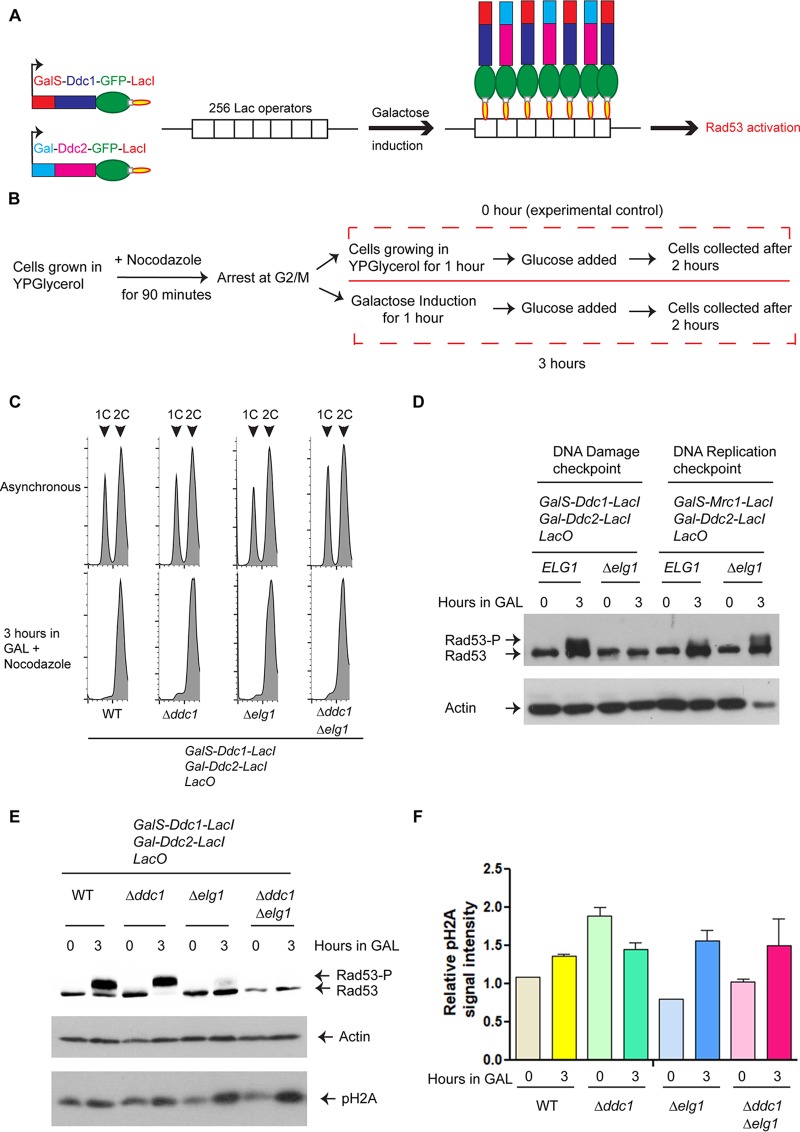
FIG 2.
The Elg1-RLC is required for the DNA damage checkpoint activation. (A) A schematic representation of the system used. From each strain, two proteins (either Ddc1 and Ddc2 or Mrc1 and Ddc2) were expressed as fusions to the Lac repressor (LacI) from a galactose (Gal)-inducible promoter (GalS or Gal). Binding of these proteins to an array of 256 copies of the Lac operator (LacO) caused induction of either the DNA damage checkpoint (DC) or the DNA replication checkpoint (RC), which can be detected as phosphorylation of Rad53. For simplicity, only the DC is represented here. (B) The schematic diagram represents the experimental regimen. Briefly, cells carrying galactose-inducible checkpoint proteins fused to the Lac repressor and LacO256 constructs were grown in YP medium containing glycerol. At an OD610 of approximately 1.0, cells were arrested at G2-M by adding nocodazole (15 μg/ml) for 90 min and were then divided into two halves; one half received galactose (final concentration, 2%) to induce expression of DDC1/MRC1 and DDC2 for 1 h. For the same duration, growth of the other half continued in YP medium containing glycerol (which served as 0 h or the experimental control). After an hour, both halves received glucose (final concentration, 2%) and were allowed to grow for another 2 h before extraction of proteins. (C) Flow cytometry analysis of the WT, Δddc1, Δelg1, and Δddc1 Δelg1 cells that were used for subsequent checkpoint induction experiments (see also Fig. S3). (D) Level of Rad53 phosphorylation in ELG1 and Δelg1 cells after induction of checkpoint fusions. (E) Level of Rad53 and histone H2A phosphorylation in WT, Δddc1, Δelg1, and Δddc1 Δelg1 cells after induction of the checkpoint. (F) Quantitation of histone H2A phosphorylation intensity (relative to actin levels). At least three independent experiments were done and results measured; error bars represent standard errors of the means (SEM). Actin was used as a loading control for each Western blot.