Abstract
PURPOSE:
The purpose of this study is to assess the outcomes of lacrimal gland injections of botulinum toxin A (BoNTA) for epiphora secondary to lacrimal drainage disorders and functional epiphora.
METHODS:
This was a retrospective interventional case series where cases were divided into functional and nonfunctional epiphora.
RESULTS:
A total of 37 eyes of 31 patients were identified: 13 males and 18 females. The mean age was 52 years (median = 53, range 29–86). The functional epiphora group had seven patients (8 eyes), subcategorized into hypersecretion (5), crocodile tears (1), and post seventh nerve palsy (1). Obstructive group (nonfunctional) had 24 patients (29 eyes), subcategorized into proximal canalicular block (12), common canalicular block (6), punctal stenosis (3), posttraumatic nasolacrimal duct obstruction (1), and partial nasolacrimal duct obstruction (1). Median preinjection Munk scores were similar in both groups (Grade 4). At 1 month, the median Munk score improved to 1 and 2 in functional and nonfunctional groups, respectively, after receiving a median dose of 4 units of BoNTA. Median reduction in Munk score was 75% in functional group versus 50% in nonfunctional group (P = 0.07). No difference in terms of complications was noted (transient ptosis).
CONCLUSIONS:
Reduction in epiphora after lacrimal gland injection of botulinum toxin is seen in cases with functional epiphora as well as those with a physical obstruction in the lacrimal drainage pathway. While the symptomatic improvement was more in functional epiphora, the difference between the two groups was not statistically significant.
Keywords: BOTOX, botulinum toxin, dacryocystorhinostomy, epiphora, facial palsy, functional, lacrimal obstruction, lacrimation, watering
Introduction
Injection of botulinum toxin A (BoNTA) into the lacrimal gland inhibits the release of neurotransmitter acetylcholine and thereby reduces basal and reflex tears secretion.[1] There are several published reports indicating its effectiveness in gustatory hyperlacrimation with minimal side effects.[2,3,4,5,6] In this regard, it is essential to understand the concept of “functional epiphora.” Functional epiphora represents tearing in the context of patent irrigation. Whittaker et al. first reported its utility for functional epiphora in 2003, and subsequently, there have been numerous studies that have reported the usage of BoNTA in canalicular obstructions, postpunctal cautery, and functional epiphora with variable results.[7,8,9,10,11,12,13] Different studies have reported varying degrees of success with differing dosages of BoNTA. It is clear that there is no consensus on the issues of dosages, indications, and treatment intervals among other things. With this background, this study was designed to find the differences, if any, in the outcomes of lacrimal gland BoNTA injections in functional and nonfunctional epiphora.
Methods
This study is a retrospective study conducted at the ophthalmic plastic surgery services of three regional referral eye centers. The medical records of all patients who received BoNTA into the lacrimal gland were reviewed. The study period ranged from January 2015 to May 2017. The study protocols at all centers adhered to the tenets of the Declaration of Helsinki. Data were collected from three referral centers where injections were performed by three fellowship-trained lacrimal surgeons (SS, AGN, and MSA). Irrigation was performed in all subjects with a 23G lacrimal cannula. Patients with patent irrigation and less than Grade 1 fluorescein dye disappearance test (FDDT) without any ocular surface disease/structural eyelid abnormality that could be attributed to causing epiphora were categorized as functional epiphora. Radiological imaging in the form of dacryoscintigraphy/dacryocystography was not performed in any of cases due to lack of insurance reimbursement or patient's unwillingness to undergo the investigation. Patients with epiphora in the presence of patent irrigation form the “functional group.” Patients with punctal stenosis, canalicular obstruction, and nasolacrimal duct obstruction were categorized under obstructive epiphora (nonfunctional group). Informed consent was obtained from all patients. Schirmer's test with anesthesia was performed to rule out evaporative dry eye disease (reflex epiphora) before injection in all cases. BOTOX®(Allergan, Dublin) containing onabotulinum toxin A reconstituted with sterile normal saline to 2.5 units/0.1 cc was used in all cases. Each patient received a drop of topical proparacaine 0.5% before injection. The lateral upper eyelid was manually elevated to expose palpebral lobe of the lacrimal gland, and BoNTA injection was given transconjunctivally in an insulin syringe with a 30G needle [Figure 1]. Two different dosages: 2.5 units and 5 units were injected based on surgeon's preference via the transconjunctival route. Indoor Munk score at baseline, 1-month follow-up, and the last visit was calculated for each patient.[14] The outcomes were reported in the form of percentage reduction in Munk score at 1 month. The parameters for which data were collected were demographic details, indication, dosage used, number of injections given, route of injection, Munk score, follow-up duration in weeks, and complications if any. Patients with <1-month follow-up were excluded from final analysis.
Figure 1.
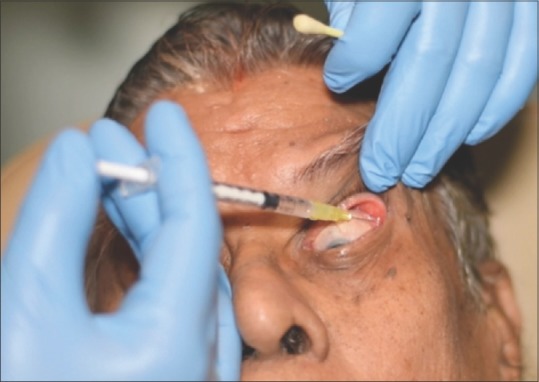
Our preferred technique for lacrimal gland botulinum toxin injection: The procedure is done under topical anesthesia with the patient looking down in adduction. The lateral upper eyelid is manually elevated to expose palpebral lobe of the lacrimal gland, and botulinum toxin A injection is given transconjunctivally in an insulin syringe with a 30G needle
Statistical analysis was performed using SPSS version 17 (IBM, Armonk, New York, USA). P <0.05 was considered statistically significant. Median values were used for statistical analysis rather than mean due to differences in numbers.
Results
A total of 37 eyes of 31 patients were identified: 13 males and 18 females. The mean age was 52 years (median = 53, range 29–86). Six cases had bilateral involvement (only one eye was included for statistical analysis). The median preinjection Munk score and Schirmer's test values were similar in both the groups. Follow-up period ranged from 1 to 20 months (mean = 6 months in the functional group and 5 months in the obstructive group). Demographics and treatment details are summarized in Table 1.
Table 1.
Distribution of variables among functional epiphora and nonfunctional epiphora groups
| Variable | Functional (n=7) | Nonfunctional (n=24) | P** |
|---|---|---|---|
| Age (years), mean±SD | 59 | 52 | 0.29* |
| Male:female | 2:5 | 11:13 | 0.67† |
| Preinjection mean Schirmer’s values | 14 | 18 | 0.93‡ |
| Preoperative Munk score, median (IQR) | 4 (3-5) | 4 (3-4) | 0.35‡ |
| Postoperative Munk score, median (IQR) | 1 (1-2) | 2 (1-3) | 0.17‡ |
| Units injected, median (IQR) | 2.5 (2.5-5) | 5 (2.5-5) | 0.28‡ |
| Outcome (% improvement), median (IQR) | 75 (50-80) | 50 (25-66) | 0.07‡ |
| Total injections given, median (IQR) | 1 (1-3) | 1.5 (1-2) | 0.91‡ |
| Follow-up (months), mean (IQR) | 6 (2-12) | 5 (1.5-7) | 0.40‡ |
| Complications (%) | 1 (14.3) | 2 (8.3) | 1.00† |
*Student’s t-test, †Fisher’s exact probability test, ‡Mann-Whitney test, **P value denotes comparison between two groups. IQR: Interquartile range, SD: Standard deviation
The functional epiphora group had seven patients (8 eyes), subcategorized into hypersecretion (5), crocodile tears (1), and lacrimal pump failure due to seventh nerve palsy (1). At 1 month, the median preinjection Munk score improved to 1 from 4 after receiving a median dose of 4 units of BoNTA. The effect lasted for an average duration of 4 months with variable follow-up ranging from 1 to 8 months. Three out of seven patients received a second injection after a mean duration of 4 months. Only one case had transient ptosis after receiving 2.5 units of BoNTA, which resolved completely in 4 weeks.
The obstructive group (nonfunctional) had 24 patients (29 eyes), subcategorized into proximal canalicular block (12), common canalicular block (6), punctal stenosis (3), posttraumatic nasolacrimal duct obstruction (1), and partial nasolacrimal duct obstruction (1). At 1 month, the median preinjection Munk score improved from 4 to 2 after receiving a median dosage of 4 units of BoNTA. Four patients showed no reduction in epiphora, of which two had received 5 units, while the other two had received 2.5 units. Twelve out of 24 patients received a repeat injection after a mean duration of 3 months. Two cases had ptosis after 2.5 and 5 units each, which resolved by 4.5 weeks. Both groups did not show any side effect in the form of dry eyes or ocular surface instability. Postoperative Schirmer's test values documentation was not available for all cases. Statistical analysis revealed no significant difference in groups based on age, sex, preoperative Munk score, units injected, number of injections given, and complications. Median reduction in Munk score was 75% in functional group compared to 50% in nonfunctional group. Improvement in Munk score showed a trend toward better results in functional group as compared to nonfunctional group but failed to achieve statistical significance (P = 0.07) [Table 1].
Discussion
In our study, reduction in epiphora after lacrimal gland BoNTA was observed more in cases of functional epiphora compared to those with physical obstruction in lacrimal drainage pathways. Detailed epiphora evaluation which includes grading of epiphora, Schirmer's testing, FDDT, syringing, eyelids examination, and tests for tear film instability should be performed to diagnose cases with true functional epiphora.
Functional epiphora has been defined very vaguely in literature, which even includes cases of partial nasolacrimal duct (NLD) stenosis in some of the studies.[15,16,17] Patent irrigation in the presence of lacrimal pump failure should be categorized as functional nasolacrimal duct obstruction. In the current study, cases with patent irrigation and no delay in FDDT/lid malposition were included in functional group, thus ruling out outflow pathway etiologies. There is a lack of clear treatment guidelines for functional epiphora. Silicone bicanalicular intubation with or without dacryocystorhinostomy has shown success rates ranging from 58% to 68%.[16,17] BoNTA injections offer a nonsurgical alternative for such cases and have shown effectiveness to the tune of 86%.[7] Theoretically, 75% median reduction in epiphora among functional group was far better than 50% median reduction among obstructive group, although statistical significance was not achieved (P = 0.07). Four patients among obstructive group reported no change in symptoms, whereas functional group had no such patients with no reported improvement. This study may serve as a pilot for designing prospective studies in future to evaluate the differences.
The mechanism of action of BoNTA once injected into the lacrimal gland is via inhibition at the neurotransmitter level, which is responsible for glandular secretion (basal or reflex). In cases with outflow obstruction, reduction in tear production is unlikely to resolve epiphora as the primary etiology has not been addressed. However, in cases aberrant re-innervation leading to increased tear production and subsequent functional epiphora, BoNTA decreases the tear production and can help in resolution of symptoms provided the outflow pathway is normal. Majority of reported literature discusses the role of BoNTA in obstructive epiphora with variable results. Wojno evaluated its role in cases with lacrimal obstruction and gustatory epiphora.[10] Improvement rates among obstructive group were 63% versus 53% in gustatory group after receiving 2.5 units of BoNTA.[10] A significant drawback in this study was that no specific objective scoring system was used for grading epiphora in their study. On the contrary, Whittaker et al. found epiphora reduction in 86% (12/14) of cases with functional epiphora lasting for up to 13 weeks, after receiving a median dose of 2.5 units.[7] Ziahosseini et al. evaluated BoNTA in proximal lacrimal system obstructions and reported 60%–90% improvement in 53% of cases.[12] Kaynak et al. reported no difference in reduction in Munk scoring after lacrimal gland BoNTA when compared to Munk scores in patients who underwent conjunctivodacryocystorhinostomy for canalicular obstructions.[13] A recent review on BoNTA for intractable lacrimal drainage disorders highlighted the need for larger studies for standardizing the concentrations, dosage, and outcome measures owing to the large variability across the published literature.[8] Furthermore, the need to formulate guidelines for specific indication-based use of botulinum toxin was stressed upon.[8] Such variability in literature can be due to differences in documentation/grading of epiphora.[14] This lacuna also highlights the need for a standardized, acceptable, and reproducible grading system to objectively and uniformly measure epiphora. Uniformity can be obtained using a validated scale for epiphora evaluation in prospectively designed studies. Subjective documentation of epiphora in the form of percentage improvement scale has been used in few studies, which has an inherent subjective bias.
Complication rates in the form of ptosis have been reported from 7% to 25% across published studies.[8] In the current study, ptosis was observed in 3/31 (10%) with no significant difference between two groups. No complaints regarding dry eyes or diplopia were noted.
Conclusions
Epiphora reduction after lacrimal gland botulinum toxin injections was noted more in functional epiphora compared to nonfunctional epiphora. Further studies with larger numbers and long-term follow-up will give us more insight into its utility in nonfunctional cases.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sellin LC. The pharmacological mechanism of botulism. Trends Pharma Sci. 1985;6:80–2. [Google Scholar]
- 2.Boroojerdi B, Ferbert A, Schwarz M, Herath H, Noth J. Botulinum toxin treatment of synkinesia and hyperlacrimation after facial palsy. J Neurol Neurosurg Psychiatry. 1998;65:111–4. doi: 10.1136/jnnp.65.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riemann R, Pfennigsdorf S, Riemann E, Naumann M. Successful treatment of crocodile tears by injection of botulinum toxin into the lacrimal gland: A case report. Ophthalmology. 1999;106:2322–4. doi: 10.1016/S0161-6420(99)90534-1. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann RJ. Treatment of Frey's syndrome (gustatory sweating) and 'crocodile tears' (gustatory epiphora) with purified botulinum toxin. Ophthalmic Plast Reconstr Surg. 2000;16:289–91. doi: 10.1097/00002341-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Falzon K, Galea M, Cunniffe G, Logan P. Transconjunctival botulinum toxin offers an effective, safe and repeatable method to treat gustatory lacrimation. Br J Ophthalmol. 2010;94:379–80. doi: 10.1136/bjo.2008.155887. [DOI] [PubMed] [Google Scholar]
- 6.Nava-Castañeda A, Tovilla-Canales JL, Boullosa V, Tovilla-y-Pomar JL, Monroy-Serrano MH, Tapia-Guerra V, et al. Duration of botulinum toxin effect in the treatment of crocodile tears. Ophthalmic Plast Reconstr Surg. 2006;22:453–6. doi: 10.1097/01.iop.0000244515.07925.99. [DOI] [PubMed] [Google Scholar]
- 7.Whittaker KW, Matthews BN, Fitt AW, Sandramouli S. The use of botulinum toxin A in the treatment of functional epiphora. Orbit. 2003;22:193–8. doi: 10.1076/orbi.22.3.193.15622. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Ali MJ, Paulsen F. A review on use of botulinum toxin for intractable lacrimal drainage disorders. Int Ophthalmol. 2018;38:2233–8. doi: 10.1007/s10792-017-0661-9. [DOI] [PubMed] [Google Scholar]
- 9.Tu AH, Chang EL. Botulinum toxin for palliative treatment of epiphora in a patient with canalicular obstruction. Ophthalmology. 2005;112:1469–71. doi: 10.1016/j.ophtha.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Wojno TH. Results of lacrimal gland botulinum toxin injection for epiphora in lacrimal obstruction and gustatory tearing. Ophthalmic Plast Reconstr Surg. 2011;27:119–21. doi: 10.1097/IOP.0b013e318201d1d3. [DOI] [PubMed] [Google Scholar]
- 11.Eustis HS, Baiuch A. Use of botulinum toxin injections to the lacrimal gland for epiphora in children with proximal obstruction of lacrimal drainage system. J Pediatr Ophthalmol Strabismus. 2012;16:e15–6. doi: 10.3928/01913913-20141120-02. [DOI] [PubMed] [Google Scholar]
- 12.Ziahosseini K, Al-Abbadi Z, Malhotra R. Botulinum toxin injection for the treatment of epiphora in lacrimal outflow obstruction. Eye (Lond) 2015;29:656–61. doi: 10.1038/eye.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaynak P, Karabulut GO, Ozturker C, Fazil K, Arat YO, Perente I, et al. Comparison of botulinum toxin-A injection in lacrimal gland and conjunctivodacryocystorhinostomy for treatment of epiphora due to proximal lacrimal system obstruction. Eye (Lond) 2016;30:1056–62. doi: 10.1038/eye.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munk PL, Lin DT, Morris DC. Epiphora: Treatment by means of dacryocystoplasty with balloon dilation of the nasolacrimal drainage apparatus. Radiology. 1990;177:687–90. doi: 10.1148/radiology.177.3.2243969. [DOI] [PubMed] [Google Scholar]
- 15.Chan W, Malhotra R, Kakizaki H, Leibovitch I, Selva D. Perspective: What does the term functional mean in the context of epiphora? Clin Exp Ophthalmol. 2012;40:749–54. doi: 10.1111/j.1442-9071.2012.02791.x. [DOI] [PubMed] [Google Scholar]
- 16.Moscato EE, Dolmetsch AM, Silkiss RZ, Seiff SR. Silicone intubation for the treatment of epiphora in adults with presumed functional nasolacrimal duct obstruction. Ophthalmic Plast Reconstr Surg. 2012;28:35–9. doi: 10.1097/IOP.0b013e318230b110. [DOI] [PubMed] [Google Scholar]
- 17.Wormald PJ, Tsirbas A. Investigation and endoscopic treatment for functional and anatomical obstruction of the nasolacrimal duct system. Clin Otolaryngol Allied Sci. 2004;29:352–6. doi: 10.1111/j.1365-2273.2004.00836.x. [DOI] [PubMed] [Google Scholar]


