Abstract
A broad pelvis is characteristic of most, if not all, pre-modern hominins. In at least some early australopithecines, most notably the female Australopithecus afarensis specimen known as “Lucy,” it is very broad and coupled with very short lower limbs. In 1991, Rak suggested that Lucy’s pelvic anatomy improved locomotor efficiency by increasing stride length through rotation of the wide pelvis in the axial plane. Compared to lengthening strides by increasing flexion and extension at the hips, this mechanism could avoid potentially costly excessive vertical oscillations of the body’s center of mass (COM). Here, we test this hypothesis. We examined 3D kinematics of walking at various speeds in 26 adult subjects to address the following questions: Do individuals with wider pelves take longer strides, and do they use a smaller degree of hip flexion and extension? Is pelvic rotation greater in individuals with shorter legs, and those with narrower pelves? Our results support Rak’s hypothesis. Subjects with wider pelves do take longer strides for a given velocity, and for a given stride length they flex and extend their hips less, suggesting a smoother pathway of the COM. Individuals with shorter legs do use more pelvic rotation when walking, but pelvic breadth was not related to pelvic rotation. These results suggest that a broad pelvis could benefit any bipedal hominin, but especially a short-legged australopithecine such as Lucy, by improving locomotor efficiency, particularly when carrying an infant or traveling in a foraging group with individuals of varying sizes.
Keywords: Australopithecus afarensis, biomechanics, body proportions, hominin, human evolution, locomotion, pelvis
INTRODUCTION
One of the most striking aspects of early hominin evolution is the tremendous variation in body proportions, both within and between species, notably in the lower limb length and in the relative and absolute dimensions of the pelvis (Ruff, 1994). This variation has been argued to reflect many aspects of the inferred adaptive niche of each hominin group, including locomotor efficiency, foraging strategies, obstetrics, infant care, habitat, and thermoregulation (e.g., Lovejoy, 1975; Berge, 1994; Ruff, 1998; Rosenberg and Trevathan, 2002; Lovejoy, 2005a; Pontzer et al., 2009; Wall-Scheffler, 2012), factors which in some cases may be in functional conflict with each other. The pelvis in particular is often described as representing a functional compromise between locomotor efficiency, childbirth, and thermoregulation (see Rosenberg, 2000; Fischer and Mitteroecker, 2015; Gruss and Schmitt, 2015, for reviews).
Although pelvic morphology varied over time both within and between species, pre-modern hominins from the Pliocene up until at least the Middle Paleolithic seem to have been characterized by pelves which were broad, with relatively wide bi-acetabular breadths and flaring iliac blades (wide bi-iliac breadth; e.g. Lovejoy, 1988; Rosenberg, 1992; Ruff, 1998; Ruff, 2010; Haeusler et al., 2016; VanSickle et al., 2016; see Gruss and Schmitt, 2015, for a review). Models concerning the functional consequences of this pelvic anatomy include arguments that pelvic breadth creates an appropriate body form for thermoregulation (Ruff, 1994), encourages successful delivery of large-brained offspring (Rosenberg, 1992), and enhances locomotor efficiency, especially in hominins with short lower limbs (Rak, 1991; Wall-Scheffler, 2012). These factors are not necessarily mutually exclusive, and therefore their relative importance, especially regarding locomotor efficiency, is difficult to resolve without robust functional tests of the role of pelvic breadth in human locomotion. The goal of this study, therefore, is to examine how the breadth of the pelvis, and its movements during walking, may have been related to aspects of locomotor adaptation in hominins of varying proportions, especially variation in lower limb length.
The pelvic anatomy of early australopithecines has been particularly well-studied in two partial skeletons: Australopithecus africanus Sts 14 and Au. afarensis A.L. 288–1 (“Lucy”), which share a mediolaterally broad, anteroposteriorly compressed pelvic inlet and outlet and notable iliac flare (Fig. 1; Tague and Lovejoy, 1986; Ruff, 1994; Haeusler and Schmid, 1995). Estimates of the medio-lateral diameter of Lucy’s pelvic inlet, which is approximately equal to bi-acetabular breadth, range between 123 mm and 132 mm (Tague and Lovejoy, 1986; Abitbol, 1991; Haeusler and Schmid, 1995), within 10mm of that of an average modern human woman (Table 1). A.L. 288–1 was smaller in stature and had much shorter lower limbs than even the smallest populations of modern people (Jungers, 1982; McHenry, 1991a; McHenry and Berger, 1998), making her pelvis remarkably broad relative to any measure of body size, but especially relative to limb length (Susman et al., 1984; Rak, 1991; Ruff, 1994). Recent discoveries of new Au. Afarensis specimens emphasize the substantial variation in body size and limb proportions (possibly between males and females) within this species (Haile-Selassie et al., 2010). The functional significance of this variation is an area of great interest for anthropologists interested in reconstructing locomotor mechanics and tradeoffs between efficiency and other aspects of anatomical adaptation. Clearly some early hominins had smaller stature and shorter lower limbs than modern humans and some did not, providing an opportunity to test specific aspects of the selective advantages of pelvic breadth relative to limb length.
Fig. 1.
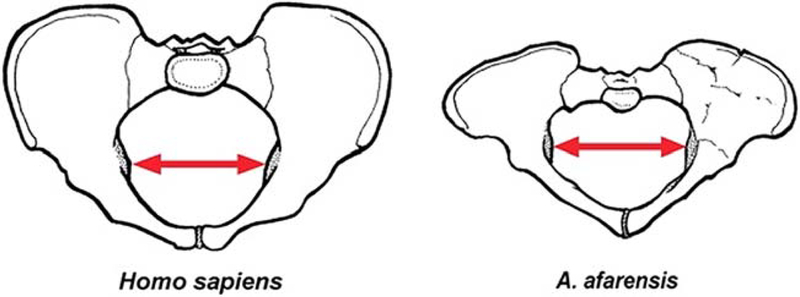
Pelvic dimensions in female modern Homo sapiens (left) and female Au. afarensis specimen A.L. 288–1 (“Lucy,” right). The two pelves are shown in in superior/anterior view and drawn to the same scale. The red arrows indicate bi-acetabular breadth, which is very similar in the two species despite the great difference in overall body size. Modified from Rosenberg and Trevathan (2002).
TABLE 1.
Pelvic dimensions relative to body size in Au. afarensis A.L. 288–1, “Lucy,” and modern human females
| Lucy (female Au. afarensis) | Modern human female | |
|---|---|---|
| Pelvic inlet width (mm) | 123–1323,4,7 | 124–1323–5,7 |
| Femur length (mm) | 280–2862,5,8 | 380–4411,2,5 |
| Inlet width/mass1/3 | 4.46 | 3.56 |
The pelvis in A.L. 288–1, although similar in medio-lateral dimensions to that of a modern human female, is much wider relative to limb length and body size. Modern human data are means from various populations.
Sources:
H€ausler and Schmid (1995).
Short lower limbs in some australopithecines could potentially have had a negative impact on their locomotor efficiency (Steudel-Numbers and Tilkens, 2004; Pontzer, 2007a; although see Kramer, 1999; Lovejoy, 2005b for contrary views), which may have had important selective consequences, since energy saved on movement may be reallocated to growth and reproduction (Wall-Scheffler, 2012). Short-legged animals generally take shorter strides than long-legged ones (Pontzer, 2007a,b), and short strides will decrease walking speed if stride frequency is kept constant. The adaptive consequences of moving slowly can be severe. For example, a short-legged hominin taking short steps and walking slowly is faced with a diminished foraging range compared to a longer-legged hominin. A short-legged individual may also affect the mobility and energy consumption of the entire foraging group (Wall-Scheffler, 2012), an especially important consideration if early australopithecines were highly dimorphic (see Richmond and Jungers, 1995; Lockwood et al., 1996; Reno and Lovejoy, 2015 for discussions of this issue). Recent work by Pontzer (2012) provides a caveat to this model: he has proposed that the selective importance of locomotor economy depends on the efficiency of an animal’s foraging behavior, and points out that among terrestrial animals, foraging range is not related to morphologies that promote locomotor efficiency.
If the short strides of a short-legged hominin like Lucy create a locomotor disadvantage by decreasing speed, the alternative-increasing stride frequency in order to maintain speed-is also energetically costly. It has been shown that cost of locomotion can be related to the frequency of foot contacts and therefore that locomotion may be described as “priced by the step,” because the greatest portion of the energetic cost of locomotion is generated by the muscles in supporting and propelling the body through each stride (Taylor et al., 1982; Alexander, 1984a; Alexander and Ker, 1990; Kram and Taylor, 1990). The cost per stride is approximately the same for animals of different sizes, but because a short-legged individual like Lucy may have to increase stride frequencies using quicker strides to travel at a given speed, the cost of transport will be higher (Kram and Taylor, 1990; Pontzer et al., 2009). Likewise, the cost to travel a given distance, whatever the speed, will be higher in an individual with shorter legs because it has to take more steps (Pontzer, 2007a, 2007b).
A potential compensatory mechanism would be for short-legged individuals to lengthen their strides by increasing the excursion of the lower limb. One way to accomplish this would be to maintain a relatively extended knee while using high degrees of flexion and extension (sagittal excursion) at the hips. However, it has been argued that this mechanism for increasing stride length could also be energetically costly because it would potentially increase the vertical excursion of the body’s center of mass (COM). Taking longer strides in this way involves more up-and-down movement of the body, which may increase muscular effort to accelerate and decelerate the COM (Rak, 1991). Furthermore, some have argued that the redirection of the COM associated with each step (called “collisions”) increases energetic costs (Donelan et al., 2002; Kuo et al., 2005; Ruina et al., 2005; Lee et al., 2013), and thus a smoother path of the COM is desirable (Rak, 1991).
The role that movements of the COM play in energetic costs is complex, however, and COM oscillations can also provide energetic benefits in locomotion. In human walking, which has been modeled as a relatively stiff springloaded inverted pendulum, appropriate oscillations of the COM allow the exchange of potential and kinetic energy to power some portion of forward locomotion, reducing muscular effort in the process (Cavagna et al., 1977; Alexander and Jayes, 1978; Alexander, 1991) and potentially increasing the range of energetically efficient walking speeds (Cavagna et al., 2000). In such a formulation, vertical COM movements would be of great value. But oscillations that are too great or too small can limit energy exchange and make the effect less useful. Low and high walking speeds, in which stride length is small or large, respectively, have lower levels of energy exchange—which forms a parabolic curve with walking speed—than the normal comfortable walking speeds for humans and other animals (Cavagna et al. 1977). Small animals and those with relatively short lower limbs may also not able to take full advantage of such mechanisms, which probably explains in part the negative relationships between cost of locomotion and body size or limb length (Biewener, 1989; Reilly et al., 2007; Bishop et al., 2008).
Rak (1991) suggested that the distinctive wide pelvis of short-legged australopithecines would have provided a unique mechanism for lengthening stride while at the same time smoothing the path of the COM. Rak (1991) based his hypothesis on a model expressed and diagrammed by Inman (Inman and Eberhart, 1953; Inman et al., 1981) in which axial pelvic rotation is a key determinant of human gait. In human locomotion, as the leading leg swings forward during swing phase, the pelvis rotates forward in the axial plane on that side, about a supero-inferior axis (Fig. 2a; Murray et al., 1964; Murray, 1967; Inman et al., 1981; Stokes et al., 1989; Wagenaar and Beek, 1992; Linley et al., 2010; Watt et al., 2010). The width of the pelvis determines how far the leading hip is brought forward as a result of this pelvic rotation. As a wider pelvis rotates, the leading hip moves farther forward during swing phase, increasing the length of the stride compared to an individual with a narrower pelvis (Inman and Eberhart, 1953; Murray, 1967; Stokes et al., 1989).
Fig. 2.
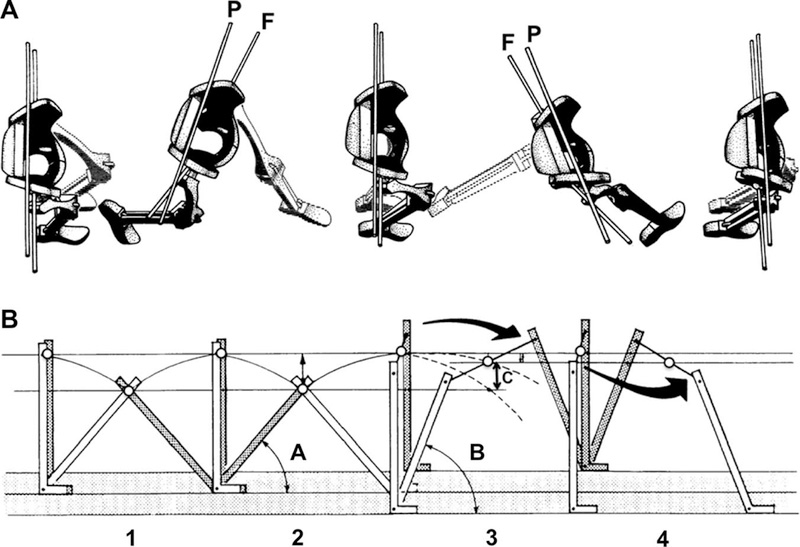
Axial rotation of the pelvis during walking. (a) Superior view of the pelvis and lower limbs during swing phase of the right leg. P is a rod showing axial rotation of the pelvis. F is a rod showing axial rotation of the left femur; a similar rod at the bottom of the image marks the axis of the right femur. The arc through which the pelvis rotates translates the right hip anteriorly during swing phase, bringing the entire limb forward and lengthening the stride. The arc of rotation of the femur is greater than that of the pelvis, bringing the right limb farther forward and increasing stride length further. A wider bi-acetabular breadth, and a longer femoral neck (wider bi-trochanteric breadth), as found in Au. afarensis, would further increase stride length via this mechanism. Modified from Inman et al. (1981). mechanism. Modified from Inman et al. (1981).
Rotation of the wide pelvis in australopithecines, in Rak’s (1991) model, would have allowed a short-legged biped to take longer strides. Their long femoral necks (Stern and Susman, 1983; Rak, 1991; Ruff, 1998) would have further contributed a small amount to the increase in stride length as the femora also rotated about their long axes. A longer stride resulting primarily from rotation of a wide pelvis could be accomplished without increased flexion and extension at the hips, thus avoiding exaggerated vertical oscillations of the COM (Fig. 2b; Inman et al., 1981; Rak, 1991), and increasing locomotor efficiency. Among early hominins, energy saved on locomotion could instead be dedicated to reproduction (see Wall-Scheffler 2012 for a review), and foraging success for the entire group may have been improved if this mechanism enabled smaller individuals to move more quickly and efficiently, increasing day range (Wall-Scheffler, 2012). Furthermore, minimization of COM movement can decrease joint reaction forces in the lower limbs (Rak, 1991), and biomechanical and anatomical analyses suggest that the relatively small lower limb joints in australopithecines must have been subjected to low joint reaction forces (Stern and Susman, 1983; Ruff, 1998; Schmitt, 2003).
To test the proposal that pelvic rotation may be an important contribution to increasing stride length in bipedal walking, Gruss et al. (2007) previously conducted a pilot study using 3D kinematic data from a sample of modern humans. This pilot study tested the hypothesis that pelvic rotation (regardless of pelvic breadth) increases with increasing walking speed and that this increase is seen most dramatically in short-legged individuals. Simple linear regressions of pelvic rotation on stride length and pelvic rotation on Froude number (a dimensionless measure of speed relative to body size; Alexander, 1984b) indicated that as short-legged people move faster and increase stride length, they do rotate their pelves more in the axial plane compared to people with longer lower limbs. In the current study, we revisit this hypothesis, including a wider variety of speeds and employing multivariate analyses to provide a more complete picture of the relationship between body proportions, pelvic movements and gait mechanics. In addition to examining the role of pelvic rotation in speed changes, here we also investigate the effects of pelvic breadth on stride length, to more directly test Rak’s (1991) hypothesis that the broad pelvis in australopithecines was an adaptation to increasing stride length.
The major questions addressed in this study are the following:
Do individuals with greater pelvic breadths (measured here as bi-trochanteric breadth, BTB, to incorporate the length of the femoral necks) take longer strides?
As individuals take longer strides, do those with relatively wider pelves (compared to limb length) have a smaller vertical movement of their center of mass (as estimated from markers on the sacrum)?
As individuals take longer strides, do those with relatively wider pelves flex and extend their hips less than those with narrower pelves?
As individuals take longer strides, do those with shorter legs rotate their pelves more than those with longer legs?
As individuals take longer strides, do those with narrower pelves rotate their pelves more? A narrower pelvis would have to rotate through more degrees than a wider pelvis to achieve a comparable increase in stride length by the same mechanism.
To answer these questions, we examined how stride length, vertical movement of the sacrum (assumed to be a close approximation for the body’s center of mass), hip excursion, and pelvic rotation change with speed in a sample of modern human subjects of varying body proportions (pelvis breadth and lower limb length).
MATERIALS AND METHODS
Subjects
Twenty-six adult subjects, 12 males and 14 females, participated in this study. All subjects were between the ages of 18 and 45, were healthy, and reported no major lower limb injuries or surgeries. This protocol for research with human subjects was approved by the Duke University Institutional Review Board. Prior to participation in the study, all subjects signed a consent form to provide informed consent.
Anthropometric Measurements
Femur length, tibia length, bi-iliac breadth (BIB), and bi-trochanteric breadth were measured on each subject at the time of data collection using a 600mm linear spreading anthropometric calipers (see Table 2 for descriptions of measurements). Calipers were placed as tightly as possible against bony landmarks without causing undue discomfort to the subjects, in order to ensure skeletal size rather than adipose tissue was measured. Each of these measurements was repeated three times by the same researcher and the average was employed in the analysis. Femur length and tibia length were summed as a measure of limb length. BTB was used both as a variable of interest in itself (since it incorporates the length of the femoral necks) and as a proxy for bi-acetabular breadth (BAB, distance between the hip joints), which cannot be measured directly from external landmarks. Reports of correlations between BTB and BAB are rare in the literature, but based on a sample of 95 individuals provided by Chris Ruff (pers. comm.) and taken from Warrener et al. (2015), the two variables are closely related (r2=0.7362, P < 0.0001). When separated by sex, the correlation improves for females (r2=0.8520, P < 0.0001) but not for males (r2=0.6660), although it remains highly significant (P < 0.0001). Although not employed in the main analysis, bi-iliac breadth (BIB) was also measured for comparison with BTB. Stature, measured with a standard medical height rod, and mass, measured using a digital scale, were also recorded at the time of the gait data collection session.
TABLE 2.
Anthropometric measurements used in this study
| Measurement | Description |
|---|---|
| Femur length | Distance between most laterally projecting point of greater trochanter and lateral epicondyle of right femur |
| Tibia length | Distance between most proximomedial point on medial condyle and most medially projecting point on medial malleolus of right tibia |
| Limb Length | Femur length1tibia length |
| Bi-iliac breadth (BIB) | Distance between most laterally projecting points of left and right iliac crests |
| Bi-trochanteric breadth (BTB) | Distance between most laterally projecting points of greater trochanters of left and right femora |
Measurements were based on external palpation of bony landmarks.
Gait Data Collection
Gait data were collected using a 3D high-speed motion capture and analysis system in the Coach Michael W. Krzyzewski Human Performance Research Laboratory (K Lab) at Duke University Medical Center’s Department of Surgery Orthopaedic Research Laboratories. The lab has a 10m rubberized walkway surrounded by six high-speed Falcon digital video cameras, equipped with 8mm lenses and infrared light rings. Cameras recorded marker data at 60 fields/sec and were calibrated before each data collection session.
Each subject attended one gait data collection session at the K Lab. Subjects wore shorts, short-sleeved or sleeveless shirts, and socks but no shoes. Adhesive, spherical, 25-mm reflective markers were attached to their bodies in a number of locations on the diaphysis and joint centers of the upper and lower limbs, following the Helen Hayes marker set (Kadaba et al., 1990), and the cameras recorded the locations of these markers as subjects walked back and forth along the walkway.
Eight to ten walking trials were recorded for each of the following speed categories: (1) Preferred speed: subjects walked along the walkway at their most comfortable walking speed. Self-selected preferred walking speeds were recorded because they may be considered physiologically equivalent (Margaria, 1938; Hoyte and Enlow, 1966; Perry et al., 1988). (2) Maximum speed: subjects were asked to walk along the walkway as fast as they could without breaking into a run. (3) Varied speeds: subjects were asked to walk back and forth along the walkway, starting very slowly and gradually increasing their speed so that the first trial was the slowest and the last was at approximately the walk-run transition. These last two categories allowed us to analyze the biomechanical mechanisms involved in increasing walking speed.
3D Movement Analysis
Trial videos were tracked digitally in 3D using Motion Analysis software (Motion Analysis Corporation, Santa Rosa, CA 95403), and completed tracks were smoothed using a second-order low-pass Butterworth filter with a cutoff frequency of 7 Hz in order to eliminate nonmovement-related noise in the data. Gait variables were calculated using OrthoTrak version 4.2.1 gait analysis software (Motion Analysis Corporation, Santa Rosa, CA 95403). For each trial, speed, stride length, and stride frequency (strides/second) were calculated from the 3D tracks. In addition, we were able to measure the vertical height of the marker attached to each subject’s sacrum. The minimum height of the sacrum during a stride was subtracted from the maximum height to give a measure of the vertical movement of the COM. Pelvic rotation range of motion (ROM) was also calculated for each trial, defined as the total rotational range of motion of the pelvis in the transverse plane, from maximum posterior rotation to maximum anterior rotation during one stride, approximately between toe-off and heelstrike. Hip sagittal ROM was defined as the total flexion-extension range of motion of one hip, from maximum extension to maximum flexion, during a single stride. Although these variables document motion that occurs primarily in the transverse plane and sagittal planes, respectively, OrthoTrak calculates joint and segment angles relative to internal axes rather than external axes. This allows an accurate assessment of the anatomical range of motion of interest regardless of the orientation of the body segments involved.
Between three and five trials were analyzed for each subject for each speed category, depending on the speeds at which the subject moved and the quality of the trials recorded at the time of data collection. For each subject an average preferred speed trial and an average maximum speed trial was created by averaging the kinematic data from three trials of each speed category. Two to three other single trials from the “varied speeds” category, chosen to maximize speed variation, were also analyzed.
Statistical Analysis
Statistical analyses were performed using JMP Pro 11.2.0 statistical software (SAS Corporation, Cary, NC 27513). The single-sex samples did not meet the requirements of normality for parametric t-tests, so nonparametric Wilcoxon sign-rank tests were used for comparisons of anthropometric measurements between males and females. T-tests were used to compare kinematic variables for preferred speed and maximum speed trials; the independent samples t-test is valid in this case because, according to the Central Limit Theorem, the sampling distribution of means for sample sizes near 30 is approximately normally distributed (n here=26).In order to answer questions 1–5 above, we constructed multiple linear regression models with stride length, vertical sacrum movement, hip sagittal ROM (flexion/extension), and pelvic rotation ROM as dependent variables to test for the effects of anthropometric and kinematic variables while taking the influence of other independent variables into account. A repeated-measures ANOVA using nominal levels of trial speed provides a viable alternative to linear regressions. For each regression model, we performed the same test using a repeated-measures ANOVA, and found that both models have comparable goodness of fit measures (AIC, BIC, −2LogLikelihood, and r2). We selected regressions, however, because they provide a higher data resolution. In both approaches, a subject random effect is necessary to account for individual variation between subjects. Under a repeated-measures ANOVA, the individual differences in acceleration between slow and fast trials would be allocated to the subject random effect, causing the variance of stride length to be lost. Thus the effect of stride length changes on pelvic ROM and sagittal ROM would be under-represented. By treating stride length as a continuous variable, we retain variance in the variable of interest, and thus obtain more accurate parameter estimates. In all multiple linear regression models, subject was added as a random effect in order to account for individual variation. Residuals were symmetric about 0 and demonstrated approximately constant variance in each case, so assumptions of linearity, independence, normality, and homoscedasticity for linear regression were met. VIFs were acceptably low, indicating low levels of multicollinearity within the independent variables. Results were considered significant where P < 0.05.
Raw data are used in summary statistics and Wilcoxon and t-tests, but in the multiple linear regression models we scaled the variables from 0 to 1 to aid interpretation of regression coefficients. Using scaled variables, it is possible to compare the relative strengths of the effect of each independent variable on the dependent variable by comparing their regression coefficients. The full sample of trials, including preferred, maximum, and varied speeds, was employed in the regression analyses in order to provide an understanding of how gait parameters change with speed.
Results
Anthropometric Variables
Summary statistics for anthropometric measurements are given in Table 3. Based on non-parametric Wilcoxon sign-rank tests, males were significantly larger than females in almost every anthropometric measurement, including BTB (Table 3). However, in this sample, there were no significant differences between males and females in almost all of the body proportions we measured: limb length or BTB relative to stature, or BTB relative to limb length. The mean and range for BTB/stature (but not BTB/LL) was higher for females than for males, but the difference between the sexes did not reach statistical significance using the Wilcoxon test (P = 0.0918). However, when standardized by body mass (BTB/mass1/3), BTB was significantly higher in females than in males (P < 0.0001). We found the same patterns of sexual dimorphism in BIB as in BTB, with the exception that the larger absolute BIB in males did not reach statistical significance. There were no significant differences between the sexes in BIB/stature or BIB/limb length, but BIB/mass1/3 was again significantly larger in females. Previous studies show inconsistent results regarding dimorphism in pelvic breadth relative to stature; statistically significant differences between the sexes seem to predominate (Ruff, 1991 and references therein; Warrener et al., 2015; Christopher Ruff, pers. comm; Cara Wall-Scheffler, pers. comm.), but some samples show a lack of dimorphism consistent with our results (Cara Wall-Scheffler, pers. comm.). Since the goal of this study was to examine the effects of variation in lower limb and pelvic proportions on locomotor kinematics, and males and females did not differ systematically in measurements of the pelvis relative to the lower limbs, the sexes were pooled for further analyses.
TABLE 3.
Anthropometric variables
| Measurement | Pooled subjects (n = 26) |
Females (n = 14) |
Males (n = 12) |
M-F comparison (Wilcoxon test) |
|---|---|---|---|---|
| Mass (kg) | ||||
| Maximum | 105.3 | 82.3 | 105.3 | M>F |
| Minimum | 50.9 | 50.9 | 62.6 | P = 0.0011 |
| Mean ± SD | 71.9 ± 16.5 | 61.9 ± 9.3 | 83.7 ± 15.4 | |
| Stature (m) | ||||
| Maximum | 1.956 | 1.759 | 1.956 | M>F |
| Minimum | 1.372 | 1.372 | 1.676 | P = 0.0009 |
| Mean ± SD | 1.690 ± 0.132 | 1.610 ± 0.105 | 1.783 ± 0.095 | |
| Limb length (m) | ||||
| Maximum | 0.896 | 0.875 | 0.896 | M>F |
| Minimum | 0.651 | 0.651 | 0.679 | P = 0.0221 |
| Mean ± SD | 0.754 ± 0.073 | 0.725 ± 0.062 | 0.788 ± 0.072 | |
| Bi-trochanteric breadth (BTB; m) | ||||
| Maximum | 0.37 | 0.37 | 0.368 | M>F |
| Minimum | 0.301 | 0.301 | 0.305 | P = 0.0192 |
| Mean ± SD | 0.331 ± 0.021 | 0.322 ± 0.017 | 0.341 ± 0.020 | |
| Bi-iliac breadth (BIB; m) | ||||
| Maximum | 0.319 | 0.294 | 0.319 | ns |
| Minimum | 0.236 | 0.236 | 0.254 | |
| Mean ± SD | 0.277 ± 0.021 | 0.269 ± 0.014 | 0.286 ± 0.024 | |
| Limb length/Stature | ||||
| Maximum | 0.499 | 0.499 | 0.476 | ns |
| Minimum | 0.399 | 0.423 | 0.399 | |
| Mean ± SD | 0.444 ± 0.022 | 0.447 ± 0.021 | 0.441 ± 0.023 | |
| BTB/Mass1/3 | ||||
| Maximum | 0.088 | 0.088 | 0.088 | M>F |
| Minimum | 0.069 | 0.076 | 0.069 | P < 0.0001 |
| Mean ± SD | 0.080 ± 0.004 | 0.082 ± 0.003 | 0.078 ± 0.005 | |
| BTB/Stature | ||||
| Maximum | 0.227 | 0.227 | 0.212 | ns1 |
| Minimum | 0.1701 | 0.185 | 0.17 | |
| Mean ± SD | 0.196 ± 0.013 | 0.201 ± 0.013 | 0.191 ± 0.012 | |
| BTB/Limb length | ||||
| Maximum | 0.532 | 0.495 | 0.532 | ns1 |
| Minimum | 0.378 | 0.378 | 0.382 | |
| Mean ± SD | 0.441 ± 0.039 | 0.446 ± 0.031 | 0.43 ± 0.047 |
Summary statistics for the pooled sex sample as well as by sex, with Wilcoxon tests for differences between the male and female samples. 1. M-F comparisons for BIB measurements give the same results as BTB measurements.
Kinematic Gait Parameters
Table 4 gives summary statistics for kinematic variables, with t-tests for differences between preferred and maximum speed trials. The mean preferred walking speed for our subjects, 1.35 m/s, is close to average energetically optimal human walking speeds calculated by other authors (e.g. Ralston, 1958; Alexander, 1980; Wall-Scheffler and Myers, 2013). Previous research has suggested that in humans and other mammals, optimal walking speed is primarily determined by limb length (e.g. Alexander, 1980), but here we find only a weak positive relationship between preferred walking speed and either lower limb length (r2=0.188; P = 0.0303) or stature (r2=0.198, P = 0.0259). Preferred walking speed is not related to mass or BTB. The mean maximum walking speed for our sample, 2.194 m/s, is just below the typical human walk/run transition speed of 2.3–2.5 m/s (Bramble and Lieberman, 2004).
TABLE 4.
Kinematic gait variables
| Variable | All trials n = 104 |
Preferred speed n = 26 |
Maximum speed n = 26 |
|---|---|---|---|
| Speed (m/s) | |||
| Maximum | 3.001 | 1.567 | 3.001 |
| Minimum | 0.887 | 1.117 | 1.79 |
| Mean ± SD | 1.609 ± 0.431 | 1.350 ± 0.117 | 2.194 ± 0.289 |
| Stride length (m) | |||
| Maximum | 1.978 | 1.704 | 1.978 |
| Minimum | 1.083 | 1.139 | 1.509 |
| Mean ± SD | 1.487 ± 0.193 | 1.383 ± 0.125 | 1.703 ± 0.121 |
| Stride length/limb length | |||
| Maximum | 2.592 | 2.139 | 2.592 |
| Minimum | 1.448 | 1.652 | 1.908 |
| Mean ± SD | 1.991 ± 0.247 | 1.852 ± 0.131 | 2.270 ± 0.172 |
| Stride length/Height | |||
| Maximum | 1.148 | 0.948 | 1.148 |
| Minimum | 0.651 | 0.735 | 0.903 |
| Mean ± SD | 0.885 ± 0.110 | 0.821 ± 0.057 | 1.011 ± 0.067 |
| Stride frequency (Hz) | |||
| Maximum | 1.82 | 1.12 | 1.82 |
| Minimum | 0.74 | 0.89 | 1.02 |
| Mean ± SD | 1.07 ± 0.18 | 0.98 ± 0.05 | 1.29 ± 0.18 |
| Pelvic rotation ROM (deg) | |||
| Maximum | 35.6 | 20.1 | 35.6 |
| Minimum | 5.9 | 5.9 | 11.1 |
| Mean ± SD | 14.4 ± 5.5 | 12.0 ± 4.3 | 17.8 ± 6.4 |
| Sacrum vertical movement (cm) | |||
| Maximum | 7.68 | 6.43 | 7.68 |
| Minimum | 1.76 | 1.76 | 2.58 |
| Mean ± SD | 4.15 ± 1.29 | 3.89 ± 1.15 | 5.03 ± 1.46 |
| Hip sagittal ROM (deg) | |||
| Maximum | 69.8 | 69.8 | 61.4 |
| Minimum | 34.5 | 38.2 | 45.4 |
| Mean ± SD | 49.1 ± 6.7 | 46.2 ± 5.1 | 54.9 ± 5.5 |
Summary statistics for the pooled-sex sample. t-Tests showed that the values for all variables were significantly greater in the maximum speed trials than in the preferred speed trials.
As expected, stride length (both absolute and relative) and stride frequency both increase at maximum walking speeds compared to preferred speeds. Stride frequency contributed almost twice as much to increasing speed as stride length did (in a multiple linear regression, the regression coefficient for stride frequency on speed was 0.799; for stride length it was only 0.441). The change in vertical position of the sacrum is significantly greater at maximum speeds than at preferred speeds, as are pelvic rotation and hip flexion/extension.
Determinants of Stride Length
Two multiple linear regression models for stride length on anthropometric and kinematic variables are shown in Table 5. In both models, speed is significantly positively related to and explains most of the variance in stride length. Limb length demonstrates a weak but significant positive effect on stride length when the effects of speed are taken into account. In Model A, we then added BTB, which likewise had a weak significant positive effect on stride length. The model was significant overall, with an adjusted r2 of 0.919. In Model B, we included relative pelvic breadth (BTB/limb length) instead of BTB. This combination of variables yielded an identical adjusted r2 (also significant), and both pelvic breadth and limb length were significantly positively related to stride length. Body mass had no effect on any of these models. A similar result was obtained by examining these relationships with a simpler statistical test. Subjects were divided into two groups based on their relative pelvic breadth (BTB/limb length)-a “wide-hipped” group above the BTB/LL mean, and a “narrow-hipped” group below it-and relative stride length (as a percentage of limb length) in the two groups for all trials was compared using a t-test (Fig. 3). Relative stride lengths were significantly higher in the wide-hipped group.
TABLE 5.
Determinants of stride length
| Dependent variable | Effect variable | Coefficient | Model adjusted r2 |
|---|---|---|---|
| A. Stride length | Speed | 0.881 | 0.918 |
| Limb length | 0.2 | ||
| BTB | 0.135 | ||
| B. Stride length | Speed | 0.879 | 0.918 |
| Limb length | 0.399 | ||
| BTB/LL | 0.214 |
Two multiple linear regression models for effects of speed, limb length and BTB on stride length, with subject as a random effect.
Fig. 3.
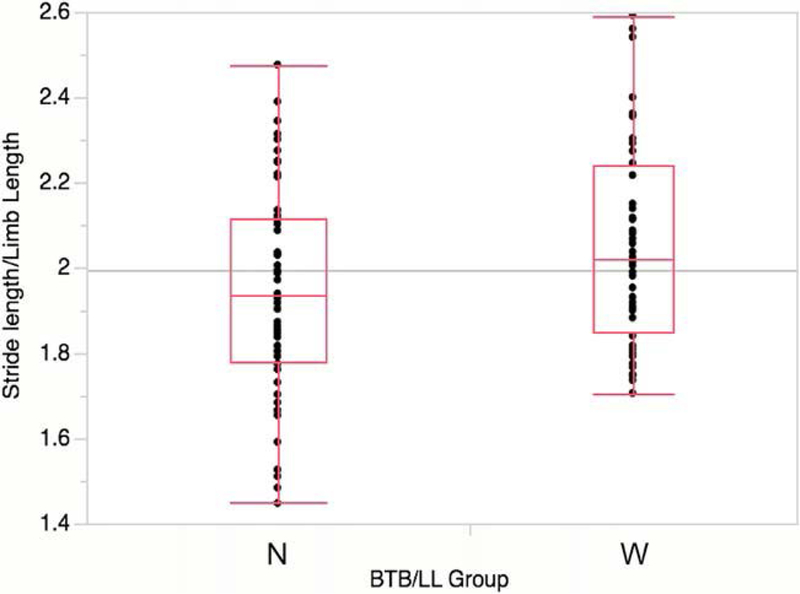
Relative stride length (compared to limb length) in subjects with wide (W) vs. narrow (N) pelves (BTB/LL). Pooled data for all speed categories is shown. The difference between the two groups is statistically significant (P = 0.0150 using a t-test).
We also tested for an effect of BTB on stride frequency using multiple linear regressions similar to those described above. Although there were slight trends toward a negative relationship (individuals with narrower pelves taking quicker steps), the effects were extremely weak (regression coefficients<0.10), and never significant, in contrast to findings by Wall-Scheffler and Myers (2013). Any effect of BTB on stride frequency disappeared when measures of body size (mass, stature or limb length) were taken into account.
Determinants of Sacrum Drop (COP Vertical Movement)
Our multiple linear regression model for the effects of stride length, limb length, and BTB on vertical movement of the sacrum (our proxy for the COM) is shown in Table 6. We used this test to determine whether the amount by which the sacrum drops during each stride varies systematically when people with varying body proportions (BTB/limb length) take relatively longer or each of the following three multiple linear regression analyses, pooled data for all speed trials are included (maximum, preferred, and varied trials). As predicted, SL/LL has a large and significantly positive effect on vertical movement of the sacrum, and BTB/LL has a significant negative effect. In addition, we found a significant negative interaction effect (SL/LL * BTB/LL), indicating that a relatively wide pelvis ameliorates the positive effect of stride length on vertical COM displacement. The overall adjusted r2 of this model was 0.793.
TABLE 6.
Determinants of change in height of the sacrum (i.e., drop in center of mass)
| Dependent variable | Effect variable | Coefficient | Model adjusted r2 |
|---|---|---|---|
| Sacrum drop | Stride length/Limb length | 0.748 | 0.793 |
| BTB/Limb length | −0.313 | ||
| SL/LL * BTB/LL | −0.631 |
Multiple linear regression model for effects of stride length, limb length, and BTB on drop in the height of the sacrum, with subject as a random effect. Pooled data for all speed categories were included in this analysis.
Determinants of Hip Flexion/Extension
A multiple linear regression model for the effects of stride length, limb length, and BTB on sagittal range of motion at the hip is given in Table 7. Here we examined whether degree of hip flexion and extension varies systematically when people with varying body proportions (BTB/limb length) take relatively longer or shorter strides (stride length/limb length). We found that stride length/limb length has a significant and large positive effect on hip sagittal ROM, while BTB/limb length has a significant negative effect. The model adjusted r2 was 0.930. Again, body mass had no effect on this model.
TABLE 7.
Determinants of hip flexion/extension
| Dependent variable | Effect variable | Coefficient | Model adjusted r2 |
|---|---|---|---|
| Hip sagittal ROM | Stride length/Limb length | 0.709 | 0.930 |
| BTB/Limb length | −0.178 |
Multiple linear regression model for effects of stride length, limb length, and BTB on hip sagittal ROM, with subject as a random effect. Pooled data for all speed categories were included in this analysis.
Determinants of Pelvic Rotation
Two different multiple linear regression models testing for effects of stride length and limb length on pelvic rotation are shown in Table 8. In Model A, we first regressed stride length against pelvic rotation ROM. Stride length has a significant and strong positive effect on pelvic rotation, with an adjusted r2 of 0.761. Limb length, when added to this regression, shows a significant negative effect on pelvic rotation. In this model, we also found a negative interaction effect of limb length * stride length: limb length moderates the positive effect of stride length on pelvic rotation. Although it does not quite reach the level of statistical significance (P = 0.0510), the influence of the interaction effect (limb length * stride length) on pelvic rotation is actually stronger than that of either limb length or stride length alone (with regression coefficients of −0.402 vs. −0.236 and 0.388, respectively). In Model B, we examined the effect of limb length on pelvic rotation when stride length is taken into account by employing stride length/limb length as the independent variable. Relative stride length is significantly positively related to pelvic rotation, with an adjusted r2 of 0.766. In either model, BTB alone does not have a significant effect on pelvic rotation when limb length and stride length are taken into account, and the addition of BTB does not improve the predictive power of these models.
TABLE 8.
Determinants of pelvic rotation
| Dependent variable | Effect variable | Coefficient | Model adjusted r2 |
|---|---|---|---|
| A. Pelvic rotation ROM | Stride length | 0.388 | 0.769 |
| Limb length | −0.236 | ||
| B. Pelvic rotation ROM | Limb length * Stride length | −0.402a | |
| Stride length/Limb length | 0.376 | 0.766 |
Two multiple linear regression models for effects of stride length and limb length on pelvic rotation, with subject as a random effect. Pooled data for all speed categories were included in this analysis. BTB does not have a significant effect on pelvic rotation.
Nearly significant at P = 0.0510.
DISCUSSION
Effects of Pelvic Breadth on Gait Kinematics
Our Question 1 asks whether individuals with wide pelves take longer strides than those with narrower pelves. This question addresses Rak’s (1991) hypothesis that the very broad bi-acetabular breadth and long femoral necks in short-legged small australopithecines such as Lucy were adaptations that could have allowed increases in stride length without exaggerating the vertical movements of the center of mass, which he contended would decrease the energetic efficiency of locomotion and increase joint reaction forces. Our analyses (Table 5) indicate that for a given speed and limb length, an individual with a wider pelvis will indeed take longer strides, in accordance with findings by Wall-Scheffler and colleagues (Wall-Scheffler et al., 2007; Wall-Scheffler and Myers, 2013). Whether short lower limbs were retained because they provided an adaptive advantage, such as in climbing (e.g. McHenry, 1991b; Susman and Stern, 1991), or simply as a non-adaptive holdover from a generalized hominoid ancestor (e.g. Latimer, 1991), the wide bi-acetabular breadth in at least some short-legged early hominins may have allowed them to maintain the stride lengths necessary for effective bipedal foraging. Adequate stride length may have been important for improving locomotor efficiency, or speed, or both. In this regard it is noteworthy that in our sample there was no relationship between pelvic breadth and the speed at which subjects chose to walk in any of the speed categories, or in the pooled sample of all trials. Likewise, we found no relationship between BTB and stride frequency at any speed. These results suggest that increased speed per se is not an advantage of a broad pelvis, but perhaps speed flexibility (see below) and efficient increases in stride length – which would allow an individual to cover a given distance with fewer steps, whatever the speed-are more important.
Question 2 directly addresses Rak’s (1991) suggestion that a broader pelvis in a short-legged hominin would have prevented excessive vertical fluctuations in the body’s center of mass as stride length increased. We found that individuals with wider pelves did indeed have a smaller excursion of the COM (as measured by sacral height) during walking, and furthermore, that this effect was stronger as stride length increased. In other words, when an individual with a relatively wider pelvis takes relatively longer strides, her sacrum doesn’t move vertically as much as it would in a person with a narrower pelvis. The presence of an observable relationship between vertical COM movement (or its proxy), pelvic breadth, and stride length in our limited sample of modern humans suggests that a stronger correlation between these variables may have existed in populations whose short limbs and wide pelves were more pronounced.
Question 3 asks whether this relationship between vertical COM movement and stride length is related to the degree of hip flexion and extension employed by individuals of differing body proportions, as proposed by Rak (1991). A greater degree of sagittal excursion of the hips during stance phase should result in a greater rise of the COM from heelstrike to mid-stance, and a greater subsequent drop (Farley and Gonzalez, 1996). Our results confirm that more hip flexion and extension does occur when individuals take strides that are longer compared to their limb length (Table 7), and that this is the dominant factor affecting hip sagittal ROM. In support of Rak’s hypothesis, we found that individuals with relatively wider pelves (again, compared to limb length) do use a smaller degree of hip flexion/extension than those with narrower pelves.
Questions 4 and 5 address the relationships between stride length, body proportions, and pelvic rotation, the subject of our earlier pilot study (Gruss et al., 2007). The positive effect of BTB on stride length suggests the corollary that for any pelvic breadth, an individual could increase their stride length (without increasing vertical displacement of the COM) by rotating the pelvis to a greater degree during each stride, thus bringing the leading hip farther forward during swing phase (see Fig. 2a). We are able to answer Question 4 in the affirmative: as individuals take longer strides they do rotate their pelves more (Table 8). Furthermore, our analysis demonstrates a significant effect of limb length and stride length together on pelvic rotation: pelvic rotation increases when the steps are longer relative to limb length. In other words, when individuals with short legs take long strides, they use a greater degree of pelvic rotation.
Question 5 follows by asking about the relationship between pelvic rotation and pelvic breadth. For a given limb length, individuals with narrower pelves may rotate their pelves more to accomplish a similar stride length to individuals with wider pelves, or conversely, individuals with wider pelves may rotate their pelves through fewer degrees to achieve a given increase in stride length. In other words, we predicted a negative relationship between BTB and pelvic rotation. However, in our analyses, BTB had no significant effect on pelvic rotation. It may be that the influence of pelvic breadth on pelvic rotation was not strong enough to be detected in our sample due to limited size and variation as compared to either the totality of modern human variation, or that found in Plio-Pleistocene hominins. It could also be that our findings reflect kinematic reality, and that pelvic rotation is simply determined by stride length and limb length, regardless of pelvic breadth. If two individuals with similar limb lengths but different pelvic breadths take strides of the same length, they may both rotate their pelves to the same degree-but the one with wider hips will be able to use less hip flexion and extension (Fig. 2b). However, our results support the hypothesis that a short-legged individual with a broad pelvis, such as Lucy, would likely have taken longer strides than an individual with a similar limb length and a narrower pelvis. This would have decreased the number of steps required to travel a given distance (at whatever speed), while limiting potentially costly vertical oscillations in the center of mass. Interestingly, a recent study by Warrener and colleagues found no relationship between bi-acetabular breadth (either absolute or relative to limb length) and locomotor cost in either walking or running (Warrener et al., 2015). This finding suggests that perhaps the long-term ability to cover foraging ranges with fewer strides would have been the main advantage conferred by a wide pelvis, rather than a reduction in the cost of locomotion per se.
It is worth acknowledging that there is considerable debate about whether early hominins like Lucy adopted a human-like extended limb gait (e.g. Lovejoy, 2005a,; Lovejoy and McCollum, 2010; Crompton et al., 2011) or used a yielding gait with flexed knees and hips (e.g. Stern and Susman, 1983; Susman and Stern, 1991; Stern, 2000; Hatala et al., 2016). After more than thirty years this debate has reached no resolution and we cannot resolve it with the data presented here. But the current study has relevance to this debate. If early hominins adopted a gait like that of modern humans, it might be modelled as an inverted pendulum. In this case, pelvic rotation could have helped early hominins with short legs maintain appropriate vertical COM oscillations for energy exchange. Conversely, if early hominins used a yielding gait, they could have extended stride length through this mechanism (Schmitt, 2003) without increasing oscillations. Pelvic rotation in that case may have been important for extending stride length when hip flexion was already maximized and hip extension was limited. In addition, if reducing collisional losses is important, in both cases pelvic rotation could reduce the magnitude of COM redirections. Though these remain interesting areas for exploration, the goal of this paper was to explicitly test Rak’s (1991) thought-provoking hypothesis.
A medio-laterally broad pelvis remained a characteristic of the general hominin bauplan until the appearance of Homo sapiens in the Middle Paleolithic (Weaver and Hublin, 2009; Ruff, 2010; Gruss and Schmitt, 2015), long after humanlike limb proportions evolved in the Plio-Pleistocene (McHenry and Coffing, 2000). Therefore, the persistence of this pelvic form in the human lineage cannot be explained solely by the need to lengthen stride in a short-legged biped. The primary adaptive explanations proposed for the pronounced pelvic width in pre-modern Homo have been thermoregulation or a primitive mechanism of childbirth (see Gruss and Schmitt, 2015 for a review), but this morphology may have also represented the intersection between locomotion and another aspect of reproduction – infant carrying. The energy savings provided by a wide pelvis may have been particularly important to female hominins throughout the evolution of the lineage, who would have spent a large proportion of their lives carrying their offspring, either in utero, in their arms, or perhaps in a sling (Wall-Scheffler, 2012). Wall-Scheffler et al. (2007) found that modern women take shorter steps when they carry babies, likely contributing to the energetic cost of transporting offspring. As discussed above, it is more costly to take a greater number of shorter strides to cover a given distance than to walk the same distance with longer strides and a lower stride frequency (Pontzer et al., 2009). In carrying an infant, the cost of supporting and vertically moving the COM through each stride would have been compounded by the added muscular effort of supporting and moving the additional weight of a child (Wall-Scheffler, 2012), and taking longer strides by using more hip flexion and extension is likely to have exacerbated this effect. Research has shown that a broader pelvis decreases the energetic cost of carrying a baby (Wall-Scheffler et al., 2007), possibly by lengthening strides without increasing hip sagittal excursion, as we found here. This advantage may have been particularly significant in species such as Au. afarensis with a high degree of body size variation (e.g. Richmond and Jungers, 1995; Haile-Selassie et al., 2010), where a broad pelvis may have been especially beneficial to short-legged females (and notably, pelvic breadth was more exaggerated in australopithecines than in later hominins; Gruss and Schmitt, 2015), but it could have been beneficial to any female biped.
However, minimization of individual mobility costs is unlikely to have been the only selective pressure acting on the biomechanics of walking in pre-modern hominins. As social primates, extinct hominins almost certainly did not forage alone, and Wall-Scheffler and colleagues have stressed the importance of walking in groups, which means traveling with individuals of different sizes and varying optimal walking speeds (Wall-Scheffler and Steudel-Numbers, 2011; Wall-Scheffler, 2012; Wall-Scheffler and Myers, 2013). Adjustments to the degree of rotation of a wide pelvis could have allowed inexpensive changes in stride length, and therefore speed, as members of groups modified their walking speeds to accommodate each other. Again, the range of body size and proportions within foraging groups may have been especially large in the case of highly dimorphic animals such as Au. afarensis, but body size variation would have been present any hominin social group.
Reproductive status would have also increased the demand for speed flexibility among members of foraging groups. At any given time some females would have been pregnant and others would have been carrying their offspring (Wall-Scheffler, 2012). In a preliminary study, Gruss et al. (2009) found that walking speed decreases when carrying an infant. Likewise, Wall-Scheffler and Myers (2013) reported that in women walking with reproductively-relevant loads, the speed at which the minimum cost of transport occurs is substantially slower than that of unburdened women; in other words, the energetically optimal walking speed is slower when carrying a baby (either externally or in utero). Therefore, the ability of individuals to walk efficiently across a variety of speeds would have been essential to both males and females finetuning their walking pace to that of other group members. Although Warrener et al. (2015) found no decrease in locomotor cost associated with a broad pelvis, their subjects were all required to walk at identical speeds on a treadmill, leaving open the possibility that speed flexibility may have been an important advantage conferred by this morphology.
The pressure to minimize the energy devoted to travel may have increased when hominins began to use greater foraging ranges, probably by the time of H. erectus (Anton, 2003), and especially when they began to spread into temperate and cold climates. Mobility is likely to have increased as hominins at higher latitudes had to travel over a larger area to obtain adequate food, and perhaps had to move more quickly to make the most of the shorter daylight hours (Wall-Scheffler, 2012). In these circumstances it would have been increasingly important to balance the energetic demands of locomotion with those of growth, reproduction, and thermoregulation, and the broad pelvis of pre-modern Homo may have been one key to maintaining this balance.
Notably, despite the many advantages that a broad pelvis may have conferred on bipedal hominins across more than three million years of evolution, pelvic breadth is significantly narrower in Homo sapiens and its antecedents beginning in the late Middle Pleistocene (see Gruss and Schmitt, 2015). This restructuring of overall pelvic morphology is rather puzzling and not completely understood, but is probably related to a combination of locomotor, energetic, and obstetric factors. It has been proposed (Weaver and Hublin, 2009; Ruff, 2010) that in high-latitude hominins such as the Neandertals, thermoregulatory pressure on pelvic shape (the need to retain a broad torso) aligned with obstetric pressure (the need to accommodate a large-brained neonate). However, as brain size increased in early Homo sapiens and its immediate ancestors in Africa, pelvic breadth could not continue to expand because of heat dissipation requirements. A change in orientation of the birth canal, along with a corresponding change in the pattern of childbirth, in which a human infant twists as it passes through the maternal pelvis, allowed the maximum diameter of the birth canal to increase without increasing the external breadth of the pelvis and interfering with thermoregulatory function (Holliday, 1997).
In summary, based on our results, we would predict that hominins with short legs and broad pelves may have been able to take longer strides than expected for a given limb length, due to the axial rotation of the pelvis. This pelvic rotation could have limited excessive flexion and extension of the hips, and large vertical excursions of the center of mass, that would have been necessary had pelvic breadth been smaller. These adaptations could have improved the efficiency or speed of walking, or both (although our results do not support the suggestion that a wider pelvis is related to a faster preferred walking speed), and may have allowed pre-modern hominins to make efficient adjustments to walking speed to accommodate slower or quicker members of their foraging groups. Although this work focused on the combination of body proportions found in some early australopithecines, a broad pelvis would have continued to confer locomotor advantages after the evolution of modern human-like body size and proportions in Pleistocene Homo.
ACKNOWLEDGMENTS
We are grateful to Jeffrey Laitman, Karen Rosenberg, and Jeremy DeSilva for the invitation to participate in this special issue of the Anatomical Record. Matt Cartmill contributed to this project through invaluable discussions on this topic and collaboration with LG and DS on the 2007 pilot study. We thank Cara Wall-Scheffler and Marcella Myers for helpful conversations about this research, and Chris Ruff for generously sharing data and insights on pelvic proportions. Three anonymous reviewers made comments and suggestions that helped improve the manuscript immensely. We are grateful to our research subjects for their participation, and Robin Queen and Stephanie Jaffe for help with data collection in the K Lab.
Grant sponsors: LSB Leakey Foundation; NIH 1PO1-AR-050245; Department of Evolutionary Anthropology, Duke University.
References
- Abitbol MM. 1991. Ontogeny and evolution of pelvic diameters in anthropoid primates and in Ausrtalopithecus-afarensis (AL-288–1). Am J Phys Anthropol 85:135–148. [DOI] [PubMed] [Google Scholar]
- Alexander RM. 1980. Optimum walking techniques for quadrupeds and bipeds. J Zool Soc Lond 192:97–117. [Google Scholar]
- Alexander RM. 1984a. Stride length and speed for adults, children, and fossil hominids. Am J Phys Anthropol 63:23–27. [DOI] [PubMed] [Google Scholar]
- Alexander RM. 1984b. The gaits of bipedal and quadrupedal animals. Int J Robot Res 3:49–59. [Google Scholar]
- Alexander RM. 1991. Energy-saving mechanisms in walking and running. J Exp Biol 160:55–69. [DOI] [PubMed] [Google Scholar]
- Alexander RM, Jayes AS. 1978. Vertical movements in walking and running. J Zool Lond 185:27–40. [Google Scholar]
- Alexander RM, Ker RF. 1990. Running is priced by the step. Nature 346:220–221. [DOI] [PubMed] [Google Scholar]
- Anton SC. 2003. Natural history of Homo erectus. Yearb Phys Anthropol 46:126–169. [DOI] [PubMed] [Google Scholar]
- Berge C 1994. How did the australopithecines walk? A biomechanical study of the hip and thigh of Australopithecus afarensis. J Hum Evol 26:259–273. [Google Scholar]
- Biewener A 1989. Scaling body support in mammals: limb posture and muscle mechanics. Science 245:45–48. [DOI] [PubMed] [Google Scholar]
- Bishop KL, Pai AK, Schmitt D. 2008. Whole body mechanics of stealthy walking in cats. PLoS One 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramble DM, Lieberman DE. 2004. Endurance running and the evolution of Homo. Nature 432:345–352. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Heglund NC, Taylor CR. 1977. Mechanical work in terrestrial locomotion two basic mechanisms for minimizing energy expenditure. Am J Physiol 233:R243–R261. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Willems PA, Heglund NC. 2000. The role of gravity in human walking: pendular energy exchange, external work and optimal speed. J Physiol 528:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton RH, Pataky TC, Savage R, D’Aouit K, Bennett MR, Day MH, Bates K, Morse S, Sellers WL. 2011. Human-like external function of the foot, and fully upright gait, confirmed in the 3.66 million year old Laetoli hominin footprints by topographic statistics, experimental footprint-formation and computer simulation. J R Soc Interface 9:707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. 2002. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J Exp Biol 205:3717–3727. [DOI] [PubMed] [Google Scholar]
- Farley CT, Gonzalez O. 1996. Leg stiffness and stride frequency in human running. J Biomech 29:181–186. [DOI] [PubMed] [Google Scholar]
- Fischer B, Mitteroecker P. 2015. Covariation between human pelvis shape, stature, and head size alleviates the obstetric dilemma. Proc Natl Acad Sci 112:5655–5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss LT, Schmitt D. 2015. The evolution of the human pelvis: changing adaptations to bipedalism, obstetrics and thermoregulation. Philos Trans R Soc Lond B: Biol Sci 370:20140063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss LT, Schmitt D, Cartmill M. 2007. Pelvic rotation and walking speed in Australopithecus and Homo. In: Annual meeting of the Paleoanthropology Society Philadelphia, PA. [Google Scholar]
- Gruss LT, Wall-Scheffler CM, Malik N. 2009. Infant carrying in humans: interactions between morphometric and gait parameters. Am J Phys Anthropol S48:182–183. [Google Scholar]
- Haeusler M, Fremondière P, Fornai C, Frater N, Mathews S, Thollon L, Marchal F. 2016. Virtual reconstruction of the MH2 pelvis (Australopithecus sediba) and obstetrical implications. Am J Phys Anthropol 159:165. [Google Scholar]
- Haile-Selassie Y, Latimer BM, Alene M, Deino AL, Gibert L, Melillo SM, Saylor BZ, Scott GR, Lovejoy CO. 2010. An early Australopithecus afarensis postcranium from Woranso-Mille, Ethiopia. Proc Natl Acad Sci U S A 107:12121–12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatala KG, Demes B, Richmond BG. 2016. Laetoli footprints reveal bipedal gait biomechanics different from those of modern humans and chimpanzees. Proc R Soc B 283:20160235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler M, Schmid P. 1995. Comparison of the pelves of Sts 14 and A.L. 288–1: implications for birth and sexual dimorphism in australopithecines. J Hum Evol 29:363–383. [Google Scholar]
- Holliday TW. 1997. Body proportions in Late Pleistocene Europe and modern human origins. J Hum Evol 32:423–448. [DOI] [PubMed] [Google Scholar]
- Hoyte DAN, Enlow DH. 1966. Wolff ‘s Law and the problem of muscle attachment on resorptive surfaces of bone. Am J Phys Anthropol 24:205–214. [DOI] [PubMed] [Google Scholar]
- Inman VT, Eberhart HD. 1953. The major determinants in normal and pathological gait. J Bone Joint Surg 35:543–558. [PubMed] [Google Scholar]
- Inman VT, Ralston HJ, Todd F. 1981. Human Walking. Baltimore: Williams & Wilkins. [Google Scholar]
- Jungers WL. 1982. Lucy’s limbs: skeletal allometry and locomotion in Australopithecus afarensis. Nature 297:676–678. [Google Scholar]
- Kadaba MP, Ramakrishnan HK, Wootten ME. 1990. Measurement of lower extremity kinematics during level walking. J Orthopaed Res 8:838–392. [DOI] [PubMed] [Google Scholar]
- Kram R, Taylor R. 1990. Energetics of running: a new perspective. Nature 346:265–267. [DOI] [PubMed] [Google Scholar]
- Kramer PA. 1999. Modelling the locomotor energetics of extinct hominids. J Exp Biol 202:2807–2818. [DOI] [PubMed] [Google Scholar]
- Kuo AD, Donelan JM, Ruina A. 2005. Energetic consequences of walking like an inverted pendulum: Step-to-step transitions. Exer Sport Sci Rev 33:88–97. [DOI] [PubMed] [Google Scholar]
- Latimer B 1991. Locomotor adaptations in Australopithecus afarensis: the issue of arboreality In: Origines de la Bipedie chez les Hominides. Paris: Editions du CNRS. [Google Scholar]
- Lee D, Comanescu T, Butcher M, Bertram J. 2013. A comparative collision-based analysis of human gait. Proc R Soc B: Biol Sci 280:20131779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley HS, Sled EA, Culham EG, Deluzio KJ. 2010. A biomechanical analysis of trunk and pelvis motion in subjects with knee osteoarthritis compared to control subjects. Clin Biomech 25:1003–1010. [DOI] [PubMed] [Google Scholar]
- Lockwood CA, Richmond BG, Jungers WL, Kimbel WH. 1996. Randomization procedures and sexual dimorphism in Australopithecus afarensis. J Hum Evol 31:537–548. [Google Scholar]
- Lovejoy CO. 1975. Biomechanical perspectives on the lower limb of early hominids In: Tuttle RH, editor. Primate Functional Morphology and Evolution. The Hague: Mouton; p 291–326. [Google Scholar]
- Lovejoy CO. 2005a. The natural history of human gait and posture. Part 1. Spine and pelvis. Gait Posture 21:95–112. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO. 2005b. The natural history of human gait and posture. Part 2. Hip and thigh. Gait Posture 21:113–124. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO. 2007. The natural history of human gait and posture: Part 3. The knee. Gait Posture 25:325–341. [DOI] [PubMed] [Google Scholar]
- Lovejoy OC, McCollum MA. 2010. Spinopelvic pathways to bipedality: why no hominids ever relied on a bent-hip–bent-knee gait. Philos Trans R Soc B: Biol Sci 365:3289–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaria R 1938. Sulla fisiologia e specialmente sul consumo energetico della marcia e della corsa a varie velocita ed inclinazioni del terreno. Atti Acca Naz Lincei Memorie 7:299–368. [Google Scholar]
- McHenry HM. 1991a. Femoral lengths and stature in Plio-Pleistocene hominids. Am J Phys Anthropol 85:149–158. [DOI] [PubMed] [Google Scholar]
- McHenry HM. 1991b. First steps? Analysis of the postcranium of early hominids In: Origine(s) de la Bipedie chez les Hominides. Paris: Editions du CNRS; p 133–141. [Google Scholar]
- McHenry HM, Berger LR. 1998. Body proportions in Australopithecus afarensis and Australopithecus africanus and the origin of the genus Homo. J Hum Evol 35:1–22. [DOI] [PubMed] [Google Scholar]
- McHenry HM, Coffing K. 2000. Australopithecus to Homo: Transformations in body and mind. Ann Rev Anthropol 29:125–146. [Google Scholar]
- Murray MP. 1967. Gait as a total pattern of movement. Am J Phys Med 46:290–333. [PubMed] [Google Scholar]
- Murray MP, Drought AB, Kory RC. 1964. Walking patterns of normal men. J Bone Joint Surg Am Vol 46:335–360. [PubMed] [Google Scholar]
- Perry AK, Blickhan R, Biewener AA, Heglund NC, Taylor CR. 1988. Preferred speeds in terrestrial verterbrates: are they equivalent? J Exp Biol 137:207–219. [DOI] [PubMed] [Google Scholar]
- Pontzer H 2007a. Effective limb length and the scaling of locomotor cost in terrestrial animals. J Exp Biol 210:1752–1761. [DOI] [PubMed] [Google Scholar]
- Pontzer H 2007b. Predicting the energy cost of terrestrial locomotion: a test of the LiMb model in humans and quadrupeds. J Exp Biol 210:484–494. [DOI] [PubMed] [Google Scholar]
- Pontzer H 2012. Relating ranging ecology, limb length, and locomotor economy in terrestrial animals. Journal of Theoretical Biology 296:6–12. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Raichlen DA, Sockol MD. 2009. The metabolic cost of walking in humans, chimpanzees, and early hominins. J Hum Evol 56:43–54. [DOI] [PubMed] [Google Scholar]
- Rak Y 1991. Lucy’s pelvic anatomy: Its role in bipedal gait. J Hum Evol 20:283–290. [Google Scholar]
- Ralston H 1958. Energy-speed relation and optimal speed during level walking. Int Z Angew Physiol Einschl Arbeitphysiol 17: 277–283. [DOI] [PubMed] [Google Scholar]
- Reilly SM, McElroy EJ, Biknevicius AR. 2007. Posture, gait and the ecological relevance of locomotor costs and energy-saving mechanisms in tetrapods. Zoology 110:271–289. [DOI] [PubMed] [Google Scholar]
- Reno PL, Lovejoy CO. 2015. From Lucy to Kadanuumuu: balanced analyses of Australopithecus afarensis assemblages confirm only moderate skeletal dimorphism. PeerJ 3:e925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond BG, Jungers WL. 1995. Size variation and sexual dimorphism in Australopithecus afarensis and living hominoids. J Hum Evol 29:229–245. [Google Scholar]
- Rosenberg KR. 1992. The evolution of modern human childbirth. Am J Phys Anthropol 35:89–124. [Google Scholar]
- Rosenberg KR. 2000. Walking, having babies and keeping warm: multiple selective pressures on human pelvic morphology. Am J Phys Anthropol Suppl 30:264. [Google Scholar]
- Rosenberg KR, Trevathan W. 2002. Birth, obstetrics and human evolution. Int J Obst Gynaecol 109:1199–1206. [DOI] [PubMed] [Google Scholar]
- Ruff CB. 1991. Climate and body shape in hominid evolution. J Hum Evol 21:81–105. [Google Scholar]
- Ruff CB. 1994. Morphological adaptation to climate in modern and fossil hominids. Am J Phys Anthropol 37:65–107. [Google Scholar]
- Ruff CB. 1998. Evolution of the hominid hip In: Strasser E, Fleagle J, Rosenberger A, McHenry H, editors. Primate Locomotion: Recent Advances. New York: Plenum Press; p 449–469. [Google Scholar]
- Ruff CB. 2010. Body size and body shape in early hominins – implications of the Gona pelvis. J Hum Evol 58:166–178. [DOI] [PubMed] [Google Scholar]
- Ruina A, Bertram JE, Srinivasan M. 2005. A collisional model of the energetic cost of support work qualitatively explains leg sequencing in walking and galloping, pseudo-elastic leg behavior in running and the walk-to-run transition. J Theoret Biol 237: 170–192. [DOI] [PubMed] [Google Scholar]
- Schmitt D 2003. Insights into the evolution of human bipedalism from experimental studies of humans and other primates. J Exp Biol 206:1437–1448. [DOI] [PubMed] [Google Scholar]
- Stern JT. 2000. Climbing to the top: a personal memoir of Australopithecus afarensis. Evol Anthropol 9:113–133. [Google Scholar]
- Stern JT, Susman RL. 1983. The locomotor anatomy of Australopithecus afarensis. Am J Phys Anthropol 60:279–317. [DOI] [PubMed] [Google Scholar]
- Steudel-Numbers KL, Tilkens MJ. 2004. The effect of lower limb length on the energetic cost of locomotion: implications for fossil hominins. J Hum Evol 47:95–109. [DOI] [PubMed] [Google Scholar]
- Stokes VP, Andersson C, Forssberg H. 1989. Rotational and translational movement features of the pelvis and thorax during adult human locomotion. J Biomech 22:43–50. [DOI] [PubMed] [Google Scholar]
- Susman RL, Stern JTJ. 1991. Locomotor behavior of early hominids: epistemology and fossil evidence In: Coppens Y, Senut B, editors. Origine(s) de la bipedie chez les hominides. Paris, France: CNRS; p 121–131. [Google Scholar]
- Susman RL, Stern JTJ, Jungers WL. 1984. Arboreality and bipedality in the Hadar hominids. Folia Primatol 43:113–156. [DOI] [PubMed] [Google Scholar]
- Tague RG, Lovejoy CO. 1986. The obstetric pelvis of A.L. 288–1 (Lucy). J Hum Evol 35:75–94. [DOI] [PubMed] [Google Scholar]
- Taylor CR, Heglund NC, Maloiy GM. 1982. Energetics and mechanics of terrestrial locomotion. I. Metabolic energy consumption as a function of speed and body size in birds and mammals. J Exp Biol 97:1–21. [DOI] [PubMed] [Google Scholar]
- Trinkaus E 1981. Neanderthal limb proportions and cold adaptation In: Stringer CB, editor. Aspects of Human Evolution. London: Taylor & Francis; p 187–224. [Google Scholar]
- VanSickle C, Cofran ZD, Garcia-Martinez D, Williams SA, Churchill SE, Berger LR, Hawks J. 2016. Primitive pelvic features in a new species of Homo. Am J Phys Anthropol 159:321–322. [Google Scholar]
- Wagenaar RC, Beek WJ. 1992. Hemiplegic gait: a kinematic analysis using walking speed as a basis. J Biomech 25:1007–1015. [DOI] [PubMed] [Google Scholar]
- Wall-Scheffler CM. 2012. Energetics, locomotion, and female reproduction: Implications for human evolution. Ann Rev Anthropol 41:71–85. [Google Scholar]
- Wall-Scheffler CM, Geiger K, Steudel-Numbers KL. 2007. Infant carrying: the role of increased locomotory costs in early tool development. Am J Phys Anthropol 133:841–846. [DOI] [PubMed] [Google Scholar]
- Wall-Scheffler CM, Myers MJ. 2013. Reproductive costs for everyone: how female loads impact human mobility strategies. J Hum Evol 64:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall-Scheffler CM, Steudel-Numbers K. 2011. The meaning of within population dimorphism for group mobility. Am J Phys Anthropol S52:303–304. [Google Scholar]
- Warrener AG, Lewton KL, Pontzer H, Lieberman DE. 2015. A wider pelvis does not increase locomotor cost in humans, with implications for the evolution of childbirth. PLoS ONE 10:e0118903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt JR, Franz JR, Jackson K, Dicharry J, Riley PO, Kerrigan DC. 2010. A three-dimensional kinematic and kinetic comparison of overground and treadmill walking in healthy elderly subjects. Clin Biomech 25:444–449. [DOI] [PubMed] [Google Scholar]
- Weaver TD, Hublin J-J. 2009. Neandertal birth canal shape and the evolution of human childbirth. Proc Natl Acad Sci USA 106:8151–8156. [DOI] [PMC free article] [PubMed] [Google Scholar]


