Abstract
The mammalian DEAD-box RNA helicase DDX5, its paralog DDX17, and their orthologs in Saccharomyces cerevisiae and Drosophila melanogaster, namely Dbp2 and Rm62, define a subfamily of DEAD-box proteins. Members from this subfamily share highly conserved protein sequences and cellular functions. They are involved in multiple steps of RNA metabolism including mRNA processing, microRNA processing, ribosome biogenesis, RNA decay, and regulation of long non-coding RNA activities. The DDX5/Dbp2 subfamily is also implicated in transcription regulation, cellular signalling pathways, and energy metabolism. One emerging theme underlying the diverse cellular functions is that the DDX5/Dbp2 subfamily of DEAD-box helicases act as chaperones for complexes formed by RNA molecules and proteins (RNP) in vivo. This RNP chaperone activity governs the functions of various RNA species through their life time. Importantly, mammalian DDX5 and DDX17 are involved in cancer progression when overexpressed through alteration of transcription and signalling pathways, meaning that they are possible targets for cancer therapy.
Graphical/Visual Abstract and Caption
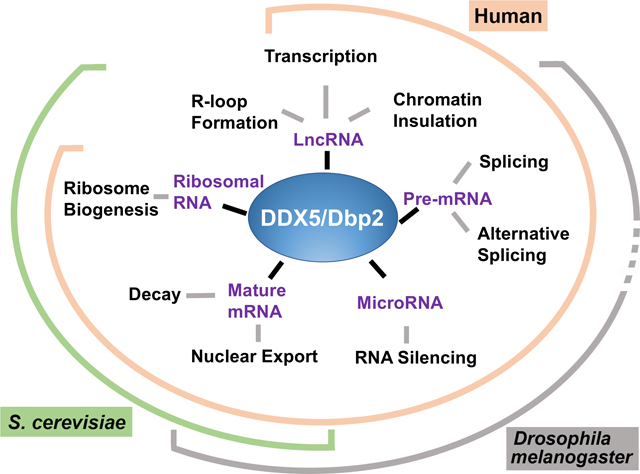
Caption: The DDX5/Dbp2 subfamily of RNA helicases act on multiple RNA substrates in a variety of cellular contexts. Some of these functions are characterized only in select organisms thus far, while others are shared across budding yeast, flies, and humans.
1. INTRODUCTION
DEAD-box proteins belong to the superfamily 2 (SF2) helicases (Linder and Jankowsky 2011). DEAD-box proteins are ATP-dependent RNA helicases and include of 38 members in humans, 25 in yeast, and 9 in bacteria (Linder and Jankowsky 2011). Similar to other SF2 RNA helicases, DEAD-box proteins contain twelve highly conserved motifs that are responsible for the RNA-dependent ATPase and ATP-dependent helicase activities (Linder and Jankowsky 2011). The name “DEAD-box” originates from the single letter amino acid code Asp (D)-Glu (E)-Ala (A)-Asp (D) that is present in the highly conserved motif II (Walker B motif) (Linder et al. 1989; Umate et al. 2011). DEAD-box proteins function throughout the lifetime of cellular RNAs from synthesis to biological activity, and to inevitable decay (Linder and Jankowsky 2011).
Since the identification of the conserved “DEAD” sequence in the DEAD-box protein family, several subfamilies have been shown to play a role in human diseases (Fuller-Pace, 2013; Lasko, 2013; Sharma & Jankowsky, 2014). The DDX5/Dbp2 subfamily is one such example. Human DDX5 and S. cerevisiae Dbp2 are both active RNA helicases in vitro (Hirling, Scheffner, Restle, & Stahl, 1989; Ma, Cloutier, & Tran, 2013; Xing, Wang, & Tran, 2017). They are highly conserved in sequence and are functionally equivalent, as ectopic expression of DDX5 fully complements a dbp2Δ yeast strain (Xing et al., 2017). Members of this subfamily function in almost all aspects of RNA metabolism, including RNA biogenesis, maturation, transport, and stability. They are also involved in differentiation, development, and cellular metabolism. However, connections between their biochemical properties and cellular functions are lacking. Here, we focus on the founding members of this subfamily including mammalian DDX5 and its paralog DDX17, Drosophila Rm62, and S. cerevisiae Dbp2. We will discuss major themes of their biochemical activities and biological functions and try to identify the relationship between biochemical properties and functionality.
2. GENERAL BIOCHEMICAL PROPERTIES OF THE DEAD-BOX PROTEINS
DEAD-box proteins can function as RNA chaperones (unwinding and refolding of RNA), RNA helicases, and RNA-protein complex (RNP) chaperones (assembling and/or disassembling of RNP) (Ballut et al. 2005; Young et al. 2013; Putnam & Jankowsky 2013; Jarmoskaite & Russell 2014). Similar to other RNA helicases, DEAD-box proteins unwind RNA duplexes in an ATP-dependent manner. However, DEAD-box proteins utilize a different unwinding mechanism from canonical RNA helicases (Rudolph and Klostermeier 2015). Most other RNA helicases use a translocation-based duplex unwinding mechanism, where the helicase first binds to a single-stranded region next to the duplex and then translocates in a unidirectional manner (Fiorini, Bagchi, Le Hir, & Croquette, 2015; Jankowsky, Gross, Shuman, & Pyle, 2000). DEAD-box proteins, on the other hand, do not unwind in a translocation-based fashion, but rather load directly onto the duplex region and separate the two strands (Yang and Jankowsky 2006; Yang et al. 2007). This is possible because DEAD-box proteins can disrupt RNA duplexes locally by bending one of the RNA strands (Rudolph & Klostermeier, 2015).
Multiple X-ray crystallographic studies of DEAD-box helicases have demonstrated that DEAD-box proteins contain a structurally conserved helicase core that consists of two globular RecA-like domains (RecA_N and RecA_C) (Sengoku et al. 2006; Andersen et al. 2006; Del Campo and Lambowitz 2009; von Moeller et al. 2009; Rudolph and Klostermeier 2015). The two domains are connected by a flexible linker to form a characteristic “dumbbell-like” core. During RNA unwinding, the two domains adopt a closed conformation upon cooperative binding of the dsRNA and ATP. This bends one strand of the RNA, which is then incompatible with the geometry of an A-form RNA duplex, and results in localized destabilization of the RNA duplex following release of the “unbent” RNA strand (Andersen et al., 2006; Banroques, Cordin, Doere, Linder, & Tanner, 2008; Del Campo & Lambowitz, 2009; Lorsch & Herschlag, 1998; Polach & Uhlenbeck, 2002; Rudolph & Klostermeier, 2015; Samatanga & Klostermeier, 2014; Sengoku, Nureki, Nakamura, Kobayashi, & Yokoyama, 2006; Theissen, Karow, Köhler, Gubaev, & Klostermeier, 2008; von Moeller et al., 2009). Upon ATP hydrolysis, the helicase dissociates from single-stranded RNA, terminating a single round of the unwinding cycle (Henn, Cao, Hackney, & De La Cruz, 2008). The DEAD-box helicase then either recycles back on the same RNA substrate or finds a new target following binding of a second ATP molecule (Fig. 1; Rudolph & Klostermeier 2015). Since each ATP hydrolysis cycle only unwinds ~ 6 base pairs of the RNA duplex (Chen et al., 2008), multiple cycles of unwinding are required to completely separate longer duplexes. Recently, Guenther et al. revealed that an enzymatically active Ded1 is required for reducing RNA structure in the 5’ untranslated regions of mRNAs on a genome-wide scale in S. cerevisiae, allowing proper translation initiation (Guenther et al., 2018). This represents the first example of RNA helicase activity associated with a DEAD-box protein in vivo.
Figure 1. DEAD-box proteins unwind duplexes non-processively via local strand separation.
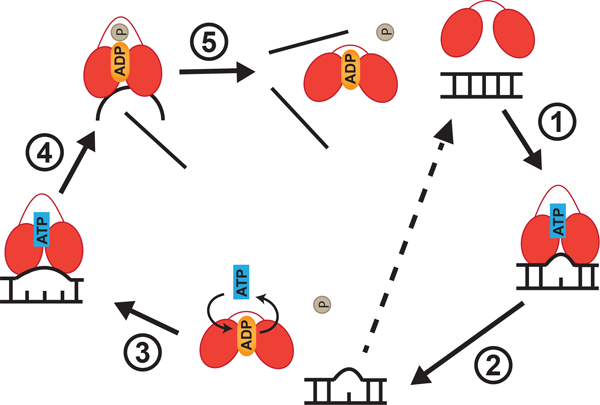
Schematic representation of the unwinding cycle of a prototypical DEAD-box RNA helicase. Lines represent RNA strands and the two ovals represent the two RecA-like domains that are connected by a flexible linker. In the absence of any nucleotide and RNA, the two RecA-like domains are farther apart and exhibit a flexible “opened, unproductive” conformation. During unwinding, the two RecA-like domains come closer together to form a “closed, productive” conformation upon binding to the double-stranded RNA (dsRNA) and ATP (step 1). Closing of the two domains bends one strand of the dsRNA and results in local duplex destabilization (~ 6 bp). Duplexes longer than 6 bp require multiple cycles of unwinding. ATP hydrolysis and inorganic phosphate release convert the two RecA-like domains back to the “opened” conformation (step 2). This causes dissociation of the helicase core from the partially opened dsRNA. The partially opened dsRNA can potentially snap back to produce a non-productive unwinding cycle (dotted arrow) or is subjected to another round of local duplex destabilization (step 3). Another round of local duplex destabilization happens after the ADP is exchanged to ATP in the DEAD-box protein or a new ATP-bound DEAD-box protein recognizes the partially opened dsRNA. This allows the DEAD-box protein to fully disrupt the partially opened duplex. Upon ATP hydrolysis, the ADP-Pi bound DEAD-box protein still associates with the bent strand whereas the non-bent strand is released (step 4). The bent strand is eventually dissociated from the DEAD-box protein once the inorganic phosphate is released (step 5) (Figure adapted from Rudolph and Klostermeier 2015).
DEAD-box proteins recognize the phosphate backbone of RNA instead of the nucleotide bases (Sengoku et al. 2006; Andersen et al. 2006; Del Campo & Lambowitz 2009; von Moeller et al. 2009), suggesting that DEAD-box proteins bind to RNA substrates in a sequence non-specific manner. This is an advantage for DEAD-box proteins that act as general RNA chaperones targeting many different RNAs (Jarmoskaite & Russell 2014). Paradoxically, 70% of DEAD-box protein genes in S. cerevisiae exhibit non-redundant biological functions, suggesting specificity inside cells (Rocak & Linder, 2004). Accessory domains of some DEAD-box proteins can confer substrate specificity (Jarmoskaite and Russell 2014). In other cases, protein cofactors provide targeting to specific biological substrates (Young et al., 2013). Accessory domains and protein cofactors likely control the substrate specificity of DEAD-box proteins in vivo.
One of the major functions of DEAD-box proteins is to act as RNP chaperones (Patrick Linder & Jankowsky, 2011). RNP chaperone activity involves both displacing and assembling RNA-binding proteins on RNA, thereby changing the structure and composition of the RNA-protein complex. Individual DEAD-box proteins alter RNP complexes by remodelling RNA structure directly to expose binding sites for RNA-binding proteins, or by functioning as high affinity, RNA-binding platforms for assembly of additional proteins. RNP chaperone activity has been demonstrated by relevant DEAD-box proteins in pre-mRNA splicing and nuclear mRNA export (Ballut et al., 2005; Tran, Zhou, Corbett, & Wente, 2007). It is well established that the DEAD-box protein eIF4AIII is a clamp that can act as a protein platform for assembly of the multicomponent exon junction complex (EJC) upstream of exon-exon junctions on mRNA (Ballut et al., 2005). DEAD-box proteins can also act as RNA chaperones to facilitate RNA folding, which involves both unwinding and annealing, to promote formation of complex tertiary structures. Several DEAD-box proteins display annealing activity independent of ATP (Rossler et al. 2001; Yang & Jankowsky 2005; Halls et al. 2007; Uhlmann-Schiffler et al. 2006; Ma et al. 2013; Young et al. 2013). This chaperone activity has been demonstrated to be biologically relevant for splicing of the Group II intron by Mss116 (Potratz, Del Campo, Wolf, Lambowitz, & Russell, 2011).
3. THE HISTORY AND SEQUENCE ANALYSIS OF THE DDX5/DBP2 SUBFAMILY
Human DDX5 (or p68) was initially identified due to its cross-reactivity with an antibody (pAb204) generated against the simian virus 40 (SV40) large T antigen (Lane & Hoeffler, 1980). Later, extensive homology was found between DDX5 and murine translation initiation factor eIF4A, leading the authors to propose a new gene family defined by these two proteins (Ford, Anton, & Lane, 1988). This gene family was named the DEAD-box helicases (Linder et al. 1989). A paralog of DDX5 exists in mammals named DDX17 (or p72) (Lamm, Nicol, Fuller-Pace, & Lamond, 1996). Human DDX5 and DDX17 are 69.7% identical and 77.6% similar (Lamm et al., 1996). Phylogenetic analysis revealed amino acid sequence diversity of 38 annotated human DEAD-box proteins (Fig. 2). This analysis also suggested that DDX43 and DDX53 paralogs are closely related to DDX5 and DDX17 (Fig. 2). DDX43 has published ATP-dependent RNA helicase activity (Talwar et al., 2017), whereas DDX53 has not been biochemically characterized to date. Similar to DDX5 and DDX17, DDX43 and DDX53 are linked to cancers (Sarkar & Ghosh, 2016). To permit genetic analysis of DDX5 function, Iggo et al. cloned yeast genes related to DDX5 and named them dbp2 in Schizosaccharomyces pombe and DBP2 in Saccharomyces cerevisiae (S. cerevisiae), for DEAD-box protein 2 (Iggo et al., 1991). The Drosophila Rm62 is the fly homolog of DDX5/DDX17, and was first described in a mutagenic screen designed to search for modifiers of retrotransposon expression, where it was named as “Lighten up (Lip)” (Csink, Linsk, & Birchler, 1994).
Figure 2. Unrooted phylogenetic tree of human DEAD-box helicases.
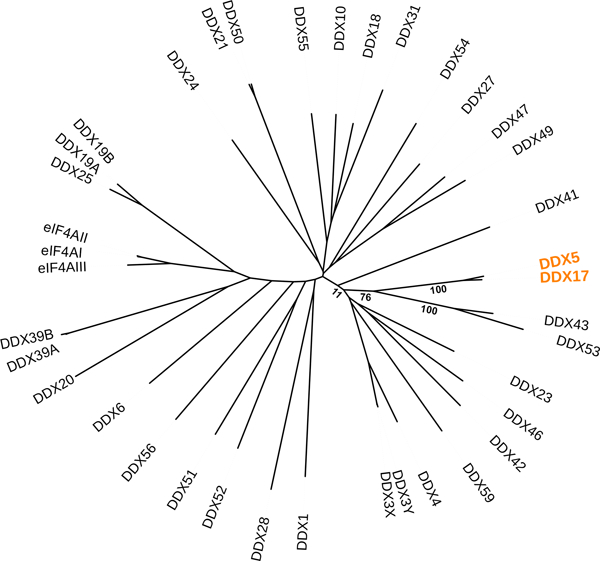
Protein sequences of 38 annotated human DEAD-box proteins were downloaded from NCBI. Multiple sequence alignment and phylogenetic analysis were conducted by MAFFT version 7 (Katoh, Rozewicki, & Yamada, 2017). Bootstrap values represent the percentage of replicates in which the same branch was recovered during 1000 times of resampling.
Human DDX5, DDX17, drosophila Rm62, and yeast Dbp2 protein sequences are highly conserved (Fig. 3). All 12 DEAD-box motifs are found in the helicase cores of these proteins, consistent with grouping into the DEAD-box family. The N- and C-terminal ends of the DDX5/Dbp2 subfamily are more diverse than their helicase cores. Several DEAD-box proteins possess an arginine and glycine (RG)-rich region at the C-terminus (Yang & Jankowsky 2005). Generally, RG-rich regions function in RNA binding, protein-protein interactions and/or protein localization (Thandapani, O’Connor, Bailey, & Richard, 2013). RG-rich regions are found adjacent to the helicase core in all four DDX5/Dbp2 subfamily members at both the N-terminus and C-terminus (Fig. 3). Interestingly, the N-terminal RG-rich region of Rm62 is longer than other subfamily members, which might account for drosophila-specific functions of Rm62. DDX17 and Rm62 have dissimilar N-terminal extensions that are absent in DDX5 and Dbp2 (Fig. 3). In fact, the N-terminal extension in DDX17 represents the major difference between DDX5 and DDX17 in terms of sequence. DDX5 and DDX17 possess mammalian-specific C-terminal extensions (CTEs) with limited sequence homology. DDX17 has a proline-rich region within the CTE (Fig. 3). Proline-rich regions generally mediate protein-protein interactions (Kay, Williamson, & Sudol, 2000). However, these N-terminal and C-terminal extensions are not yet characterized.
Figure 3. Sequence alignment of the founding DDX5/Dbp2 subfamily members.
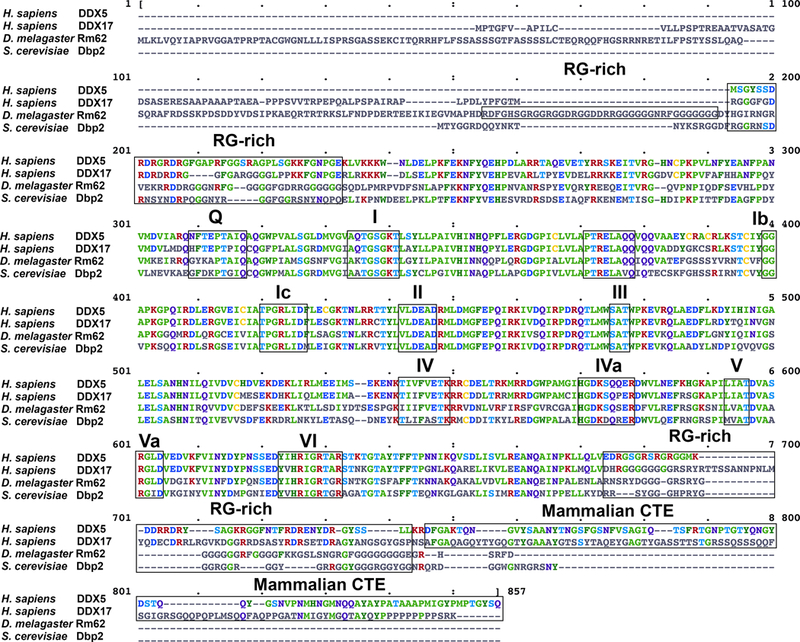
Multiple sequence alignment of human DDX5 (NP_001307525.1), DDX17 (NP_006377.2), Drosophila Rm62 (NP_524243.2), and S. cerevisiae Dbp2 (NP_014287.3) was conducted using the Clustal Omega web service (McWilliam et al., 2013). 12 conserved domains in the helicase core, the RG-rich regions, and the mammalian-specific CTE are highlighted with black boxes.
4. ENZYMATIC ACTIVITIES OF DDX5 AND DBP2
4.1. DDX5 and Dbp2 are RNA-dependent ATPases and ATP-dependent RNA helicases
The RNA helicase activity of DDX5 was first described almost 30 years ago, shortly after discovery of the DDX5 protein (Hirling et al., 1989). DDX5 purified from human 293 cells possesses RNA-stimulated ATPase activity and ATP-dependent RNA unwinding activity in vitro (Hirling et al., 1989). Surprisingly, this initial study showed that DDX5 unwound a 162 bp duplex (Hirling et al., 1989), a substrate considerably longer than the ~ 12–20 bp unwound by other members of the DEAD-box protein family (Cordin, Banroques, Tanner, & Linder, 2006). This indicates that DDX5 and DDX17 act processively, an activity that is unprecedented for DEAD-box proteins due to the localized mode of unwinding (Cordin et al., 2006). Later, Rossler and colleagues purified recombinant DDX5 and DDX17 from Sf9 insect cells and showed that both DDX5 and DDX17 require 3’ overhangs for duplex unwinding and unwind in a 3’−5’ directional manner (Rossler et al., 2001). The authors also showed that DDX5 and DDX17 unwind RNA duplexes in single-turnover conditions, indicating processivity of these enzymes. However, more recent work showed non-directional unwinding for recombinant DDX5 when purified from bacteria and a lack of processivity (Huang & Liu, 2002; Xing et al., 2017, Xing et al., unpublished). These later reports reveal an activity more consistent with other DEAD-box proteins (Rudolph & Klostermeier, 2015), and argue against a processive role for DDX5.
Direct comparison of bacterially expressed DDX5 and Dbp2 unwinding kinetics using a 16 bp blunt-end RNA duplex showed that DDX5 displays faster unwinding as compared to Dbp2 (Xing et al., 2017). Dbp2 anneals this RNA duplex in an ATP-independent manner, whereas DDX5 did not display any annealing activity (Xing et al., 2017). Interestingly, truncation of the mammalian-specific CTE of DDX5 reduces unwinding rates and restores some annealing activity (Xing et al., 2017), suggesting that this extended region impacts the conformation of the helicase core and its interactions with the RNA duplex substrate. Not surprisingly, the CTE of DDX5 is not required for DDX5-dependent complementation of a dbp2Δ S. cerevisiae strain, consistent with the fact that this domain is not present in Dbp2 (Xing et al., 2017). Future structural characterization of full length DDX5 is necessary to understand the contribution of the CTE to duplex unwinding.
5. FUNCTIONS OF THE DDX5/DBP2 SUBFAMILY IN RNA METABOLISM AND GENE EXPRESSION
In line with the lack of stringent sequence specificity in vitro (see section 4.1), the DDX5/Dbp2 subfamily function in multiple steps of RNA metabolism from post-transcriptional gene regulation to ribosome biogenesis (see below). Members from this subfamily appear to function as RNP chaperones in most cases, regardless of the nature of their RNA targets (Fig. 4). It is clear that mammalian DDX5 and DDX17 paralogs share some functional redundancy, however, paralog-specific functions have also been reported (see below). It is important to note that, while most studies to date have focused on DDX5, some of these studies have used simultaneous knockdown of both DDX5 and DDX17, complicating interpretation of the results and the specific contribution of DDX5 and DDX17 to the phenotype. We will note this below in our discussion of DDX5 and DDX17 paralogs.
Figure 4. The DDX5/Dbp2 subfamily acts as RNP chaperone in vivo.
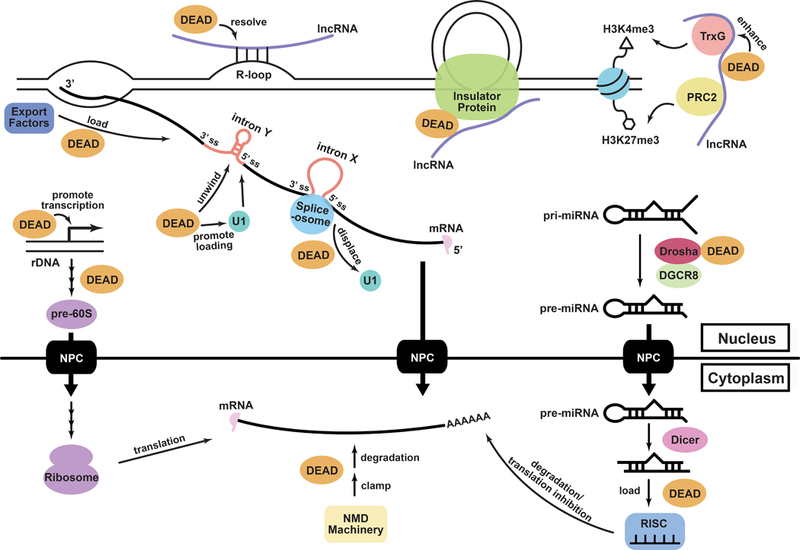
The DDX5/Dbp2 subfamily (orange circles, “DEAD”) functions as RNP chaperones in a variety of processes. This includes regulation of lncRNA activities (Cloutier et al., 2016; Yao et al., 2010; Zhang et al., 2016), pre-mRNA splicing (Liu 2002), alternative splicing (Lee et al., 2018), mRNA export (Ma et al., 2016), miRNA processing (Remenyi et al., 2016), nonsense mediated decay (NMD) (Geißler et al., 2013), and ribosome biogenesis (Saporita et al., 2011).
5.1. Cellular localization of DDX5
In eukaryotes, the nucleus and cytoplasm are separated by a double-membrane structure termed the nuclear envelope (Cautain, Hill, de Pedro, & Link, 2015). The nuclear pore complex (NPC) spans the nuclear envelope and functions as gate for trafficking of macromolecules (Cautain et al., 2015). Transport of proteins through the NPC is mediated by the β-karyopherins in an ATP-dependent manner. β-karyopherins are receptor proteins that recognize sequence motifs in the protein cargoes termed the nuclear localization signals (NLS) or nuclear export signals (NES) for nuclear import and export, respectively (Cautain et al., 2015).
DDX5, DDX17 and Dbp2 are predominantly nuclear proteins (Cloutier, Ma, Nguyen, & Tran, 2012; Iggo et al., 1991; Kahlina, Goren, Pfeilschifter, & Frank, 2004; Lamm et al., 1996). However, the localization of DDX5 is not static, but, rather, depends on cell cycle status, cell type, and post-translational modifications. DDX5 displays a diffuse granular nuclear staining pattern during interphase and is localized to nascent nucleoli during telophase (Iggo et al., 1991; Nicol, Causevic, Prescott, & Fuller-Pace, 2000). During the G2/M phase, DDX5 becomes more cytoplasmic (Choi & Lee, 2012). Interestingly, DDX5 localization is also cell type-specific and heterogeneous within a population of the same type of cells, varying from exclusively nuclear to predominantly cytoplasmic (Rossow & Janknecht, 2003). Phosphorylation of tyrosine 593 (Tyr593) in DDX5 by c-Abl promotes the nuclear export of DDX5, explaining the latter subcellular shift in localization (Yang et al. 2006; Arun et al. 2012). Cytoplasmic localization is thought to alter protein-protein interactions in the Wnt signalling pathway (Yang et al., 2006). Consistent with both nuclear and cytoplasmic localization, Wang and colleagues showed that DDX5 is a nucleocytoplasmic shuttling protein using heterokaryon assays (Wang, Gao, Huang, Yang, & Liu, 2009). Amino acid sequences 351–363 and 482–502 of DDX5 are nuclear localization signals (NLS), while sequences 282–308 and 446–461 serve as nuclear export signals (NES) (Wang et al. 2009). Tyr593 does not reside in either of the NLS or NES signals, suggesting that phosphorylation of this residue may influence cellular localization by inducing a conformational change.
5.2. The DDX5/Dbp2 subfamily functions in transcription regulation
RNA polymerase II transcription in eukaryotes is tightly regulated by transcription factors (Lelli, Slattery, & Mann, 2012). Interestingly, transcriptional roles of DDX5 and DDX17 have been linked to cancers. DDX5 and DDX17 coactivate oncogenic transcription factors including estrogen receptor (ER) and androgen receptor (AR), which are important in the development of breast cancer and prostate cancer, respectively (Zhao et al. 2008; Wortham et al. 2009; Clark et al. 2008; Clark et al. 2013). Paradoxically, DDX5 also acts as a coactivator for the tumor suppressor p53 (Bates et al., 2005; Nicol et al., 2013). Depletion of DDX5, but not DDX17, inhibits the expression of p53-responsive cell cycle arrest gene p21 (Bates et al., 2005; Nicol et al., 2013). However, DDX5 is dispensable for the expression of p53-responsive pro-apoptotic genes (Nicol et al., 2013). This suggests that DDX5 has selective coactivator activity for p53 and may modulate the decision between cell cycle arrest and apoptosis, favoring cell survival. In addition to transcription co-activation, DDX5/DDX17 co-repress the Repressor Element 1-silencing transcription factor (REST), which is required for turning off neuronal genes in non-neuronal cells (Baldelli & Meldolesi, 2015; Lambert et al., 2018). Moreover, DDX5 and DDX17 repress transcription from specific promoters when associated with histone deacetylase 1 (HDAC1) (Wilson et al., 2004).
5.2.1. DDX5 and DDX17 regulate lncRNPs formed by lncRNAs and histone modifiers
Recent studies have also connected transcription regulation by the DDX5/Dbp2 subfamily to long noncoding RNAs (lncRNAs) (Rinn & Chang, 2012). LncRNAs are noncoding transcripts that are greater than 200 nt in length. They are transcribed by RNA polymerase II, and regulate gene expression by binding and scaffolding different protein factors, and/or directly interacting with the genomic DNA (Engreitz et al. 2016). The DDX5/Dbp2 subfamily regulates chromatin environment and transcription by controlling the activities of lncRNAs. Both DDX5 and DDX17 associate with the steroid receptor RNA activator (SRA) lncRNA, which then binds to the trithorax group complex (TrxG) and the polycomb repressive complex 2 (PRC2) (Wongtrakoongate, Riddick, Fucharoen, & Felsenfeld, 2015). These are protein complexes that deposit histone H3 lysine 4 tri-methylation (H3K4me3, mark for active transcription) and lysine 27 tri-methylation (H3K27me3, repressive histone mark), respectively. Therefore, TrxG and PRC2 can create bivalent chromatin domains consisting of two functionally opposing histone modifications (Voigt, Tee, & Reinberg, 2013). Bivalent chromatin domains usually associate with developmental genes, rendering these genes poised for activation (Bernstein et al., 2006; Voigt et al., 2013). DDX5 and DDX17 enhance the interaction between SRA and TrxG, thereby increasing H3K4me3 at genomic regions bound by both DDX5 and SRA. However, the interaction between SRA and PRC2 is independent of DDX5 and DDX17 (Wongtrakoongate et al., 2015).
Interestingly, PRC2 interacts with DDX5 through another lncRNA, the Hox transcript antisense intergenic RNA (HOTAIR) (Zhang et al., 2016). DDX5 stabilizes the SUZ12 subunit of PRC2, likely by displacing the RNA-binding E3 ligase Mex3b from the HOTAIR/PRC2 complex. This promotes PRC2-mediated gene repression (Zhang et al., 2016). RNA binding, but not ATP hydrolysis, is required for this activity (Zhang et al., 2016).
5.2.2. The DDX5/Dbp2 subfamily functions in chromatin insulation
DDX5 and DDX17 associate with the CCCTC-binding factor (CTCF), a DNA binding protein involved in chromatin insulator activity, through the lncRNA SRA (Yao et al., 2010). Chromatin insulators are DNA-protein complexes that bridge long distance interactions and create insulated chromatin domains (Phillips-Cremins & Corces, 2013). DDX5, DDX17, and SRA are required for CTCF insulator activity (Yao et al., 2010). Interestingly, the interaction between DDX5 and CTCF requires the N and C-terminal domains, but does not require the helicase core (Yao et al., 2010). This suggests that these accessory domains are sufficient to mediate lncRNA-dependent complex formation in this case.
Rm62 is also involved in insulator function in Drosophila (Lei & Corces, 2006). Rm62 physically interacts with DNA-binding centrosomal protein 190 (CP190), the gypsy insulator associated protein in Drosophila, in an RNA-dependent manner (Lei & Corces, 2006). Different from DDX5, Rm62 loss of function promotes the activity of the gypsy insulator (Lei & Corces, 2006), suggesting negative regulation and context-dependent roles of the DDX5/Dbp2 subfamily proteins in chromatin insulation.
5.2.3. Dbp2 antagonizes the GAL lncRNAs in S. cerevisiae
Dbp2 functions in concert with the GAL lncRNAs in S. cerevisiae, but in a significantly different manner from what has been established for mammalian DDX5. The GAL lncRNAs are transcribed from the GAL gene cluster, which encodes the GAL genes required for galactose metabolism (Houseley, Rubbi, Grunstein, Tollervey, & Vogelauer, 2008; Pinskaya, Gourvennec, & Morillon, 2009; van Dijk et al., 2011). In the presence of glucose, the preferred carbon source for yeast, the GAL genes are shut off transcriptionally. While the GAL genes are repressed, the GAL lncRNAs are expressed and form RNA/DNA hybrids (R-loops) with the genomic DNA (Cloutier et al., 2012, 2016). R-loops are generated by base paring of an RNA molecule to one strand of the genomic DNA, leaving the other strand free. The GAL R-loops prepare the GAL genes for faster induction during a nutritional switch to glucose-depleted, galactose-containing media (Cloutier et al., 2016; Cloutier, Wang, Ma, Petell, & Tran, 2013). Dbp2 antagonizes the function of the GAL lncRNAs: deletion of DBP2 promotes the GAL lncRNA R-loop formation, thereby displacing transcriptional repressors and speeding up transcriptional activation (Cloutier et al., 2016). Recent studies revealed that the in vivo RNA binding sites of Dbp2 tend to form R-loops (Tedeschi, Cloutier, Tran, & Jankowsky, 2018), indicating that Dbp2 may have broader impact on these nucleic acid structures. It is rational to propose that Dbp2 may resolve R-loops directly using its RNA helicase activity, since Dbp2 is capable of unwinding an RNA-DNA hybrid in vitro (Ma et al., 2013). However, the weaker RNA-DNA hybrid unwinding activity (observed unwinding rate ~0.13 min−1, unwinding amplitude ~60%) compared to a pure RNA duplex (observed unwinding rate ~0.22 min−1, unwinding amplitude~100%) suggests that Dbp2 may not unwind R-loops in vivo (Ma et al., 2013). Further studies of Dbp2 function in vivo are necessary to understand how loss of this enzyme promotes formation of these nucleic acid structures in the cell.
5.3. The DDX5/Dbp2 subfamily promotes mRNA processing
Messenger RNA is processed in the cells through 5’ capping, 3’ polyadenylation, splicing of precursor mRNAs (pre-mRNAs), and mRNA export, all of which are coupled with transcription to some extent (Bentley, 2014). The DDX5/Dbp2 subfamily of DEAD-box proteins, are involved in many steps of mRNP assembly and mRNA processing.
5.3.1. The DDX5/Dbp2 subfamily functions in pre-mRNA splicing and alternative splicing
Eukaryotic pre-mRNAs typically contain introns that are excised through two transesterification reactions catalyzed by a multi-megadalton RNP termed the spliceosome (Shi, 2017). The spliceosome consists of U1, U2, U4, U5, and U6 small nuclear ribonucleoproteins (snRNP). Assembly of the spliceosome on pre-mRNA starts with recognition of the 5’ splice site (5’ ss) by the U1 snRNP, followed by subsequent association of the other four snRNPs (Shi, 2017). Many RNA helicases, including the DDX5/Dbp2 subfamily proteins, have been linked to splicing (Cordin & Beggs, 2013). Drosophila Rm62 was identified as a core component of spliceosome in large-scale RNA interference (RNAi) screens (Park et al. 2004; Brooks et al. 2015). Proteomic analysis also showed that DDX5 and DDX17 co-purifies with the human spliceosome (Rappsilber, Ryder, Lamond, & Mann, 2002; Zhou, Licklider, Gygi, & Reed, 2002). In addition, DDX5 crosslinks with the transient U1–5’ ss complex (Liu et al. 1998). This suggests a direct role for DDX5, and possibly DDX17, in spliceosome assembly. Indeed, DDX5 promotes the dissociation of U1 from the 5’ ss, which allows the formation of a catalytically active spliceosome (Liu 2002). The enzymatic activities of DDX5 is required for this process.
DDX5 and DDX17 are also implicated in alternative splicing, a process that contributes to protein diversity and is often aberrant in human diseases (Lee & Rio, 2015). Downregulation of DDX5 leads to the inclusion of the c-H-ras gene alternative exon IDX that harbors an in-frame stop codon (Guil et al., 2003). Inclusion of exon IDX leads to production of a shorter H-Ras protein, which induces a G1/S phase delay (Camats, Kokolo, Heesom, Ladomery, & Bach-Elias, 2009). DDX5 and DDX17 depletion also affect the alternative splicing of a histone variant macroH2A1 (mH2A1), leading to increased levels of the mH2A1.1 isoform, which alters the expression of genes involved in redox metabolism (Dardenne et al., 2012). Large-scale analysis showed that DDX5 and/or DDX17 depletion affect alternative splicing patterns globally (Dardenne et al., 2014; Lee, Wang, & Rio, 2018). The binding targets of DDX5, and pre-mRNAs that require this enzyme for specific splicing events, largely overlap with those of hnRNPA1, another RNA binding protein involved in alternative splicing (Lee et al., 2018). This suggests that DDX5 and hnRNPA1 function in similar pathways to regulate alternative splicing. DDX5 is also found in a large multiprotein complex LASR (large assembly of splicing regulators) that recruits the alternative splicing Rbfox proteins in mammalian brain (Damianov et al., 2016), suggesting tissue-specific roles for this enzyme in splicing.
DDX5 and DDX17 seem to target specific sequence elements to regulate alternative splicing. Overexpression of DDX17, but not DDX5, increases inclusion of the cell-surface glycoprotein CD44 exons V4 and V5, by recognizing the AC-rich enhancer elements within the alternative exons (Honig, Auboeuf, Parker, O’Malley, & Berget, 2002). DDX5 and DDX17 may also regulate alternative spicing by resolving RNA structure (Dardenne et al. 2014; Kar et al. 2011). Simultaneous knockdown of DDX5 and DDX17 results in exclusion of exons that have predicted downstream RNA G-quadruplexes, stable tertiary structures formed at G-rich regions (Dardenne et al., 2014). Exon exclusion may be manifested by reduced hnRNP H/F binding to these G-rich regions that are otherwise exposed in the presence of DDX5 and/or DDX17 (Dardenne et al., 2014). Consistently, DDX5 and DDX17 were found to bind the RNA G-quadruplexes of the NRAS oncogene in vivo (Herdy et al., 2018). However, we do not know if DDX5/DDX17 actively unwind G-quadruplexes or if they are biologically connected to G-quadruplexes. DDX5 also regulates alternative splicing of the tau gene encoding a neuronal microtubule-stabilizing protein (Kar et al. 2011). DDX5 triggers an open conformation of the stem-loop structure at the 5’ splice site of tau exon 10, allowing binding of U1 snRNP and promoting exon 10 inclusion (Kar et al. 2011). Predictably, the enzymatic activities of DDX5 and DDX17 are required in all three cases discussed above. However, it is not known how does DDX5/DDX17 recognize these sequence elements.
5.3.2. The DDX5/Dbp2 subfamily facilitates mRNA export
In the nucleus, mRNA is bound by proteinaceous export factors that enable mRNA transport to the cytoplasm through the nuclear pore complex (Bjork & Wieslander, 2014). DDX5 co-immunoprecipitates with the export factors ALY and TAP, with depletion leading to reduced association of TAP with oncogenic c-fos mRNA, resulting in the nuclear accumulation of this mRNA (Zonta et al., 2013). In S. cerevisiae, DBP2 genetically interacts with mRNA export factors YRA1 (S. cerevisiae ALY) and MEX67 (S. cerevisiae TAP), and is required for efficient association of Yra1 and Mex67 with poly(A)+ RNA (Ma et al., 2013). Therefore, DDX5/Dbp2 facilitate the loading of export factors onto mRNA. Interestingly, Yra1 also interacts directly with Dbp2 and this association inhibits the single-stranded (ss) RNA binding activity of Dbp2 (Ma et al., 2016). This suggests that Yra1 promotes release of Dbp2 from mRNA after a round of RNA duplex unwinding and mRNP assembly (Ma et al., 2016), emphasizing the fact that mRNP assembly is a dynamic process and that activities of RNP chaperones are also regulated by RNPs.
Drosophila Rm62 is found at polytene chromosome puffs, sites of active transcription (Buszczak & Spradling, 2006). Loss of Rm62 results in accumulation of the heat shock hsp70 mRNA at its sites of synthesis (Buszczak & Spradling, 2006). Export factors NXF1 (Drosophila TAP) and ALY are redistributed from the nuclear rim to the nucleoplasm in Rm62 mutant cells, suggesting a block in release of assembled mRNPs from transcription sites in the absence of Rm62 (Buszczak & Spradling, 2006). Taken together, this suggests that the DDX5/Dbp2 subfamily may not only promote proper assembly of export factors onto mRNA co-transcriptionally, but also facilitate the movement of the processed mRNP toward nuclear pores.
5.4. The DDX5/Dbp2 subfamily functions in nonsense-mediated decay (NMD)
Nonsense-mediated decay (NMD) is a surveillance pathway that targets a selection of mRNAs for degradation (Lykke-Andersen & Jensen, 2015). Canonical targets for NMD are mRNAs containing premature termination codons (PTC), however, normal transcripts can be affected by the NMD pathway as well (Hug, Longman, & Caceres, 2016). DDX5/DDX17 and Dbp2 have been implicated in different stages of NMD (see below).
DDX5 and DDX17 physically interact with hUPF3, an essential component of the NMD machinery (Geißler, Altmeyer, Stein, Uhlmann-Schiffler, & Stahl, 2013). Depletion of DDX5 promotes the accumulation of transcripts harboring long 3’ UTRs, such as the DDX17 and SMG5 transcripts, likely by inhibiting NMD (Geißler et al., 2013). Consistently, DDX5 binds to these endogenous transcripts and a reporter mRNA containing the 3’ UTR of SMG5 in vivo. It is worth noting that SMG5 is also a component of the NMD machinery (Hug et al., 2016), indicating an intricate feedback loop controlling the expression levels of DDX5 and DDX17.
Interestingly, the RNA helicase activity of DDX5 is not required for NMD per se, because overexpression of a DDX5 Walker B motif mutant (DDX5-E249Q) inhibits the expression of DDX17 similar to overexpression of wild type DDX5 (Geißler et al., 2013). However, DDX5 Walker B motif mutants likely retain RNA binding activity (Zhang et al., 2016, and Table 1). Thus, DDX5 may act as an RNA-binding protein that promotes or stabilizes the assembly of the NMD machinery onto their RNA targets. This is reminiscent of the DEAD-box helicase eIF4AIII, which acts as a high affinity RNA-binding protein and multiprotein scaffold to “clamp” the exon-junction-complex at exon junctions and enable recognition of PTCs (Ballut et al., 2005). Interestingly, loss of DDX5/DDX17 does not affect PTC-containing transcripts in a reporter assay, but, instead changes the decay mRNAs with long 3’UTRs (Geißler et al., 2013). It is possible that DDX5 and/or DDX17 play functionally analogous roles in NMD as eIF4AIII but in a non-canonical NMD pathway.
Table 1. Biochemically characterized DDX5/Dbp2 mutants.
The biochemical properties of DEAD-box proteins are not necessarily coupled. We observe that the literature sometimes uses “helicase-dead” mutants to demonstrate that the RNA helicase activities are not required for certain biological functions of the DDX5/Dbp2 subfamily. However, while such statements are true, these mutants may still be partially active. ATP hydrolysis is required for recycling DEAD-box proteins, allowing multiple rounds of duplex separation, but is not necessary for RNA-binding, or a single round of strand separation (Liu et al. 2008). Therefore, a mutant lacking ATP hydrolysis may still possess RNA-binding activity. For example, DDX5-D248N, a frequently used “enzymatically-dead” mutant that harbors a point mutation in motif II (Walker B motif), possesses equal RNA binding activity and reduced ATPase activity as compared to wild type DDX5. This suggests that the DDX5-D248N mutant can act as an “RNA clamp” that binds to RNA but does not readily dissociate. Taking the ensemble of biochemical properties into account is necessary to decipher the roles of DEAD-box proteins in biological processes. n.d. stands for not determined.
| Mutants | Motif | ATP binding |
ATP hydrolysis |
RNA binding |
RNA unwinding |
RNA annealing |
References |
|---|---|---|---|---|---|---|---|
| DDX5-K144N | I | None | None | Reduced | None | n.d. |
Zhang et al., 2016, Jalal et al., 2007 |
| DDX5-D248N | II | n.d. | Partial | Wild-type | n.d. | n.d. | Zhang et al., 2016 |
| DDX5-E249Q | II | Wild-type | None | n.d. | None | n.d. | Jalal et al., 2007 |
| DDX5-R403L | V | Wild-type | None | n.d. | None | n.d. | Lin et al., 2005 |
| DDX5-R428L | VI | Wild-type | None | n.d. | None | n.d. | Lin et al., 2005 |
| Dbp2-K163N | I | n.a. | None | n.a. | n.a. | n.a. | Cloutier et al., 2012 |
| Dbp2-D268Q | II | n.a. | None | n.a. | n.a. | n.a. | Cloutier et al., 2012 |
In S. cerevisiae, DBP2 genetically interacts with UPF1, a central component of the NMD pathway (Bond, Mangus, He, & Jacobson, 2001; He & Jacobson, 1995). Distinct from the observations in human cells, PTC-containing transcripts accumulate in dbp2Δ, suggesting a role of Dbp2 in canonical NMD (Bond et al., 2001). In addition, a Walker B mutation in DBP2 abolishes NMD, suggesting a requirement for enzymatically active Dbp2 (Bond et al., 2001). Consistent with the differences of DDX5 and Dbp2 in this specific pathway, ectopic expression of DDX5 in dbp2Δ does not restore NMD (Bond et al., 2001). This also agrees with the fact that the pathways for NMD between yeast and humans are different (Hug et al., 2016).
In addition to protein-coding transcripts, it was recently found that Xrn1-sensitive unstable transcripts (XUTs) containing long 3’ extensions are degraded through the NMD pathway in a DBP2-dependent manner (Wery et al., 2016). The 3’ extensions of XUTs are reminiscent of the long 3’ UTRs that renders NMD dependent on DDX5 (Geißler et al., 2013). Therefore, DDX5 and Dbp2 may share a conserved role in the recruitment of the NMD machinery onto RNAs harboring as of yet undefined elements within long 3’ ends.
5.5. The DDX5/Dbp2 subfamily promotes microRNA processing
MicroRNAs (miRNAs) are non-coding RNAs that target mRNAs for silencing (Ha & Kim, 2014). Primary-miRNAs (pri-miRNAs) are transcribed by RNA polymerase II or RNA polymerase III and undergo two major steps for maturation that are catalyzed by enzymes in the RNAse III family. Pri-miRNAs are processed by Drosha in the nucleus into precursor-miRNAs (pre-miRNAs). Pre-miRNAs are then cleaved by Dicer in the cytoplasm and become the mature miRNA (Ha & Kim, 2014).
The DDX5/Dbp2 subfamily functions in pri-miRNA processing. Drosophila Rm62 binds to pri-miRNA stem loops in vivo (Moy et al., 2014). In human cells, DDX5/DDX17 associate with Drosha and are required for the maturation of a subset of pri-miRNAs (Gregory et al., 2004; Lambert et al., 2018; Li et al., 2017; Remenyi, Bajan, Fuller-Pace, Arthur, & Hutvagner, 2016; Salzman, Shubert-Coleman, & Furneaux, 2007; Wang, Huang, & Hu, 2012). DDX5/DDX17 appear to interact with accessory proteins that regulate pri-miRNA processing. For example, the signal transducer proteins SMADs interact with Drosha through DDX5, thereby promoting the processing of a subset of miRNAs in response to growth factors (Davis, Hilyard, Lagna, & Hata, 2008; Davis, Hilyard, Nguyen, Lagna, & Hata, 2010). Similarly, p53 associates with Drosha and promotes the processing of miR-16–1 and miR-143 through DDX5/DDX17 (Suzuki et al., 2009). The interaction between p53 and Drosha is sensitive to RNAse treatment, suggesting a direct role for RNA molecules in the assembly of a p53-Drosha-DDX5/DDX17 complex. Association with SMADs or p53 confer different pri-miRNA specificities to the Drosha-DDX5/Drosha-DDX17 complex, but pri-mRNA processing was enhanced by these factors in both cases (Davis et al., 2008, 2010; Suzuki et al., 2009). In contrast, Cyclooxygenase 2 (COX-2), an enzyme involved in inflammation, represses miR-183 processing by interacting with the Drosha-DDX5 complex (Motiño et al., 2015). Taken together, DDX5/DDX17 may be important for modulating miRNA maturation in a context-dependent manner.
The DDX5/Dbp2 subfamily also functions in the miRNA-mediated silencing process itself. Rm62 associates with Argonaute 2 (AGO2), an RNA binding protein that associates with the mature miRNA to form the RNA-induced silencing complex, and is required for efficient RNA interference (Ishizuka, Siomi, & Siomi, 2002; Zhou et al., 2008). In line with this, DDX5 unwinds the let-7 miRNA duplex, presumably allowing the loading of let-7 miRNA into the RISC complex (Salzman et al., 2007). Thus, DDX5/Dbp2 function at multiple steps along the miRNA pathway from synthesis to RNA-dependent gene regulation.
5.6. The DDX5/Dbp2 subfamily promotes ribosome biogenesis
The ribosome is a massive ribonucleoprotein complex (RNP), whose assembly requires cooperative work of numerous (> 200) transiently associated proteins and small RNAs (Jalal, Uhlmann-Schiffler, & Stahl, 2007; Thomson, Ferreira-Cerca, & Hurt, 2013). DDX5 associates with the ribosomal DNA (rDNA) promoter, and ectopically overexpressed DDX5 increases the amount of primary rRNA transcript (Saporita et al., 2011). This suggests that DDX5 may promote the transcription from rDNA. Interestingly, R-loops have been observed in the rDNA locus (Amon & Koshland, 2016; El Hage, French, Beyer, & Tollervey, 2010), which block RNA polymerase I transcription when not removed efficiently (El Hage et al., 2010). Given that Dbp2 likely antagonizes R-loops in S. cerevisiae (Cloutier et al., 2016; Tedeschi et al., 2018), it is possible that DDX5 promote the clearance of R-loops to allow proper transcription in the rDNA locus. However, a connection between DDX5 and R-loops are not been established to date.
DDX5 also functions in processing of 32S rRNA to mature 28S rRNA in mammalian cells, a role which appears to be shared with DDX17 (Jalal et al., 2007; Saporita et al., 2011). Dbp2 appears to function similarly in S. cerevisiae, as processing of the 35S pre-rRNA into mature 25S rRNA is reduced in dbp2Δ S. cerevisiae cells, along with the levels of 60S subunits, monosomes, polysomes, and protein synthesis activity (Bond et al., 2001). Ectopic expression of DDX5 rescues the ribosome biogenesis defects in dbp2Δ cells (Bond et al., 2001), suggesting conserved roles of DDX5 and Dbp2 in ribosome biogenesis. It is possible that DDX5/Dbp2 promotes the assembly of RNPs that are necessary for rRNA processing and ribosome function. One type of these hypothetical DDX5/Dbp2-dependent RNPs are small nucleolar (sno) RNPs, since snoRNAs compromise 9% of Dbp2’s RNA targets in S. cerevisiae (Tedeschi et al., 2018). Given that Dbp2 has been shown to bind 60S pre-ribosomal particles in S. cerevisiae (Nissan, Bassler, Petfalski, Tollervey, & Hurt, 2002), we also predict that members from the DDX5/Dbp2 subfamily will be identified as partners of more ribosomal proteins and/or pre-ribosomal factors in the future.
6. BROADER ROLES OF THE DDX5/DBP2 SUBFAMILY IN CELL GROWTH AND ENERGY HOMEOSTASIS
Consistent with the implication in ribosome biogenesis, the DDX5/Dbp2 subfamily is implicated in cell growth through regulating multiple signalling pathways that integrate environmental cues and proliferation (section 6.1). This includes the target of rapamycin (TOR) signalling (Du et al., 2017). Interestingly, TOR regulates ribosome biogenesis in mammals and yeast, respectively (Iadevaia, Liu, & Proud, 2014; Martin, Powers, & Hall, 2006). Thus, the functions of DDX5 and Dbp2 in ribosome biogenesis may be at least partially mediated though TOR. In addition, DDX5 and Dbp2 are recently linked to glucose metabolism (section 6.2), another cellular process regulated by TOR signalling. This indicates that TOR connects DDX5 and Dbp2’s roles in cell growth and energy homeostasis. In some cases, hyperactivated pro-proliferative signalling pathways and aberrant energy metabolism lead to dysregulated growth of cells that cause cancer (Liberti & Locasale, 2016; Sever & Brugge, 2015). Indeed, overexpression of DDX5 is frequently found in cancers (section 6.3).
6.1. DDX5 is involved in various signaling pathways
DDX5 activates pro-growth signaling pathways including Wnt, Notch, and mTOR pathways (Du et al., 2017; Lin et al., 2013; Taniguchi et al., 2016; Wang et al., 2015; Yang et al., 2006). First, in colon cancer cells and NSCLCs, Tyr593 phosphorylated DDX5 is translocated to the cytoplasm to facilitate the nuclear translocation of β-catenin, which activates Wnt target genes including Cyclin D1 and c-Myc (Yang et al. 2006; Yang et al. 2007; Shin et al. 2007; Wang et al. 2015). These Wnt targets then promote cell proliferation and tumorigenesis. Furthermore, inhibition of DDX5 decreases the expression of Notch target genes and hinders the growth of T cell acute lymphoblastic leukemia (T-ALL) cells (Lin et al., 2013), consistent with the roles of Notch signaling in proliferation and differentiation (Andersson, Sandberg, & Lendahl, 2011). Moreover, DDX5 depletion inhibits the mTOR pathway in prostate cancer cells, as evidenced by reduced phosphorylation of ribosomal protein S6 kinase 1 (S6K1) (Taniguchi et al., 2016). Increased or reduced S6K1 phosphorylation represents the hallmark of mTOR activation or suppression (Saxton & Sabatini, 2017). Consistently, ectopic overexpression of DDX5 promotes mTOR and S6K1 phosphorylation, as well as cell proliferation in gastric cancers (Du et al., 2017). Importantly, mTOR activation is necessary for the pro-proliferative roles of DDX5 in gastric cancers (Du et al., 2017).
6.2. The newly identified link between the DDX5/Dbp2 subfamily and glucose metabolism
The DDX5/Dbp2 subfamily has recently implicated in glucose metabolism (Guo et al., 2010; Mazurek et al., 2014; Wang et al., 2017; Xing et al., 2017), a process that is altered in human diseases, including cancers. In normal cells, the end product of glycolysis, pyruvate, is usually oxidized through oxidative phosphorylation. When oxygen is limited in some scenarios, pyruvate is converted to lactate. However, cancer cells often convert pyruvate to lactate regardless of the oxygen availability, a phenomenon termed aerobic glycolysis, or the Warburg effect (Liberti & Locasale, 2016). Although the roles of Warburg effect are still unclear, one of the theories is that glycolysis supports biosynthesis, which is essential for rapid proliferation of cancer cells (Liberti & Locasale, 2016).
Depletion of DDX5 inhibits the expression of genes involved in glucose metabolism and increases reactive oxygen species (ROS) levels in Acute Myeloid Leukemia cells, leading to apoptosis (Mazurek et al., 2014). Moreover, downregulation of DDX5 in human hepatocyte AML12 cells reduces glucose uptake and glycolysis and upregulates respiration (Xing et al., 2017). In S. cerevisiae, the nuclear localization of Dbp2 is responsive to nutrient availability (Beck et al., 2014); Dbp2 is rapidly shuttled to the cytoplasm within 10 min of glucose depletion. Interestingly, DDX5 localization is regulated similarly when ectopically expressed in yeast (Xing et al., unpublished). Although the upstream events of Dbp2 glucose-dependent nucleocytoplasmic shuttling are not yet defined, several factors that are linked to glucose deprivation are not required for Dbp2 relocalization following glucose deprivation (Beck et al., 2014). These include the AMP-activated protein kinase SNF1, the mitogen-activated protein kinase HOG1, and the TOR signaling pathway (Beck et al., 2014). Given that DDX5 functions upstream of mTOR in mammalian cells (see section 6.1), it is possible that DDX5/Dbp2 senses nutrient availability directly through an as-of-yet unidentified mechanism.
6.3. DDX5 overexpression is linked to cancer
It is well established that DDX5 and/or DDX17 are overexpressed in various types of cancers including colon cancers (Causevic et al., 2001; Shin et al., 2007), prostate cancer (Clark et al., 2008), breast cancers (Wortham et al., 2009), brain cancers (Wang et al. 2012), lung cancers (Wang et al., 2015), gastric cancers (Du et al., 2017), and leukemias (Lin et al., 2013). Depletion of DDX5/DDX17 in many of these cancers inhibit the growth of tumor cells, suggesting requirement of DDX5/DDX17 in cancer development. Importantly, overexpression of DDX5 promotes growth of gastric cancers and non-small cell lung cancer (NSCLC) cells both in vitro and in vivo, indicating that DDX5 may be a driver for cellular transformation (Du et al., 2017; Wang et al., 2015). A small molecule inhibitor for DDX5, Supinoxin™ (RX-5902), has been developed for cancer therapy, and is currently in a clinical trial in patients with metastatic triple negative breast cancer (ClinicalTrials.gov identifier: NCT02003092). This suggests that DDX5 is a promising drug target for cancers.
Conclusion
A large body of evidence suggests that the DDX5/Dbp2 subfamily functions in a variety of distinct pathways in eukaryotes, many of which are conserved. Members of this subfamily function as RNP chaperones in key steps of gene expression. They regulate transcription, promote mRNA processing (splicing and nuclear export), regulate mRNA levels (NMD and RNA interference), and promote ribosome biogenesis (Fig. 4). We propose that the broader cellular roles of DDX5/Dbp2 in glucose metabolism and carcinogenesis are largely manifested through regulation of gene expression and that these diverse biological roles are not due to different biochemical mechanisms, but, rather, are dictated by the wide range of RNA targets acted on by DDX5/Dbp2. The enzymatic roles of the DDX5/Dbp2 subfamily are fundamentally similar, however, context-dependent alterations to RNA structure and/or RNP conformation can have different outcomes on cellular pathways. Further studies are needed to define how these family members select RNA targets and how these enzymes are regulated. These include defining:
-
(i)
Substrate specificity of the DDX5/Dbp2 subfamily. DDX5 and Dbp2 bind and unwind RNA duplexes with random sequences in vitro (Ma et al., 2013; Xing et al., 2017). Moreover, members from this subfamily target a variety of RNA species in vivo (section 5). However, it is not clear if they recognize specific nucleotide sequences or RNA structures. Techniques that are used to identify in vivo targets of RNA binding proteins (eg. iCLIP-Seq) and to map native RNA structures (eg. DMS-Seq) will help answer such questions. Furthermore, substrate specificity of DEAD-box proteins may also be determined by protein co-factors (Young et al., 2013). However, we do not know if this is true for the DDX5/Dbp2 subfamily.
-
(ii)
Targets of DDX5/DDX17 in cancers. DDX5 and DDX17 are heavily implicated in cancers ((Fuller-Pace & Moore, 2011) and section 6). However, many studies neglect the known biochemical roles of these RNA helicases. Furthermore, mutants that are not biochemical characterized were used in the literature, assuming that they are enzymatically dead. This is not a safe assumption since some of these mutants may be partially active (section 4). Such assumption may lead to incorrect conclusions that the enzymatic activities of DDX5/DDX17 are dispensable for cancers in some cases. Therefore, it will be of great interest to define the RNA targets and enzymatic roles of DDX5/DDX17 in cancers.
-
(iii)
Nutrient-dependent cellular trafficking mechanisms and DDX5/Dbp2-dependent roles in metabolism. DDX5 and Dbp2 are nucleocytoplasmic shuttling proteins with condition-dependent cellular localization (section 5.1). Particularly, Dbp2 displays glucose-dependent nuclear localization (Beck et al., 2014). It will be interesting to examine if DDX5 and DDX17 shuttle in response to glucose in humans, and to determine the upstream signals for DDX5/Dbp2 re-localization. Furthermore, DDX5/Dbp2 was the first subfamily of RNA helicases shown to be involved in glucose metabolism (Wang et al., 2017; Xing et al., 2017). However, the underlying mechanism is largely unknown. Defining factors required for DDX5/Dbp2-mediated metabolic regulation will be required.
Acknowledgments
We would like to thank Dr. Siwen Wang for making the electronic version of Figure 4. This work was supported by National Institutes of Health R01GM097332 to E.J.T. and an American Heart Association Predoctoral Fellowship 16PRE31170005 to Z.X.
Footnotes
No conflicts of interest.
Contributor Information
Zheng Xing, Department of Biochemistry, Purdue University, West Lafayette, Indiana 47906, USA; Purdue Center for Cancer Research, Purdue University, West Lafayette, Indiana 47906, USA.
Wai, Kit Ma, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, USA.
Elizabeth, J Tran, Department of Biochemistry, Purdue University, West Lafayette, Indiana 47906, USA; Purdue Center for Cancer Research, Purdue University, West Lafayette, Indiana 47906, USA.
References
- Amon JD, & Koshland D (2016). RNase H enables efficient repair of R-loop induced DNA damage. ELife, 5 10.7554/eLife.20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen CB, Ballut L, Johansen JS, Chamieh H, Nielsen KH, Oliveira CL, … Andersen GR (2006). Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science, 313(5795), 1968–1972. https://doi.org/1131981 [pii] 10.1126/science.1131981 [DOI] [PubMed] [Google Scholar]
- Andersson ER, Sandberg R, & Lendahl U (2011). Notch signaling: simplicity in design, versatility in function. Development (Cambridge, England), 138(17), 3593–3612. 10.1242/dev.063610 [DOI] [PubMed] [Google Scholar]
- Arun G, Akhade VS, Donakonda S, & Rao MRS (2012). mrhl RNA, a long noncoding RNA, negatively regulates Wnt signaling through its protein partner Ddx5/p68 in mouse spermatogonial cells. Molecular and Cellular Biology, 32(15), 3140–3152. 10.1128/MCB.00006-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldelli P, & Meldolesi J (2015). The Transcription Repressor REST in Adult Neurons: Physiology, Pathology, and Diseases. ENeuro, 2(4). 10.1523/ENEURO.0010-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballut L, Marchadier B, Baguet A, Tomasetto C, Seraphin B, & Le Hir H (2005). The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat Struct Mol Biol, 12(10), 861–869. https://doi.org/nsmb990 [pii] 10.1038/nsmb990 [DOI] [PubMed] [Google Scholar]
- Banroques J, Cordin O, Doere M, Linder P, & Tanner NK (2008). A conserved phenylalanine of motif IV in superfamily 2 helicases is required for cooperative, ATP-dependent binding of RNA substrates in DEAD-box proteins. Mol Cell Biol, 28(10), 3359–3371. https://doi.org/MCB.01555-07 [pii] 10.1128/MCB.01555-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates GJ, Nicol SM, Wilson BJ, Jacobs A-MF, Bourdon J-C, Wardrop J, … Fuller-Pace FV (2005). The DEAD box protein p68: a novel transcriptional coactivator of the p53 tumour suppressor. The EMBO Journal, 24(3), 543–553. 10.1038/sj.emboj.7600550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck ZT, Cloutier SC, Schipma MJ, Petell CJ, Ma WK, & Tran EJ (2014). Regulation of glucose-dependent gene expression by the RNA helicase Dbp2 in Saccharomyces cerevisiae. Genetics, 198(3), 1001–1014. 10.1534/genetics.114.170019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley DL (2014, March). Coupling mRNA processing with transcription in time and space. Nature Reviews. Genetics. England. 10.1038/nrg3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, … Lander ES (2006). A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell, 125(2), 315–326. 10.1016/j.cell.2006.02.041 [DOI] [PubMed] [Google Scholar]
- Bjork P, & Wieslander L (2014). Mechanisms of mRNA export. Seminars in Cell & Developmental Biology, 32, 47–54. 10.1016/j.semcdb.2014.04.027 [DOI] [PubMed] [Google Scholar]
- Bond AT, Mangus DA, He F, & Jacobson A (2001). Absence of Dbp2p alters both nonsense-mediated mRNA decay and rRNA processing. Molecular and Cellular Biology, 21(21), 7366–7379. 10.1128/MCB.21.21.7366-7379.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buszczak M, & Spradling AC (2006). The Drosophila P68 RNA helicase regulates transcriptional deactivation by promoting RNA release from chromatin. Genes & Development, 20(8), 977–989. 10.1101/gad.1396306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camats M, Kokolo M, Heesom KJ, Ladomery M, & Bach-Elias M (2009). P19 H-ras induces G1/S phase delay maintaining cells in a reversible quiescence state. PloS One, 4(12), e8513 10.1371/journal.pone.0008513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causevic M, Hislop RG, Kernohan NM, Carey FA, Kay RA, Steele RJ, & Fuller-Pace FV (2001). Overexpression and poly-ubiquitylation of the DEAD-box RNA helicase p68 in colorectal tumours. Oncogene, 20(53), 7734–7743. 10.1038/sj.onc.1204976 [DOI] [PubMed] [Google Scholar]
- Cautain B, Hill R, de Pedro N, & Link W (2015). Components and regulation of nuclear transport processes. The FEBS Journal, 282(3), 445–462. 10.1111/febs.13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Potratz JP, Tijerina P, Del Campo M, Lambowitz AM, & Russell R (2008). DEAD-box proteins can completely separate an RNA duplex using a single ATP. Proc Natl Acad Sci U S A, 105(51), 20203–20208. https://doi.org/0811075106 [pii] 10.1073/pnas.0811075106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y-J, & Lee S-G (2012). The DEAD-box RNA helicase DDX3 interacts with DDX5, co-localizes with it in the cytoplasm during the G2/M phase of the cycle, and affects its shuttling during mRNP export. Journal of Cellular Biochemistry, 113(3), 985–996. 10.1002/jcb.23428 [DOI] [PubMed] [Google Scholar]
- Clark EL, Coulson A, Dalgliesh C, Rajan P, Nicol SM, Fleming S, … Robson CN (2008). The RNA helicase p68 is a novel androgen receptor coactivator involved in splicing and is overexpressed in prostate cancer. Cancer Research, 68(19), 7938–7946. 10.1158/0008-5472.CAN-08-0932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EL, Hadjimichael C, Temperley R, Barnard A, Fuller-Pace FV, & Robson CN (2013). p68/DdX5 supports beta-catenin & RNAP II during androgen receptor mediated transcription in prostate cancer. PloS One, 8(1), e54150. 10.1371/journal.pone.0054150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier SC, Ma WK, Nguyen LT, & Tran EJ (2012). The DEAD-box RNA helicase Dbp2 connects RNA quality control with repression of aberrant transcription. The Journal of Biological Chemistry, 287(31), 26155–26166. 10.1074/jbc.M112.383075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier SC, Wang S, Ma WK, Al Husini N, Dhoondia Z, Ansari A, … Tran EJ (2016). Regulated Formation of lncRNA-DNA Hybrids Enables Faster Transcriptional Induction and Environmental Adaptation. Molecular Cell, 61(3), 393–404. 10.1016/j.molcel.2015.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier SC, Wang S, Ma WK, Petell CJ, & Tran EJ (2013). Long noncoding RNAs promote transcriptional poising of inducible genes. PLoS Biology, 11(11), e1001715. 10.1371/journal.pbio.1001715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordin O, Banroques J, Tanner NK, & Linder P (2006). The DEAD-box protein family of RNA helicases. Gene, 367, 17–37. 10.1016/j.gene.2005.10.019 [DOI] [PubMed] [Google Scholar]
- Cordin O, & Beggs JD (2013). RNA helicases in splicing. RNA Biology, 10(1), 83–95. 10.4161/rna.22547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csink AK, Linsk R, & Birchler JA (1994). The Lighten up (Lip) gene of Drosophila melanogaster, a modifier of retroelement expression, position effect variegation and white locus insertion alleles. Genetics, 138(1), 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damianov A, Ying Y, Lin C-H, Lee J-A, Tran D, Vashisht AA, … Black DL (2016). Rbfox Proteins Regulate Splicing as Part of a Large Multiprotein Complex LASR. Cell, 165(3), 606–619. 10.1016/j.cell.2016.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne E, Pierredon S, Driouch K, Gratadou L, Lacroix-Triki M, Espinoza MP, … Auboeuf D (2012). Splicing switch of an epigenetic regulator by RNA helicases promotes tumor-cell invasiveness. Nature Structural & Molecular Biology, 19(11), 1139–1146. 10.1038/nsmb.2390 [DOI] [PubMed] [Google Scholar]
- Dardenne E, Polay Espinoza M, Fattet L, Germann S, Lambert M-P, Neil H, … Auboeuf D (2014). RNA helicases DDX5 and DDX17 dynamically orchestrate transcription, miRNA, and splicing programs in cell differentiation. Cell Reports, 7(6), 1900–1913. 10.1016/j.celrep.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, & Hata A (2008). SMAD proteins control DROSHA-mediated microRNA maturation. Nature, 454(7200), 56–61. 10.1038/nature07086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Nguyen PH, Lagna G, & Hata A (2010). Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Molecular Cell, 39(3), 373–384. 10.1016/j.molcel.2010.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo M, & Lambowitz AM (2009). Structure of the Yeast DEAD box protein Mss116p reveals two wedges that crimp RNA. Molecular Cell, 35(5), 598–609. 10.1016/j.molcel.2009.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo M, & Lambowitz AM (2009). Structure of the Yeast DEAD box protein Mss116p reveals two wedges that crimp RNA. Mol Cell, 35(5), 598–609. https://doi.org/S1097-2765(09)00591-7 [pii] 10.1016/j.molcel.2009.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Li D-Q, Li N, Chen L, Li S-S, Yang Y, … Zheng Z-D (2017). DDX5 promotes gastric cancer cell proliferation in vitro and in vivo through mTOR signaling pathway. Scientific Reports, 7, 42876 10.1038/srep42876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage A, French SL, Beyer AL, & Tollervey D (2010). Loss of Topoisomerase I leads to R-loop-mediated transcriptional blocks during ribosomal RNA synthesis. Genes & Development, 24(14), 1546–1558. 10.1101/gad.573310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, … Lander ES (2016). Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature, 539(7629), 452–455. 10.1038/nature20149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijo Delgado F, Cermak N, Hecht VC, Son S, Li Y, Knudsen SM, … Manalis SR (2013). Intracellular water exchange for measuring the dry mass, water mass and changes in chemical composition of living cells. PloS One, 8(7), e67590. 10.1371/journal.pone.0067590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini F, Bagchi D, Le Hir H, & Croquette V (2015). Human Upf1 is a highly processive RNA helicase and translocase with RNP remodelling activities. Nature Communications, 6, 7581 10.1038/ncomms8581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MJ, Anton IA, & Lane DP (1988). Nuclear protein with sequence homology to translation initiation factor eIF-4A. Nature, 332(6166), 736–738. 10.1038/332736a0 [DOI] [PubMed] [Google Scholar]
- Fuller-Pace FV (2013). The DEAD box proteins DDX5 (p68) and DDX17 (p72): multi-tasking transcriptional regulators. Biochimica et Biophysica Acta, 1829(8), 756–763. 10.1016/j.bbagrm.2013.03.004 [DOI] [PubMed] [Google Scholar]
- Fuller-Pace FV, & Moore HC (2011). RNA helicases p68 and p72: multifunctional proteins with important implications for cancer development. Future Oncology (London, England), 7(2), 239–251. 10.2217/fon.11.1 [DOI] [PubMed] [Google Scholar]
- Geißler V, Altmeyer S, Stein B, Uhlmann-Schiffler H, & Stahl H (2013). The RNA helicase Ddx5/p68 binds to hUpf3 and enhances NMD of Ddx17/p72 and Smg5 mRNA. Nucleic Acids Research, 41(16), 7875–7888. 10.1093/nar/gkt538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RI, Yan K-P, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, & Shiekhattar R (2004). The Microprocessor complex mediates the genesis of microRNAs. Nature, 432(7014), 235–240. 10.1038/nature03120 [DOI] [PubMed] [Google Scholar]
- Guenther U-P, Weinberg DE, Zubradt MM, Tedeschi FA, Stawicki BN, Zagore LL, … Jankowsky E (2018). The helicase Ded1p controls use of near-cognate translation initiation codons in 5’ UTRs. Nature, 559(7712), 130–134. 10.1038/s41586-018-0258-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil S, Gattoni R, Carrascal M, Abian J, Stevenin J, & Bach-Elias M (2003). Roles of hnRNP A1, SR proteins, and p68 helicase in c-H-ras alternative splicing regulation. Molecular and Cellular Biology, 23(8), 2927–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Hong F, Loke J, Yea S, Lim CL, Lee U, … Friedman SL (2010). A DDX5 S480A polymorphism is associated with increased transcription of fibrogenic genes in hepatic stellate cells. The Journal of Biological Chemistry, 285(8), 5428–5437. 10.1074/jbc.M109.035295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha M, & Kim VN (2014). Regulation of microRNA biogenesis. Nature Reviews. Molecular Cell Biology, 15(8), 509–524. 10.1038/nrm3838 [DOI] [PubMed] [Google Scholar]
- Halls C, Mohr S, Del Campo M, Yang Q, Jankowsky E, & Lambowitz AM (2007). Involvement of DEAD-box proteins in group I and group II intron splicing. Biochemical characterization of Mss116p, ATP hydrolysis-dependent and -independent mechanisms, and general RNA chaperone activity. J Mol Biol, 365(3), 835–855. https://doi.org/S0022-2836(06)01307-6 [pii] 10.1016/j.jmb.2006.09.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, & Jacobson A (1995). Identification of a novel component of the nonsense-mediated mRNA decay pathway by use of an interacting protein screen. Genes & Development, 9(4), 437–454. [DOI] [PubMed] [Google Scholar]
- Henn A, Cao W, Hackney DD, & De La Cruz EM (2008). The ATPase cycle mechanism of the DEAD-box rRNA helicase, DbpA. Journal of Molecular Biology, 377(1), 193–205. 10.1016/j.jmb.2007.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdy B, Mayer C, Varshney D, Marsico G, Murat P, Taylor C, … Balasubramanian S (2018). Analysis of NRAS RNA G-quadruplex binding proteins reveals DDX3X as a novel interactor of cellular G-quadruplex containing transcripts. Nucleic Acids Research. 10.1093/nar/gky861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirling H, Scheffner M, Restle T, & Stahl H (1989). RNA helicase activity associated with the human p68 protein. Nature, 339(6225), 562–564. 10.1038/339562a0 [DOI] [PubMed] [Google Scholar]
- Honig A, Auboeuf D, Parker MM, O’Malley BW, & Berget SM (2002). Regulation of alternative splicing by the ATP-dependent DEAD-box RNA helicase p72. Molecular and Cellular Biology, 22(16), 5698–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseley J, Rubbi L, Grunstein M, Tollervey D, & Vogelauer M (2008). A ncRNA modulates histone modification and mRNA induction in the yeast GAL gene cluster. Molecular Cell, 32(5), 685–695. 10.1016/j.molcel.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, & Liu Z-R (2002). The ATPase, RNA unwinding, and RNA binding activities of recombinant p68 RNA helicase. The Journal of Biological Chemistry, 277(15), 12810–12815. 10.1074/jbc.M200182200 [DOI] [PubMed] [Google Scholar]
- Hug N, Longman D, & Caceres JF (2016). Mechanism and regulation of the nonsense-mediated decay pathway. Nucleic Acids Research, 44(4), 1483–1495. 10.1093/nar/gkw010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadevaia V, Liu R, & Proud CG (2014). mTORC1 signaling controls multiple steps in ribosome biogenesis. Seminars in Cell & Developmental Biology, 36, 113–120. 10.1016/j.semcdb.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Iggo RD, Jamieson DJ, MacNeill SA, Southgate J, McPheat J, & Lane DP (1991). p68 RNA helicase: identification of a nucleolar form and cloning of related genes containing a conserved intron in yeasts. Molecular and Cellular Biology, 11(3), 1326–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka A, Siomi MC, & Siomi H (2002). A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes & Development, 16(19), 2497–2508. 10.1101/gad.1022002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal C, Uhlmann-Schiffler H, & Stahl H (2007). Redundant role of DEAD box proteins p68 (Ddx5) and p72/p82 (Ddx17) in ribosome biogenesis and cell proliferation. Nucleic Acids Research, 35(11), 3590–3601. 10.1093/nar/gkm058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky E, Gross CH, Shuman S, & Pyle AM (2000). The DExH protein NPH-II is a processive and directional motor for unwinding RNA. Nature, 403(6768), 447–451. 10.1038/35000239 [DOI] [PubMed] [Google Scholar]
- Jarmoskaite I, & Russell R (2014). RNA helicase proteins as chaperones and remodelers. Annu Rev Biochem, 83, 697–725. 10.1146/annurev-biochem-060713-035546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlina K, Goren I, Pfeilschifter J, & Frank S (2004). p68 DEAD box RNA helicase expression in keratinocytes. Regulation, nucleolar localization, and functional connection to proliferation and vascular endothelial growth factor gene expression. The Journal of Biological Chemistry, 279(43), 44872–44882. 10.1074/jbc.M402467200 [DOI] [PubMed] [Google Scholar]
- Kar A, Fushimi K, Zhou X, Ray P, Shi C, Chen X, … Wu JY (2011). RNA helicase p68 (DDX5) regulates tau exon 10 splicing by modulating a stem-loop structure at the 5’ splice site. Molecular and Cellular Biology, 31(9), 1812–1821. 10.1128/MCB.01149-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar A, Fushimi K, Zhou X, Ray P, Shi C, Chen X, … Wu JY (2011). RNA helicase p68 (DDX5) regulates tau exon 10 splicing by modulating a stem-loop structure at the 5’ splice site. Mol Cell Biol, 31(9), 1812–1821. https://doi.org/MCB.01149-10 [pii] 10.1128/MCB.01149-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Rozewicki J, & Yamada KD (2017). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics. 10.1093/bib/bbx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay BK, Williamson MP, & Sudol M (2000). The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 14(2), 231–241. [PubMed] [Google Scholar]
- Lambert M-P, Terrone S, Giraud G, Benoit-Pilven C, Cluet D, Combaret V, … Bourgeois CF (2018). The RNA helicase DDX17 controls the transcriptional activity of REST and the expression of proneural microRNAs in neuronal differentiation. Nucleic Acids Research, 46(15), 7686–7700. 10.1093/nar/gky545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm GM, Nicol SM, Fuller-Pace FV, & Lamond AI (1996). p72: a human nuclear DEAD box protein highly related to p68. Nucleic Acids Research, 24(19), 3739–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DP, & Hoeffler WK (1980). SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature, 288(5787), 167–170. [DOI] [PubMed] [Google Scholar]
- Lasko P (2013). The DEAD-box helicase Vasa: evidence for a multiplicity of functions in RNA processes and developmental biology. Biochimica et Biophysica Acta, 1829(8), 810–816. 10.1016/j.bbagrm.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Wang Q, & Rio DC (2018). Coordinate regulation of alternative pre-mRNA splicing events by the human RNA chaperone proteins hnRNPA1 and DDX5. Genes & Development, 32(15–16), 1060–1074. 10.1101/gad.316034.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, & Rio DC (2015). Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annual Review of Biochemistry, 84, 291–323. 10.1146/annurev-biochem-060614-034316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei EP, & Corces VG (2006). RNA interference machinery influences the nuclear organization of a chromatin insulator. Nature Genetics, 38(8), 936–941. 10.1038/ng1850 [DOI] [PubMed] [Google Scholar]
- Lelli KM, Slattery M, & Mann RS (2012). Disentangling the many layers of eukaryotic transcriptional regulation. Annual Review of Genetics, 46, 43–68. 10.1146/annurev-genet-110711-155437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lai P, Jia J, Song Y, Xia Q, Huang K, … Yao H (2017). RNA Helicase DDX5 Inhibits Reprogramming to Pluripotency by miRNA-Based Repression of RYBP and its PRC1-Dependent and -Independent Functions. Cell Stem Cell, 20(4), 462–477.e6. 10.1016/j.stem.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Liberti MV, & Locasale JW (2016). The Warburg Effect: How Does it Benefit Cancer Cells? Trends in Biochemical Sciences, 41(3), 211–218. 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Tian L, Shen H, Gu Y, Li J-L, Chen Z, … Wu L (2013). DDX5 is a positive regulator of oncogenic NOTCH1 signaling in T cell acute lymphoblastic leukemia. Oncogene, 32(40), 4845–4853. 10.1038/onc.2012.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder P, & Jankowsky E (2011). From unwinding to clamping - the DEAD box RNA helicase family. Nature Reviews. Molecular Cell Biology, 12(8), 505–516. 10.1038/nrm3154 [DOI] [PubMed] [Google Scholar]
- Linder P, Lasko PF, Ashburner M, Leroy P, Nielsen PJ, Nishi K, … Slonimski PP (1989, January). Birth of the D-E-A-D box. Nature. England. 10.1038/337121a0 [DOI] [PubMed] [Google Scholar]
- Liu F, Putnam A, & Jankowsky E (2008). ATP hydrolysis is required for DEAD-box protein recycling but not for duplex unwinding. Proceedings of the National Academy of Sciences of the United States of America, 105(51), 20209–20214. 10.1073/pnas.0811115106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z-R (2002). p68 RNA helicase is an essential human splicing factor that acts at the U1 snRNA-5’ splice site duplex. Molecular and Cellular Biology, 22(15), 5443–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZR, Sargueil B, & Smith CW (1998). Detection of a novel ATP-dependent cross-linked protein at the 5’ splice site-U1 small nuclear RNA duplex by methylene blue-mediated photo-cross-linking. Molecular and Cellular Biology, 18(12), 6910–6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorsch JR, & Herschlag D (1998). The DEAD box protein eIF4A. 2. A cycle of nucleotide and RNA-dependent conformational changes. Biochemistry, 37(8), 2194–2206. 10.1021/bi9724319 [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen S, & Jensen TH (2015). Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nature Reviews Molecular Cell Biology, 16, 665 Retrieved from 10.1038/nrm4063 [DOI] [PubMed] [Google Scholar]
- Ma WK, Cloutier SC, & Tran EJ (2013). The DEAD-box protein Dbp2 functions with the RNA-binding protein Yra1 to promote mRNP assembly. Journal of Molecular Biology, 425(20), 3824–3838. 10.1016/j.jmb.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WK, Paudel BP, Xing Z, Sabath IG, Rueda D, & Tran EJ (2016). Recruitment, Duplex Unwinding and Protein-Mediated Inhibition of the Dead-Box RNA Helicase Dbp2 at Actively Transcribed Chromatin. Journal of Molecular Biology, 428(6), 1091–1106. 10.1016/j.jmb.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DE, Powers T, & Hall MN (2006). Regulation of ribosome biogenesis: where is TOR? Cell Metabolism, 4(4), 259–260. 10.1016/j.cmet.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Mazurek A, Park Y, Miething C, Wilkinson JE, Gillis J, Lowe SW, … Stillman B (2014). Acquired dependence of acute myeloid leukemia on the DEAD-box RNA helicase DDX5. Cell Reports, 7(6), 1887–1899. 10.1016/j.celrep.2014.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, … Lopez R (2013). Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Research, 41(Web Server issue), W597–600. 10.1093/nar/gkt376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol SM, Bray SE, Black HD, Lorimore SA, Wright EG, Lane DP, … Fuller-Pace FV (2013). The RNA helicase p68 (DDX5) is selectively required for the induction of p53-dependent p21 expression and cell-cycle arrest after DNA damage. Oncogene, 32(29), 3461–3469. 10.1038/onc.2012.426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol SM, Causevic M, Prescott AR, & Fuller-Pace FV (2000). The nuclear DEAD box RNA helicase p68 interacts with the nucleolar protein fibrillarin and colocalizes specifically in nascent nucleoli during telophase. Experimental Cell Research, 257(2), 272–280. 10.1006/excr.2000.4886 [DOI] [PubMed] [Google Scholar]
- Nissan TA, Bassler J, Petfalski E, Tollervey D, & Hurt E (2002). 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. The EMBO Journal, 21(20), 5539–5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Cremins JE, & Corces VG (2013). Chromatin insulators: linking genome organization to cellular function. Molecular Cell, 50(4), 461–474. 10.1016/j.molcel.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinskaya M, Gourvennec S, & Morillon A (2009). H3 lysine 4 di- and tri-methylation deposited by cryptic transcription attenuates promoter activation. The EMBO Journal, 28(12), 1697–1707. 10.1038/emboj.2009.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polach KJ, & Uhlenbeck OC (2002). Cooperative binding of ATP and RNA substrates to the DEAD/H protein DbpA. Biochemistry, 41(11), 3693–3702. [DOI] [PubMed] [Google Scholar]
- Potratz JP, Del Campo M, Wolf RZ, Lambowitz AM, & Russell R (2011). ATP-dependent roles of the DEAD-box protein Mss116p in group II intron splicing in vitro and in vivo. Journal of Molecular Biology, 411(3), 661–679. 10.1016/j.jmb.2011.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam AA, & Jankowsky E (2013). DEAD-box helicases as integrators of RNA, nucleotide and protein binding. Biochim Biophys Acta, 1829(8), 884–893. https://doi.org/S1874-9399(13)00024-2 [pii] 10.1016/j.bbagrm.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappsilber J, Ryder U, Lamond AI, & Mann M (2002). Large-scale proteomic analysis of the human spliceosome. Genome Research, 12(8), 1231–1245. 10.1101/gr.473902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi J, Bajan S, Fuller-Pace FV, Arthur JSC, & Hutvagner G (2016). The loop structure and the RNA helicase p72/DDX17 influence the processing efficiency of the mice miR-132. Scientific Reports, 6, 22848 10.1038/srep22848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn JL, & Chang HY (2012). Genome regulation by long noncoding RNAs. Annual Review of Biochemistry, 81, 145–166. 10.1146/annurev-biochem-051410-092902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocak S, & Linder P (2004). DEAD-box proteins: the driving forces behind RNA metabolism. Nature Reviews. Molecular Cell Biology, 5(3), 232–241. 10.1038/nrm1335 [DOI] [PubMed] [Google Scholar]
- Rossler OG, Straka A, & Stahl H (2001). Rearrangement of structured RNA via branch migration structures catalysed by the highly related DEAD-box proteins p68 and p72. Nucleic Acids Research, 29(10), 2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow KL, & Janknecht R (2003). Synergism between p68 RNA helicase and the transcriptional coactivators CBP and p300. Oncogene, 22(1), 151–156. 10.1038/sj.onc.1206067 [DOI] [PubMed] [Google Scholar]
- Rudolph MG, & Klostermeier D (2015). When core competence is not enough: functional interplay of the DEAD-box helicase core with ancillary domains and auxiliary factors in RNA binding and unwinding. Biological Chemistry, 396(8), 849–865. 10.1515/hsz-2014-0277 [DOI] [PubMed] [Google Scholar]
- Salzman DW, Shubert-Coleman J, & Furneaux H (2007). P68 RNA helicase unwinds the human let-7 microRNA precursor duplex and is required for let-7-directed silencing of gene expression. The Journal of Biological Chemistry, 282(45), 32773–32779. 10.1074/jbc.M705054200 [DOI] [PubMed] [Google Scholar]
- Samatanga B, & Klostermeier D (2014). DEAD-box RNA helicase domains exhibit a continuum between complete functional independence and high thermodynamic coupling in nucleotide and RNA duplex recognition. Nucleic Acids Research, 42(16), 10644–10654. 10.1093/nar/gku747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz LA, Hartono SR, Lim YW, Steyaert S, Rajpurkar A, Ginno PA, … Chedin F (2016). Prevalent, Dynamic, and Conserved R-Loop Structures Associate with Specific Epigenomic Signatures in Mammals. Molecular Cell, 63(1), 167–178. 10.1016/j.molcel.2016.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saporita AJ, Chang H-C, Winkeler CL, Apicelli AJ, Kladney RD, Wang J, … Weber JD (2011). RNA helicase DDX5 is a p53-independent target of ARF that participates in ribosome biogenesis. Cancer Research, 71(21), 6708–6717. 10.1158/0008-5472.CAN-11-1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M, & Ghosh MK (2016). DEAD box RNA helicases: crucial regulators of gene expression and oncogenesis. Frontiers in Bioscience (Landmark Edition), 21, 225–250. [DOI] [PubMed] [Google Scholar]
- Saxton RA, & Sabatini DM (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell, 168(6), 960–976. 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengoku T, Nureki O, Nakamura A, Kobayashi S, & Yokoyama S (2006). Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell, 125(2), 287–300. 10.1016/j.cell.2006.01.054 [DOI] [PubMed] [Google Scholar]
- Sengoku T, Nureki O, Nakamura A, Kobayashi S, & Yokoyama S (2006). Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell, 125(2), 287–300. https://doi.org/S0092-8674(06)00376-X [pii] 10.1016/j.cell.2006.01.054 [DOI] [PubMed] [Google Scholar]
- Sever R, & Brugge JS (2015). Signal transduction in cancer. Cold Spring Harbor Perspectives in Medicine, 5(4). 10.1101/cshperspect.a006098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, & Jankowsky E (2014). The Ded1/DDX3 subfamily of DEAD-box RNA helicases. Critical Reviews in Biochemistry and Molecular Biology, 49(4), 343–360. 10.3109/10409238.2014.931339 [DOI] [PubMed] [Google Scholar]
- Shi Y (2017). Mechanistic insights into precursor messenger RNA splicing by the spliceosome. Nature Reviews. Molecular Cell Biology, 18(11), 655–670. 10.1038/nrm.2017.86 [DOI] [PubMed] [Google Scholar]
- Shin S, Rossow KL, Grande JP, & Janknecht R (2007). Involvement of RNA helicases p68 and p72 in colon cancer. Cancer Research, 67(16), 7572–7578. 10.1158/0008-5472.CAN-06-4652 [DOI] [PubMed] [Google Scholar]
- Suzuki HI, Yamagata K, Sugimoto K, Iwamoto T, Kato S, & Miyazono K (2009). Modulation of microRNA processing by p53. Nature, 460(7254), 529–533. 10.1038/nature08199 [DOI] [PubMed] [Google Scholar]
- Talwar T, Vidhyasagar V, Qing J, Guo M, Kariem A, Lu Y, … Wu Y (2017). The DEAD-box protein DDX43 (HAGE) is a dual RNA-DNA helicase and has a K-homology domain required for full nucleic acid unwinding activity. The Journal of Biological Chemistry, 292(25), 10429–10443. 10.1074/jbc.M117.774950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T, Iizumi Y, Watanabe M, Masuda M, Morita M, Aono Y, … Sakai T (2016). Resveratrol directly targets DDX5 resulting in suppression of the mTORC1 pathway in prostate cancer. Cell Death & Disease, 7, e2211 10.1038/cddis.2016.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi FA, Cloutier SC, Tran EJ, & Jankowsky E (2018). The DEAD-box protein Dbp2p is linked to non-coding RNAs, the helicase Sen1p, and R-loops. RNA (New York, N.Y.). 10.1261/rna.067249.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thandapani P, O’Connor TR, Bailey TL, & Richard S (2013). Defining the RGG/RG motif. Molecular Cell, 50(5), 613–623. 10.1016/j.molcel.2013.05.021 [DOI] [PubMed] [Google Scholar]
- Theissen B, Karow AR, Köhler J, Gubaev A, & Klostermeier D (2008). Cooperative binding of ATP and RNA induces a closed conformation in a DEAD box RNA helicase. Proceedings of the National Academy of Sciences of the United States of America, 105(2), 548–553. 10.1073/pnas.0705488105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson E, Ferreira-Cerca S, & Hurt E (2013). Eukaryotic ribosome biogenesis at a glance. Journal of Cell Science, 126(Pt 21), 4815–4821. 10.1242/jcs.111948 [DOI] [PubMed] [Google Scholar]
- Tran EJ, Zhou Y, Corbett AH, & Wente SR (2007). The DEAD-box protein Dbp5 controls mRNA export by triggering specific RNA:protein remodeling events. Mol Cell, 28(5), 850–859. https://doi.org/S1097-2765(07)00631-4 [pii] 10.1016/j.molcel.2007.09.019 [DOI] [PubMed] [Google Scholar]
- Uhlmann-Schiffler H, Jalal C, & Stahl H (2006). Ddx42p--a human DEAD box protein with RNA chaperone activities. Nucleic Acids Research, 34(1), 10–22. 10.1093/nar/gkj403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umate P, Tuteja N, & Tuteja R (2011). Genome-wide comprehensive analysis of human helicases. Communicative & Integrative Biology, 4(1), 118–137. 10.4161/cib.4.1.13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk EL, Chen CL, d’Aubenton-Carafa Y, Gourvennec S, Kwapisz M, Roche V, … Morillon A (2011). XUTs are a class of Xrn1-sensitive antisense regulatory non-coding RNA in yeast. Nature, 475(7354), 114–117. 10.1038/nature10118 [DOI] [PubMed] [Google Scholar]
- Voigt P, Tee W-W, & Reinberg D (2013). A double take on bivalent promoters. Genes & Development, 27(12), 1318–1338. 10.1101/gad.219626.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moeller H, Basquin C, & Conti E (2009). The mRNA export protein DBP5 binds RNA and the cytoplasmic nucleoporin NUP214 in a mutually exclusive manner. Nature Structural & Molecular Biology, 16(3), 247–254. 10.1038/nsmb.1561 [DOI] [PubMed] [Google Scholar]
- Wang D, Huang J, & Hu Z (2012). RNA helicase DDX5 regulates microRNA expression and contributes to cytoskeletal reorganization in basal breast cancer cells. Molecular & Cellular Proteomics : MCP, 11(2), M111.011932. 10.1074/mcp.M111.011932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gao X, Huang Y, Yang J, & Liu Z-R (2009). P68 RNA helicase is a nucleocytoplasmic shuttling protein. Cell Research, 19(12), 1388–1400. 10.1038/cr.2009.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Jiao Z, Li R, Yue H, & Chen L (2012). p68 RNA helicase promotes glioma cell proliferation in vitro and in vivo via direct regulation of NF-kappaB transcription factor p50. Neuro-Oncology, 14(9), 1116–1124. 10.1093/neuonc/nos131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Xing Z, Pascuzzi PE, & Tran EJ (2017). Metabolic Adaptation to Nutrients Involves Coregulation of Gene Expression by the RNA Helicase Dbp2 and the Cyc8 Corepressor in Saccharomyces cerevisiae. G3 (Bethesda, Md.), 7(7), 2235–2247. 10.1534/g3.117.041814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Luo Z, Zhou L, Li X, Jiang T, & Fu E (2015). DDX5 promotes proliferation and tumorigenesis of non-small-cell lung cancer cells by activating beta-catenin signaling pathway. Cancer Science, 106(10), 1303–1312. 10.1111/cas.12755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wery M, Descrimes M, Vogt N, Dallongeville A-S, Gautheret D, & Morillon A (2016). Nonsense-Mediated Decay Restricts LncRNA Levels in Yeast Unless Blocked by Double-Stranded RNA Structure. Molecular Cell, 61(3), 379–392. 10.1016/j.molcel.2015.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BJ, Bates GJ, Nicol SM, Gregory DJ, Perkins ND, & Fuller-Pace FV (2004). The p68 and p72 DEAD box RNA helicases interact with HDAC1 and repress transcription in a promoter-specific manner. BMC Molecular Biology, 5, 11 10.1186/1471-2199-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongtrakoongate P, Riddick G, Fucharoen S, & Felsenfeld G (2015). Association of the Long Non-coding RNA Steroid Receptor RNA Activator (SRA) with TrxG and PRC2 Complexes. PLoS Genetics, 11(10), e1005615. 10.1371/journal.pgen.1005615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortham NC, Ahamed E, Nicol SM, Thomas RS, Periyasamy M, Jiang J, … Fuller-Pace FV (2009). The DEAD-box protein p72 regulates ERalpha-/oestrogen-dependent transcription and cell growth, and is associated with improved survival in ERalpha-positive breast cancer. Oncogene, 28(46), 4053–4064. 10.1038/onc.2009.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Wang S, & Tran EJ (2017). Characterization of the mammalian DEAD-box protein DDX5 reveals functional conservation with S. cerevisiae ortholog Dbp2 in transcriptional control and glucose metabolism. RNA (New York, N.Y.), 23(7), 1125–1138. 10.1261/rna.060335.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Lin C, & Liu Z-R (2006). P68 RNA helicase mediates PDGF-induced epithelial mesenchymal transition by displacing Axin from beta-catenin. Cell, 127(1), 139–155. 10.1016/j.cell.2006.08.036 [DOI] [PubMed] [Google Scholar]
- Yang L, Lin C, Zhao S, Wang H, & Liu Z-R (2007). Phosphorylation of p68 RNA helicase plays a role in platelet-derived growth factor-induced cell proliferation by up-regulating cyclin D1 and c-Myc expression. The Journal of Biological Chemistry, 282(23), 16811–16819. 10.1074/jbc.M610488200 [DOI] [PubMed] [Google Scholar]
- Yang Q, Del Campo M, Lambowitz AM, & Jankowsky E (2007). DEAD-box proteins unwind duplexes by local strand separation. Mol Cell, 28(2), 253–263. https://doi.org/S1097-2765(07)00559-X [pii] 10.1016/j.molcel.2007.08.016 [DOI] [PubMed] [Google Scholar]
- Yang Q, & Jankowsky E (2005). ATP- and ADP-dependent modulation of RNA unwinding and strand annealing activities by the DEAD-box protein DED1. Biochemistry, 44(41), 13591–13601. 10.1021/bi0508946 [DOI] [PubMed] [Google Scholar]
- Yang Q, & Jankowsky E (2005). ATP- and ADP-dependent modulation of RNA unwinding and strand annealing activities by the DEAD-box protein DED1. Biochemistry, 44(41), 13591–13601. 10.1021/bi0508946 [DOI] [PubMed] [Google Scholar]
- Yang Q, & Jankowsky E (2006). The DEAD-box protein Ded1 unwinds RNA duplexes by a mode distinct from translocating helicases. Nat Struct Mol Biol, 13(11), 981–986. https://doi.org/nsmb1165 [pii] 10.1038/nsmb1165 [DOI] [PubMed] [Google Scholar]
- Yao H, Brick K, Evrard Y, Xiao T, Camerini-Otero RD, & Felsenfeld G (2010). Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes & Development, 24(22), 2543–2555. 10.1101/gad.1967810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CL, Khoshnevis S, & Karbstein K (2013). Cofactor-dependent specificity of a DEAD-box protein. Proc Natl Acad Sci U S A, 110(29), E2668–76. https://doi.org/1302577110 [pii] 10.1073/pnas.1302577110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xing Z, Mani SKK, Bancel B, Durantel D, Zoulim F, … Andrisani O (2016). RNA helicase DEAD box protein 5 regulates Polycomb repressive complex 2/Hox transcript antisense intergenic RNA function in hepatitis B virus infection and hepatocarcinogenesis. Hepatology (Baltimore, Md.), 64(4), 1033–1048. 10.1002/hep.28698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Watanabe M, Yano T, Yanagisawa J, Nakagawa S, Oishi H, … Taketani Y (2008). Analysis of the status of the novel estrogen receptor alpha (ERalpha) coactivator p72 in endometrial cancer and its cross talk with erbB-2 in the transactivation of ERalpha. Molecular Medicine Reports, 1(3), 387–390. [PubMed] [Google Scholar]
- Zhou R, Hotta I, Denli AM, Hong P, Perrimon N, & Hannon GJ (2008). Comparative analysis of argonaute-dependent small RNA pathways in Drosophila. Molecular Cell, 32(4), 592–599. 10.1016/j.molcel.2008.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Licklider LJ, Gygi SP, & Reed R (2002). Comprehensive proteomic analysis of the human spliceosome. Nature, 419(6903), 182–185. 10.1038/nature01031 [DOI] [PubMed] [Google Scholar]
- Zonta E, Bittencourt D, Samaan S, Germann S, Dutertre M, & Auboeuf D (2013). The RNA helicase DDX5/p68 is a key factor promoting c-fos expression at different levels from transcription to mRNA export. Nucleic Acids Research, 41(1), 554–564. 10.1093/nar/gks1046 [DOI] [PMC free article] [PubMed] [Google Scholar]


