Abstract
Objective
Parasitic infections are the commonest infections affecting 3.5 billion people leading 450 million illnesses. Parasites are major public health problems in developing countries. This study was aimed to assess the prevalence and associated factors of parasitic infections among patients. A cross sectional study was conducted on 364 patients, attending Shahura Health Center, Northwest Ethiopia. Stool specimens were collected and examined using formol-ether concentration technique. Socio-demographic data collected using questionnaire. Binary and multivariable logistic regression analyses were conducted to calculate the strength of association between variables.
Result
The overall prevalence of intestinal parasitosis was 56.9%. The most prevalent parasite was Entamoeba histolytica/dispar 32.4% followed by Hookworm species 11.8% and Giardia lamblia 7.4% singly or mixed with other parasites. Furthermore, double and triple parasitic infections were observed in 3% and 1.4% patients respectively. Being male in gender (P = 0.049), age group interval between 1 and 20 years of old (P = 0.012), having stomach pain (P = 0.032) and having diarrhea (P = 0.007) were found to be significantly associated with parasitic infection. In conclusion, prevalence of parasitic infection in the area is high. Therefore, ensuring provision of clean potable water and minimizing the contamination of vegetables are recommended.
Electronic supplementary material
The online version of this article (10.1186/s13104-019-4377-y) contains supplementary material, which is available to authorized users.
Keywords: Intestinal parasitic infections, Shahura Health Center, Intestinal helminths
Introduction
Intestinal parasitic infections are amongst the most common infections affecting approximately 3.5 billion people causing over 450 million ill health problems annually [1]. The ten global parasitic diseases are; Amoebiasis, Ascariasis, Hookworm infection and Trichuriasis. The three major soil-transmitted helminths of global health concern are; Ascaris lumbricoides, Trichuris trichiura and Hookworm. They cause over one billion infections and two billions are at risk of infection [2]. Furthermore, protozoan infections are another serious public health concerns and responsible for iron deficiency anemia, growth retardation, physical and mental health problems among children [3]. Infections with these parasites usually lead nutritional depletion, poor immunity in infants, mucosal loss and lymphatic leakage and local hemorrhage [4].
Parasitic infections affect the poorest and deprived communities of low and middle-income countries of the tropical and subtropical regions [5]. The main reasons for the high prevalence of parasite infections in tropical and subtropical countries were increasing population density, poor sanitation conditions, poor public health practices, inadequate toilet facilities, contaminated food and water, malnutrition, low host resistance and environmental changes [6].
Lack of safe water supplies and poor environmental sanitation situations are the main reasons for approximately 800 million expected cases of diarrheal incidences and the occurrence of 4.5 million mortalities in developing countries [7]. Behavioral, biological, environmental, socio-economic, health systems factors and local conditions such as quality of domestic and village infrastructure; household income, employment, occupational and social factors influence the risk of parasitic infections, disease transmission and associated morbidity and mortality [8]. Infection with pathogenic intestinal protozoa and helminths result in considerable morbidity, malnutrition and mortality worldwide, particularly among young children in developing countries and immune-compromised individuals [9–11].
In Ethiopia, the major health problems of Ethiopia are mainly preventable communicable parasitic diseases [12]. There are several reports which indicate the burden of parasitic diseases in Ethiopia. However, there are several localities including the current study area in which prevalence yet not determined. Therefore, this study was aimed to determine the prevalence of intestinal parasitic infections and associated factors among patients visiting Shahura Health Center, Northwest, Ethiopia.
Main text
Materials and methods
Study area
The study was conducted at Shahura town Health Center in Alefa Woreda, North Gondar Zone, Northwest, Ethiopia. The town is located 150 km Southwest of Gondar town at latitude of 12°361N and longitude of 37°281E with an elevation of 2133 m above sea level. In Shahura town (Alefa Woreda), the climate is warm and temperate. Alefa Woreda has 48 rural Kebeles with an estimated population of 240,000. In this district, majority of the fertile land is covered by cereals like teff, sorghum and maize. Permanent crops like coffee, hops and fruit trees are also grown. Most farmers both raise crops and livestock, while some only grow crops and very few only raise livestock. In addition there are also rivers crossing Shahura town and Alefa Woreda. Shahura is a small town with 58,441 dwellers. The town has one Health Center which provides different health services to the dwellers of Shahura town and surrounding rural Kebele populations.
Study design, population and sampling technique
A cross-sectional study design was used to assess the prevalence of intestinal parasitic infections and associated factors among patients visiting Shahura Health Center from June 2018 to September 2018. The study included patients who visited Shahura Health Center complaining gastrointestinal problems. Single population proportion formula was used to calculate the sample size. Study participants were consecutively enrolled until the targeted sample size three hundred sixty-four was achieved.
The aims of the study and benefits of participation were clearly explained to the participants prior to data collection. Participation was on voluntarily basis and they have told them it is their right to withdraw from the study any time during the course of data collection. A questionnaire based on known and possible factors was developed to explore the objectives of the study and pre-tested. Data’s on socio-demographic and associated factors were collected according to local culture and norm.
Stool specimen collection and examination
Fresh stool specimens were collected by experienced laboratory technologists who were selected and trained for the purpose of this study. Patients were instructed to collect the stool specimens into a leak-proof clean stool cup. All specimens were subjected to direct wet mount and formol-ether concentration technique [13]. A drop of normal saline or Lugol’s iodine and about 2 mg of stool were mixed on a microscopic glass slide, covered with cover slip and examined using microscope. After completion of direct stool examination, samples were emulsified in a 10% formalin solution and transported to University of Gondar, School of Biomedical and Laboratory Sciences Parasitology Laboratory to perform formol-ether concentration technique [13]. All standard procedures were strictly followed during stool sample examination to ensure the quality and sensitivity of the test result.
Data analysis
Data were checked for completeness and entered to EPI-Info version-7 and exported to SPSS version-20. The baseline characteristics of the study population were summarized using frequencies, mean and standard deviation. An internal comparison was made using binary logistic regression to determine the independent effect of the variables by calculating the strength of the association between IPIs and associated factors using odds ratio (OR) and 95% confidence interval (CI). Adjusted OR was computed using multivariable logistic regression to control the confounding variables. A P value less than 0.05 were considered statistically significant.
Result
Socio-demographic characteristics
A total of 364 study participants were included in the present study. Of the total participants, 51.4% (187/364) were males while the remaining 48.6% (177/364) were females. Age of the study participants were ranged from 1 to 82 years with a mean age of 27.08 (SD + 12.851) years. Most of the study participants, 55.8% (203/364) were found in the age group of 18–30 years of old and nearly 60% (218/364) of the participants were rural residents. Among the study participants, 57.7% (210/364) had a family size fewer than five members and 36% (131/364) of the study participants were unable to read and write. Furthermore, majority of the study participants, 95.1% (346/364) were followers of Orthodox Christian religion and 79.1% (288/364) of the participants live with their family. Finally, 50.5% (184/364) of the participants were farmers in their profession (Additional file 1: Table S1).
Prevalence of intestinal parasitosis
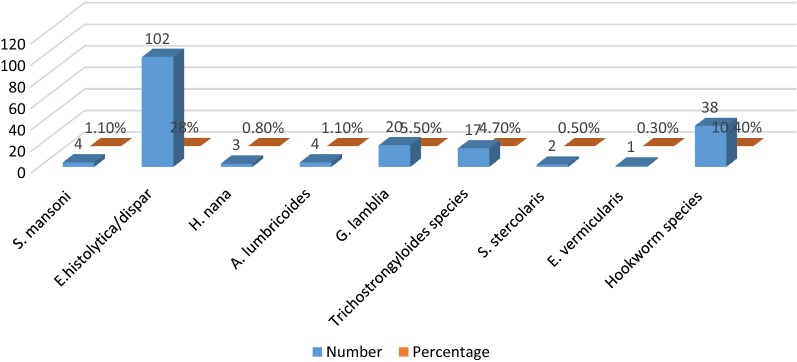
Based on the microscopic examination of stool specimens, nine species of parasites were identified. Hence, the overall prevalence for at least one parasite is 56.9% (207/364). Of these detected parasites, Entamoeba histolytica/dispar, 32.4% (118/364) was the most common parasite followed by Hookworm species 11.8% (43/364), G. lamblia 7.4% (27/364), Trichostrongyloides species 4.7% (17/364), A. lumbricoides 2.2% (8/364) and S. mansoni 1.4% (5/364) singly or mixed with other parasites. Furthermore, double and triple parasitic infections were observed in 3% (11/364) and 1.4% (5/364) patients respectively. Additionally, one quadruple infection was detected (Table 1 and Fig. 1).
Table 1.
Distribution of intestinal parasites species among patients (n = 364) at Shahura Health Center, Northwest Ethiopia, 2018
| Type of intestinal parasite | Number of male +ve for IP (%) | Number of female +ve for IP (%) | Total number of +ve (%) |
|---|---|---|---|
| Single infection (n = 190) | |||
| E. histolytica/dispar | 61 (16.8) | 41 (11.3) | 102 (28) |
| Hookworm species | 18 (4.9) | 20 (5.5) | 38 (10.4) |
| G. lamblia | 9 (2.3) | 11 (3) | 20 (5.5) |
| Trichostrongyloides species | 8 (2.2) | 9 (2.3) | 17 (4.7) |
| A. lumbricoides | 3 (0.8) | 1 (0.8) | 4 (1.1) |
| S. mansoni | 2 (0.5) | 2 (0.5) | 4 (1.1) |
| H. nana | 3 (0.8) | 0 (0.0) | 3 (0.8) |
| S. stercolaris | 1 (0.3) | 1 (0.3) | 2 (0.5) |
| Double infection (n = 11) | |||
| E. histolytica/dispar and G. lamblia | 3 (0.8) | 2 (0.5) | 5 (1.4) |
| E. histolytica/dispar and A. lumbricoides | 1 (0.3) | 2 (0.5) | 3 (0.8) |
| E. histolytica/dispar and H. nana | 1 (0.3) | 1 (0.3) | 2 (0.5) |
| A. lumbricoides and Hookworm species | 1 (0.3) | 0 (0.0) | 1 (0.3) |
| Triple infection (n = 5) | |||
| E. histolytica/dispar, S. stercolaris and Hookworm species | 1 (0.3) | 1 (0.3) | 2 (0.5) |
| E. histolytica/dispar, S. mansoni and Hookworm species | 2 (0.5) | 0 (0.0) | 2 (0.5) |
| E. histolytica/dispar, G. lamblia and E. vermicularis | 1 (0.3) | 0 (0.0) | 1 (0.3) |
| Quadruple infection (n = 1) | |||
| E. histolytica/dispar, G. lamblia, S. mansoni and H. nana | 0 (0.0) | 1 (0.3) | 1 (0.3) |
| Total | 115 (31.6) | 92 (25.3) | 207 (56.9) |
Fig. 1.
Prevalence of intestinal parasites by species among patients at Shahura Health Center, Northwest Ethiopia, 2018
The most prevalent intestinal parasites in double infection was E. histolytica/dispar and G. lamblia (5 patients; 1.4%), followed by E. histolytica/dispar and A. lumbricoides (3 patients; 0.8%); E. histolytica/dispar and H. nana (2 patients; 0.55%); and A. lumbricoides and Hookworm species (1 patients; 0.3%). Furthermore, 5 patients (1.4%) were infected by three species of parasites and one patient (0.3%) was parasitized by four species of parasites (E. histolytica/dispar, G. lamblia, S. mansoni and H. nana) (Table 1 and Additional file 2: Figure S1).
As it is depicted in Tables 1 and 2, 31.6% (115/364) of intestinal parasite infected participants were males. Highest intestinal parasitosis, 31.3% (114/364) was found among in the age groups 21–40 years of old followed by 1–20 years of old, 15.9% (58/364). The prevalence is higher in rural (131/364; 36%) than urban dwellers (76/364; 20.9%). Among the 207 infected patients, highest burden (112/364; 30.8%) of infection was found in patients who are illiterate and those who can read and write. Furthermore, intestinal parasitosis is high in patients those who have a family size between 1 and 5 (159/364; 43.7%).
Table 2.
Factors associated with intestinal parasitosis among patients at Shahura Health Center, Northwest Ethiopia, 2018
| Factors associated with intestinal parasitosis | Number (%) | Number of +ve for IP (%) | Number of −ve for IP (%) | Crude OR (95% CI) | P value | Adjusted OR (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| Hand wash reason before meal | |||||||
| Yes | 313 (86) | 181 (57.8) | 132 (42.2) | 0.758 [0.419–1.372] | 0.361 | – | – |
| No | 51 (14) | 26 (51) | 15 (49) | 1 | – | – | |
| Hand washing habit before meal | |||||||
| Always | 202 (55.5) | 110 (54.5) | 92 (45.5) | 1.045 [0.273–4.007] | 0.948 | – | – |
| Mostly | 23 (6.3) | 12 (52.2) | 11 (47.8) | 1.146 [0.244–5.391] | – | – | |
| Often | 6 (1.6) | 1 (16.7) | 5 (83.3) | 6.250 [0.504–77.494] | – | – | |
| Sometimes | 124 (34.1) | 79 (63.7) | 45 (36.3) | 0.712 [0.182–2.788] | – | – | |
| Rarely | 9 (2.5) | 5 (55.6) | 4 (44.4) | 1 | – | – | |
| Source of drinking water | |||||||
| Pipe | 173 (47.5) | 94 (54.3) | 79 (45.7) | 1.513 [0.908–2.520] | 0.112 | – | – |
| River | 56 (15.4) | 32 (57.1) | 24 (42.9) | 1.350 [0.690–2.642] | – | – | |
| Pond | 37 (10.2) | 18 (48.6) | 19 (51.4) | 1.900 [0.883–4.086] | – | – | |
| Unprotected spring | 98 (26.9) | 63 (64.3) | 35 (35.7) | 1 | – | – | |
| Storage of water in wide-open pot | |||||||
| Yes | 108 (29.7) | 60 (55.6) | 48 (44.4) | 1.079 [0.686–1.698] | 0.743 | – | – |
| No | 256 (70.3) | 147 (57.4) | 109 (42.6) | 1 | – | – | |
| Contact with water bodies | |||||||
| Yes | 73 (20.1) | 42 (57.5) | 31 (42.5) | 0.967 [0.575–1.624] | 0.898 | – | – |
| No | 291 (79.9) | 165 (56.7) | 126 (43.3) | 1 | – | – | |
| Habit of eating raw vegetable | |||||||
| Yes | 97 (26.6) | 60 (61.9) | 37 (38.1) | 0.755 [0.470–1.215] | 0.248 | – | – |
| No | 267 (73.4) | 147 (55.1) | 120 (44.9) | 1 | – | – | |
| Habit of eating raw meat | |||||||
| Yes | 98 (26.9) | 58 (59.2) | 40 (40.8) | 0.878 [0.549–1.405] | 0.588 | – | – |
| No | 266 (73.1) | 149 (56) | 117 (44) | 1 | – | – | |
| Have latrine | |||||||
| Yes | 316 (86.8) | 182 (57.6) | 134 (42.4) | 0.800 [0.435–1.471] | 0.473 | – | – |
| No | 48 (13.2) | 25 (52.1) | 23 (47.9) | 1 | – | – | |
| Type of latrine | |||||||
| Family | 299 (82.1) | 174 (58.2) | 125 (41.8) | 0.678 [0.336–1.368] | 0.279 | ||
| Public | 30 (8.2) | 16 (53.3) | 14 (46.7) | 0.826 [0.311–2.195] | – | – | |
| Field | 35 (9.7) | 17 (48.6) | 18 (51.4) | 1 | – | – | |
| Latrine usage habit | |||||||
| Always | 223 (61.3) | 124 (55.6) | 99 (44.4) | 0.748 [0.353–1.588] | 0.450 | – | – |
| Sometimes | 110 (30.2) | 68 (61.8) | 42 (38.2) | 0.579 [0.260–1.292] | – | – | |
| Rarely | 31 (8.5) | 15 (48.4) | 16 (51.6) | 1 | – | – | |
| Hand washing habit after toilet visit | |||||||
| Always | 171 (47) | 90 (52.6) | 81 (47.4) | 1.050 [0.523–2.109] | 0.891 | – | – |
| Mostly | 25 (6.9) | 14 (56) | 11 (44) | 0.917 [0.334–2.517] | – | – | |
| Often | 19 (5.2) | 14 (73.7) | 5 (26.3) | 0.417 [0.126–1.383] | – | – | |
| Sometimes | 110 (30.2) | 68 (61.8) | 42 (38.2) | 0.721 [0.345–1.507] | – | – | |
| Rarely | 39 (10.7) | 21 (53.8) | 18 (46.2) | 1 | – | – | |
| Presence of domestic animal | |||||||
| Yes | 234 (64.3) | 139 (59.4) | 95 (40.6) | 1.334 [0.866–2.055] | 0.191 | – | – |
| No | 130 (35.7) | 68 (52.3) | 62 (47.7) | 1 | – | – | |
| Presence of cat or dog | |||||||
| Yes | 216 (59.3) | 134 (62) | 82 (38) | 0.596 [0.390–0.910] | 0.016 | – | – |
| No | 148 (40.7) | 73 (49.3) | 75 (50.7) | 1 | – | – | |
| Presence of stomach pain | |||||||
| Yes | 337 (92.6) | 196 (58.2) | 141 (41.8) | 0.455 [0.223–0.989] | 0.048 | 0.401 [0.174–0.925] | 0.032 |
| No | 27 (7.4) | 11 (40.7) | 16 (59.3) | 1 | 1 | ||
| Presence of diarrhea | |||||||
| Yes | 170 (46.7) | 89 (52.4) | 81 (47.6) | 0.413 [0.931–0.844] | 0.041 | 0.474 [0.191–0.948] | 0.007 |
| No | 194 (53.3) | 118 (60.8) | 76 (39.2) | 1 | 1 | ||
| Presence of bloody diarrhea | |||||||
| Yes | 42 (11.5) | 27 (64.3) | 15 (35.7) | 0.704 [0.361–1.374] | 0.304 | – | – |
| No | 322 (88.5) | 180 (55.9) | 142 (44.1) | 1 | – | – | |
| Habit of trimming nail | |||||||
| Yes | 289 (79.4) | 169 (58.5) | 120 (41.5) | 0.729 [0.438–1.214] | 0.225 | – | – |
| No | 75 (20.6) | 38 (50.7) | 37 (49.3) | 1 | – | – | |
| Presence of dirt on the nail | |||||||
| Yes | 131 (36) | 81 (61.8) | 50 (38.2) | 0.727 [0.470–1.125] | 0.152 | – | – |
| No | 233 (64) | 126 (54.1) | 107 (45.9) | 1 | – | – | |
| Have protective shoes | |||||||
| Yes | 341 (93.7) | 194 (56.9) | 147 (43.1) | 0.985 [0.420–2.309] | 0.972 | – | – |
| No | 23 (6.3) | 13 (56.5) | 10 (43.5) | 1 | – | – | |
| Habit of wearing shoes | |||||||
| Always | 230 (63.2) | 122 (53) | 108 (47) | 1.012 [0.355–2.882] | 0.983 | – | – |
| Sometimes | 119 (32.7) | 77 (64.7) | 42 (35.3) | 0.623 [0.211–1.839] | – | – | |
| Rarely | 15 (4.1) | 8 (53.3) | 7 (46.7) | 1 | – | – | |
| Personal hygiene | |||||||
| Good | 262 (72) | 143 (54.6) | 119 (45.4) | 1.402 [0.877–2.241] | 0.159 | – | – |
| Poor | 102 (28) | 64 (62.7) | 38 (37.3) | 1 | – | – | |
Regarding the study variables, 86% (313/364) of them know why they wash their hands before meal and 55.5% (202/364) of the participants are always wash their hands before eating; 70.3% (256/364) do not put water in wide-open pot or plastic container, and 73.4% (267/364) and 73.1% (266/364) of the participants were do not have habit of eating raw vegetables and meat, respectively. In addition, 47.5% (173/364) of the study participants were obtain drinking water from pipe. Study participants those had latrine are 86.8% (316/364) and only 61.3% (223/364) of them are always use latrine. Among the study participants, 79.4% (289/364) have habit of trimming their finger nail regularly. Furthermore, 93.7% (341/364) of them have shoes and only 63.2% (230/364) of them are always wear their shoes. In this study, 92.6% (337/364) of the participants had stomach pain and 46.7% (170/364) of the patients were diarrheic. Finally, 64.3% (234/364) of study participants have at least one domestic animal and 59.3% (216/364) have at least one pet (Table 2).
The distribution of parasites among study variables were shown in Table 2. Hence, 16.5% (60/364) of the participants those have habit of eating raw vegetables and raw meat 15.9% (58/364) were infected by parasites. Of the participants, 50% (182/364) positive cases have latrine at their home. Furthermore, 53.8% (196/364) of the positive patient cases had stomach pain and 24.5% (89/364) of parasite infected patients were diarrheic.
Factors associated with intestinal parasites
In this study several factors were considered to assess factors that contribute to intestinal parasite infection. Binary and multivariable logistic analyses were calculated. Multivariable logistic analysis was conducted after adjusting variables which were statistically significant in binary logistic analysis and from the factors associated with intestinal parasites; being male in gender (P = 0.049; AOR = 0.645; CI 0.416–0.998), age group interval between 1 and 20 years of old (P = 0.012; AOR = 0.645; CI 0.458–0.908), having stomach pain (P = 0.032; AOR = 0.401; CI 0.174–0.925) and having diarrhea (P = 0.007; AOR = 0.474; CI 0.191–0.948) were found to be significantly associated with parasitic infections. However, majority of the study variables were non-significantly associated with intestinal parasitic infections with a P-value of > 0.05 (Table 2).
Discussion
Intestinal parasitic infections are distributed virtually throughout the world and results in an impact on human health and development [1]. Developing countries are more affected by parasites than bacterial infections [4]. In this study, the overall prevalence of intestinal parasitosis was 56.9%. The finding of this study was higher than prevalence of parasites at Workmeda Health Center, Ethiopia (27.7%), Uganda (32.8%), Nigeria (41.2%), India (49.38%) and United Arab Emirates (7.74%) [14–19]. On the other hand, this study had showed a lower prevalence of parasitic infection than the reports in Southwest Ethiopia (83%), Tseda Health Center, Northwest Ethiopia (62.2%) and India (92.32%) [9, 20–22]. These variations might be due to the difference in the characteristics of the study population, geographical distribution and diagnostic techniques.
In this study, among the 207 infected patients, 115/364 (31.6%) were males which indicated that higher proportion of intestinal parasitic infections among male than female patients, 92/364 (25.3%). The higher prevalence in males might be due to everyday participation of males in outdoor activities is than females which make them more vulnerable to parasitic infections. Intestinal parasitic infections were higher in rural 36% (131/364) than urban patients 20.1% (76/364). This might be due to poor personal hygiene, over-crowding and the frequent contamination of water bodies and animals in rural than urban community.
The prevalence of E. histolytica/dispar in this study is higher when compared to other detected intestinal parasites. The possible reason might be due to contamination of potable water, poor handling of food stuff, contamination of food, and eating food without washing hands. The prevalence of intestinal parasites in direct wet mount and formol-ether sedimentation technique was quite different. This variation of prevalence of intestinal parasites in direct wet mount and formol-ether sedimentation technique might be due to the difference in the characteristics of intestinal parasites; more trophozoite stages of protozoan parasite detected in direct wet mount technique but not in formol-ether sedimentation technique. It might be due to the highly fragile nature of the trophozoite and the effect of preservatives in formol-ether sedimentation technique.
Several studies showed that socio-demographic characteristics and associated factors contribute a lot to contract intestinal parasitic infections [13, 23, 24]. The result of this study showed that being male in gender was significantly associated with intestinal parasitic infections. The possible reason might be involvement of males in farming activities than females which exposes them to more animal contact, water body contact and soil contamination. Age category of study subjects belonging to 1–20 years of old was a significant factor for intestinal parasitic infections which showed that being young in age, the more likely to develop intestinal parasitic infections. The possible reasons might be due to frequent contact among each other, playing with soil and water bodies, over-crowding in classroom or day-care centers, poor personal hygiene, immuno-compromised condition and habit of sharing of materials which facilitates the spread of the parasites.
Stomach pain in this study was a statistically significant factor to intestinal parasitic infections. Our study showed that study participants having stomach pain were more likely to be positive for parasites than those who have no stomach pain. The possible reason might be due to the abdominal cramps, bloating, nausea and watery diarrhea during parasitic infections. Most intestinal parasitic infections were associated with diarrhea. In this study having diarrhea also was significantly associated with parasitic infections. The possible reason might be due to diarrhea causing nature of intestinal parasites.
Conclusion
The prevalence of intestinal parasitic infection in our study was 56.9%. Being male in gender, age less than 20 years, having stomach pain and having diarrhea were associated factors for intestinal parasite infection. Ensuring provision of clean potable water and minimizing the contamination of vegetables are recommended. Furthermore, health education on wearing protective shoe and deworming of carriers should also be done. Finally, large community-based studies assessing all associated risk factors and continuous surveillance should be conducted to halt the spread of intestinal parasite infections in Shahura town and Alefa Woreda.
Limitations of the study
Due to resource constraints, we did not perform molecular techniques like PCR to identify and discriminate the true pathogenic E. histolytica from E. dispar. Thus, over diagnosis of Amoebiasis might occur. And also we did not perform other sensitive methods specific for some intestinal parasites such as Kato-Katz method, Trichome and modified Ziehl–Neelsen staining methods.
Additional files
Additional file 1: Table S1. Socio-demographic characteristics of study participants among patients at Shahura Health Center, Northwest Ethiopia, 2018.
Additional file 2: Figure S1. The overall burden of intestinal parasites in terms of their frequency per individual among patients at Shahura Health Center, Northwest Ethiopia, 2018.
Acknowledgements
We would like to thank all the study participants, University of Gondar, directors of Alefa Takusa health office and staff of Shahura Health Center for their cooperation.
Abbreviations
- AIDS
Acquired Immune Deficiency Syndrome
- CI
confidence interval
- GIT
gastrointestinal tract
- GOs
Governmental Organizations
- HIV
human immunodeficiency virus
- IPs
intestinal parasites
- IPIs
intestinal parasitic infections
- NGOs
Non-Governmental Organizations
- OR
odds ratio
- SPSS
Statistical Package of Social Science
- WHO
World Health Organization
Authors’ contributions
AT* conceptualized and designed the study. AT*, ST, MA and KA carried out data collection and laboratory works. KA entered the data. AT* and ST analyzed and interpreted the data, drafted the manuscript and critically reviewed the manuscript. KA and MA assisted in analyzing, drafting and reviewing the manuscript. All authors read and approved the final manuscript.
Funding
There was no funding for this research
Availability of data and materials
All data generated or analyzed during this study are included in this article. Data that support the findings of this study are also available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Ethical clearance was obtained from University of Gondar ethical review committee. Written permission was obtained from Alefa Takusa health office prior to data collection. Written informed consent from adults was obtained. Assent was also obtained from parents or legal guardian for under 16 years of old participants. Additionally, confidentiality of information was assured.
Full names of ethical review committee of University of Gondar
Professor Feleke Moges……………………. Chairperson
Professor Moges Tiruneh…………………… Member
Mr. Gashaw Neberu………………………… Member
Mr. Niguse Yigzaw…………………………. Secretary
Mr. Genanew Agitew………………………. Member
Mr. Abiyot Endale………………………….. Member
Dr. Misaye Mulate………………………….. Member
Dr. Kassahun Denekew…………………….. Member
Mr. Wondimenew Kassa…………………… Member
The Reference Number of approval is: O/V/P/RCS/05/481/2017.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abiye Tigabu, Phone: +251-918-192721, Email: abty12@gmail.com.
Solomon Taye, Email: tayesolomon38@gmail.com.
Melak Aynalem, Email: melak.aynalem1234@gmail.com.
Kasaw Adane, Email: kasawadane@gmail.com.
References
- 1.Tesema A. Prevalence of intestinal parasitic infections among patients who attended Tikur Anbessa University Hospital, Ethiopia. MSc thesis, 2011.
- 2.Mamie-Eleanor S. Prevalence, risk factors and consquenceses for child growth, iron status and development in rural Ecuador. 2001.
- 3.Mengistu A, Gebre-Selassie S, Kassa T. Prevalence of intestinal parasitic infections among urban dwellers in southwest Ethiopia. Ethiop J Health Dev. 2007;21(1):1021–6790. [Google Scholar]
- 4.Mandakini MP, Prashant RP, Bhavna G, Jigna M, Suresh P. Prevalence of intestinal parasites infestation in Surat City Of South Gujarat. Natl J Commun Med. 2014;5(3):273. [Google Scholar]
- 5.Thomas K, Fomefret L, Emmanuel E, et al. Prevalence and risk factors of intestinal helminths and protozoa infections. Am J Epidemiol Infect Dis. 2015;3(2):36–44. [Google Scholar]
- 6.Unasho A. An investigation of intestinal parasitic infections among the asymptomatic children in, Southern Ethiopia. Int J Child Health Nutr. 2013;2:212–222. [Google Scholar]
- 7.Tigabu E, Petros B, Endeshaw T. Prevalence of Giardiasis and Cryptosporidiosis among children in relation to water sources in Selected Village of Pawi Special District in Benishangul-Gumuz Region, Northwestern Ethiopia. Ethiop J Health Develop. 2010;24(3):1021–6790. [Google Scholar]
- 8.Gelaw A, Anagaw B, Nigussi B, et al. Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School. BMC Public Health. 2013;13:304. doi: 10.1186/1471-2458-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haftu D, Deyessa N, Agedew E. Prevalence and determinant factors of intestinal parasites among school children in Arba Minch town, Southern Ethiopia. Am J Health Res. 2014;2(5):247–254. doi: 10.11648/j.ajhr.20140205.15. [DOI] [Google Scholar]
- 10.Benjamin S, Hanspeter M, Shaali M, et al. Prevalence of intestinal protozoa infection among school-aged children on Pemba Island, Tanzania. BioMed Central. 2013;6:1756–2330. doi: 10.1186/1756-3305-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayalew D. Association of Cryptosporidium Parvum, Giardia Lamblia and Entamoeba histolytica/dispar infection with drinking water sources among children in rural part of Dire-Dawa, Eastern Ethiopia. MSc thesis. 2006.
- 12.Firdu T, Abunna F, Girma M. Intestinal protozoal parasites in diarrheal children and associated risk factors at Yirgalem Hospital, Ethiopia: a case-control study. Int Scholarly Res Not. 2014;357:126–128. doi: 10.1155/2014/357126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tulu B, Taye S, Amsalu E. Prevalence and its associated risk factors of intestinal parasitic infections among Yadot primary school children of South Eastern Ethiopia: a cross-sectional study. BMC Res Notes. 2014;7:848. doi: 10.1186/1756-0500-7-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wegayehu T, Tsalla T, Seifu B, Teklu T. Prevalence of intestinal parasitic infections among highland and lowland dwellers in Gamo area, South Ethiopia. BMC Public Health. 2013;13:151. doi: 10.1186/1471-2458-13-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brig Hemant K, CaptKalpana J, Maj Rahul J. Prevalence of intestinal worm infestation and efficacy of anthelminthic drugs. Med J Armed Forces India. 2014;70:144–148. doi: 10.1016/j.mjafi.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nihar D, Mansour A-Z, Khurshid A, Debadatta P. Prevalence of intestinal parasitic infections in Sharjah, United Arab Emirates. Hum Parasit Dis. 2010;2:21–24. doi: 10.4137/HPD.S5081. [DOI] [Google Scholar]
- 17.Buzigi E. Prevalence of intestinal parasites, and its association with severe acute malnutrition related diarrhoea. J Biol Agric Healthcare. 2015;5:2. [Google Scholar]
- 18.Egbuobi R, Nwagbaraocha M, Dike-Ndudim J, et al. Incidence of intestinal parasites among food handlers (Hawkers) around the University of Nigeria Teaching Hospital Enugu, Enugu State, Nigeria. Open J Med Microbiol. 2014;4:23–28. doi: 10.4236/ojmm.2014.41004. [DOI] [Google Scholar]
- 19.Abate A, Kibret B, Bekalu E, et al. Cross-sectional study on the prevalence of intestinal parasites and associated risk factors in Tseda Health Centre, Northwest Ethiopia. Hindawi Publishing Corporation. 2013;757451:5. doi: 10.5402/2013/757451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alamir M, Awoke W, Feleke A. Intestinal parasites infection and associated factors among school children. Health. 2013;5(10):1697–1701. doi: 10.4236/health.2013.510228. [DOI] [Google Scholar]
- 21.Pandey B, Kumar D, Verma D. Epidemiological study of parasitic infestations in rural women of Terai belt of Bihar. India Ann Biol Res. 2013;4(10):30–33. [Google Scholar]
- 22.Hailu T. Current prevalence of intestinal parasites emphasis on Hookworm and Schistosoma mansoni infections among patients at Workemeda Health Center, Northwest Ethiopia. Clin Microbiol. 2014;3:4. doi: 10.4172/2327-5073.1000155. [DOI] [Google Scholar]
- 23.Barbara M, Mohion B, Gulzira K, et al. Prevalence and risk factors of helminths and intestinal protozoa infections among children from primary schools in western Tajikistan. Parasites Vectors. 2011;4:195. doi: 10.1186/1756-3305-4-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayalew A, Debebe T, Worku A. Prevalence and risk factors of intestinal parasites among Delgi school children, North Gondar. Ethiop J Parasitol Vector Biol. 2011;3(5):75–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Socio-demographic characteristics of study participants among patients at Shahura Health Center, Northwest Ethiopia, 2018.
Additional file 2: Figure S1. The overall burden of intestinal parasites in terms of their frequency per individual among patients at Shahura Health Center, Northwest Ethiopia, 2018.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Data that support the findings of this study are also available from the corresponding author upon reasonable request.