Abstract
Introduction:
Laparoscopic repeat liver resection (LRLR) is a safe and effective treatment in recurrent hepatocellular carcinoma (rHCC) in particular patients. However, there are less reports about surgery strategy of LRLR for rHCC. The aim of this study was to perform a systematic strategy for bleeding of liver to increase the safety and feasibility of LRLR for rHCC.
Methods:
In this study, a total of 13 cases of LRLR for rHCC, including 8 males and 5 females; aged 28–72 years, mean age 54 years, who were received at least one laparotomy due to HCC. We employ to block the local blood flow, ligation of the left or right hepatic artery and/or approach of Pringle according to the assessment of the degree of adhesions in the abdominal and the first hepatic portal, the location of the tumour (edge/central).
Results:
Three cases were less adhesions, nine cases were dense adhesions but 1 case was serious adhesions. Two cases were employed to block the local blood flow, 3 cases were employed to ligation of the left or right hepatic artery and 7 cases were employed to approach of Pringle. Twelve cases were successfully completed by LRLR whereas 1 case was completed by transfer to the open resection, including massive resection in 3 cases (the diameter of resection ≥3 cm), small hepatectomy in 10 cases (the diameter of resection <3 cm), no severe perioperative complication. The average operative time was (142 ± 34) min, the average intraoperative blood loss was (251 ± 92) ml and the average post-operative hospital time was (9 ± 3) d. The mean follow-up time was 25 months. Until the last follow-up, 11 cases survived while 2 cases died because of tumour recurrence.
Conclusions:
It can improve the safety and feasibility of LRLR for rHCC, according to the degree of adhesion of the peritoneal adhesions and the first hepatic portal, then selecting the appropriate technique to control the bleeding of the hepatectomy.
Keywords: Bleeding, hepatocellular carcinoma, laparoscopic hepatectomy, recurrence, strategy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumour worldwide, accounting for 5.6% of all human cancers.[1] It is also the third most common cause of cancer-related deaths worldwide.[2] Liver resection (LR) for HCC is now considered to be a safe procedure and has been expanded to include major hepatectomies and resection of the posterosuperior segments.[3,4,5,6] Although the recurrence rate of HCC is as high as 70% after 5 years,[7,8] there is a relatively good prognosis for recurrent HCC (rHCC) on repeat LR (RLR).[9,10,11] These resections are typically performed through the traditional open RLR (ORLR) method; however, laparoscopic RLR (LRLR) for rHCC has been shown to have several feasibility and safety advantages.[12,13,14]
Further research is needed to evaluate the extent of the advantages of LRLR. To the best of our knowledge, this is the first report of an LRLR surgery strategy that combined removal of adhesions with control of bleeding, two factors that contribute to the difficulty of RLR procedures. The aim of this study was to improve the safety and feasibility of LRLR for rHCC, according to the extent of the peritoneal adhesions and the first hepatic portal, and also to select the appropriate technique to control the hepatectomy bleeding.
METHODS
Patients and inclusion criteria
A total of 13 cases of LRLR for rHCC, who were admitted to the Department of Hepatobiliary Surgery of the Affiliated Hospital of Guilin Medical and The First Affiliated Hospital of Guangxi Medical University in China for a period from 1 January, 2013 to 25 March, 2017, including 8 males and 5 females; aged 28–72 years, mean age 54 years. Thirteen cases were received at least one laparotomy due to HCC. These were as shown in Table 1 and Partial images were as shown in Figure 1.
Table 1.
Information of patient of laparoscopic repeat liver resection for recurrent hepatocellular carcinoma
| Case | Gender | Age (year) | The liver segment in the first operation | Recurrence time (min) | Recurrence of the liver segment | The diameter of recurrence tumour (mm) | The operative time (min) | The intra-operative blood loss (ml) | The post-operative hospital time (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 47 | 4 | 18 | 2 | 30 | 130 | 180 | 7 |
| 2 | Female | 42 | 1,4 | 60 | 4 | 15 | 110 | 280 | 8 |
| 3 | Male | 41 | 7 | 12 | 7 | 20 | 180 | 300 | 12 |
| 4 | Male | 56 | 2 | 3 | 2 | 10 | 120 | 120 | 6 |
| 5 | Male | 65 | 5,6 | 16 | 3 | 15 | 110 | 150 | 6 |
| 6 | Female | 64 | 7 | 10 | 6 | 35 | 180 | 350 | 10 |
| 7 | Female | 57 | 3 | 52 | 4 | 20 | 120 | 200 | 9 |
| 8 | Male | 28 | 8 | 21 | 5 | 20 | 190 | 360 | 12 |
| 9 | Male | 68 | 8 | 42 | 8 | 15 | 200 | 360 | 13 |
| 10 | Female | 72 | 2,3 | 30 | 5 | 45 | 160 | 350 | 12 |
| 11 | Male | 45 | 6 | 24 | 4 | 15 | 120 | 300 | 10 |
| 12 | Male | 53 | 5 | 3 | 3 | 15 | 110 | 160 | 7 |
| 13 | Female | 60 | 6,7 | 10 | 2 | 20 | 120 | 150 | 6 |
Figure 1.
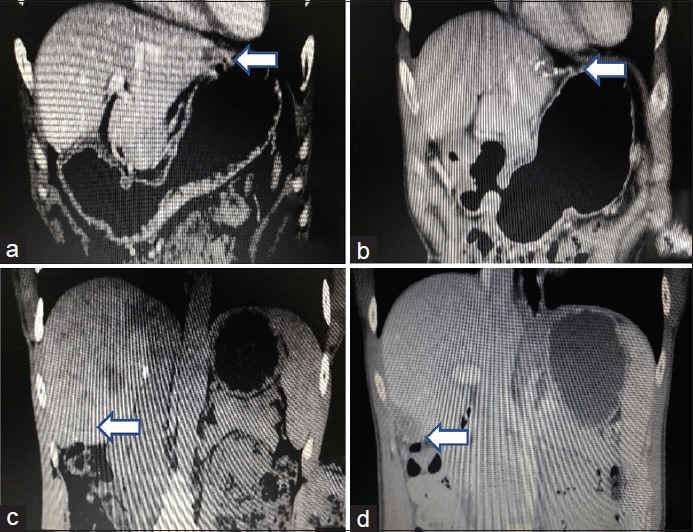
(a) Tumour in the left lobe in liver on the first pre-operative day. (b) Resection of tumour on the first post-operative day. (c) Tumor in the right lobe in liver on the second pre-operative day. (d) Resection of tumour in the second post-operative
The inclusion criteria were as follows: (1) rHCC after laparotomy; (2) rHCC located in the left lateral lobe or S5,6 segment of the right lobe or near the surface of S7,8 segment of the liver, without surgical contraindications; (3) no major vessel or bile duct tumour invasion or metastasis; (4) Grade A and no accompanied by severe cirrhosis and (5) Patients could generally tolerate hepatectomy besides disease of heart and lung and so on.
Surgical position and trocar approaches
A total of 13 patients were anaesthetized and intubated. They were placed in a supine, low position, with their legs spread apart. A six-way access approach was used, in which the surgeon stood between the patient's legs, the first surgical assistant stood on the left side of the patient, the second assistant stood on the right side of the patient and the laparoscope-supporting assistant stood next to the first surgical assistant.
First, a small incision (1.0 cm in length) was made along the upper edge of the navel, as far from the adhesions as possible, and then, a 10-mm trocar was inserted and pneumoperitoneum was established. Pressure was maintained at 12 mmHg. The laparoscope was inserted to explore the liver tumour and peritoneal adhesions. Five additional access points were created: a 5-mm trocar was inserted at 2 cm below the right costal margin; a 10-mm trocar was inserted lateral and parallel to the umbilicus and used as the main surgical access; a 5-mm trocar was inserted underneath the left costal margin and a 10-mm trocar was inserted for the primary surgical access located at laterally and upper with the umbilicus. Moreover, the last was a 10-mm trocar located 2 cm below the xyphoid for the Pringle manoeuvre and the surgeon separated the adhesions.
The grading criteria for the peritoneal adhesions were as follows: P1 = mild adhesion that can be bluntly separated; P2 = mild adhesion that can be sharply separated; P3 = more tissue and vascular adhesions requiring sharp separation and P4 = dense organ, intestinal adhesions, possibility of inducing injury during the process of separation. The grading criteria for adhesions of the first hepatic portal were as follows: H1 = adhesion of the first hepatic portal can be easily separated; H2 = more tissue adhesions and the left or right hepatic artery can be separated; H3 = adhesion can be separated and the first hepatic portal can be managed by the Pringle approach and H4= dense adhesions, and the first hepatic portal cannot be separated. Adhesions were divided into stages according to the above combination. These were as shown in Table 2 and Figure 2.
Table 2.
Adhesive stages according to criteria
| H1 | H2 | H3 | H4 | |
|---|---|---|---|---|
| P1 | I | I | III | IV |
| P2 | I | I | III | IV |
| P3 | II | II | III | IV |
| P4 | III | III | IV | IV |
Figure 2.
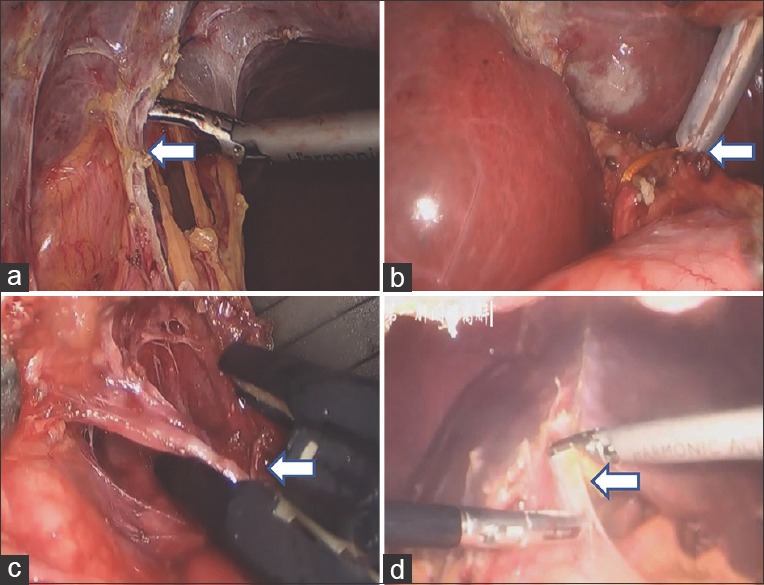
(a) Peritoneal adhesions by laparoscopic. (b) Adhesion can be separated and the first hepatic portal can be handled by approach of Pringle. (c) More tissue adhesions and the left hepatic artery can be separated. (d) Dense adhesions and the first hepatic portal cannot be separated
Surgical procedure
The location and incision edges of the tumour were identified by the surgeon using laparoscopic ultrasound guidance. First, the local blood flow was blocked through ligation of the left or right hepatic artery and/or the Pringle approach, based on the assessment of the degree of the abdominal and first hepatic portal adhesions, taking into consideration the location of the tumour (edge/central) and the relationship between the tumour and the main blood vessels of the liver. Next, we used an ultrasound scalpel to separate the liver parenchyma, followed by absorbable ligating clips to stop the bleeding from the main vessels. The surgeon then resected the tumour and used argon plasma coagulation to stop minor bleeding in the operating field. Finally, we placed an abdominal drainage tube per the surgical site.
Post-surgery follow-up
The patients were to return routinely every month for 12 months, and then every 2–3 months after the first 12 months. Evaluations included abdominal ultrasound, computed tomography, magnetic resonance imaging, liver function tests, alpha-fetoprotein levels and other evaluations as needed.
RESULTS
A total of 13 cases of rHCC were successfully treated, including 1 case that was completed using ORLR. Three cases had less adhesions, 9 cases were more adhesions with 1 case having serious adhesions. Two cases required that the local blood flow be blocked because the tumours were on the edge of the liver and the adhesions, according to the criteria, were less than Stage II. Three cases used ligation of the left or right hepatic artery because the adhesions were Stage II, but the tumours were at the centre of the liver parenchyma. Seven cases used the Pringle approach because the adhesions were Stage III. The remaining case was completed by transfer to the ORLR method because the adhesions were Stage IV. Three of the cases were mass resection (diameter ≥3 cm), the others were small hepatectomies (diameter <3 cm). No severe perioperative complications occurred. The average time for surgery was 142 ± 34 min; the average intra-operative blood loss was 251 ± 92 ml and the average post-operative hospital time was 9 ± 3 days. The mean follow-up time was 25 months. At the last follow-up, 11 of the 13 patients were surviving; two patients died because of tumour recurrence.
DISCUSSION
Currently, treatments of rHCC mainly include transarterial chemoembolization, radiofrequency ablation and molecular-targeted therapies. A relatively good prognosis is also achieved using RLR for rHCC. However, only 7% of patients with rHCC are suitable for resection.[15,16] The dense peritoneal adhesions and the first hepatic portal can increase the risk and difficulty of repeated open surgery. Laparoscopic surgery, however, can significantly reduce adhesions and therefore make repeated resections more viable.[17,18] LRLR has been widely used in the treatment of HCC due to the advantages of being minimally invasive, but LRLR is still not the typical procedure used for rHCC. If it can be proved that LRLR has similar validity and safety as ORLR, then it may become more widely used. Some reports indicate that the patients had also made by LRLR[19,20] Yu et al.[21] reported that they confirmed that it was the feasibility of LRLR because they made 14 patients by LRLR who had received resection of HCC.
We reported 13 cases of LRLR as the surgery strategy for rHCC. The primary purpose of this study was to improve the safety and feasibility of LRLR for rHCC based on the degree of severity of the peritoneal adhesions and the first hepatic portal, then to select the appropriate technique to control the bleeding of the hepatectomy.
In general, because of the complexity of post-operative adhesions from the first open operation for HCC, laparoscopic surgery has not been recommended for repeat surgery in the past. Compared with LRLR, ORLR can lead to more severe abdominal cavity adhesions. RLR can also induce many complications in the process of separating the adhesions, such as injury of the bowel loops or blood vessels. To reduce complications, we formulated a surgical strategy. First, the first trocar was placed in a site away from the adhesion; then pneumoperitoneum was established and the rest of the trocars were placed in a laparoscopic observation. Second, we separated the adhesions by taking advantage of the fixed and familiar anatomical signs, avoiding blind separation. Third, separation of the adhesions was made close to the abdominal wall and liver, but as far away as possible from the bowel and blood vessels. And finally, the severity of the adhesions in the abdominal cavity had to be determined before separation, and if they were severe, we took care to avoid creating a secondary injury. To control the bleeding of the LR, we dissected either the left or right hepatic artery. If the adhesion was serious and Stage IV, we completed the resection by ORLR.
We had initially confirmed that LRLR was safe and feasible for rHCC after rigorous indications and recommended that it should be done by a surgical team with extensive experience in the liver and endoscopic surgery.
CONCLUSIONS
We found that LRLR was safe and feasible for rHCC, and we recommend that it should be more widely used in clinical practice. The severity of the adhesions in the abdominal cavity needs to be evaluated before separation, and the appropriate technique for control of the bleeding following the hepatectomy needs to be determined. This was a small study and a randomized study with a larger sample size is needed to confirm the results.
Financial support and sponsorship
This study was financially supported by the Fund of the Science and Technology, Commission of Guangxi Province, China (grant number 2015GXNSFAA139218).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chacko S, Samanta S. Hepatocellular carcinoma: A life-threatening disease. Biomed Pharmacother. 2016;84:1679–88. doi: 10.1016/j.biopha.2016.10.078. [DOI] [PubMed] [Google Scholar]
- 2.Bishayee A, Darvesh AS. Angiogenesis in hepatocellular carcinoma: A potential target for chemoprevention and therapy. Curr Cancer Drug Targets. 2012;12:1095–118. [PubMed] [Google Scholar]
- 3.Oba A, Takahashi S, Kato Y, Gotohda N, Kinoshita T, Shibasaki H, et al. Usefulness of resection for hepatocellular carcinoma with macroscopic bile duct tumor thrombus. Anticancer Res. 2014;34:4367–72. [PubMed] [Google Scholar]
- 4.Komatsu S, Brustia R, Goumard C, Perdigao F, Soubrane O, Scatton O, et al. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: A matched pair analysis. Surg Endosc. 2016;30:1965–74. doi: 10.1007/s00464-015-4422-4. [DOI] [PubMed] [Google Scholar]
- 5.Cho JY, Han HS, Yoon YS, Shin SH. Experiences of laparoscopic liver resection including lesions in the posterosuperior segments of the liver. Surg Endosc. 2008;22:2344–9. doi: 10.1007/s00464-008-9966-0. [DOI] [PubMed] [Google Scholar]
- 6.Teo JY, Kam JH, Chan CY, Goh BK, Wong JS, Lee VT, et al. Laparoscopic liver resection for posterosuperior and anterolateral lesions-a comparison experience in an Asian centre. Hepatobiliary Surg Nutr. 2015;4:379–90. doi: 10.3978/j.issn.2304-3881.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CC, Cheng SB, Yeh DC, Wang J, P’eng FK. Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg. 2009;96:1049–57. doi: 10.1002/bjs.6690. [DOI] [PubMed] [Google Scholar]
- 8.Kishi Y, Shimada K, Nara S, Esaki M, Kosuge T. Role of hepatectomy for recurrent or initially unresectable hepatocellular carcinoma. World J Hepatol. 2014;6:836–43. doi: 10.4254/wjh.v6.i12.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kishi Y, Saiura A, Yamamoto J, Koga R, Seki M, Morimura R, et al. Repeat treatment for recurrent hepatocellular carcinoma: Is it validated? Langenbecks Arch Surg. 2011;396:1093–100. doi: 10.1007/s00423-011-0837-0. [DOI] [PubMed] [Google Scholar]
- 10.Sung PS, Yang H, Na GH, Hwang S, Kang D, Jang JW, et al. Long-term outcome of liver resection versus transplantation for hepatocellular carcinoma in a region where living donation is a main source. Ann Transplant. 2017;22:276–84. doi: 10.12659/AOT.904287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai WC, Cheung TT. Strategic overview on the best treatment option for intrahepaitc hepatocellular carcinoma recurrence. Expert Rev Anticancer Ther. 2016;16:1063–72. doi: 10.1080/14737140.2016.1226136. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Sui C, Li B, Yin Z, Tan Y, Yang J, et al. Repeat hepatectomy for recurrent hepatocellular carcinoma: A local experience and a systematic review. World J Surg Oncol. 2010;8:55. doi: 10.1186/1477-7819-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu M, Zhao G, Xu D, Liu R. Laparoscopic repeat resection of recurrent hepatocellular carcinoma. World J Surg. 2011;35:648–55. doi: 10.1007/s00268-010-0919-0. [DOI] [PubMed] [Google Scholar]
- 14.Ahn KS, Han HS, Yoon YS, Cho JY, Kim JH. Laparoscopic liver resection in patients with a history of upper abdominal surgery. World J Surg. 2011;35:1333–9. doi: 10.1007/s00268-011-1073-z. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita Y, Shirabe K, Tsuijita E, Takeishi K, Ikegami T, Yoshizumi T, et al. Third or more repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery. 2013;154:1038–45. doi: 10.1016/j.surg.2013.04.046. [DOI] [PubMed] [Google Scholar]
- 16.Hadjittofi C, Athanasopoulos PG, Koti RS, Konstantinidou SK, Davidson BR. Long-term survival with repeated resections of recurrent hepatocellular carcinoma in a non-cirrhotic liver: Case report and brief review of the literature. Ann Transl Med. 2016;4:112. doi: 10.21037/atm.2016.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razmaria AA, Marchetti PE, Prasad SM, Shalhav AL, Gundeti MS. Does robot-assisted laparoscopic ileocystoplasty (RALI) reduce peritoneal adhesions compared with open surgery? BJU Int. 2014;113:468–75. doi: 10.1111/bju.12284. [DOI] [PubMed] [Google Scholar]
- 18.Mais V. Peritoneal adhesions after laparoscopic gastrointestinal surgery. World J Gastroenterol. 2014;20:4917–25. doi: 10.3748/wjg.v20.i17.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belli G, Cioffi L, Fantini C, D’Agostino A, Russo G, Limongelli P, et al. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: Feasibility, safety, and results. Surg Endosc. 2009;23:1807–11. doi: 10.1007/s00464-009-0344-3. [DOI] [PubMed] [Google Scholar]
- 20.Brytska N, Han HS, Shehta A, Yoon YS, Cho JY, Choi Y, et al. Laparoscopic liver resection for hepatitis B and C virus-related hepatocellular carcinoma in patients with child B or C cirrhosis. Hepatobiliary Surg Nutr. 2015;4:373–8. doi: 10.3978/j.issn.2304-3881.2015.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu D, Shang C, Xiang Q, Cao J, Li W, Zhang L, et al. Laparoscopic hepatectomy for recurrent hepatocellular carcinoma after previous open hepatectomy. Zhonghua Wai Ke Za Zhi. 2014;52:405–8. [PubMed] [Google Scholar]


