Abstract
Background
Epidural use can prevent peri-operative neuro-endocrine stress responses, mitigate pain after surgery, and reduce opioid use, which all lead to immunosuppression.
Methods
Forty patients with gastric cancer were ultimately enrolled into the study. Patients who received general anaesthesia (GA group, n = 20) or a combination of general anaesthesia and peri-operative epidural use (EGA group, n = 20) were given intravenous analgesia or epidural analgesia, respectively. We collected visual analogue scale (VAS) scores, length of hospital stay, the time of the first passage of flatus and incidence of postoperative nausea and vomiting (PONV). We also collected data on the cluster of differentiation markers (CD)3+, CD4+, CD8+, CD4+/CD8+, interleukin (IL)-4, IL-6, and interferon (IFN)-γ the day before surgery as well as on postoperative days 1, 3, and 7.
Results
VAS scores and PONV in the GA group were higher than in the EGA group on postoperative day 3. CD3+, CD4+, and CD4+/CD8+ T cells declined on postoperative day 3 and nearly recovered to baseline seven days after surgery in both groups. CD3+ T cells decreased more in the GA group than in the EGA group. IL-4, IL-6, and IFN-γ increased on postoperative day 3 and nearly recovered to baseline seven days after surgery in both groups. IL-4 and IL-6 increased more in the GA group than in the EGA group. IFN-γ increased more in the EGA group than in the GA group.
Conclusions
A combination of general anaesthesia and peri-operative epidural use can relieve postoperative pain and PONV. A combination of general anaesthesia and peri-operative epidural use decreases immunosuppression in gastric cancer resection.
Trial registration
The study procedures were approved by the Ethics Committee of The Harbin Medical University Cancer Hospital. This study was registered prospectively at http://www.chictr.org.cn/index.aspx on October 10, 2017 (Registered ChiCTR-INR-17012939).
Keywords: Epidural anaesthesia, General anaesthesia, Patient-controlled analgesia, Immune function, Cluster of differentiation, Cytokines
Background
The stress response induced by surgery can activate the immune regulation mechanism during a systemic inflammatory reaction [1–4]. There are many important contributors to the tumor microenvironment, such as cytokines, chemokines, inflammatory mediators, which exists in many stages of progression to metastasis [5–7]. Some clinical factors, such as general anaesthetics, postoperative pain, and opioid analgesia, have been recognized as immunosuppressive and have influenced the development of tumours [8–14]. In some studies, epidural anaesthesia (EA) was associated with improved overall survival in patients with gastric cancer; in other studies, EA did not improve overall survival [15–17]. EA may reduce cytokines and neuro-endocrine stress immune stimulation, prevent nerve impulses, decrease excitability of the sympathetic adrenal medulla axis, reduce cortisol production, and improve the function of T lymphocytes [18, 19].
Among cytokines, interleukin (IL)-4, IL-6, and interferon (IFN)-γ play prominent roles in chronic inflammation, autoimmunity, infectious diseases and cancer, where they often act as diagnostic or prognostic indicators of disease activity and response to therapy [20, 21]. Therefore, we designed this study to explore the interaction between epidural use and the aforementioned cytokines in patients with gastric cancer.
Methods
Patient identification and exclusion
This study was a single-centre, randomized, observer-blinded study. The study procedures were approved by the Ethics Committee of The Harbin Medical University Cancer Hospital. This study was registered prospectively at http://www.chictr.org.cn/index.aspx on October 10, 2017 (Registered ChiCTR-INR-17012939). After the written consent was obtained from study participants, 50 American Society of Anesthesiologists (ASA) I-II patients aged 20–80 yr. who underwent radical resection of gastric cancer in our hospital were enrolled in this study between November 12, 2017, and December 15, 2017. Exclusion criteria were: (1) emergency operations, (2) laparoscopic procedures,(3) neoadjuvant treatment,(4) abnormally white blood cell count, (5) peri-operative transfusion, (6) severe heart, lung, liver, kidney, or endocrine diseases, After confirming understanding of the recruiter’s description of the trial, all the patients signed informed consent forms. Anaesthesia and analgesia protocols were standardized as follows. Patients were randomly assigned to the general anaesthesia (GA) group or epidural anaesthesia (EGA) group by computer-generated codes on the beginning of the study.
Sample size calculation and masking method
The clear primary outcome of this study was inflammatory response, which was evaluated via the concentration of IL-6. Previously published studies on IL-6 suggested that its standard deviation (SD) in vivo is in the order of 7.6 pg/ml. Twenty patients were calculated based on detecting a reduction of 6.8 pg/ml deviation on α-value 0.05 and a power of 0.8. To compensate for potential dropouts, we enrolled Twenty-five patients.
The patients were randomly divided into two groups by computer-generated codes on the time of the study onset. Physicians who completed postoperative assessment and biological detection were blinded to the group assignments throughout the study period until follow-up was completed for final analysis.
Anaesthesia technique and grouping method
On arrival to the operating room, patients were monitored via electrocardiogram and blood pressure as well as pulse oximetry. Patients randomly assigned to the GA group underwent induction of balanced GA with 0.05 mg/kg midazolam (Enhua Pharmaceutical Co., Jiangsu, China), 0.2 μg/kg sufentanil (Renfu Pharmaceutical Co., Beijing, China), 1–1.5 mg/kg propofol (AstraZeneca Pharmaceutical Co., Shanghai, China), and 0.15 mg/kg cisatracurium (Hengrui Medicine Co., Jiangsu, China). Anaesthesia was maintained with propofol when the bispectral index (BIS) was 40–60, and intraoperative analgesia consisted of remifentanil (Enhua Pharmaceutical Co., Jiangsu, China). Oesophageal temperature was monitored and maintained above 36 °C. Patient-controlled intravenous analgesia (PCIA) with sufentanil (0.5 μg/ml) was available for 72–120 h. The PCIA protocol of the sufentanil group (S group) consisted of 0.5 μg/ml sufentanil (total 300 ml) with a background infusion rate of 4 ml h− 1, a bolus dose of 3 ml, and a lockout time of 15 min. Patients in the EGA group were given T8–10 epidural anaesthesia before general anaesthesia. An infusion of 0.5% ropivacaine (AstraZeneca Pharmaceutical Co., Shanghai, China) was administered during surgery, and the loading dose (0.5% ropivacaine, 5-7 ml) depended on the height and weight of the patient. GA was induced with 0.05 mg/kg midazolam, 0.2 μg/kg sufentanil, 1–1.5 mg/kg propofol, and 0.15 mg/kg cisatracurium, and anaesthesia was maintained with propofol when the bispectral index (BIS) was 40–60. Patient-controlled epidural analgesia (PCEA) with a combination of 0.2% ropivacaine and 0.5 μg/ml sufentanil was available for 72–120 h. Acute rescue analgesic medications could be used when the VAS score was more than 4 and a bolus of 100 mg tramadol, administered via intravenous injection, was required more than 3 times.
Indicator and data
A physician who was blinded to the group assignment assessed postoperative pain intensity using the visual analogue scale (VAS) on postoperative days 1, 2, and 3. The demographic data, cancer stage, degree of differentiation, duration of the operation, length of hospital stay, time of the first passage of flatus and incidence of postoperative nausea and vomiting (PONV) were recorded. A physician who was blinded to the group assignment collected data on cluster of differentiation markers (CD)3+, CD4+, CD8+, and CD4+/CD8+, Interleukin (IL)-4, IL-6, and Interferon (IFN)-γ through peripheral blood (10 ml) on the day before surgery, day (d) 0, and on postoperative days 1, 3, and 7.CD3+, CD4+, CD8+ were measured by flow cytometric analysis. The plasma level of IL-4, IL-6, and Interferon IFN-γ were measured by enzyme-linked immunosorbent assay (ELISA). All samples were measured using three independent experiments.
Statistical approach
Statistical analysis was performed using SPSS version 22.0 for Windows (IBM Corp., USA). Normally distributed data were expressed as the means ± SDs. Categorical variables were described using frequencies and were analysed using the χ2 test. Fisher’s exact test was used for small sample sizes (expected frequencies < 5). We checked for normality of the data with the Shapiro Wilk test and used a one-way ANOVA between the two groups. The results with P < 0.05 were considered statistically significant.
Results
Patient characteristics
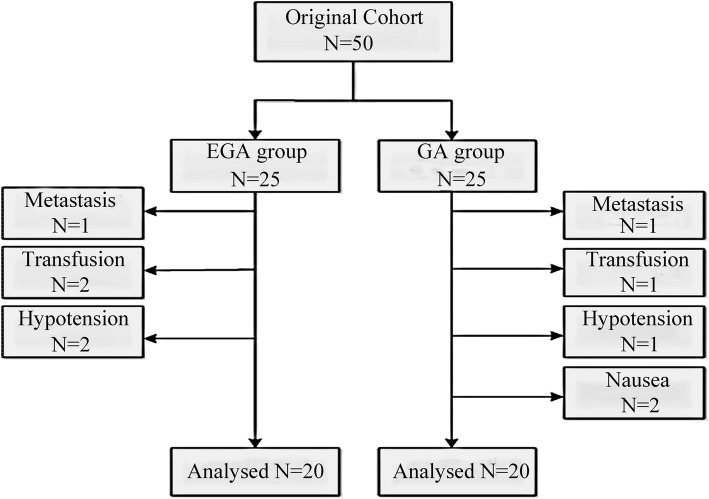
Between November 12, 2017, and December 15, 2017, fifty patients were screened for this study, and forty patients were ultimately included in this study. Five patients with metastases or peri-operative transfusions were excluded. Five patients had their analgesic regimen modified because of hypotension and severe nausea (Fig. 1). The two groups were similar with respect to age, height, weight, gender, ASA grade, cancer stage and degree of differentiation, duration of surgery, surgical procedure and surgical manner between the two groups (Table 1).
Fig. 1.
Patient identification and exclusion
Table 1.
Baseline and surgical characteristics of general anesthesia group and epidural combined general anesthesia group
| Characteristics | GA group (n = 20) | EGA group (n = 20) | P value |
|---|---|---|---|
| Age (year) | 59.3 ± 6.8 | 59.7 ± 8.5 | 0.870 |
| Height (cm) | 164.0 ± 6.1 | 164.5 ± 8.7 | 0.832 |
| Weight (kg) | 66.5 ± 8.4 | 64.3 ± 7.7 | 0.394 |
| Gender (male) | 11 (55%) | 10 (50%) | 0.752 |
| ASA grade | |||
| I | 2 (10%) | 2 (10%) | 1.000 |
| II | 16 (80%) | 17 (85%) | |
| III | 2 (10%) | 1 (5%) | |
| Cancer stage | |||
| I | 3 (15%) | 4 (20%) | 0.925 |
| II | 2 (10%) | 2 (10%) | |
| III | 12 (60%) | 10 (50%) | |
| Cancer stageIV | 3 (15%) | 4 (20%) | |
| Degree of differentiation | |||
| 1 | 7 (35%) | 5 (25%) | 0.915 |
| 2 | 10 (50%) | 11 (55%) | |
| 3 | 2 (10%) | 2 (10%) | |
| 4 | 1 (5%) | 2 (10%) | |
| Duration of surgery (h) | 2.25 (2.00,3.75) | 2.25 (2.00,3.50) | 0.577 |
| Surgical procedure | |||
| open | 20 (100%) | 20 (100%) | 1.000 |
| minimal invasive | 0 (0%) | 0 (0%) | |
| Surgical manner | |||
| total | 20 (100%) | 20 (100%) | 1.000 |
| partial | 0 (0%) | 0 (0%) | |
GA = general anesthesia group
EGA = epidural anesthesia combined with general anesthesia group
ASA = American Society of Anesthesiologists
Cancer stages: I grade-T1, N0, M0/T2, N0, M0/T1, N1, M0; II grade-T3, N0, M0/T4a, N1, M0/T3, N1, M0/T2, N2, M0/T1, N3, M0; III grade-T2, N3, M0/T3, N2, M0/T3, N3, M0/T4a, N2, M0/T4a, N3, M0/ any T4b, any N, M0; IV grade, any T, any N, M1
Degrees of differentiation: Degree1, poorly differentiated; Degree2, moderately differentiated; Degree3, well differentiated; Degree 4, other/unknown differentiated
Association between epidural use and postoperative variables
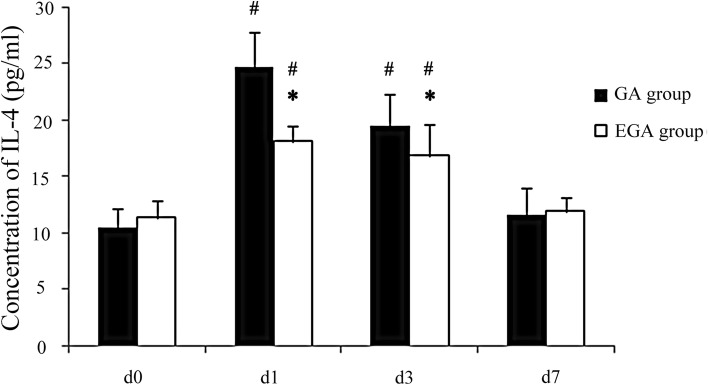
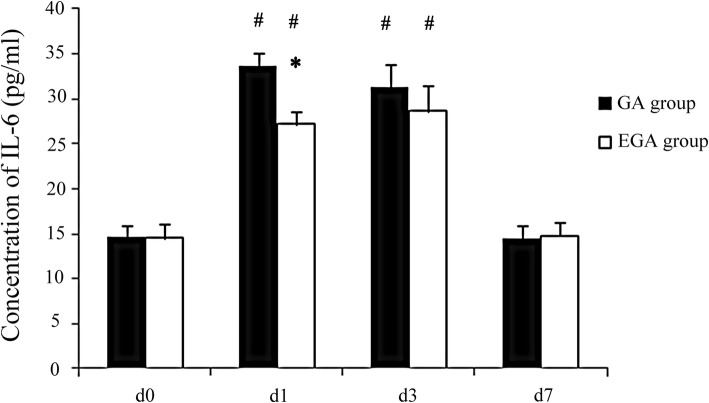
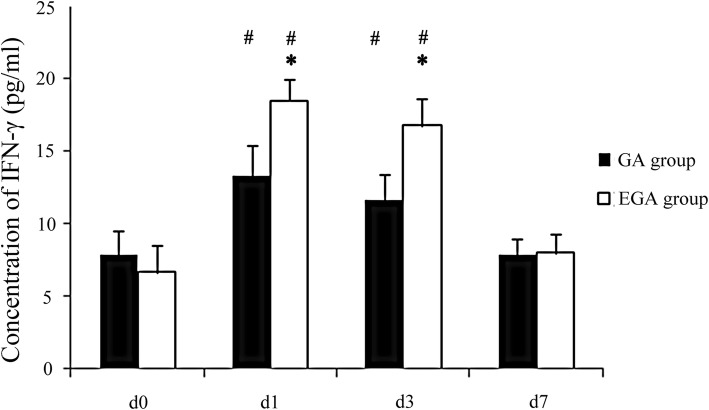
The VAS scores in the GA group were higher than in the EGA group on postoperative day 3, and the incidence of PONV in the EGA group was lower (P < 0.05, Table 2). There were no differences in days of analgesia, the time of the first passage of flatus, and length of hospital stay. CD3+, CD4+, and CD4+/CD8+ T cells declined on postoperative day 3 (P < 0.05) and nearly recovered to baseline seven days after surgery in both groups. CD3+ T cells decreased more in the GA group than in the EGA group (P < 0.05, Table 3). IL-4, IL-6, and IFN-γ increased on postoperative day 3 (P < 0.05; Figs. 2, 3, and 4) and nearly recovered to baseline seven days after surgery in both groups. IL-4 and IL-6 increased more in the GA group than in the EGA group (P < 0.05, Figs. 2 and 3). IFN-γ increased more in the EGA group than in the GA group (P < 0.05, Fig. 4).
Table 2.
The VAS scores of patients in the postoperative days 1, 2, 3 between general anesthesia group and epidural combined general anesthesia group
| Characteristics | GA group (n = 20) | EGA group (n = 20) | P value |
|---|---|---|---|
| VAS scores | |||
| POD1 | 3 (2,5) | 2 (1,4) | 0.004* |
| POD2 | 2 (1,4) | 1 (0,3) | 0.003* |
| POD3 | 1 (0,3) | 0 (0,2) | 0.003* |
| Days of analgesia | 3 (3,5) | 3 (3,5) | 0.527 |
| Days of flatus time | 3 (3,4) | 3 (2,4) | 0.764 |
| Nausea and vomiting (%) | 8(40%) | 1 (5%) | 0.020* |
| Length of stay | 11 (9,12) | 11 (9,12) | 0.795 |
VAS=Visual analogue scale (VAS) scores
POD = Postoperative day
*: Compare between two groups, P < 0.05
Table 3.
Comparison of T lymphocyte subsets of patients in general anesthesia group and epidural combined general anesthesia group
| Characteristics | Group | d0 | d1 | d3 | d7 |
|---|---|---|---|---|---|
| CD3+ (%) | GA | 54.3 ± 3.5 | 42.5 ± 2.3# | 44.6 ± 3.2# | 51.8 ± 6.7 |
| EGA | 55.6 ± 4.1 | 46.1 ± 2.9#* | 48.1 ± 2.2#* | 51.2 ± 6.9 | |
| CD4+ (%) | GA | 32.4 ± 4.3 | 21.5 ± 3.4# | 23.2 ± 1.6# | 31.7 ± 2.8 |
| EGA | 34.5 ± 3.5 | 23.4 ± 3.5# | 24.5 ± 2.1# | 33.0 ± 2.0 | |
| CD8+ (%) | GA | 21.1 ± 1.8 | 20.7 ± 1.8 | 21.0 ± 1.9 | 20.9 ± 1.3 |
| EGA | 21.5 ± 1.2 | 21.2 ± 1.1 | 22.9 ± 2.0 | 21.3 ± 1.5 | |
| CD4+/CD8+ | GA | 1.5 ± 0.5 | 1.0 ± 0.2# | 1.0 ± 0.1# | 1.5 ± 0.2 |
| EGA | 1.6 ± 0.5 | 1.2 ± 0.2# | 1.2 ± 0.3# | 1.5 ± 0.1 |
CD = Clusters of Differentiation
#: Compare to d0 P < 0.05, *:Compare between two groups, P < 0.05
Fig. 2.
Comparison of IL-4 between the general anaesthesia group and epidural combined with general anaesthesia group on day (d) 0, the day before surgery, and on postoperative days 1, 3, and 7
Fig. 3.
Comparison of IL-6 between the general anaesthesia group and epidural combined with general anaesthesia group on day (d) 0, the day before surgery, and on postoperative days 1, 3, and 7
Fig. 4.
Comparison of IFN-γ between the general anaesthesia group and epidural combined with general anaesthesia group on day (d) 0, the day before surgery, and postoperative days 1, 3, and 7
Discussion
In our study, we found a beneficial effect of epidural use. The VAS scores in the GA group were higher than those in the EGA group on postoperative day three. The incidence of PONV was higher in the GA group. It has been proven that epidural anaesthesia can provide better analgesia after gastric cancer surgery, which was consistent with our study [22]. Epidural administration of anaesthesia and analgesia is considered a technique with risk of complications, such as neuraxial haematoma, hypotension, pruritus; the subjective experience of anaesthetists often leads to the failure of epidural anaesthesia and analgesia [23, 24]. In our study, there were two patients in the EGA group and one patient in the GA group who could not use EA because of hypotension.
Many studies have reported that opioids impair the peri-operative immune system and increase vascular permeability, and the use of epidural anaesthesia may prevent these peri-operative immunosuppressive changes during major surgery because it can decrease neuro-endocrine stress responses [18, 25–28]. Immune surveillance is the primary indicator for stopping the metastasis of tumours, and immunosuppression may destroy the defensive barrier [29]. Clinical events that may lead to immunosuppressive changes facilitated by surgery include injury, pain, and use of anaesthetic medications [30–32]. CD3+ T cells, CD4+ T cells, and CD8+ T cells are the main cells which take part in antitumour immunity [33]. The ratio of CD4+/CD8+ cells decreases as the serum cortisol levels increase [34, 35]. In our study, CD3+, CD4+, and CD4+/CD8+ T cells were inhibited and had a negative trend. All patients’ CD3+ and CD4+ T cells decreased to different degrees on the first postoperative day, but in the GA group, patients’ CD3+ T cells significantly decreased and recovered to preoperative levels until the seventh postoperative day. The change in CD8+ T cells was not statistically significant. These results are consistent with some prior studies [36, 37],they described a lymphocyte depressing factor that was present in the serum of patients in the GA group but was absent in the serum of patients in the EGA group.
Many cytokines can modulate the immune system. Pro-inflammatory cytokines may favour tumour progression. Activated biological cascades lead to immunosuppression, which affects the immune response and decreases IL-4 and IFN-γ [38, 39]. As a pro-inflammatory factor, IL-4 stimulates the proliferation of B cells and participates in the differentiation of Th2 cells. The combination of the cytokine IL-6 with prostaglandin 2 can reduce the production of the immune factor IL-2 by Th1 cells and affect the activation of NK cells [40]. IL-6 has a major influence on the proliferation, survival and metastatic properties of cancerous cells, and studies using preclinical mouse models indicate that treatments targeting IL-6 or its receptor display therapeutic efficacy as anticancer agents [20].In our study, the pro-inflammatory cytokines IL-4 and IL-6 increased more in the GA group than in the EGA group, which may indicate that immune function was less suppressed in the EGA group. IFN-γ can kill tumour cells directly and transform Th0 cells into Th1 cells, which play a large role in the activation of NK cells and T cells. The level of IFN-γ expression embodies the memory ability of this antitumour cytokine [41, 42]. In our study, IFN-γ increased more in the EGA group than in the GA group, which may indicate that immune function was less suppressed in the EGA group. Therefore, in our study, EA was able to relieve postoperative pain and PONV, was able to decrease immunosuppression, and may have reduced inflammatory-associated metastasis in gastric cancer.
There were a few limitations in our study. First, we only measured some T cell subsets and some important immune factors. The immune system is a complex system. There are many immune cells, such as NK cells, and other immune factors, such as IL-2 and IL-12, which may be investigated in the future because these antitumourigenic cytokines were increased by EA. Second, these patients’ long-term outcomes, such as long-term survival, recurrence rates and metastasis rates, could not be obtained at present. We will evaluate these results in 3 to 5 years.
Conclusions
A combination of general anaesthesia and peri-operative epidural use can relieve postoperative pain and PONV. A combination of general anaesthesia and peri-operative epidural use decreases immunosuppression in gastric cancer resection.
Acknowledgements
Not applicable.
Abbreviations
- (NK) cell
Natural killer cell
- ASA
American Society of Anesthesiologists
- BIS
Bispectral index
- EGA
Epidural general anaesthesia
- GA
General anaesthesia
- IFN
Interferon
- IL
Interleukin
- PCEA
Patient-controlled epidural analgesia
- PCIA
Patient-controlled intravenous analgesia
- PONV
Postoperative nausea and vomiting
- VAS
Visual analogue scale
Authors’ contributions
Y.W. and L.W. contributed to the study conception and the designing and drafting of the manuscript. L.W., S.L., C.H. and Y.X. contributed to acquisition and interpretation of the data. H.C. and L.W. were responsible for the revision of important intellectual content and final approval of the version to be published. All authors read and approved the final manuscript.
Funding
This study was supported by the Education Office of Hei Longjiang (the Fundamental Research Funds for the Provincial Universities, Grant#2017LCZX90) and the Postdoctoral Office of Heilongjiang Province (Hei Long Jiang Postdoctoral Foundation Grant#LBH-Z17181).
Availability of data and materialS
All data generated or analysed during this study are included in this published article, and can be freely available to any scientists wishing to use. The data supporting our findings can be found in http://www.chictr.org.cn/index.aspx (Registered ChiCTR-INR-17012939), but identifying patient data would not be shared. All the authors agreed to share their data.
Ethics approval and consent to participate
The study procedures were approved by the Ethics Committee of The Harbin Medical University Cancer Hospital. This study was registered prospectively at http://www.chictr.org.cn/index.aspx on October 10, 2017 (Registered ChiCTR-INR-17012939). The purpose of the study was explained to the family of patients under the study. The written consent was obtained from study participants. The patients were informed that the care to be given was not be compromised in any way and confidentiality was assured. Name and other identifying information were not used in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liping Wang and Si Liang contributed equally to this work.
Contributor Information
Liping Wang, Email: applew708@126.com.
Si Liang, Email: 154938096@qq.com.
Hong Chen, Email: 517891483@qq.com.
Yang Xu, Email: 5106829@qq.com.
Yu Wang, Phone: 86-0451-86298029, Email: littlepigdiudiu@163.com.
References
- 1.Cassinello F, Prieto I, del OM, Rivas S, Strichartz GR. Cancer surgery: how may anesthesia influence outcome. J Clin Anesth. 2015;27(3):262–272. doi: 10.1016/j.jclinane.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Shakhar G, Ben-Eliyahu S. Potential prophylactic measures against postoperative immunosuppression: could they reduce recurrence rates in oncological patients. Ann Surg Oncol. 2003;10(8):972–992. doi: 10.1245/ASO.2003.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Xu YJ, Li SY, Cheng Q, et al. Effects of anaesthesia on proliferation, invasion and apoptosis of LoVo colon cancer cells in vitro. Anaesthesia. 2016;71(2):147–154. doi: 10.1111/anae.13331. [DOI] [PubMed] [Google Scholar]
- 4.O’Dwyer MJ, Owen HC, Torrance HD. The perioperative immune response. Curr Opin Crit Care. 2015;21(4):336–342. doi: 10.1097/MCC.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 5.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6(3):297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Tan W, Zhang W, Strasner A, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470(7335):548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou M, Zhou NB, Li H, et al. Morphine and ketamine inhibit immune function of gastric cancer patients by increasing percentage of CD4(+)CD25(+)Foxp3(+) regulatory T cells in vitro. J Surg Res. 2016;203(2):306–312. doi: 10.1016/j.jss.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Oka M, Hazama S, Suzuki M, et al. Depression of cytotoxicity of nonparenchymal cells in the liver after surgery. Surgery. 1994;116(5):877–882. [PubMed] [Google Scholar]
- 9.Müller-Edenborn B, Frick R, Piegeler T, et al. Volatile anaesthetics reduce neutrophil inflammatory response by interfering with CXC receptor-2 signalling. Br J Anaesth. 2015;114(1):143–149. doi: 10.1093/bja/aeu189. [DOI] [PubMed] [Google Scholar]
- 10.Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for Cancer surgery: a retrospective analysis. Anesthesiology. 2016;124(1):69–79. doi: 10.1097/ALN.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 11.Cata JP, Chavez-MacGregor M, Valero V, et al. The impact of paravertebral block analgesia on breast Cancer survival after surgery. Reg Anesth Pain Med. 2016;41(6):696–703. doi: 10.1097/AAP.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 12.Yi W, Li D, Guo Y, Zhang Y, Huang B, Li X. Sevoflurane inhibits the migration and invasion of glioma cells by upregulating microRNA-637. Int J Mol Med. 2016;38(6):1857–1863. doi: 10.3892/ijmm.2016.2797. [DOI] [PubMed] [Google Scholar]
- 13.Connolly C, Buggy DJ. Opioids and tumour metastasis: does the choice of the anesthetic-analgesic technique influence outcome after cancer surgery. Curr Opin Anaesthesiol. 2016;29(4):468–474. doi: 10.1097/ACO.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 14.Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev. 2016. [DOI] [PubMed]
- 15.Hiller JG, Hacking MB, Link EK, Wessels KL, Riedel BJ. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta Anaesthesiol Scand. 2014;58(3):281–290. doi: 10.1111/aas.12255. [DOI] [PubMed] [Google Scholar]
- 16.Cummings KC, Patel M, Htoo PT, Bakaki PM, Cummings LC, Koroukian S. A comparison of the effects of epidural analgesia versus traditional pain management on outcomes after gastric cancer resection: a population-based study. Reg Anesth Pain Med. 2014;39(3):200–207. doi: 10.1097/AAP.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-González O, Cuéllar-Guzmán LF, Navarrete-Pacheco M, Ortiz-Martínez JJ, Williams WH, Cata JP. Impact of regional anesthesia on gastroesophageal Cancer surgery outcomes: a systematic review of the literature. Anesth Analg. 2018;127(3):753–758. doi: 10.1213/ANE.0000000000003602. [DOI] [PubMed] [Google Scholar]
- 18.Ben-David B. Anaesthesia in Cancer surgery: can it affect Cancer survival. Curr Clin Pharmacol. 2016;11(1):4–20. doi: 10.2174/1574884711666160122093154. [DOI] [PubMed] [Google Scholar]
- 19.Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis. Can J Anaesth. 2016;63(2):184–192. doi: 10.1007/s12630-015-0523-8. [DOI] [PubMed] [Google Scholar]
- 20.Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18(12):773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 21.Cui M, Gong C, Jiang B, et al. Evaluation of immune responses of gastric cancer patients treated by laparoscopic and open gastrectomy. Med Oncol. 2015, 32(11):253. [DOI] [PubMed]
- 22.Yanagimoto Y, Takiguchi S, Miyazaki Y, et al. Comparison of pain management after laparoscopic distal gastrectomy with and without epidural analgesia. Surg Today. 2016;46(2):229–234. doi: 10.1007/s00595-015-1162-y. [DOI] [PubMed] [Google Scholar]
- 23.Salicath JH, Yeoh EC, Bennett MH. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev. 2018;(8):CD010434. [DOI] [PMC free article] [PubMed]
- 24.Wang L, Li X, Chen H, Liang J, Wang Y. Effect of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia on postoperative pain management and short-term outcomes after gastric cancer resection: a retrospective analysis of 3,042 consecutive patients between 2010 and 2015. J Pain Res. 2018;11:1743–1749. doi: 10.2147/JPR.S168892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hole A, Terjesen T, Breivik H. Epidural versus general anaesthesia for total hip arthroplasty in elderly patients. Acta Anaesthesiol Scand. 1980;24(4):279–287. doi: 10.1111/j.1399-6576.1980.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 26.Rem J, Brandt MR, Kehlet H. Prevention of postoperative lymphopenia and granulocytosis by epidural analgesia. Lancet. 1980;1(8163):283–284. doi: 10.1016/S0140-6736(80)90780-1. [DOI] [PubMed] [Google Scholar]
- 27.Neeman Elad, Ben-Eliyahu Shamgar. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain, Behavior, and Immunity. 2013;30:S32–S40. doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahlers O, Nachtigall I, Lenze J, et al. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth. 2008;101(6):781–787. doi: 10.1093/bja/aen287. [DOI] [PubMed] [Google Scholar]
- 29.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Sekandarzad MW, van Zundert AA, Lirk PB, Doornebal CW, Hollmann MW. Perioperative anesthesia care and tumor progression. Anesth Analg. 2016. [DOI] [PubMed]
- 31.Buckley A., McQuaid S., Johnson P., Buggy D.J. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. British Journal of Anaesthesia. 2014;113:i56–i62. doi: 10.1093/bja/aeu200. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y.J., Chen W.K., Zhu Y., Wang S.L., Miao C.H. Effect of thoracic epidural anaesthesia on serum vascular endothelial growth factor C and cytokines in patients undergoing anaesthesia and surgery for colon cancer. British Journal of Anaesthesia. 2014;113:i49–i55. doi: 10.1093/bja/aeu148. [DOI] [PubMed] [Google Scholar]
- 33.Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents. Lancet Oncol. 2003;4(10):605–615. doi: 10.1016/S1470-2045(03)01220-8. [DOI] [PubMed] [Google Scholar]
- 34.Hosokawa T. Effect of anesthesia and surgery on cell-mediated immunity--the application of two color analysis to classification of functional lymphocyte subpopulation. Masui. 1987;36(6):956–961. [PubMed] [Google Scholar]
- 35.Hosokawa T, Hori Y, Ohtsuka T, Nakagawa H, Miyazaki M. Effect of anesthesia and surgery on immunity in patients with benign and malignant diseases. Masui. 1989;38(3):343–349. [PubMed] [Google Scholar]
- 36.Hashimoto T, Hashimoto S, Hori Y, Nakagawa H, Hosokawa T. Epidural anaesthesia blocks changes in peripheral lymphocytes subpopulation during gastrectomy for stomach cancer. Acta Anaesthesiol Scand. 1995;39(3):294–298. doi: 10.1111/j.1399-6576.1995.tb04064.x. [DOI] [PubMed] [Google Scholar]
- 37.Hole A, Unsgaard G. The effect of epidural and general anaesthesia on lymphocyte functions during and after major orthopaedic surgery. Acta Anaesthesiol Scand. 1983;27(2):135–141. doi: 10.1111/j.1399-6576.1983.tb01923.x. [DOI] [PubMed] [Google Scholar]
- 38.Page GG, Blakely WP, Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90(1–2):191–199. doi: 10.1016/S0304-3959(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 39.Greenfeld K, Avraham R, Benish M, et al. Immune suppression while awaiting surgery and following it: dissociations between plasma cytokine levels, their induced production, and NK cell cytotoxicity. Brain Behav Immun. 2007;21(4):503–513. doi: 10.1016/j.bbi.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Goldfarb Y, Ben-Eliyahu S. Surgery as a risk factor for breast cancer recurrence and metastasis: mediating mechanisms and clinical prophylactic approaches. Breast Dis. 2006;26:99–114. doi: 10.3233/BD-2007-26109. [DOI] [PubMed] [Google Scholar]
- 41.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98(5):1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Carpenter RL, Neal JM. Epidural anesthesia and analgesia. Their role in postoperative outcome. Anesthesiology. 1995;82(6):1474–1506. doi: 10.1097/00000542-199506000-00019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article, and can be freely available to any scientists wishing to use. The data supporting our findings can be found in http://www.chictr.org.cn/index.aspx (Registered ChiCTR-INR-17012939), but identifying patient data would not be shared. All the authors agreed to share their data.