Abstract
Objective
To review long-term certolizumab pegol (CZP) safety across all approved indications: rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), psoriasis (PSO) and Crohn’s disease (CD).
Methods
Data were pooled across 49 UCB-sponsored CZP clinical trials (27 RA, one axSpA, one PsA, five PSO, 15 CD) to August 2017. Serious adverse events (SAEs) of interest (infections, malignancies, autoimmunity/hypersensitivity events, major adverse cardiovascular events (MACE), gastrointestinal (GI) perforations, psoriasis events, laboratory abnormalities) and deaths were medically reviewed by an external expert committee, using predefined case rules. Incidence rates (IRs)/100 patient-years (PY) are presented by indication; standardised mortality and malignancy rates were calculated using WHO/GLOBOCAN/SEER databases. Pregnancies with maternal CZP exposure are also reported.
Results
Of 11 317 CZP-treated patients across indications (21 695 PY CZP exposure; maximum: 7.8 years), infections were the most common SAEs (overall IR: 3.62/100 PY; IRs ranged from 1.50/100 PY(PSO) to 5.97/100 PY(CD)). The IR for malignancies was 0.82/100 PY, including lymphoma (0.06/100 PY). MACE and GI perforation IRs in CZP-treated patients were 0.47/100 PY and 0.08/100 PY and were highest in RA and CD, respectively. Patients with PSO had the lowest SAE rates. The incidence of deaths and malignancies aligned with expected general population data.
Conclusion
This extensive overview of the CZP safety profile in clinical trials, across all indications, provides large-scale confirmation of previous reports. No new safety signals or relevant non-disease-related laboratory abnormalities were identified. The study demonstrated some indication-specific differences in certain SAE rates that may be attributable to the underlying inflammatory disease.
Keywords: anti-tnf, ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis, dmards (biologic)
Key messages.
What is already known about this subject?
Certolizumab pegol (CZP) has an established positive benefit–risk ratio and regulatory approval for the treatment of rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, psoriasis and Crohn’s disease.
Potential safety concerns exist with anti-tumour necrosis factor medications due to their immunosuppressive action, particularly around serious infectious events and other important risks identified for biologics.
What does this study add?
This is the largest review of long-term CZP safety in clinical trials to date and the first across multiple indications, totaling 21 695 patient-years of CZP exposure, with some patients exposed for >7 years.
A range of serious adverse events (SAEs) most relevant to biologics were examined, demonstrating indication-specific differences in the rates of certain SAEs. No new safety signals were identified and the long-term safety profile of CZP across indications was comparable to previous reports for CZP.
How might this impact on clinical practice or future developments?
Comprehensive, long-term safety data across different indications and patient subpopulations help to distinguish study drug effects from disease-associated events and help clinicians to balance the potential benefits and risks of biologic medications, including CZP.
Introduction
Anti-tumour necrosis factor (anti-TNF) drugs are used to treat a range of moderate-to-severe immune-mediated inflammatory diseases (IMIDs) encompassing rheumatology, dermatology and gastroenterology. Comprehensive long-term safety data across different IMIDs and patient subpopulations may help physicians to distinguish study drug effects from disease-associated events. Analyses of pooled long-term safety data from clinical trials can provide valuable information for decision-making in clinical practice, where the potential benefits and risks of anti-TNF medications must be balanced for individual patients. Certolizumab pegol (CZP) is an Fc-free, PEGylated anti-TNF agent approved to treat adults with rheumatoid arthritis (RA), Crohn’s disease (CD), axial spondyloarthritis (axSpA; including ankylosing spondylitis/radiographic axSpA (AS/r-axSpA) and non-radiographic axSpA (nr-axSpA)), psoriatic arthritis (PsA) and plaque psoriasis (PSO).1 2 Although CZP and other anti-TNF medications have well-established benefit–risk profiles in IMIDs,3–7 their immunosuppressive action remains a potential safety concern, especially regarding serious infectious events (SIEs) and malignancies.8–15
As of 2018, CZP is approved in 66 countries worldwide, with cumulative exposure estimated at >420 000 patient-years (PY).16 Previous indication-specific safety reports from randomised controlled trials (RCTs) and open-label extensions (OLEs) in RA and CD showed that the safety profile of CZP is consistent with other anti-TNF medications.17 18 Since then, new data have become available from additional study populations, including trials in early, progressive RA,19 a head-to-head trial in patients with established RA,20 4-year data from trials in axSpA and PsA21 22 and trials in moderate-to-severe PSO.23 24 In addition, two studies focusing on pregnant and postpartum women have been completed, showing evidence of no to minimal placental transfer of CZP from mothers to infants25 and minimal transfer into breast milk.26 These two studies provide safety evidence for CZP treatment during breastfeeding and, if clinically necessary, during pregnancy.1 2
The data reported here represent the largest review to date of long-term CZP safety outcomes from clinical trials spanning RA, axSpA, PsA, PSO and CD, representing 21 695 PY of exposure (December 1998–August 2017) from study sites worldwide. We report differences in the risk of specific serious adverse events (SAEs) across disease indications, including infections (and opportunistic infections (OIs)), malignancies, autoimmunity/hypersensitivity events, major adverse cardiovascular events (MACE), gastrointestinal (GI) perforations, psoriasis events, relevant laboratory abnormalities and deaths. We also report on pregnancies with maternal CZP exposure during the included trials, these data being both relevant and under-reported for women of childbearing age.
Methods
Data sources and patient populations
Data were pooled across 49 clinical trials of CZP: 27 RA, one axSpA, one PsA, five PSO and 15 CD. RA trials included one open-label, single-dose pharmacokinetic study, 18 RCTs, seven OLEs and one head-to-head study (online supplementary figure S1), encompassing data up to August 2017. Four-year safety data from the RAPID-axSpA and RAPID-PsA trials (both to April 2016) were included (online supplementary figure S2),21 22 and safety data for moderate-to-severe PSO were pooled across five RCTs and OLEs up to August 2017 (online supplementary figure S3).23 24 27 CD safety data up to April 2012 were reported previously and pooled across 15 CZP trials (online supplementary figure S4).17
rmdopen-2019-000942supp001.pdf (109.2KB, pdf)
rmdopen-2019-000942supp002.pdf (47.2KB, pdf)
rmdopen-2019-000942supp003.pdf (54.4KB, pdf)
rmdopen-2019-000942supp004.pdf (94.9KB, pdf)
The approved CZP dosage comprises three 400 mg loading doses (Weeks 0, 2, and 4) in all indications, followed by maintenance dosing of 200 mg every 2 weeks (Q2W) or 400 mg every 4 weeks (Q4W), in RA, axSpA and PsA and 400 mg Q4W in CD.2 In Europe, patients with PSO can receive 200 mg Q2W or 400 mg Q2W if the response is inadequate.1 The standard dose for PSO in the USA is 400 mg Q2W, with the option to prescribe 200 mg Q2W after the loading regimen for patients ≤90 kg.2 In this safety analysis, CZP dosing varied by indication, study and treatment arm.
Patients randomised to CZP or placebo (PBO), respectively, are denoted as RCT CZP and RCT PBO. RCT+OLE represents all patients exposed to CZP during RCTs or OLEs, including patients withdrawn from RCTs who re-consented to CZP treatment for an OLE. Where studies involved patients switching between CZP and another anti-TNF (EXXELERATE in RA;20 CIMPACT in PSO24), only events during CZP treatment in patients originally randomised to CZP were included (with data censored at the time of switch).
Events requiring compulsory patient withdrawal from RCTs varied between trials, but typically included a positive pregnancy test or tuberculosis (TB) screening result (post-baseline TB screening was annual at minimum).
External medical review of all SAEs of potential concern
A retrospective external safety data review was conducted by a committee of independent experts with backgrounds in safety reporting and rheumatology (JRC, KW, XM, CGV, TKK and VPB), dermatology (AB) or gastroenterology (WJS), selected from a range of geographic locations.
Experts agreed by consensus on the events of potential concern, and thereafter, with the support of the sponsor’s medical team, identified the lists of terms to be searched for in the study databases. SAEs of potential concern (reviewer subcommittees) included SIEs (JRC, KW), TB (XM, KW), malignancies (TKK, XM), autoimmunity/hypersensitivity events (AB, CGV, TKK, XM), MACE (VPB, JRC, TKK), GI perforations (JRC, WJS), psoriasis events (AB, TKK), deaths (CGV, VPB) and laboratory abnormalities (CGV). SAEs adjudicated for the previous RA safety update, which used a similar approach and involved some of the same experts,18 were carried forward without repeat review. Additional TB events reviewed for a previous publication were also carried forward.28
As per recommendations, all SAEs were recorded using standardised Council for International Organisations of Medical Sciences (CIOMS) documentation. Experts could access the narratives from the CIOMS forms recorded in the sponsor’s pharmacovigilance database; when provided by the reporting body, follow-up information on final outcomes (sometimes beyond the 70-day follow-up period), detailed biochemistry and pathology reports, and diagnostic imaging results could be used to aid the experts’ adjudication.
Reviewed SAEs were classified as diagnosis ‘confirmed’, ‘doubtful’, ‘rejected’ or ‘unassessable’. Psoriasis events were further classified as new onset or worsening in patients with pre-existing psoriasis. Confirmed TB cases were further categorised as ‘pulmonary’, ‘non-pulmonary’, ‘disseminated’ or ‘unassessable’.
Fatal events were reviewed to assess whether the reported AE was the likely cause of death. Additionally, experts provided primary (and, if applicable, secondary) possible causes of death, selecting from ‘infection’, ‘myocardial infarction’, ‘other cardiac’, ‘malignancy’, ‘sudden death’ (if sudden deaths were unexplained—some sudden deaths were attributable to cardiac causes) or ‘other’.
Safety assessments
Safety assessments included all AEs that occurred between the first dose and 70 days after the last dose of study drug (5× the half-life of CZP2 29), study withdrawal (for any reason, including withdrawal of consent or loss to follow-up) or death. AEs and SAEs were categorised according to the Medical Dictionary for Regulatory Activities (MedDRA) version 18.1.30 Standardised criteria for SAEs were used in this review of CZP safety, consistent with the review conducted previously.18 Across all clinical trials, SAEs included all medical occurrences that were life-threatening or led to death, hospitalisation, congenital anomalies or birth defects or resulted in persistent or significant disability.31 Infections requiring treatment with intravenous antibiotics were classified as serious. Events deemed serious by the clinical investigator could also be recorded as SAEs (regardless of severity). The expert review process helped apply consistency in confirming SAEs.
Expert consensus was that appendicitis was not an infectious event, being commonly secondary to a mechanical obstruction of the appendix.32 These events were still included as SAEs. OIs were identified using the definition proposed by the Opportunistic Infections Consensus Committee15 (OICC; both definite and probable terms were included, online supplementary table S1), aligned with appropriate MedDRA coded terms and approved by KW. TB screening methods have been well documented.28 All suspected TB cases reported since the last TB update,28 including latent TB, underwent expert review.
rmdopen-2019-000942supp005.pdf (178.9KB, pdf)
For malignancies, cases were also subcategorised into lymphoma, melanoma and non-melanoma skin cancer (NMSC). For NMSCs, all serious and non-serious cases were considered, to more accurately reflect their true occurrence across the patient population, as the majority were recorded as non-serious AEs. MACE included fatal and non-fatal myocardial infarction, serious cerebrovascular events and serious congestive heart failure, based on MedDRA terms and medical review.
GI perforations included all SAEs associated with the MedDRA high-level term (HLT) ‘gastrointestinal ulcers and perforation, site unspecified’ and potential cases identified using MedDRA lowest level terms of perforation, abscess and fistula. Abscess and fistula events were also reviewed as potential SIEs, because of the inferred presence of associated infection in abscess formation, or as a precursor to/sequela of a fistula. Potential autoimmune and vasculitis events were identified by manual data review and included a selection of MedDRA terms (online supplementary table S2). A Standardised MedDRA Query (SMQ) identified potential hypersensitivity SAEs, including an algorithm to identify anaphylactic reactions.33 Deep vein thrombosis and pulmonary embolism (PE) events were identified as SAEs of potential concern, but reviewed by sponsor medical personnel only. These are collectively reported as venous thromboembolism (VTE). Serious psoriasis events identified for expert review were those with the MedDRA preferred terms (PTs) ‘dermatitis psoriasiform’, ‘erythrodermic psoriasis’, ‘guttate psoriasis’, ‘psoriasis’ and ‘pustular psoriasis’. Biochemistry and haematology laboratory results externally reviewed for potential safety signals included alanine aminotransferase (ALT) or aspartate aminotransferase (AST) elevated to ≥3× the upper limit of normal (ULN), accompanied by an elevated bilirubin of ≥2× ULN (using Hy’s law34), as well as relevant isolated increases in ALT, AST or bilirubin. White blood cell counts <1.5x109/L, neutrophil counts <1.0x109/L or platelet counts <5.0x1010/L were also externally reviewed (CGV). Pregnancy data consisted of pregnancies with maternal CZP exposure, prospectively reported up to August 2017.
Statistical analysis
Statistical analysis used SAS® version 9.4. Incidence rates (IRs) were calculated per 100 PY with 95% CIs. Event rates (ERs) per 100 PY were also calculated for SIEs (to include repeat events in the same patients).
For all AE categories that underwent expert review (SIEs (including TB and other OIs), malignancies, MACE, GI perforations, autoimmunity/hypersensitivity, psoriasis), the number and rates of ‘confirmed’ events are reported. Malignancies diagnosed within 4 weeks of starting the study drug were not included, due to the likelihood that these were present at enrollment.
Excepting NMSC, standardised IRs (SIRs) of expert-confirmed malignancies were calculated using age-matched and gender-matched rates from the WHO GLOBOCAN database (part of the International Agency for Research on Cancer [IARC] Global Cancer Observatory [GCO]) and the US Surveillance, Epidemiology and End Results (SEER) programme. Standardised mortality rates (SMRs) were calculated as the ratio of observed deaths to expected deaths (based on a WHO general population standardised by age, gender and country).35
Time-to-event Kaplan-Meier analyses were performed for expert-confirmed SIEs. Cox proportional hazards models of time to first SIE in the RCT+OLE population were developed—adjusting for age, gender, baseline corticosteroid use, body mass index (BMI) category and geographic region—to quantify the relative risk of SIEs by disease indication, using RA as the comparator. Sensitivity analyses were also conducted for SIEs, including or excluding GI-specific infections (GI-specific MedDRA PTs are listed in online supplementary table S3).
Results
Patient population
A total of 11 317 patients (21 695 PY of exposure) were treated with CZP, including 6927 patients with RA, 315 patients with axSpA, 393 patients with PsA, 1112 patients with PSO and 2570 patients with CD (table 1).
Table 1.
Population baseline characteristics for CZP-treated patients (all doses) in the combined RCT and OLE periods (RCT+OLE)
| Overall (n=11 317; 21 695 PY) |
RA (n=6927; 13 542 PY) |
axSpA (n=315; 978 PY) |
PsA (n=393; 1316 PY) |
PSO (n=1112; 1481 PY) |
CD (n=2570; 4378 PY) |
||
| Mean age, years (SD) | 48.1 (13.9) | 53.0 (12.2) | 39.8 (11.9) | 47.7 (11.3) | 45.4 (13.0) | 37.1 (12.2) | |
| Age category, n (%) |
≥18 to <45 years | 4382 (38.7) | 1609 (23.2) | 203 (64.4) | 154 (39.2) | 529 (47.6) | 1887 (73.4) |
| ≥45 to <65 years | 5575 (49.3) | 4131 (59.6) | 104 (33.0) | 214 (54.5) | 505 (45.4) | 621 (24.2) | |
| ≥65 years | 1354 (12.0) | 1187 (17.1) | 8 (2.5) | 25 (6.4) | 78 (7.0) | 56 (2.2) | |
| Female, n (%) | 7637 (67.5) | 5491 (79.3) | 119 (37.8) | 218 (55.5) | 373 (33.5) | 1436 (55.9) | |
| Female ≥18 to <45 years, n (%) | 2705 (23.9) | 1345 (19.4) | 80 (25.4) | 78 (19.8) | 169 (15.2) | 1033 (40.2) | |
| Geographic region, n (%) | North America | 4098 (36.2) | 2784 (40.2) | 82 (26.0) | 94 (23.9) | 360 (32.4) | 778 (30.3) |
| Western and Central Europe | 4698 (41.5) | 2332 (33.7) | 202 (64.1) | 242 (61.6) | 752 (67.6) | 1170 (45.5) | |
| Eastern Europe | 840 (7.4) | 594 (8.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 246 (9.6) | |
| Asia | 371 (3.3) | 195 (2.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 176 (6.8) | |
| Japan | 860 (7.6) | 771 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 89 (3.5) | |
| Latin America | 365 (3.2) | 251 (3.6) | 31 (9.8) | 57 (14.5) | 0 (0.0) | 26 (1.0) | |
| South Africa | 85 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 85 (3.3) | |
| Mean BMI, kg/m2 (SD) | 27.2 (6.7) | 27.8 (6.6) | 27.6 (5.9) | 29.8 (6.5) | 30.1 (6.9) | 24.0 (5.5) | |
| BMI class, n (%) |
<25 kg/m2 | 4804 (42.4) | 2661 (38.4) | 112 (35.6) | 90 (22.9) | 237 (21.3) | 1704 (66.3) |
| ≥25 to <30 kg/m2 | 3349 (29.6) | 2149 (31.0) | 105 (33.3) | 144 (36.6) | 408 (36.7) | 543 (21.1) | |
| ≥30 kg/m2 | 3136 (27.7) | 2096 (30.3) | 93 (29.5) | 158 (40.2) | 467 (42.0) | 322 (12.5) | |
| Mean disease duration, years (SD) | 8.1 (8.7) | 6.4 (6.9) | 6.8 (7.5) | 8.6 (8.3) | 18.4 (12.3) | 8.5 (8.1) | |
| Baseline systemic steroid use, n (%) | 4132 (36.5) | 3200 (46.2) | 160 (50.8) | 99 (25.2) | 37 (3.3) | 636 (24.7) | |
| Baseline MTX use, n (%) | 5782 (51.1) | 5435 (78.5) | 55 (17.5) | 250 (63.6) | 1 (0.1) | 41 (1.6) | |
| Prior anti-TNF use, n (%) | 2515 (22.2) | 1283 (18.5) | 49 (15.6) | 75 (19.1) | 148 (13.3) | 960 (37.4) | |
| Date of treatment initiation, n (%)* | Pre-2007 | 4602 (40.7) | 2367 (34.2) | 0 | 0 | 117 (10.5) | 2118 (82.4) |
| 2007 onwards | 6715 (59.3) | 4560 (65.8) | 315 (100.0) | 393 (100.0) | 995 (89.5) | 452 (17.6) | |
*Before 2007, the threshold for a positive result on the PPD tuberculin skin test varied (from ≥5 to ≥20 mm) according to geographic region. Since 2007, CZP recommendations internationally mandate that all patients with PPD ≥5 mm receive treatment for latent tuberculosis infection. North America: Canada, USA; Western and Central Europe: Austria, Belgium, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Norway, Poland, Portugal, Slovakia, Slovenia, Spain, Sweden, Switzerland, Netherlands, UK; Eastern Europe: Belarus, Croatia, Georgia, Israel, Romania, Russia, Serbia, Ukraine; Asia: Australia, Hong Kong, Republic of Korea, New Zealand, Singapore; Latin America: Argentina, Brazil, Chile, Colombia, Mexico.
axSpA, axial spondyloarthritis; BMI, body mass index; CD, Crohn’s disease;CZP, certolizumab pegol; MTX, methotrexate; OLE, open-label extension; PPD, purified protein derivative; PsA, psoriatic arthritis; PSO, psoriasis; PY, patient-years; RA, rheumatoid arthritis; RCT, randomised controlled trial; TNF, tumour necrosis factor.
Mean (SD) CZP exposure in the RCT+OLE population was 700 (±656) days (online supplementary table S4); the longest CZP exposure was 7.8 years. For RCT CZP patients (n=6467), mean CZP exposure was 170 (±109) days, with 3017 PY total exposure. For RCT PBO patients (n=3092), mean exposure was 140 (±96) days and total exposure was 1184 PY.
Mean age ranged from 37.1 (±12.2) years in CD (2% ≥65 years) to 53.0 (±12.2) years in RA (17% ≥65 years) (median age range: 35.0 years (CD)–54.0 years (RA)). The proportion of female patients was higher in RA (79%), PsA (56%) and CD (56%), compared with PSO (34%) and axSpA (38%). BMI varied across indications: in PsA and PSO over 40% of patients had a BMI ≥30 kg/m2, whilst 66.3% of patients with CD had a BMI of <25 kg/m2. Mean disease duration for PSO (18.4±12.3 years) was approximately double that of other indications (table 1).
Approximately one-third of CZP-treated patients took corticosteroids at baseline, most commonly in RA (46%) and axSpA (52%). Around half of CZP-treated patients were on methotrexate (MTX) at baseline (with the lowest baseline MTX use in PSO (0.1%) and CD (1.6%)) and the majority of CZP-treated patients (78%) were naive to anti-TNF medications (table 1).
AEs and SAEs
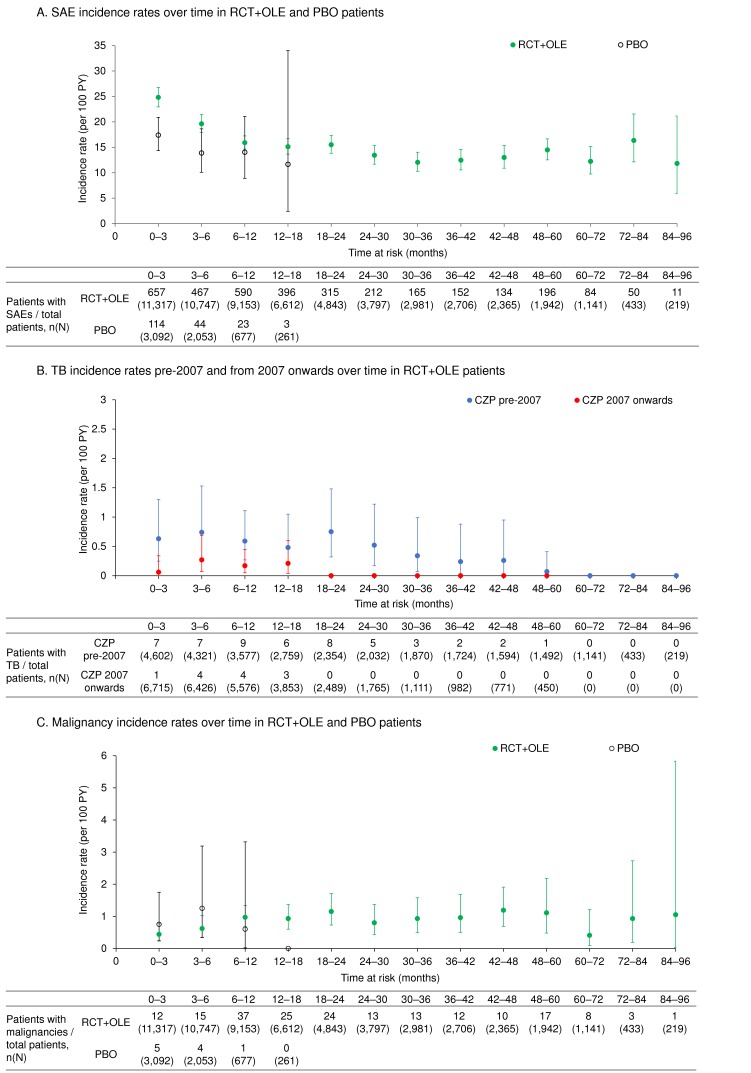
The overall incidence of AEs in RCT+OLE was 212/100 PY and the majority of events (94.7%) were mild or moderate. The IR of SAEs as reported by study investigators in RCT+OLE ranged from 7.8/100 PY to 24.2/100 PY across indications (online supplementary table S4, table 2,figure 1A). In RCTs, the overall SAE IR was 18.0/100 PY in CZP-treated patients and 15.5/100 PY in PBO patients (online supplementary table S4). Events in the MedDRA system organ class ‘Infections and infestations’ were most frequently reported.
Table 2.
Summary of AEs of interest reported for CZP-treated patients (all doses) in the combined RCT and OLE periods (RCT+OLE)
| Overall (n=11 317; 21 695 PY) |
RA (n=6927; 13 542 PY) |
axSpA (n=315; 978 PY) |
PsA (n=393; 1316 PY) |
PSO (n=1112; 1481 PY) |
CD (n=2570; 4378 PY) |
||
| Mean exposure (years) | 1.92 | 1.95 | 3.10 | 3.35 | 1.33 | 1.70 | |
| Median exposure (years) | 1.15 | 1.13 | 3.75 | 3.99 | 1.51 | 0.86 | |
| IR/100 PY [n (%)] | |||||||
| SIEs* | 3.62 [757 (6.7)] |
3.44 [450 (6.5)] |
1.67 [16 (5.1)] |
1.64 [21 (5.3)] |
1.50 [22 (2.0)] |
5.97 [248 (9.6)] |
|
| OIs including TB disease | 0.39 [85 (0.8)] |
0.51 [69 (1.0)] |
0.10 [1 (0.3)] |
0.08 [1 (0.3)] |
0.14 [2 (0.2)] |
0.27 [12 (0.5)] |
|
| All TB disease | 0.29 [62 (0.5)] |
0.38 [51 (0.7)] |
0.10 [1 (0.3)] |
0 | 0.14 [2 (0.2)] |
0.18 [8 (0.3)] |
|
| TB disease by date of treatment initiation† | Pre-2007 | 0.42 [50 (1.1)] |
0.52 [42 (1.8)] |
N/A | N/A | 1.46 [1 (0.9)] |
0.19 [7 (0.3)] |
| 2007 onwards | 0.12 [12 (0.2)] |
0.17 [9 (0.2)] |
0.10 [1 (0.3)] |
0 | 0.07 [1 (0.1)] |
0.13 [1 (0.2)] |
|
| Herpes zoster | 0.06 [14 (0.1)] |
0.07 [10 (0.1)] |
0.10 [1 (0.3)] |
0.08 [1 (0.3)] |
0 | 0.05 [2 (0.1)] |
|
| All malignancies | 0.82 [178 (1.6)] |
0.93 [125 (1.8)] |
0.51 [5 (1.6)] |
0.46 [6 (1.5)] |
0.68 [10 (0.9)] |
0.73 [32 (1.2)] |
|
| All malignancies excluding NMSC | 0.66 [144 (1.3)] |
0.77 [104 (1.5)] |
0.41 [4 (1.3)] |
0.46 [6 (1.5)] |
0.47 [7 (0.6)] |
0.53 [23 (0.9)] |
|
| Melanoma | 0.06 [12 (0.1)] |
0.06 [8 (0.1)] |
0 | 0 | 0 | 0.09 [4 (0.2)] |
|
| Lymphoma, including Hodgkin’s disease‡ | 0.06 [13 (0.1)] |
0.07 [10 (0.1)] |
0 | 0.08 [1 (0.3)] |
0.07 [1 (0.1)] |
0.02 [1 (0.0)] |
|
| NMSC | 0.17 [37 (0.3)] |
0.16 [22 (0.3)] |
0.10 [1 (0.3)] |
0 | 0.20 [3 (0.3)] |
0.25 [11 (0.4)] |
|
| MACE | 0.47 [101 (0.9)] |
0.62 [84 (1.2)] |
0.10 [1 (0.3)] |
0.54 [7 (1.8)] |
0.27 [4 (0.4)] |
0.11 [5 (0.2)] |
|
| GI perforations | 0.08 [17 (0.2)] |
0.04 [5 (0.1)] |
0 | 0 | 0 | 0.27 [12 (0.5)] |
|
| New onset or worsening psoriasis§ | 0.03 [6 (0.1)] |
0 | 0.10 [1 (0.3)] |
0 | 0.27 [4 (0.4)] |
0.02 [1 (0.0)] |
|
| Venous thromboembolism¶ | 0.23 [49 (0.4)] |
0.27 [37 (0.5)] |
0 | 0.31 [4 (1.0)] |
0.14 [2 (0.2)] |
0.14 [6 (0.2)] |
|
| Pulmonary embolism (SAEs only) | 0.09 [20 (0.2)] |
0.11 [15 (0.2)] |
0 | 0.23 [3 (0.8)] |
0.07 [1 (0.1)] |
0.02 [1 (0.0)] |
|
n (%) refers to the number of patients with events; zeros indicate that there were no cases. NMSC includes serious and non-serious cases.
*Including the five appendicitis events confirmed as SIEs during the previous safety update in RA.18
†Before 2007, a positive TB result on the PPD tuberculin skin test varied (from ≥5 to ≥20 mm) according to geographic region. Since 2007, CZP recommendations internationally mandate all patients with PPD ≥5 mm receive treatment for latent TB infection. There were no patients with axSpA or PsA enrolled prior to 2007.
‡Lymphoma cases include two cases of Hodgkin’s disease, one in RA and one in PSO.
§Worsening psoriasis defined as psoriasis reported as an AE in a patient enrolled in a PSO study; new-onset psoriasis defined as psoriasis in a patient enrolled in a non-PSO study.
¶Includes serious and non-serious deep vein thrombosis and pulmonary embolism events.
AE, adverse event; axSpA, axial spondyloarthritis; CD, Crohn’s disease; CZP, certolizumab pegol; GI, gastrointestinal; IR, incidence rate (the number of new cases per 100 PY, with the denominator being the exposure duration up to the first occurrence of a particular AE); MACE, major adverse cardiovascular events;NMSC, non-melanoma skin cancer;OI, opportunistic infection;OLE, open-label extension; PPD, purified protein derivative; PsA, psoriatic arthritis; PSO, psoriasis; PY, patient-years; RA, rheumatoid arthritis;RCT, randomised controlled trial;SAE, serious adverse event;SIE, serious infectious event;TB, tuberculosis.
Figure 1.
Incidence rates over time of SAEs, malignancies and TB pre-2007 and post-2007 for RCT PBO and CZP-treated patients (all doses) in the combined RCT and OLE periods (RCT+OLE). (A) SAE incidence rates over time in RCT+OLE and PBO patients. (B) TB disease incidence rates pre-2007 and from 2007 onwards over time in RCT+OLE patients. (C) Malignancy incidence rates over time in RCT+OLE and PBO patients. Pre-2007 and 2007 onwards refer to the date of treatment initiation with CZP. Before 2007, the threshold for a positive result on the PPD tuberculin skin test varied (from ≥5 to ≥20 mm) according to geographic region. Since 2007, CZP recommendations internationally mandate that all patients with PPD ≥5 mm receive treatment for latent TB infection. axSpA, axial spondyloarthritis; CD, Crohn’s disease; CZP, certolizumab pegol; IR, incidence rate; OLE, open-label extension; PBO, placebo; PPD, purified protein derivative; PsA, psoriatic arthritis; PSO, psoriasis; PY, patient-years; RA, rheumatoid arthritis; SAE, serious adverse event; TB, tuberculosis.
AEs leading to treatment withdrawal
In RCT+OLE, the IR (95% CI) of AEs leading to treatment withdrawal was 8.65 (95% CI 8.26 to 9.05)/100 PY. For RCT CZP patients, the IR was 12.05 (95% CI 10.83 to 13.37)/100 PY, compared to 15.61 (95% CI 13.42 to 18.06)/100 PY for RCT PBO patients (online supplementary table S4).
Deaths
In the 11 317 patients exposed to CZP across all studies, there were 87 deaths (IR: 0.40/100 PY) (online supplementary table S4). The most common primary cause of death was infection (0.08/100 PY), followed by malignancy (0.07/100 PY), myocardial infarction (0.07/100 PY) and other cardiac causes (0.03/100 PY). The IRs of sudden/unexplained death and death from other causes were 0.03/100 PY and 0.10/100 PY, respectively. The primary cause of death was unassessable for two patients. Most deaths were recorded in patients with RA (n=68; IR: 0.50/100 PY). There were nine deaths in patients with CD (0.21/100 PY), six in PsA (0.46/100 PY) and four in PSO (0.27/100 PY). No deaths were reported in the axSpA population. There were 15 deaths in total in the RCT CZP population (0.50/100 PY) and three in PBO (0.25/100 PY).
The SMR (95% CI) for RA was 0.50 (95% CI 0.38 to 0.63), with an observed IR lower than the expected rate in the general population over the same time period. SMRs were also comparatively low for PsA (0.70 (95% CI 0.26 to 1.53)), PSO (0.35 (95% CI 0.09 to 0.89)) and CD (0.41 (95% CI 0.20 to 0.75)). The SMR for PBO patients across all indications was 0.33 (95% CI 0.07 to 0.96), in the context of a shorter mean exposure.
SIEs
The IR of confirmed SIEs for the RCT+OLE group across all indications was 3.62 (95% CI 3.36 to 3.88)/100 PY (table 2). ERs of confirmed SIEs in RCT+OLE were 4.17/100 PY overall, 4.01/100 PY in RA, 1.74/100 PY in axSpA, 1.90/100 PY in PsA, 1.76/100 PY in PSO and 6.72/100 PY in CD, including GI-specific infections. Overall, the onset of SIEs in RCT+OLE was highest during the first 3 months of CZP treatment (IR: 3.69/100 PY) and decreased with longer CZP exposure.
In RCT CZP, IRs of confirmed SIEs across indications ranged from 2.03/100 PY (axSpA) to 8.15/100 PY (CD) (table 3). The IR of SIEs in patients receiving CZP 200 mg Q2W was 4.40 (95% CI 3.48 to 5.49)/100 PY; for CZP 400 mg Q2W it was 5.44 (95% CI 3.74 to 7.64)/100 PY (online supplementary table S5). For RCT PBO patients, the IR of SIEs was 2.46/100 PY across indications.
Table 3.
Summary of AEs of interest reported for PBO-controlled and CZP-treated patients (all doses) in the RCT period
| Overall | RA | axSpA | PsA | PSO | CD | ||||||||
| RCT PBO (n=3092; 1184 PY) |
RCT CZP (n=6467; 3017 PY) |
RCT PBO (n=1759; 775 PY) |
RCT CZP (n=4248; 2260 PY) |
RCT PBO (n=107; 39 PY) |
RCT CZP (n=218; 99 PY) |
RCT PBO (n=136; 51 PY) |
RCT CZP (n=273; 122 PY) |
RCT PBO (n=215; 59 PY) |
RCT CZP (n=809; 237 PY) |
RCT PBO (n=875; 262 PY) |
RCT CZP (n=919; 299 PY) |
||
| Mean exposure (years) | 0.38 | 0.47 | 0.44 | 0.53 | 0.36 | 0.45 | 0.37 | 0.45 | 0.27 | 0.29 | 0.30 | 0.33 | |
| Median exposure (years) | 0.31 | 0.38 | 0.31 | 0.46 | 0.31 | 0.46 | 0.32 | 0.46 | 0.31 | 0.31 | 0.23 | 0.31 | |
| IR/100 PY[n(%)] | |||||||||||||
| SIEs | 2.46 [29 (0.9)] |
4.83 [144 (2.2)] |
2.08 [16 (0.9)] |
4.88 [109 (2.6)] |
0 | 2.03 [2 (0.9)] |
1.98 [1 (0.7)] |
3.30 [4 (1.5)] |
0 | 2.11 [5 (0.6)] |
4.63 [12 (1.4)] |
8.15 [24 (2.6)] |
|
| OIs including TB disease | 0.08 [1 (0.0)] |
0.76 [23 (0.4)] |
0.13 [1 (0.1)] |
0.89 [20 (0.5)] |
0 | 0 | 0 | 0.82 [1 (0.4)] |
0 | 0.42 [1 (0.1)] |
0 | 0.34 [1 (0.1)] |
|
| All TB disease | 0 | 0.46 [14 (0.2)] |
0 | 0.53 [12 (0.3)] |
0 | 0 | 0 | 0 | 0 | 0.42 [1 (0.1)] |
0 | 0.34 [1 (0.1)] |
|
| TB disease by date of treatment initiation* | Pre-2007 | 0 | 0.86 [11 (0.4)] |
0 | 0.91 [9 (0.5)] |
N/A | N/A | N/A | N/A | 0 | 3.81 [1 (0.9)] |
0 | 0.39 [1 (0.1)] |
| 2007 onwards | 0 | 0.17 [3 (0.1)] |
0 | 0.24 [3 (0.1)] |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Herpes zoster | 0 | 0.13 [4 (0.1)] |
0 | 0.13 [3 (0.1)] |
0 | 0 | 0 | 0.82 [1 (0.4)] |
0 | 0 | 0 | 0 | |
| All malignancies | 0.76 [9 (0.3)] |
0.63 [19 (0.3)] |
0.65 [5 (0.3)] |
0.71 [16 (0.4)] |
0 | 0 | 1.98 [1 (0.7)] |
0 | 0 | 0.42 [1 (0.1)] |
1.15 [3 (0.3)] |
0.67 [2 (0.2)] |
|
| All malignancies excluding NMSC | 0.68 [8 (0.3)] |
0.46 [14 (0.2)] |
0.52 [4 (0.2)] |
0.58 [13 (0.3)] |
0 | 0 | 1.98 [1 (0.7)] |
0 | 0 | 0 | 1.15 [3 (0.3)] |
0.33 [1 (0.1)] |
|
| Melanoma | 0.08 [1 (0.0)] |
0.03 [1 (0.0)] |
0.13 [1 (0.1)] |
0.04 [1 (0.0)] |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Lymphoma, including Hodgkin’s disease† | 0.08 [1 (0.0)] |
0.07 [2 (0.0)] |
0 | 0.09 [2 (0.0)] |
0 | 0 | 0 | 0 | 0 | 0 | 0.38 [1 (0.1)] |
0 | |
| NMSC | 0.08 [1 (0.0)] |
0.17 [5 (0.1)] |
0.13 [1 (0.1)] |
0.13 [3 (0.1)] |
0 | 0 | 0 | 0 | 0 | 0.42 [1 (0.1)] |
0 | 0.34 [1 (0.1)] |
|
| MACE | 0.34 [4 (0.1)] |
0.76 [23 (0.4)] |
0.52 [4 (0.2)] |
0.84 [19 (0.4)] |
0 | 0 | 0 | 2.47 [3 (1.1)] |
0 | 0.42 [1 (0.1)] |
0 | 0 | |
| GI perforations | 0.08 [1 (0.0)] |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.38 [1 (0.1)] |
0 | |
| New onset or worsening psoriasis‡ | 0 | 0.03 [1 (0.0)] |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.42 [1 (0.1)] |
0 | 0 | |
| Venous thromboembolism§ | 0.42 [5 (0.2)] |
0.30 [9 (0.1)] |
0.52 [4 (0.2)] |
0.35 [8 (0.2)] |
0 | 0 | 0 | 0 | 1.71 [1 (0.5)] |
0 | 0 | 0.33 [1 (0.1)] |
|
| Pulmonary embolism (SAEs only) | 0.25 [3 (0.1)] |
0.10 [3 (0.0)] |
0.39 [3 (0.2)] |
0.09 [2 (0.0)] |
0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.33 [1 (0.1)] |
|
n (%) refers to the number of patients with events; zeros indicate that there were no cases. NMSC includes serious and non-serious cases.
*Before 2007, a positive TB result on the PPD tuberculin skin test varied (from ≥5 to ≥20 mm) according to geographic region. Since 2007, CZP recommendations internationally mandate that all patients with PPD ≥5 mm receive treatment for latent TB infection. There were no patients with axSpA or PsA enrolled prior to 2007.
†Lymphoma cases include one case of Hodgkin’s disease in a CZP-treated CD patient.
‡Worsening psoriasis defined as psoriasis reported as an adverse event in a patient enrolled in a PSO study; new-onset psoriasis defined as psoriasis in a patient enrolled in a non-PSO study.
§Includes serious and non-serious deep vein thrombosis and pulmonary embolism events.
AE, adverse events; axSpA, axial spondyloarthritis; CD, Crohn’s disease; CZP, certolizumab pegol; GI, gastrointestinal;IR, incidence rate (the number of new cases per 100 PY, with the denominator being the exposure duration up to the first occurrence of a particular AE);MACE, major adverse cardiovascular events;NMSC, non-melanoma skin cancer;OI, opportunistic infection;OLE, open-label extension;PBO, placebo; PPD, purified protein derivative; PsA, psoriatic arthritis; PSO, psoriasis; PY, patient-years; RA, rheumatoid arthritis;RCT, randomised controlled trial;SAE, serious adverse event;SIE, serious infectious event;TB, tuberculosis.
In RCT+OLE, pneumonia was the most frequent SIE across indications, apart from CD (overall IR: 0.62 (95% CI 0.52 to 0.74)/100 PY). In RA, the rates of lower respiratory tract and lung infections (IR: 0.89/100 PY; ER: 1.00/100 PY) were higher than upper respiratory tract infections (0.21/100 PY; 0.23/100 PY), largely due to the frequency of pneumonia (106 events; IR: 0.74/100 PY; ER: 0.78/100 PY). In CD, the most common SIE was anal abscess (IR: 1.08/100 PY; ER: 1.19/100 PY).
In RCT+OLE, the IR of GI-specific SIEs in patients with CD was 2.96 (95% CI 2.47 to 3.52)/100 PY. Rates across other indications were much lower: 0.24 (95% CI 0.16 to 0.33)/100 PY in RA, 0.31 (95% CI 0.06 to 0.90)/100 PY in axSpA, 0.08 (95% CI 0.00 to 0.42)/100 PY in PsA and 0.27 (95% CI 0.07 to 0.69)/100 PY in PSO. IRs of non-GI-specific SIEs were in RA: 3.26 (95% CI 2.96 to 3.59)/100 PY; axSpA: 1.35 (95% CI 0.72 to 2.31)/100 PY; PsA: 1.56 (95% CI 0.95 to 2.41)/100 PY; PSO: 1.29 (95% CI 0.78 to 2.02)/100 PY; and 3.16 (95% CI 2.65 to 3.75)/100 PY in CD.
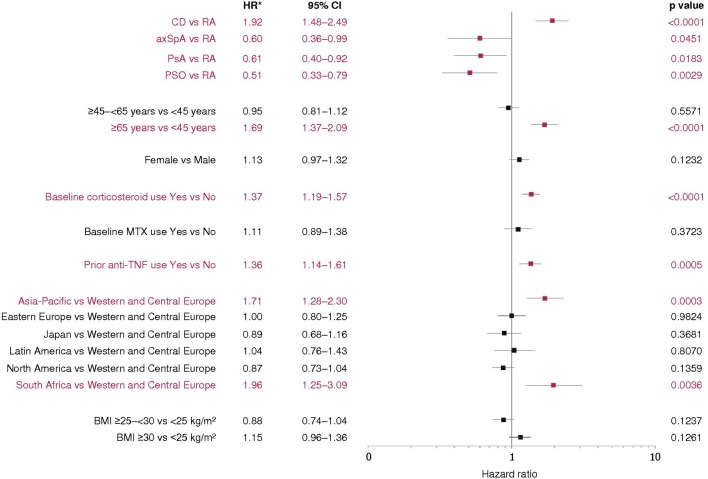
In the adjusted Cox proportional hazards model of time to first SIE (figure 2, online supplementary file 7), patients with CD had twice the risk of SIEs (including GI-specific SIEs) compared with RA; SIE risk in axSpA, PsA and PSO was 40%–50% lower compared with RA. Age ≥65 years was associated with a ~70% increase in SIE risk relative to age <45 years. Baseline corticosteroid use and prior anti-TNF use increased SIE risk by approximately 35%–40%. Patients in Asia-Pacific (3% of RCT+OLE) and South Africa (CD patients only, 1% of RCT+OLE) had an increased risk of SIEs compared with Western and Central Europe (42%); no other geographic regions influenced the risk of SIEs. BMI did not have an important impact on SIE risk.
Figure 2.
Multivariate Cox proportional hazards model of time to first serious infectious events. For some subgroups, the small sample size may preclude accurate assessment of results. The patient population in South Africa included CD patients only. All reported p values and CIs are nominal and can only be interpreted in an exploratory manner. *Derived from model parameter estimates. axSpA, axial spondyloarthritis; BMI, body mass index; CD, Crohn’s disease; MTX, methotrexate; PsA, psoriatic arthritis; PSO, psoriasis; RA, rheumatoid arthritis; TNF, tumour necrosis factor.
rmdopen-2019-000942supp007.pdf (77.9KB, pdf)
Opportunistic infections
IRs for all confirmed OIs in RCT+OLE ranged from 0.08/100 PY in PsA to 0.51/100 PY in RA. The most commonly reported OI was tuberculous infection, followed by herpes virus infection (tables 2 and 3).
In RCT+OLE, the overall IR of TB (excluding latent TB) was 0.29 (95% CI 0.22 to 0.37)/100 PY (table 2), with a profound decrease in TB rates after a screening protocol amendment for CZP trials in 2007 (figure 1B and online supplementary figure S5B; amendment described previously28). There were 62 TB cases overall, mostly in RA (51 cases), with eight cases in CD, one in axSpA, two in PSO and none in PsA. Overall, 40/62 TB cases were pulmonary (IR: 0.18 (95% CI 0.13 to 0.25)/100 PY), 22/62 were non-pulmonary or disseminated TB (0.10 (95% CI 0.06 to 0.15)/100 PY), including one patient with concurrent pulmonary TB and GI TB reported on the same day.
rmdopen-2019-000942supp006.pdf (47.7KB, pdf)
Overall, 14 serious cases of herpes zoster were reported in RCT+OLE patients (IR: 0.06/100 PY). Six cases were in Central Europe, three in Western Europe, one in North America and four in Japan (Japan IR: 0.21/100 PY vs 0.05/100 PY in the rest of the world). There was one confirmed OI of herpes simplex (oral and genital). The incidence of postherpetic neuralgia was also low, with three non-serious cases reported.
Fungal OIs were rare, with one case each of candidiasis (oral) and Aspergillus infection (both in RA). There was one expert-reviewed case of blood-borne viral hepatitis—a suspected hepatitis B reactivation in a patient with RA.
Malignancies
In RCT+OLE, there were 147 confirmed malignancies excluding NMSC (IR: 0.66/100 PY overall, ranging from 0.41/100 PY in axSpA to 0.77/100 PY in RA) (table 2). During RCTs, the overall IR of malignancy excluding NMSC was 0.46/100 PY for CZP and 0.68/100 PY for PBO (table 3). The most common non-NMSC malignancies were breast (25 cases), respiratory tract (11 cases) and colorectal malignancies (10 cases). Overall, there were 13 cases of lymphoma (IR: 0.06 (95% CI 0.03 to 0.10)/100 PY), including two cases of Hodgkin’s disease (0.01 (95% CI 0.00 to 0.03)/100 PY; one in RA and one in PSO) (table 2). The IR of malignancy in RCT+OLE remained largely stable over time (figure 1C and online supplementary figure S5C). Age-matched and gender-matched SIR data for each malignancy type are presented in figure 3.
Figure 3.
SIR data for all CZP-treated patients (RCT+OLE) for each malignancy type, standardised to the general population by age and gender (GLOBOCAN and SEER). (A) All malignancies excluding NMSC (GLOBOCAN data). (B) Lymphatic and haematopoietic malignancies (GLOBOCAN data). (C) All malignancies excluding NMSC (SEER data). *There were no lymphatic or haematopoietic malignancies in the axSpA subpopulation. Forest plots show SIRs with 95% CIs. PY calculated using total years of CZP exposure from the first dose of CZP to end of AE exposure period (if no malignancy) or to first malignancy. AE, adverse event; axSpA, axial spondyloarthritis; CD, Crohn’s disease; CZP, certolizumab pegol; NA, not applicable; NMSC, non-melanoma skin cancer; OLE, open-label extensions; PsA, psoriatic arthritis; PSO, psoriasis; PY: patient-years; RA, rheumatoid arthritis; RCT, randomised controlled trials; SIR, standardised incidence ratio.
Major adverse cardiovascular events (MACE)
IRs for MACE in RCT+OLE ranged from 0.10/100 PY in axSpA to 0.62/100 PY in RA (table 2). During RCTs, the IR of MACE was 0.76/100 PY for CZP and 0.34/100 PY for PBO (table 3). There were 51 SAEs (IR: 0.23/100 PY) under the HLT ‘Ischaemic coronary artery disorders’. ‘Acute myocardial infarction’ (17 events, IR: 0.08/100 PY) and ‘Myocardial infarction’ (25 events, IR: 0.11/100 PY) were the most common MedDRA PTs. There were 19 events (IR: 0.08/100 PY) under the HLT ‘Central nervous system haemorrhages and cerebrovascular accidents’.
Other events of potential concern
Overall, there were 17 confirmed GI perforations in RCT+OLE (IR: 0.08/100 PY): 12 cases in CD (0.27/100 PY, including three of five cases of ‘large intestine perforation’ that occurred after recent instrumentation) and five in RA (IR: 0.04/100 PY: one oesophageal, one 'intestinal', one large intestine and two unspecified). There were no GI perforations in axSpA, PsA or PSO. There was one GI perforation in RCT PBO (IR: 0.08/100 PY).
There were five confirmed demyelination events in RCT+OLE (IR: 0.02/100 PY): two in RA, one in CD and two in PSO (one PSO case was likely pre-existing, as patient history indicated that symptoms pre-dated study entry). In RCT+OLE, there were three confirmed SAEs of lupus-like syndrome (IR: 0.01/100 PY), one confirmed SAE of pulmonary immune disease (‘idiopathic pulmonary fibrosis’ in a patient with RA taking MTX at baseline, possibly representing RA-interstitial lung disease), nine confirmed cases of vasculitis (IR: 0.04/100 PY; seven cases in RA, two in CD) and three SAEs of new onset sarcoidosis (IR: 0.01/100 PY; two in RA, one in CD). There were 60 cases of VTE during RCT+OLE (IR: 0.23/100 PY). Of these 60 cases, 22 were PE events (0.10/100 PY), with three in RCT (0.10/100 PY) and three in PBO (0.25/100 PY). Indication-specific IRs of VTE and SAEs of PE are reported in tables 2 and 3 and online supplementary table S5.
There were 20 cases of hypersensitivity and allergic reaction in RCT+OLE, possibly related to CZP, including two considered as anaphylactic reactions (IR: 0.01/100 PY), of which one case required hospitalisation, and treatment with antihistamines and low dose corticosteroids.
There were six confirmed serious psoriasis events in RCT+OLE across all indications (IR: 0.03/100 PY). Worsening psoriasis was confirmed in four CZP-treated patients within PSO trials (two psoriasis, one guttate psoriasis and one erythrodermic psoriasis) and one patient with axSpA with pre-existing psoriasis. There was one case of new-onset psoriasis, in CD.
Forty-two pregnancies with known maternal CZP exposure were recorded across all CZP studies: 15 in RA, four in axSpA, one in PsA, five in PSO and 17 in CD. This is a limited subset of the much larger number of prospectively reported pregnancies (528 with known outcomes) in the most recent review of the sponsor’s global pharmacovigilance database,36 where the safety of CZP was more robustly evaluated.
Laboratory values
Eight patients, including one PBO patient, met postbaseline liver function test criteria of elevated bilirubin (≥2× ULN) and elevated AST or ALT (≥3× ULN). None were considered to fulfil Hy’s Law, as each patient had either a significant medical history, concurrent illness or comedication that could have contributed to these measurements. Expert review of other clinically significant alterations in bilirubin, AST, ALT, white blood cell counts, neutrophil counts or platelet counts did not reveal any abnormalities unrelated to a concurrent AE.
Discussion
This study, the largest report of pooled CZP safety data to date, has comprehensively evaluated CZP safety in 11 317 CZP-treated patients in clinical trials, comprising 21 695 PY, with individual patient exposures up to 7.8 years. This is the first pooled CZP safety update including all indications approved as of 2018. Compared with the last major review of CZP safety in RA,18 this population benefited from additional patients from phase 3 trials in Japan, phase 4 studies in the USA, plus a trial of CZP in DMARD-naïve, early RA patients and the first head-to-head study of CZP versus another anti-TNF.20 The addition of patients with axSpA, PsA, PSO and CD to this review enables a comparison of patients being treated across rheumatology, dermatology and gastroenterology.
The retrospective independent expert review process helped align the data, according to predefined criteria, to reduce AE reporting differences between study investigators across specialties and geographic locations. This helped to ensure events were appropriately classified, alongside systematic or algorithm-based AE assessment. Similar approaches have been taken for other biologics, such as adalimumab and tofacitinib.37 38
The mean patient age at baseline was broadly similar (37–53 years), and the female majority (68%) was driven principally by the RA population (79% female). As previously reported,18 the IR of SAEs peaked during the first 3–6 months of treatment (for both CZP and PBO) before decreasing to a plateau. CZP dose did not impact SAE risk. As expected, infections were the most commonly reported SAE.
The observation that SIE rates peaked in the first 3 months of CZP treatment, decreasing with longer exposure, is consistent with the previous pooled RA safety report.18 SIE rates in RA patients (3.4/100 PY) were similar to rates observed for CZP in the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA). In this extensive real-world RA registry (19 282 patients, 46 771 PY exposure), the SIE rate in 1446 patients taking CZP were numerically lower (IR: 3.80 (95% CI 2.97 to 4.85)/100 PY) than the IR for all patients starting a new biologic (5.51 (95% CI 5.29 to 5.71)/100 PY) and the lowest of all individual drugs studied.39 Previous data suggest that patients with IMIDs are at greater risk of SIEs, with known predictors including older age and corticosteroid use.40–42 This was consistent with our adjusted Cox proportional hazards model and a previous CZP study in RA, which revealed that age ≥65 years and baseline corticosteroid use were associated with a greater risk of SIEs.40 The highest IR of SIEs was in patients with CD; the risk associated with this disease was also reflected in the Cox model and may have been due to clustering of GI tract SIEs in CD patients (10-fold higher incidence than in other indications), supporting the argument that disease-specific factors contribute to SIE risk. A higher burden of inflammation in certain conditions (systemic inflammation in RA and particularly in the GI tract for CD) could play a role. Importantly, the SIE rate did not increase with the higher CZP doses used for the loading regimen and currently approved dosing for PSO. Notably, PSO patients had the lowest infection rates.
TB was the most commonly reported OI, with IRs for confirmed TB cases comparable to previous reports.18 28 As expected and observed with other anti-TNFs, TB rates declined steeply after stricter baseline screening criteria were introduced in 2007, in line with updated WHO guidance.28 43 These new criteria included a purified protein derivative cut-off of 5 mm and a TB-specific questionnaire to help identify patients at risk.28 Some later studies incorporated an Interferon Gamma Release Assay (IGRA) test (QuantiFERON-TB GOLD).
RA has been shown to increase the risk of herpes zoster,44 and an elevated risk has been observed in patients with RA and CD receiving Janus kinase (JAK) inhibitors.45–47 Similar to other published long-term anti-TNF safety data, confirmed herpes zoster and post-herpetic neuralgia rates in our analysis were low.38
Certain cancers are more common in patients with IMIDs compared with the general population, for example, non-Hodgkin’s lymphoma in RA;48 49 colorectal cancer in CD,50 though up-to-date control data are lacking. In this study, the SIR of malignancies was 1.03 (95% CI 0.87 to 1.20) when compared with the SEER population, but increased to 1.45 (95% CI 1.23 to 1.69) relative to the GLOBOCAN population. Although other biologic safety studies have used SEER for comparison, the worldwide GLOBOCAN healthy population could be more appropriate, and caution is needed over the long-term risk of cancer with any biologic. However, the IR of any malignancy in CZP-treated patients by 3-month or 6-month follow-up intervals remained largely constant over time. As expected in RA, lymphatic and haematopoietic cancer incidence (principally lymphoma) was increased compared with the GLOBOCAN/SEER general population (SIR: 2.13 (95% CI 1.17 to 3.58)) but remained very low across other indications. Overall, IRs for lymphoma and other malignancy types, including NMSC and melanoma in RA patients, were similar to previous safety updates of CZP in RA18 and across indications. No melanomas were reported in PSO trials.
Data suggest that patients with RA and PSO have a clinically significant increased risk of cardiovascular death compared with the general population,51–53 although published findings for PSO suggest that treatment with anti-TNF medications may lower cardiovascular risk.54 The IR of MACE in PSO was 0.27/100 PY. The higher IRs of MACE in patients with RA and PsA in our RCT+OLE population (0.62/100 PY and 0.54/100 PY, respectively) may reflect age as well as disease duration, the latter indicating how long the patient may have had untreated inflammatory disease.
As expected, GI perforations were more prevalent in CD and included post-procedure cases. Of the five patients with RA who had perforations, four were known to be taking corticosteroids and two of these were also on non-steroidal anti-inflammatory drugs. The overall IR of GI perforations was low compared with published safety data for tocilizumab55 and similar to available data for adalimumab in RA.56 In line with long-term safety data for other anti-TNF medications, IRs of other SAEs, including autoimmune conditions, demyelination events and psoriasis, were very low in our CZP study population.38 57
The advantage of reporting safety events using a large amount of RCT data is that all events occurring during a trial are recorded, regardless of the potential relationship with the study drug, generally providing a more stringent view of drug safety. However, while it is possible to infer that some AEs are related to the study drug, other AEs will occur independently of treatment. Differential adherence in RCTs versus real-world practice may affect the numbers of reported AEs to an unknown extent. Moreover, patients in RCTs/OLEs are often different to those treated in real-world settings. Real-world patients can be older, with more comorbidities and concomitant medications, and may have received multiple previous biologics. This treatment refractoriness suggested by multiple switches may serve as a proxy indicator of SAE risk, for disease or comorbidity-related reasons.
This review of pooled CZP safety data was limited by the fact that some RCTs (eg, in RA in China, PSO OLEs and other axSpA studies) were ongoing at the time of cut-off, with subsequent results not included in this analysis. Furthermore, patients with axSpA were not separated into AS and nr-axSpA populations. Thus, the overview of CZP clinical data here is extensive, though not complete. The current work focuses on well-known important identified risks for biologics; very rare SAEs may not be reported. For some subgroups, the small sample size may preclude accurate assessment of results.
By comparing safety data across all approved indications, we were able to identify differences in the risks of specific SAEs that may be driven by patient factors, including age, concomitant medications, disease state and comorbidities. In conclusion, no new or unexpected safety signals have emerged in this long-term cross-indication pooled safety analysis of CZP across 49 clinical trials. The RA data reported are consistent with the previous pooled CZP safety update,18 and overall data are in line with post-marketing data for CZP and other biologic medications.39
Acknowledgments
This study, including medical writing and editorial assistance, was funded by UCB Pharma. The authors thank the patients and their caregivers in addition to the investigators and their teams who contributed to this study. The authors also acknowledge Pauline Ralston, MSc, and Nicola Tilt, MSc, UCB Pharma, Brussels, Belgium, for statistical assistance, Christina Popova, MD, and Catherine Arendt, MD, PharmD, UCB Pharma, Brussels, Belgium, for assistance with medical review of adverse events, plus Debbie Nixon, DPhil, UCB Pharma, UK, for publication coordination and Lucy Berry, MBBS, and Emma Phillips, PhD, Costello Medical, UK, for medical writing and editorial assistance.
Footnotes
Contributors: JRC, XM, CGV, AB, TKK, WJS, KW, MdL, IH and VPB contributed to the conception, design, execution or analysis and interpretation of the data. All authors approved the final version to be published after critically revising the manuscript for important intellectual content.
Funding: UCB Pharma sponsored the study and the development of this manuscript, and reviewed the text to ensure that from UCB perspective, the data presented in the publication are scientifically, technically and medically supportable, that they do not contain any information that has the potential to damage the intellectual property of UCB, and that the publication complies with applicable laws, regulations, guidelines and good industry practice. The authors approved the final version to be published after critically revising the manuscript for important intellectual content.
Competing interests: JRC: Grant/research and consultancy fees from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Crescendo, Genentech, Janssen, Roche, Pfizer and UCB Pharma. XM: Consultant for Bristol-Myers Squibb, GlaxoSmithKline, Janssen, LFB Pharmaceuticals, Pfizer and UCB Pharma. Grant/research support from: Biogen, Pfizer and UCB Pharma. CGV: Speaking and/or consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, Merck-Serono, Medac, Nordic Pharma, Novartis, Pfizer, Roche, Sandoz, Sanofi and UCB Pharma. AB: Consulting honoraria and clinical investigator: AbbVie, Aclaris, Akros, Allergan, Almirall, Amgen, Arena, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dermavant, Dermira Inc., Eli Lilly, Genentech/Roche, GlaxoSmithKline, Janssen, Leo, Meiji, Merck, Novartis, Pfizer, Purdue Pharma, Regeneron, Revance, Sandoz, Sanofi Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB Pharma, Valeant and Vidac. TKK: Speaking and/or consulting fees from Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celltrion, Eli Lilly, Epirus, Hospira, Merck-Serono, Novartis, Orion Pharma, Pfizer, Sandoz and UCB Pharma. WJS: Research grants from Atlantic Healthcare Limited, AbbVie, Amgen, Celgene/Receptos, Genentech, Eli Lilly, Gilead Sciences, Janssen and Takeda; Consulting fees from AbbVie, Allergan, Amgen, Boehringer Ingelheim, Celgene, Conatus, Cosmo, Eli Lilly, Escalier Biosciences, Ferring, Genentech, Gilead, Gossamer Bio, Janssen, Miraca Life Sciences, Nivalis Therapeutics, Novartis Nutrition Science Partners, Oppilan Pharma, Otsuka, Paul Hastings, Pfizer, Precision IBD, Progenity, Prometheus Laboratories, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust or HART), Salix, Shire, Seres Therapeutics, Sigmoid Biotechnologies, Takeda, Tigenix, Tillotts Pharma, UCB Pharma and Vivelix; Stock options from Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Precision IBD, Progenity and Ritter Pharmaceuticals. KW: Consultant fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, Pfizer, Roche and UCB Pharma. MdL: Employee of UCB Pharma. IH: Employee of UCB Pharma. VPB: Consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Genentech, Pfizer, Regeneron, Scipher and UCB Pharma.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1.European Medicines Agency Cimzia summary of product characteristics, 2018. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001037/WC500069763.pdf [Accessed 22 May 2018].
- 2.United States Food and Drug Administration Cimzia prescribing information, 2018. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125160s283lbl.pdf [Accessed 22 May 2018].
- 3.Singh JA, Hossain A, Tanjong Ghogomu E, et al. . Biologics or tofacitinib for rheumatoid arthritis in incomplete responders to methotrexate or other traditional disease-modifying anti-rheumatic drugs: a systematic review and network meta-analysis. Cochrane Database Syst Rev 2016;(5):CD012183 10.1002/14651858.CD012183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ash Z, Gaujoux-Viala C, Gossec L, et al. . A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26. 10.1136/ard.2011.150995 [DOI] [PubMed] [Google Scholar]
- 5.Callhoff J, Sieper J, Weiß A, et al. . Efficacy of TNFα blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Ann Rheum Dis 2015;74:1241–8. 10.1136/annrheumdis-2014-205322 [DOI] [PubMed] [Google Scholar]
- 6.Miligkos M, Papamichael K, Vande Casteele N, et al. . Efficacy and safety profile of anti-tumor necrosis factor-α versus anti-integrin agents for the treatment of Crohn's disease: a network meta-analysis of indirect comparisons. Clin Ther 2016;38:1342–58. 10.1016/j.clinthera.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 7.Sbidian E, Chaimani A, Garcia-Doval I, et al. . Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 2017;12:CD011535 10.1002/14651858.CD011535.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bongartz T, Sutton AJ, Sweeting MJ, et al. . Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275–85. 10.1001/jama.295.19.2275 [DOI] [PubMed] [Google Scholar]
- 9.Bonovas S, Minozzi S, Lytras T, et al. . Risk of malignancies using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug Saf 2016;15:35–54. 10.1080/14740338.2016.1238458 [DOI] [PubMed] [Google Scholar]
- 10.Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol 2012;9:496–503. 10.1038/nrgastro.2012.125 [DOI] [PubMed] [Google Scholar]
- 11.Kemanetzoglou E, Andreadou E. CNS demyelination with TNF-α blockers. Curr Neurol Neurosci Rep 2017;17:36 10.1007/s11910-017-0742-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh JA, Wells GA, Christensen R, et al. . Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev 2011CD008794 10.1002/14651858.CD008794.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Nagpal SJS, Murad MH, et al. . Inflammatory bowel disease is associated with an increased risk of melanoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:210–8. 10.1016/j.cgh.2013.04.033 [DOI] [PubMed] [Google Scholar]
- 14.Winthrop KL. Risk and prevention of tuberculosis and other serious opportunistic infections associated with the inhibition of tumor necrosis factor. Nat Clin Pract Rheumatol 2006;2:602–10. 10.1038/ncprheum0336 [DOI] [PubMed] [Google Scholar]
- 15.Winthrop KL, Novosad SA, Baddley JW, et al. . Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann Rheum Dis 2015;74:2107–16. 10.1136/annrheumdis-2015-207841 [DOI] [PubMed] [Google Scholar]
- 16.European Medicines Agency Cimzia assessment report, 2018. Available: https://www.ema.europa.eu/documents/variation-report/cimzia-h-c-1037-ii-0065-epar-assessment-report-variation_en.pdf [Accessed 15 November 2018].
- 17.Loftus EV, Colombel J-F, Schreiber S, et al. . Safety of long-term treatment with certolizumab pegol in patients with Crohn's disease, based on a pooled analysis ofdata from clinical trials. Clin Gastroenterol Hepatol 2016;14:1753–62. 10.1016/j.cgh.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 18.Bykerk VP, Cush J, Winthrop K, et al. . Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015;74:96–103. 10.1136/annrheumdis-2013-203660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emery P, Bingham CO, Burmester GR, et al. . Certolizumab pegol in combination with dose-optimised methotrexate in DMARD-naïve patients with early, active rheumatoid arthritis with poor prognostic factors: 1-year results from C-EARLY, a randomised, double-blind, placebo-controlled phase III study. Ann Rheum Dis 2017;76:96–104. 10.1136/annrheumdis-2015-209057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolen JS, Burmester G-R, Combe B, et al. . Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet 2016;388:2763–74. 10.1016/S0140-6736(16)31651-8 [DOI] [PubMed] [Google Scholar]
- 21.van der Heijde D, Deodhar A, FitzGerald O, et al. . 4-year results from the RAPID-PsA phase 3 randomised placebo-controlled trial of certolizumab pegol in psoriatic arthritis. RMD Open 2018;4:e000582 10.1136/rmdopen-2017-000582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Heijde D, Dougados M, Landewé R, et al. . Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology 2017;56:1498–509. 10.1093/rheumatology/kex174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottlieb AB, Blauvelt A, Thaçi D, et al. . Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol 2018;79:302–14. 10.1016/j.jaad.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 24.Lebwohl M, Blauvelt A, Paul C, et al. . Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). J Am Acad Dermatol 2018;79:266–76. 10.1016/j.jaad.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 25.Mariette X, Förger F, Abraham B, et al. . Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis 2018;77:228–33. 10.1136/annrheumdis-2017-212196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clowse MEB, Förger F, Hwang C, et al. . Minimal to no transfer of certolizumab pegol into breast milk: results from CRADLE, a prospective, postmarketing, multicentre, pharmacokinetic study. Ann Rheum Dis 2017;76:1890–6. 10.1136/annrheumdis-2017-211384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reich K, Ortonne J-P, Gottlieb AB, et al. . Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab′ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol 2012;167:180–90. 10.1111/j.1365-2133.2012.10941.x [DOI] [PubMed] [Google Scholar]
- 28.Mariette X, Vencovsky J, Lortholary O, et al. . The incidence of tuberculosis in patients treated with certolizumab pegol across indications: impact of baseline skin test results, more stringent screening criteria and geographic region. RMD Open 2015;1:e000044 10.1136/rmdopen-2014-000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito S. Pharmacokinetics 101. Paediatr Child Health 2011;16:535–6. 10.1093/pch/16.9.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. MedDRA. Introductory guide MedDRA version 18.1, 2015. Available: https://www.meddra.org/sites/default/files/guidance/file/intguide_18_1_english.pdf [Accessed 21 Feb 2019].
- 31.FDA Reporting serious problems to the FDA: what is a serious adverse event? 2012. Available: https://www.fda.gov/safety/medwatch/howtoreport/ucm053087.htm [Accessed 21 February 2019].
- 32.Brunicardi F, Andersen D, Billiar T, et al. . Schwartz's principles of surgery. 10th ed McGraw Hill Professional, 2014: 1119–37. [Google Scholar]
- 33. MedDRA. Introductory guide for standardised MedDRA queries (SMQs) version 18.1, 2015. Available: https://www.meddra.org/sites/default/files/guidance/file/smq_intguide_18_1_english.pdf [Accessed 21 February 2019].
- 34.Temple R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidem. Drug Safe. 2006;15:241–3. 10.1002/pds.1211 [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization Global health observatory visualizations, indicator metadata registry, 2016. Available: http://apps.who.int/gho/data/node.wrapper.imr?x-id=1 [Accessed 21 February 2019].
- 36.Clowse MEB, Scheuerle AE, Chambers C, et al. . Pregnancy outcomes after exposure to certolizumab pegol: updated results from a pharmacovigilance safety database. Arthritis Rheumatol 2018;70:1399–407. 10.1002/art.40508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen SB, Tanaka Y, Mariette X, et al. . Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. 10.1136/annrheumdis-2016-210457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burmester GR, Panaccione R, Gordon KB, et al. . Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis 2013;72:517–24. 10.1136/annrheumdis-2011-201244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutherford AI, Subesinghe S, Hyrich KL, et al. . Serious infection across biologic-treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2018;17:905–10. 10.1136/annrheumdis-2017-212825 [DOI] [PubMed] [Google Scholar]
- 40.Curtis JR, Winthrop K, O'Brien C, O’Brien C, et al. . Use of a baseline risk score to identify the risk of serious infectious events in patients with rheumatoid arthritis during certolizumab pegol treatment. Arthritis Res Ther 2017;19:276 10.1186/s13075-017-1466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon WG, Suissa S, Hudson M. The association between systemic glucocorticoid therapy and the risk of infection in patients with rheumatoid arthritis: systematic review and meta-analyses. Arthritis Res Ther 2011;13:R139 10.1186/ar3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacaille D, Guh DP, Abrahamowicz M, et al. . Use of nonbiologic disease-modifying antirheumatic drugs and risk of infection in patients with rheumatoid arthritis. Arthritis Rheum 2008;59:1074–81. 10.1002/art.23913 [DOI] [PubMed] [Google Scholar]
- 43.Arkema EV, Jonsson J, Baecklund E, et al. . Are patients with rheumatoid arthritis still at an increased risk of tuberculosis and what is the role of biological treatments? Ann Rheum Dis 2015;74:1212–7. 10.1136/annrheumdis-2013-204960 [DOI] [PubMed] [Google Scholar]
- 44.Winthrop KL, Furst DE. Rheumatoid arthritis and herpes zoster: risk and prevention in those treated with anti-tumour necrosis factor therapy. Ann Rheum Dis 2010;69:1735–7. 10.1136/ard.2010.133843 [DOI] [PubMed] [Google Scholar]
- 45.Winthrop KL, Yamanaka H, Valdez H, et al. . Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol 2014;66:2675–84. 10.1002/art.38745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smolen JS, Genovese MC, Takeuchi T, et al. . Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019;46:7–18. 10.3899/jrheum.171361 [DOI] [PubMed] [Google Scholar]
- 47.Winthrop KL, Melmed GY, Vermeire S, et al. . Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2018;24:2258–65. 10.1093/ibd/izy131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naz SM, Symmons DPM. Mortality in established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2007;21:871–83. 10.1016/j.berh.2007.05.003 [DOI] [PubMed] [Google Scholar]
- 49.Fallah M, Liu X, Ji J, et al. . Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol 2014;25:2025–30. 10.1093/annonc/mdu365 [DOI] [PubMed] [Google Scholar]
- 50.Stidham RW, Higgins PDR. Colorectal cancer in inflammatory bowel disease. Clin Colon Rectal Surg 2018;31:168–78. 10.1055/s-0037-1602237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahlehoff O, Gislason GH, Charlot M, et al. . Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med 2011;270:147–57. 10.1111/j.1365-2796.2010.02310.x [DOI] [PubMed] [Google Scholar]
- 52.Aviña-Zubieta JA, Choi HK, Sadatsafavi M, et al. . Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum 2008;59:1690–7. 10.1002/art.24092 [DOI] [PubMed] [Google Scholar]
- 53.Peters MJL, van Halm VP, Voskuyl AE, et al. . Does rheumatoid arthritis equal diabetes mellitus as an independent risk factor for cardiovascular disease? A prospective study. Arthritis Rheum 2009;61:1571–9. 10.1002/art.24836 [DOI] [PubMed] [Google Scholar]
- 54.Wu JJ, Joshi AA, Reddy SP, et al. . Anti-inflammatory therapy with tumour necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis. J Eur Acad Dermatol Venereol 2018. doi: 10.1111/jdv.14951 [Epub ahead of print]. 10.1111/jdv.14951 [DOI] [PubMed] [Google Scholar]
- 55.Schiff MH, Kremer JM, Jahreis A, et al. . Integrated safety in tocilizumab clinical trials. Arthritis Res Ther 2011;13:R141 10.1186/ar3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monemi S, Berber E, Sarsour K, et al. . Incidence of gastrointestinal perforations in patients with rheumatoid arthritis treated with tocilizumab from clinical trial, postmarketing, and real-world data sources. Rheumatol Ther 2016;3:337–52. 10.1007/s40744-016-0037-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kay J, Fleischmann R, Keystone E, et al. . Golimumab 3-year safety update: an analysis of pooled data from the long-term extensions of randomised, double-blind, placebo-controlled trials conducted in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. Ann Rheum Dis 2015;74:538–46. 10.1136/annrheumdis-2013-204195 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2019-000942supp001.pdf (109.2KB, pdf)
rmdopen-2019-000942supp002.pdf (47.2KB, pdf)
rmdopen-2019-000942supp003.pdf (54.4KB, pdf)
rmdopen-2019-000942supp004.pdf (94.9KB, pdf)
rmdopen-2019-000942supp005.pdf (178.9KB, pdf)
rmdopen-2019-000942supp007.pdf (77.9KB, pdf)
rmdopen-2019-000942supp006.pdf (47.7KB, pdf)