| Present | 43 (44%) | 38 (17%) | 49 (58%) | 46 (19%) | ||
| Primary tumor (pT) stage | ||||||
| T1 | 6 (6%) | 110 (49%) | <0.001a | 6 (7%) | 110 (46%) | <0.001a |
| T2 | 28 (29%) | 71 (32%) | 26 (31%) | 73 (31%) | ||
| T3 | 64 (65%) | 42 (19%) | 52 (62%) | 54 (23%) | ||
| Regional node (pN) stage | n = 96 | n = 172 | n = 83 | n = 185 | ||
| N0 | 39 (41%) | 129 (75%) | <0.001a | 32 (39%) | 136 (74%) | <0.001a |
| N1 | 57 (59%) | 43 (25%) | 51 (61%) | 49 (26%) | ||
| Synchronous metastases | ||||||
| Absent | 61 (62%) | 209 (94%) | <0.001a | 54 (64%) | 216 (91%) | <0.001a |
| Present | 37 (38%) | 14 (6%) | 30 (36%) | 21 (9%) | ||
| Metachronous metastases | n = 61 | n = 209 | n = 54 | n = 216 | ||
| Absent | 28 (46%) | 200 (96%) | <0.001a | 25(46%) | 203 (94%) | <0.001a |
| Present | 33 (54%) | 9 (4%) | 29 (54%) | 13 (6%) | ||
| ALT | ||||||
| Negative | 0 (0%) | 223 (94%) | <0.001a | |||
| Positive | 84 (100%) | 14 (6%) |
Indicates that the value in question is statistically significantly better than the relevant control, where significance is defined by P < 0.05.
Prognostic significance of ALT and loss of DAXX/ATRX expression in primary PanNETs
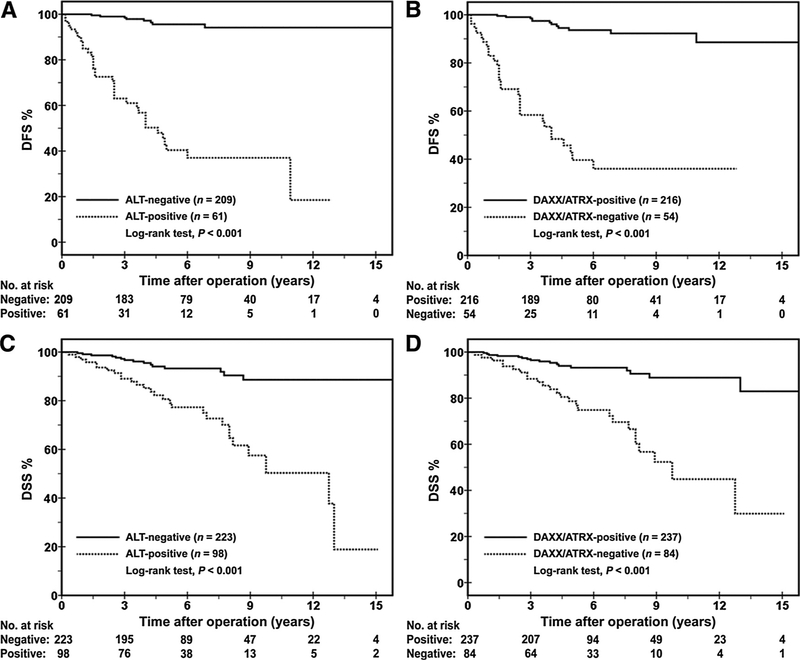
Patients whose tumors demonstrated ALT had shorter DFS and DSS. Among ALT-positive PanNETs, the DFS rates were 63% at 3 years and 40% at 5 years. The DSS rates were 81% at 5 years and 50% at 10 years. In comparison, patients with ALT-negative PanNETs had significantly longer DFS (99% at 3 years and 96% at 5 years; P < 0.001) and better DSS (93% at 5 years and 89% at 10 years, P < 0.001) rates (Fig. 3). No statistically significant differences in DFS and DSS between ALT-positive PanNETs and DAXX/ATRX-negative PanNETs were identified.
Figure 3.
Kaplan–Meier curves comparing the cumulative probabilities of DFS and DSS after surgical resection among PanNET patients with respect to ALT and DAXX/ATRX status. Patients with ALT-positive and DAXX/ATRX-negative PanNETs were associated with shorter DFS (A, B) and shorter DSS (C, D), as compared with patients with ALT-negative and DAXX/ATRX-positive PanNETs.
Results from univariate Cox regression analysis for DFS and DSS in relation to various clinicopathologic features, including ALT status are shown in Table 2. Shorter DFS and poor DSS were associated with tumor size >2.0 cm (P < 0.001 and P < 0.001), G2 to G3 WHO grade (P < 0.001 and P < 0.001, respectively), lymphovascular invasion (P < 0.001 and P < 0.001, respectively), perineural invasion (P < 0.001 and P < 0.001, respectively), advanced tumor stage (P < 0.001 and P < 0.001, respectively), lymph node metastasis (P < 0.001 and P < 0.001, respectively), and ALT (P < 0.001 and P < 0.001, respectively). Age also correlated with shorter DFS (P = 0.006), but not DSS (P =0.063). Multivariate analysis was used to determine the prognostic significance of ALT for DFS and DSS and included tumor size >2.0 cm, WHO grade, and regional lymph node (pN) metastasis. Although ALT was an independent prognostic factor for DFS (HR = 7.12, P < 0.001), it was not for DSS (HR = 1.35,P = 0.388; Table 2). Similar results were seen with loss of DAXX/ATRX expression when substituted for ALT.
Table 2.
Univariate and multivariate Cox regression analysis for DFS and DSS
| Univariate Cox regression analysis | Multivariate Cox regression analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient or tumor characteristics | DFS HR (95% CI) | P | DSS HR (95% CI) | P | DFS HR (95% CI) | P | DSS HR (95% CI) | P |
| Gender, male vs. female | 1.12 (0.61–2.05) | 0.726 | 1.03 (0.57–1.88) | 0.914 | ||||
| Age, years | 1.04 (1.01–1.06)a | 0.006a | 1.02 (0.99–1.05) | 0.063 | ||||
| Functional vs. nonfunctional | 0.17 (0.02–1.26) | 0.084 | 0.58 (0.18–1.88) | 0.367 | ||||
| Location, head and uncinate vs. body and tail | 0.45 (0.17–1.16) | 0.098 | 0.57 (0.31–1.03) | 0.062 | ||||
| Tumor size, >2.0 cm vs. ≤2.0 cm | 36.47 (5.02–265.24)a | <0.001a | 26.84 (3.69–195.03)a | <0.001a | 11.60 (1.57–85.89)a | 0.016a | 8.51 (1.15–63.15)a | 0.036a |
| WHO grade, G2 or G3 vs. G1 | 4.69 (2.45–8.97)a | <0.001a | 5.75 (2.86–11.54)a | <0.001a | 1.65 (0.80–3.41) | 0.175 | 2.87 (1.34–6.16)a | 0.007a |
| Lymphovascular invasion, presence vs. absence | 6.97 (3.53–13.75)a | <0.001a | 12.42 (5.13–30.05)a | <0.001a | ||||
| Perineural invasion, presence vs. absence | 5.99 (3.21–11.16)a | <0.001a | 4.75 (2.58–8.75)a | <0.001a | ||||
| Tumor stage (pT), pT3 vs. pT1 and pT2 | 5.62 (3.04–10.40)a | <0.001a | 7.87 (3.92–15.82)a | <0.001a | ||||
| Lymph node metastasis (pN), pN1 vs. pNO | 6.35 (3.25–12.42)a | <0.001a | 5.86 (2.95–11.64)a | <0.001a | 2.49 (1.24–5.00)a | 0.010a | 3.09 (1.51–6.33)a | 0.002a |
| ALT, positive vs. negative | 20.55 (9.45–44.66)a | <0.001a | 4.76 (2.53–8.94)a | <0.001a | 7.12 (3.06–16.56)a | <0.001a | 1.35 (0.68–2.66) | 0.388 |
Indicates that the value in question is statistically significantly better than the relevant control, where significance is defined by P < 0.05.
Assessment and correlation of ALT and loss of DAXX/ATRX expression in distant metastases
Considering ALT and loss of DAXX/ATRX expression correlated with the development of distant metastases, the status of ALT and DAXX/ATRX was assessed in paired resected primary and distant metastases from 52 patients (Table 3) within the study cohort that had sufficient pathologic material for further ancillary studies. Twenty-eight (54%) patients had synchronous metastases, 15 (29%) had metachronous metastases, and 9 (17%) had both. In total, 191 distant metastases were evaluated and consisted of 111 (58%) synchronous and 80 (42%) metachronous metastases. The sites of metastases varied widely and included 167 (87%) liver, 14 (7%) nonregional lymph nodes, 3 (2%) diaphragm, 2 (1%) omentum, 1 remnant pancreas, 1 small bowel serosa, 1 ovary, 1 adrenal gland, and 1 epidural space. ALT and loss of DAXX/ATRX expression was detected in 35 (67%) and 27 (52%) of patients with metastatic PanNETs, respectively (Supplementary Fig. S1). No differences in the status of ALT and DAXX/ATRX expression were found among metastatic PanNETs from the same patient, regardless of whether they were synchronous and/or metachronous metastases. A comparison of ALT and DAXX/ATRX status between metastases and corresponding primary PanNET from the same patient identified 4 discordant cases. In these 4 cases, the primary PanNET was ALT negative and had preserved expression for DAXX/ATRX, while the metastases were ALT positive and had loss of DAXX and/or ATRX. However, evaluation of DAXX/ATRX IHC and telomere FISH using additional sections of the patient’s primary PanNET revealed heterogeneous ALT positivity and DAXX/ATRX loss (Fig. 2).
Table 3.
Clinical and pathologic comparison of metastatic PanNETs with respect to ALT and DAXX/ATRX status
| Patient or tumor characteristics | ALT-positive, n = 35 (67%) | ALT-negative, n = 17 (33%) | P | DAXX/ATRX-negative, n = 27 (52%) | DAXX/ATRX-positive, n = 25 (48%) | P |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 13 (37%) | 12 (71%) | 0.038a | 8 (30%) | 17 (68%) | 0.012a |
| Male | 22 (63%) | 5 (29%) | 19 (70%) | 8 (32%) | ||
| Mean age at initial presentation | 58.2 (31–82) | 57.8 (32–77) | 0.921 | 58.3 (31–82) | 57.8 (32–77) | 0.874 |
| (range), years | ||||||
| Mean primary tumor size (range), cm | 6.3 (1.0–18) | 5.2 (1.3–17.0) | 0.294 | 6.0 (1.0–15.0) | 5.9 (1.3–18.0) | 0.952 |
| Primary tumor, WHO grade | ||||||
| Low (G1) | 10 (29%) | 4 (24%) | 1.000 | 6 (22%) | 8 (32%) | 0.536 |
| Intermediate (G2) | 25 (71%) | 13 (76%) | 21 (78%) | 17 (68%) | ||
| Metastatic tumor(s), highest | ||||||
| WHO grade | ||||||
| Low (G1) | 2 (6%) | 4 (24%) | 0.204 | 0 | 6 (24%) | 0.030a |
| Intermediate (G2) | 26 (74%) | 10 (59%) | 21 (78%) | 15 (60%) | ||
| High (G3) | 7 (20%) | 3 (17%) | 6 (22%) | 4 (16%) | ||
| Primary tumor, ALT status | ||||||
| Positive | 35 (100%)b | 0 | <0.001a | 27 (100%)b | 8 (32%) | <0.001a |
| Negative | 0 | 17 (100%) | 0 | 17 (68%) | ||
| Primary tumor, DAXX/ATRX status | ||||||
| Negative | 27 (77%)b | 0 | <0.001a | 27 (100%)b | 0 | <0.001a |
| Positive | 8 (23%) | 17 (100%) | 0 | 25 (100%) | ||
| Timing of metastatic tumor(s) | ||||||
| Synchronous | 16 (46%) | 12 (71%) | 0.051 | 14 (52%) | 14 (56%) | 0.651 |
| Metachronous | 10 (29%) | 5 (29%) | 7 (26%) | 8 (32%) | ||
| Both | 9 (25%) | 0 | 6 (22%) | 3 (12%) | ||
| Metastatic sites | n = 135 | n = 56 | n = 108 | n = 83 | ||
| Liver | 114 (84%) | 53 (94%) | 0.932 | 89 (82%) | 78 (94%) | 0.719 |
| Nonregional lymph node | 12 (9%) | 2 (4%) | 10 (9%) | 4 (5%) | ||
| Diaphragm | 2 (2%) | 1 (2%) | 2 (2%) | 1 (1%) | ||
| Omentum | 2 (2%) | 0 | 2 (2%) | 0 | ||
| Remnant pancreas | 1 (1%) | 0 | 1 (1%) | 0 | ||
| Small-bowel serosa | 1 0%) | 0 | 1 (1%) | 0 | ||
| Ovary | 1 (1%) | 0 | 1 (1%) | 0 | ||
| Adrenal gland | 1 (1%) | 0 | 1 (1%) | 0 | ||
| Epidural space | 1 (1%) | 0 | 1 (1%) | 0 |
Indicates that the value in question is statistically significantly better than the relevant control, where significance is defined by P < 0.05.
For 4 PanNETs, a representative tumor section for each case was ALT negative and DAXX/ATRX positive; however, staining of additional tumor sections identified a small subclone that was both ALT positive and DAXX/ATRX negative.
Similar to their primary counterparts, ALT-positive metastatic PanNETs were associated with a predilection for male patients (P = 0.038), but no statistically significant difference between ALT status and mean patient age (P = 0.921), mean primary tumor size (P = 0.294), primary tumor WHO grade (P = 1.000), metastatic tumor(s) WHO grade (P 0.204), chronologic presentation with respect to the patient’s primary PanNET (synchronous vs. meta-chronous vs. both; P = 0.051), and metastatic site (P = 0.932). DAXX/ATRX-negative metastatic PanNETs had essentially identical clinicopathologic features as ALT-positive metastatic PanNETs. In addition, no differences in patient DSS rates were detected with respect to ALT and DAXX/ATRX status as assessed from the time after resection of the patient’s primary PanNET (Supplementary Fig. S2), but the number of cases within this cohort may be too small to be conclusive.
Discussion
The activation of a telomere maintenance mechanism is a central hallmark of human cancers (17). While the majority of cancers rely on the reverse transcriptase telomerase, a significant proportion of neoplasms maintain their telomere lengths utiliz ing the homologous recombination–based mechanism, known as ALT (18). A characteristic finding of ALT is the accumulation of large amounts of telomeric DNA, which is the basis for the telomere-specific FISH assay. Loss of nuclear expression for DAXX and ATRX coincides with ALT, and thus, both proteins are considered to be suppressors of ALT (8).
Within our study, the prevalence of ALT and loss of DAXX/ATRX expression in resected, primary PanNETs was 31% and 26%, respectively. All DAXX/ATRX-negative PanNETs were ALT positive, but 14% of ALT-positive cases had preserved expression for DAXX/ATRX. This finding suggests the presence of other suppressors of ALT in PanNETs. We also found ALT and loss of DAXX/ATRX expression can have a heterogeneous distribution within PanNETs. ALT and DAXX/ATRX loss was associated with larger tumor size, advanced pathologic tumor stage, regional lymph node metastasis, and distant metastasis. Moreover, the prevalence of ALT and DAXX/ATRX loss in metastatic PanNETs was 2-fold higher than in primary PanNETs. Considering de Wilde and colleagues reported the absence of ALT in pancreatic neuroendocrine microadenomas, our observations would support that ALT and loss of DAXX/ATRX expression are late events in the pathogenesis of PanNETs (14). In addition, ALT and DAXX/ATRX loss within distant metastases correlated with ALT and DAXX/ATRX loss within the corresponding primary PanNET. In many of these patients, only a small subpopulation of neoplastic cells was ALT positive and DAXX/ATRX negative. Furthermore, no differences in the status of ALT and DAXX/ATRX were identified among meta-static PanNETs from the same patient, regardless of whether they were synchronous or metachronous distant metastases. On the basis of these findings, although ALT and loss of DAXX/ATRX expression in PanNETs are late events, they occur prior to the development of metastatic disease and likely play a significant role in driving tumor metastasis.
In addition to homologous recombination–mediated telomere maintenance, ALT-positive tumors, including PanNETs, are characterized by complex karyotypes with extensive numerical and structural chromosomal instability (19–22). These chromosomal alterations are often clustered at specific genomic sites (19). It is plausible that ALT may lead to secondary deletions in tumor suppressor genes and oncogenic gains or rearrangements, which can potentiate metastasis formation. Extensive epigenetic modifications are also a feature of ALT-positive tumors. Both DAXX and ATRX are components of a heterochromatic/chromatin remodeling complex involved in the deposition of histone H3.3 to nucleosomes at pericentromeric and telomeric regions (7, 23). Histone H3.3 enrichment coincides with histone methylation and transcriptional repression at these sites (24–26). Therefore, loss of DAXX/ATRX could result in transcriptional activation of putative oncogenes involved in metastatic spread.
Regardless of their role in tumor metastasis, the prognostic and therapeutic implications of ALT and loss of DAXX/ATRX expression in PanNETs should be underscored. Currently, the WHO recommends classification of neuroendocrine neoplasms into 3 grades (G1, G2, and G3), based on proliferative index using mitotic rate and Ki67 immunolabeling (10). The WHO grade of a neuroendocrine neoplasm provides important prognostic information that is independent of tumor stage (27–29). PanNETs are typically categorized as G1 or G2, whereas G3 neoplasms are synonymous with poorly differentiated neuroendocrine carcinomas (PanNEC). PanNETs and PanNECs are distinct neoplasms that differ in their etiology, genetics, treatment, and outcome, and therefore, the accurate measurement of mitotic rate and Ki67 immunolabeling is critical (6, 30, 31). However, scoring mitotic figures suffers from poor interobserver reproducibility and is time consuming in high volume centers (32–34). Ki67 staining is also labor intensive and less reflective of the true proliferation index because it not only stains neoplastic cells within the M phase, but those in S, G1, and G2 phases of the cell cycle as well (35, 36). In addition, it has become increasingly recognized that G3 pancreatic neuroendocrine neoplasms not only include PanNECs, but also PanNETs (37, 38). Considering G3 PanNETs in the absence of a G1 or G2 component can be morphologically indistinguishable from PanNECs, WHO grade alone is insufficient for disease assessment in pancreatic neuroendocrine neoplasms (38).
In addition to the quantification of mitotic rate and Ki67, telomere-specific FISH for ALT and DAXX/ATRX IHC represent useful adjunct tests to the assessment of PanNETs. Consistent with the results published by Marinoni and colleagues, the presence of large, ultrabright telomere FISH signals indicative of ALT and loss of DAXX/ATRX expression in PanNETs within our study cohort correlated with shorter DFS and DSS. The 5-year DFS and 10-year DSS of patients with ALT-positive and DAXX/ATRX-negative PanNETs were 40% and 50%, respectively, as compared with 96% and 89%, respectively, among patients with ALT-negative and DAXX/ATRX-positive PanNETs. In multivariate analysis, ALT and DAXX/ATRX loss were negative, independent prognostic factors for DFS. Moreover, as opposed to other prognostic parameters and markers, ALT and DAXX/ATRX status reflects the underlying molecular pathogenesis of these neoplasms. Yachida and colleagues performed comparative molecular and immunohistochemical analysis of PanNETs and PanNECs and demonstrated preserved expression for DAXX/ATRX in PanNECs, while loss in a subset of PanNETs (30). However, in contrast to Ki67, the presence of ALT and loss of DAXX/ATRX was not an independent prognostic factor for DSS. Thus, the assessment of ALT and DAXX/ATRX expression in conjunction with WHO grading can further refine current prognostic classification of pancreatic neuroendocrine neoplasms and, in cases of G3 neuroendocrine neoplasms, improve selection of appropriate treatment. In fact, based on the results reported herein and those by Marinoni and colleagues, DAXX/ATRX IHC has been integrated into the routine evaluation of resected pancreatic neuroendocrine neoplasms at our institution.
Nonetheless, the current study is not without limitations. It is retrospective by design and not all patients received the same form of treatment. While a pancreaticoduodenectomy or distal pancreatectomy was performed in the majority of cases, 9% of patients underwent an enucleation or central pancreatectomy, and thus, regional lymph node sampling may be inadequate. Removing these patients from our analysis would have little impact on the statistical associations and prognostic findings of ALT and DAXX/ATRX loss. Of note, enucleation and central pancreatectomy procedures are typically done in the setting of small PanNETs (≤ 2.0 cm) because these tumors often have anindolent natural history (39). However, studies including an analysis of the SEER database suggest that a subset of small PanNETs can pursue a more aggressive course (40–42). In fact, within our study cohort, 7% of PanNETs that measured 2.0 cm in size were ALT positive and showed loss of DAXX/ATRX expression. Although further studies are required, the identification of ALT and DAXX/ATRX loss in preoperative biopsies could indicate an increased risk of developing metastatic disease and, in turn, prompt a change in surgical management to ensure complete regional lymph node dissection. Another point of contention is that DAXX and ATRX, within this study, were evaluated by IHC rather than mutational analysis. In many scenarios, protein expression does not accurately mirror the status of the corresponding gene. Heaphy and colleagues demonstrated a strong correlation between DAXX/ATRX mutations and loss of DAXX/ATRX protein expression (8). Furthermore, DAXX/ATRX loss can occur in the absence of detectable genetic alterations, suggesting the presence of other inhibitory mechanisms, such as promoter methylation, and thus, the assessment of protein expression is likely the ideal method of evaluating these genes (8). Regardless, telomere-specific FISH for ALT was analyzed in all cases.
In summary, we report the comprehensive assessment of ALT and DAXX/ATRX status in a large, multi-institutional cohort of primary and metastatic PanNETs. Patients with ALT-positive and DAXX/ATRX-negative PanNETs had shorter DFS and DSS. In addition, ALT and loss of DAXX/ATRX expression is a negative, independent prognostic factor for DFS. Furthermore, based on our analysis of paired primary PanNETs and their corresponding distant metastases, ALT and DAXX/ATRX loss are late events in the pathogenesis of PanNETs and occur prior to the development of metastatic disease. Although further studies are required, ALT and loss of DAXX/ATRX expression likely play a significant role in driving distant metastases in patients with PanNETs.
Supplementary Material
Translational Relevance.
A significant challenge in the management of pancreatic neuroendocrine tumors (PanNET) is predicting their behavior. Clinicopathologic grading and staging systems and biomarker development for PanNETs have evolved considerably over the past few decades, but for a subset of cases, may be subjective in interpretation and may not take into account the underlying biology of PanNETs. Whole-exome studies have identified recurrent mutations in the genes DAXX and ATRX. Mutations in these genes correlate with loss of protein expression by IHC and alternative lengthening of telomeres (ALT) by telomerespecific FISH. Both ALT and DAXX/ATRX loss in PanNETs are associated with shorter DFS and DSS. Therefore, telomerespecific FISH for ALT and DAXX/ATRX IHC is a useful adjunct to current prognostic classification systems and reflects the biological behavior of these neoplasms. As a result of this study, DAXX/ATRX IHC has been integrated into the routine evaluation of resected pancreatic neuroendocrine neoplasms at our institution.
Acknowledgments
The authors would like to thank Mrs. Robyn L. Roche for outstanding administrative assistance. In addition, the authors thank Drs. Ralph H. Hruban and Raja R. Seethala for helpful comments and suggestions.
Grant Support
This project was supported in part by a grant from the National Pancreas Foundation, Western Pennsylvania Chapter (to A.D. Singhi).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
A. Slivka is a consultant/advisory board member for Boston Scientific. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72. [DOI] [PubMed] [Google Scholar]
- 2.Fraenkel M, Kim MK, Faggiano A, Valk GD. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol 2012;26:691–703. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1–18. [DOI] [PubMed] [Google Scholar]
- 4.Halfdanarson TR, Rubin J, Farnell MB, Grant CS, Petersen GM. Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer 2008;15:409–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011;331:1199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Sullivan RJ, Almouzni G. Assembly of telomeric chromatin to create ALTernative endings. Trends Cell Biol 2014;24:675–85. [DOI] [PubMed] [Google Scholar]
- 8.Heaphy CM, de Wilde RF, Jiao Y, Klein AP, Edil BH, Shi C, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science 2011; 333:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marinoni I, Kurrer AS, Vassella E, Dettmer M, Rudolph T, Banz V, et al. Loss of DAXX and ATRX are associated with chromosome instability and reduced survival of patients with pancreatic neuroendocrine tumors. Gastroenterology 2014;146:453–60.e5. [DOI] [PubMed] [Google Scholar]
- 10.Bosman FT, Carneiro F, Hruban RH, Theise ND. World Health Organization (WHO) Classification of Tumours of the Digestive System. Lyon, France: IARC Press; 2010. [Google Scholar]
- 11.Reid MD, Bagci P, Ohike N, Saka B, Erbarut Seven I, Dursun N, et al. Calculation of the Ki67 index in pancreatic neuroendocrine tumors: a comparative analysis of four counting methodologies. Mod Pathol 2015; 28:686–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York, NY: Springer; 2010. [Google Scholar]
- 13.Cesare AJ, Heaphy CM, O’Sullivan RJ. Visualization of telomere integrity and function in vitro and in vivo using immunofluorescence techniques. Curr Protoc Cytom 2015;73:12.40.1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Wilde RF, Heaphy CM, Maitra A, Meeker AK, Edil BH, Wolfgang CL, et al. Loss of ATRX or DAXX expression and concomitant acquisition of the alternative lengthening of telomeres phenotype are late events in a small subset of MEN-1 syndrome pancreatic neuroendocrine tumors. Mod Pathol 2012;25:1033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dogeas E, Karagkounis G, Heaphy CM, Hirose K, Pawlik TM, Wolfgang CL, et al. Alternative lengthening of telomeres predicts site of origin in neuroendocrine tumor liver metastases. J Am Coll Surg 2014;218:628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaphy CM, Subhawong AP, Hong SM, Goggins MG, Montgomery EA, Gabrielson E, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol 2011;179:1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 18.Dilley RL, Greenberg RA. ALTernative telomere maintenance and cancer. Trends Cancer 2015;1:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakellariou D, Chiourea M, Raftopoulou C, Gagos S. Alternative lengthening of telomeres: recurrent cytogenetic aberrations and chromosome stability under extreme telomere dysfunction. Neoplasia 2013;15: 1301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nabetani A, Ishikawa F. Alternative lengthening of telomeres pathway: recombination-mediated telomere maintenance mechanism in human cells. J Biochem 2011;149:5–14. [DOI] [PubMed] [Google Scholar]
- 21.Gagos S, Chiourea M, Christodoulidou A, Apostolou E, Raftopoulou C, Deustch S, et al. Pericentromeric instability and spontaneous emergence of human neoacrocentric and minute chromosomes in the alternative pathway of telomere lengthening. Cancer Res 2008;68:8146–55. [DOI] [PubMed] [Google Scholar]
- 22.Lovejoy CA, Li W, Reisenweber S, Thongthip S, Bruno J, de Lange T, et al. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet 2012;8:e1002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis PW, Elsaesser SJ, Noh KM, Stadler SC, Allis CD. Daxx is an H3.3-specific histone chaperone and cooperates with ATRX in replication-independent chromatin assembly at telomeres. Proc Natl Acad Sci U S A 2010;107:14075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udugama M, M Chang FT, Chan FL, Tang MC, Pickett HA, R McGhie JD, et al. Histone variant H3.3 provides the heterochromatic H3 lysine 9 trimethylation mark at telomeres. Nucleic Acids Res 2015;43:10227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng RK, Gurdon JB. Epigenetic memory of an active gene state depends on histone H3.3 incorporation into chromatin in the absence of transcription. Nat Cell Biol 2008;10:102–9. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg AD, Banaszynski LA, Noh KM, Lewis PW, Elsaesser SJ, Stadler S, et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 2010;140:678–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst 2012;104:764–77. [DOI] [PubMed] [Google Scholar]
- 28.Liu TC, Hamilton N, Hawkins W, Gao F, Cao D. Comparison of WHO Classifications (2004, 2010), the Hochwald grading system, and AJCC and ENETS staging systems in predicting prognosis in locoregional well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2013; 37:853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellison TA, Wolfgang CL, Shi C, Cameron JL, Murakami P, Mun LJ, et al. A single institution’s 26-year experience with nonfunctional pancreatic neuroendocrine tumors: a validation of current staging systems and a new prognostic nomogram. Ann Surg 2014;259:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol 2012;36:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi C, Klimstra DS. Pancreatic neuroendocrine tumors: pathologic and molecular characteristics. Semin Diagn Pathol 2014;31:498–511. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Tang LH, Klimstra DS. Gastroenteropancreatic neuroendocrine neoplasms: historical context and current issues. Semin Diagn Pathol 2013; 30:186–96. [DOI] [PubMed] [Google Scholar]
- 33.Tsuta K, Liu DC, Kalhor N, Wistuba II, Moran CA. Using the mitosis-specific marker anti-phosphohistone H3 to assess mitosis in pulmonary neuroendocrine carcinomas. Am J Clin Pathol 2011;136:252–9. [DOI] [PubMed] [Google Scholar]
- 34.Voss SM, Riley MP, Lokhandwala PM, Wang M, Yang Z. Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. Am J Surg Pathol 2015;39:13–24. [DOI] [PubMed] [Google Scholar]
- 35.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984;133:1710–5. [PubMed] [Google Scholar]
- 36.Remes SM, Tuominen VJ, Helin H, Isola J, Arola J. Grading of neuroendocrine tumors with Ki-67 requires high-quality assessment practices. Am J Surg Pathol 2012;36:1359–63. [DOI] [PubMed] [Google Scholar]
- 37.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 2015; 39:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang LH, Untch BR, Reidy DL, O’Reilly E, Dhall D, Jih L, et al. Well-differentiated neuroendocrine tumors with a morphologically apparent high-grade component: a pathway distinct from poorly differentiated neuroendocrine carcinomas. Clin Cancer Res 2016;22: 1011–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulke MH, Shah MH, Benson ABIII, Bergsland E, Berlin JD, Blaszkowsky LS, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw 2015;13:78–108. [DOI] [PubMed] [Google Scholar]
- 40.Kuo EJ, Salem RR. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol 2013;20:2815–21. [DOI] [PubMed] [Google Scholar]
- 41.Haynes AB, Deshpande V, Ingkakul T, Vagefi PA, Szymonifka J, Thayer SP, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg 2011;146:534–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherenfant J, Stocker SJ, Gage MK, Du H, Thurow TA, Odeleye M, et al. Predicting aggressive behavior in nonfunctioning pancreatic neuroendocrine tumors. Surgery 2013;154:785–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.