Abstract
BACKGROUND:
Achievement of diabetes care goals is suboptimal globally. Diabetes-focused quality improvement (QI) is effective, but remains untested in South Asia.
OBJECTIVE:
To assess whether a multi-component QI strategy (non-physician care coordinator [CC] and decision-support electronic health records [DS-EHR]) vs. usual care improves cardio-metabolic profiles in poorly-controlled diabetes.
DESIGN:
Parallel open-label randomized controlled pragmatic trial.
SETTING:
Diabetes clinics in India and Pakistan
PATIENTS:
1146 (575 intervention; 571 usual care) poorly-controlled patients with type 2 diabetes (glycated hemoglobin [HbA1c]≥8% and either systolic blood pressure [SBP]≥140mmHg or/and low-density lipoprotein cholesterol [LDLc]≥130mg/dl)
INTERVENTION:
Multi-component QI: non-physician CCs (to motivate patients) and DS-EHR (recommended care prompts to physicians).
MEASUREMENTS:
Proportions achieving HbA1c<7.0% and BP<130/80mmHg or/and LDLc<100mg/dl (primary outcome); mean risk factor reductions, health-related quality of life (HRQL), and treatment satisfaction (secondary outcomes).
RESULTS:
At baseline, intervention and usual care participants were similar: mean age 54.2±9.2 years; 45.9% male; median diabetes duration 7.0 years; 6.8% and 39.4% had pre-existing cardiovascular and microvascular disease, respectively; and mean HbA1c, BP, and LDLc were 9.9%, 143.3/81.7mmHg, and 122.4mg/dl, respectively. Over a median 28 months, twice as many intervention as usual care participants achieved the primary outcome (18.2% vs. 8.7%; relative risk 2.24 [95% CI:1.71, 2.92]). Compared to usual care, intervention participants achieved larger reductions for HbA1c (−0.50%; −0.69,−0.32), SBP (−4.04mmHg; −5.85,−2.22), diastolic BP (−2.03mmHg; −3.00,−1.05), LDLc (−7.86mg/dl; −10.90,−4.81), and reported higher HRQL and treatment satisfaction. Achievement of the primary outcome was better among college-educated and highest-income participants and no different across age, sex, baseline metabolic subgroups, insulin use, nor between public and private clinics.
LIMITATIONS:
Findings confined to urban specialist diabetes clinics.
CONCLUSIONS:
Multi-component QI improves achievement of diabetes care goals, even in resource-challenged clinics.
TRIAL REGISTRATION:
INTRODUCTION
Diabetes is one of the fastest growing, burdensome, and costly chronic diseases, and affects an estimated 387 million people worldwide.(1) Diabetes management focuses on reducing patients’ risk of microvascular (retinopathy, nephropathy, neuropathy) and macrovascular complications (coronary, cerebrovascular, and peripheral vascular diseases) through controlling blood glucose,(2–5) blood pressure (BP),(6–8) lipids,(9, 10) and tobacco avoidance.(11, 12) Though evidence-based guidelines (13, 14) strongly recommend comprehensively controlling these cardio-metabolic parameters,(15, 16) large gaps exist in achieving care goals in actual practice globally.(17–21)
To address gaps that cannot be fully achieved with new drugs or devices, quality improvement (QI) interventions directed at patients (e.g., reminders), providers (e.g., guideline prompts), and health systems (e.g., institutionalizing a “culture of quality”)(22) can improve adherence, risk factor control, and patient satisfaction.(23, 24) Indeed, QI may have contributed to encouraging data from the United States showing risk factor improvements and parallel reductions in diabetes complications over the past two decades.(25, 26) However, gaps remain (19) and are even more substantial in low-and middle-income countries (LMIC)(20, 21) that shoulder large and growing diabetes populations and have fewer resources.(1) This is especially true in South Asia where patient- (e.g., low motivation), provider- (e.g., clinical inertia), and system-level (e.g., fragmentation of care) barriers result in small proportions of patients achieving diabetes care goals.(21) Additionally, most QI evidence comes from trials of single QI interventions.(27) In reality, however, care gaps occur due to multiple interacting factors: poor patient adherence and lack of treatment intensification by providers each explain roughly half of poor control among patients with diabetes.(28, 29)
We evaluated a multi-component diabetes QI strategy, addressing patient and provider barriers, in a pragmatic implementation trial in South Asia, a challenging LMIC region with 76 million people affected by diabetes and the highest number of annual diabetes-related deaths worldwide.(1) We tested whether multi-component QI, compared to usual care, could improve achievement of diabetes care goals over 2.5 years among patients with multiple poorly-controlled cardio-metabolic parameters.
METHODS
Design Overview
The Center for cArdio-metabolic Risk Reduction in South Asia (CARRS) Trial was a multi-center prospective, parallel randomized, controlled, open-label pragmatic trial where investigators were blinded to endpoints. Patients with poorly-controlled type 2 diabetes attending ten outpatient diabetes clinics in India and Pakistan were randomized to a multicomponent care model – consisting of non-physician care coordinators (CC) and decision-support electronic health record software (DS-EHR)– or usual care in a 1:1 ratio. Detailed methods used in the CARRS Trial have been published separately.(30)(clinicaltrials.gov registration: NCT01212328).
Institutional ethics committees at each participating site and the coordinating centers (Public Health Foundation of India, New Delhi, India and Emory University, Atlanta, USA) approved the study and all eligible patients gave written informed consent prior to enrollment. Participants were enrolled from January 2011 to June 2012 and final follow-up visits were in July 2014.
Setting and Participants
CARRS Trial sites were selected to include a diverse mix of publicly-funded, semi-private, and private outpatient clinics in India and Pakistan (please see Acknowledgements).
Men and women aged ≥35 years with type 2 diabetes and poor cardio-metabolic profiles (glycated hemoglobin [HbA1c]≥8% and either: systolic BP≥140mmHg and/or low-density lipoprotein cholesterol [LDLc]≥130mg/dl) who had attended the recruiting clinic for ≥3 months were eligible. We excluded individuals with type 1 diabetes, rare forms of diabetes, or those experiencing a documented myocardial infarction, unstable angina, or stroke in past 12 months.
Site investigators identified potentially eligible patients based on elevated HbA1c values and referred these patients to a screening officer (usually a clinic physician) for further baseline eligibility assessments. Subsequent annual assessments were conducted by a site physician. Since this was a pragmatic trial, site physicians were not purposefully blinded to the patient’s intervention status and treated participants in both arms.
Randomization and Interventions
Following baseline assessment, study staff at each clinic accessed each eligible participant’s randomization allocation –either receiving the intervention (CC+DS-EHR) or usual care– from a password-protected web-based data management system (Interactive Web Response System [IWRS]). IWRS randomized participants in blocks of four and allocation was stratified by site.
The choice of QI components for the intervention was based on extensive literature searches, conversations among the investigators who designed the study, and conversations with clinic site investigators regarding feasibility and acceptability. To our knowledge, none of the components of or the package of QI strategies that we employed have been tested in South Asia.
The intervention, as a whole, focused on improving patient self-care and facilitating better monitoring and treatment intensification by providers. Usual care participants continued to be treated at the discretion of their clinic physicians.
Intervention participants were supported by non-physician CCs, in addition to their usual physicians. CCs individualized patient follow-up based on patients’ risk level and adherence. At a minimum, per the study protocol, the CC was responsible for following up with intervention patients at least every three months for setting up laboratory or clinic appointments and contacted patients telephonically at least once a month to discuss diabetes self-management, adherence to diet plans, exercise, tobacco cessation, medication use, self-monitoring of glucose (if taking insulin), and stress management.
Treatments were aligned with evidence-based guidelines through individualized computer-generated clinical prompts –the DS-EHR integrated patients’ consultation and laboratory data and used adapted algorithms based on prevailing clinical guidelines (31) with a preference towards low-cost generic medications. CCs had a distinct access level in the DS-EHR and would record their interactions with patients. To encourage responsive and appropriate treatment intensification by physicians, CCs were responsible for entering updated patient indicators into the DS-EHR; CCs would then print management prompts generated by the DS-EHR and would review these with the treating physician imminently, instead of waiting for a subsequent patient visit. Physicians could, at their discretion, accept or reject care prompts and modify management plans based on clinical judgment, so long as justification was documented in the DS-EHR.
With scalability in low-resource settings in mind, selection criteria for CCs included: non-physicians with training in allied health fields (e.g., dietetics, social work); ≥six months healthcare experience; and good organizational and basic computing skills. CCs received intensive training (2.5 days prior to enrollment) with half-day refresher sessions at annual study meetings related to diabetes, barriers to treatment, supporting treatment changes, and motivational interviewing. CCs also had two teleconference sessions with an experienced endocrinologist to discuss common barriers and strategies to overcome them.
Outcomes and Follow-up
The primary outcome was the proportion from each group achieving multiple care targets: HbA1c<7% AND either BP<130/80mmHg or LDLc<100mg/dl (≤70 mg/dl if prior history of cardiovascular disease [CVD]) or both. We also examined between-group differences in secondary outcomes: achieving individual risk factor targets, mean risk factor changes, and patient reported outcomes (PRO) (e.g., health-related quality of life [HRQL] and treatment satisfaction scores). Other secondary outcomes assessed included: processes of care, medication use, self-care activities, acceptability of the intervention from provider and patient perspectives, and cost-effectiveness, all of which will be reported in future.
Participants in both arms attended baseline (prior to randomization), annual, and end of study (EOS) data collection visits. The EOS visit occurred between 24 months and 36 months postrandomization. As this was a pragmatic trial focused on generalizability, data collection visits were paid for by the study, but costs of interim follow-up visits, testing, medications, or procedures advised by physicians for either group were borne by patients themselves or their clinics in the case of publicly-funded settings.
Annual data collection visits included HbA1c, BP, and LDLc measurements. Blood pressure was collected using electronic devices (Omron T9P) and paper printouts were logged. Blood samples were analyzed by local laboratories which were enrolled in an external quality assurance scheme. Participants reported self-care activities (diet and exercise) and adherence to medications as the number of days diet/exercise plan was followed or medication was taken in the week prior to the visit (value between 0–7 days), respectively. The research team also asked participants to rate their HRQL (using the EQ-5D Visual Analogue Scale; score range: 0–100) and treatment satisfaction (using the Diabetes Treatment Satisfaction Questionnaire; score range: 0–32). Participants were asked open-ended questions about macro- and microvascular diabetes complications and any adverse (e.g., hypoglycemia) or serious adverse (e.g., myocardial infarction) events or hospitalizations. The severity (mild, moderate and severe) of all reported adverse events were assessed using the Hartwig and Seigel scale.(32) In addition, site investigators assessed the likelihood of whether serious adverse events were causally related to the study intervention using the Uppsala monitoring center guidelines.(33) Full study measures and definitions were published in greater detail elsewhere.(30)
Statistical Analysis
A priori sample size calculations demonstrated that 1,120 participants (560 intervention, 560 usual care) offered over 85% power (α=0.05) to detect a 40% relative difference (28% vs. 20% in absolute terms) in achieving the primary outcome, accounting for an expected 20% loss to follow-up.
All analyses were based on intention-to-treat (ITT) principles. Two-sided P<0.05 was used to indicate statistical significance. We used STATA version 14.0 (College Station, Texas USA) for all analyses.
We described participant characteristics in the intervention and usual care groups at baseline, and compared them using Student’s t-tests or Wilcoxon-rank sum tests (for normally distributed and skewed continuous variables, respectively) and chi-square or Fishers’ exact tests (for categorical variables with adequate and sparse cell counts, respectively). For the primary analysis, to determine the relative risks (RR) of achieving multiple risk factor control using all longitudinal timepoints available, we estimated log-binomial models with a generalized estimating equation (GEE) approach to account for correlation of observations within participants over time. Mean differences in continuously specified HbA1c, SBP, DBP, LDL-c were determined using linear regression with a GEE approach. Both sets of models adjusted for baseline values, treatment group, time, treatment*time interaction, and site.
We examined for heterogeneity of effect across sites and by age (35–49, 50–64, ≥65 years), sex, education (≤primary, secondary, ≥college-educated), income (<US$200, US$200–399, ≥US$400 per month), public or private clinic, body mass index (BMI: <25, 25–29.9, ≥30kg/m2), duration of diabetes (<7 years, ≥7 years based on median duration of follow-up), prior history of CVD, prior history of microvascular complications, insulin use, baseline HbA1c (≥9.0 vs. <9.0%), baseline systolic BP (≥140 vs. <140mmHg), and baseline LDLc (≥130 vs. <130mg/dl). For each of the aforementioned subgroups, we repeated the primary analysis with the addition of the subgroup variable along with its interaction with treatment. Heterogeneity was assessed based on the significance of the interaction term.
We estimated within-group baseline-to-EOS changes in HbA1c, BP, LDLc, BMI, weight, waist circumference (WC), creatinine, albumin to creatinine ratio, HRQL, and treatment satisfaction scores using paired t-tests/Wilcoxon sign rank tests. We estimated mean differences by treatment group in baseline-to-EOS changes using analysis of covariance adjusting for baseline values.
We assessed the mechanism of missingness by using Little’s test.(34) We also conducted additional analyses to examine sensitivity of our primary results to missing outcome data by applying two methods, inverse probability weighting (IPW)(35) and multiple imputation using chained equations.(36) The IPW approach weighted the analysis by the inverse of the predicted probability of being observed at any given visit. The predicted probability of being observed was computed based on a logistic model with treatment group, site, and all baseline risk factors as predictors. For multiple imputation, we used a fully conditional specification method to impute missing outcome data. We used a linear regression model that contained the variables: treatment group, age, sex, education, site, and baseline values (height, weight, waist circumference, plasma glucose, HbA1c, SBP, DBP, triglycerides, LDL-c, and HDL-c). We also assessed convergence by plotting means and standard deviation by iteration and imputation. We conducted 10 imputations and analysed the results following convention.(37)
Role of the Funding Source
This study was funded by the National Heart Lung Blood Institute, National Institutes of Health, USA; and UnitedHealth Group, USA. The funding agencies were not involved in the study design, data collection, interpretation, or writing of the report.
RESULTS
Of 1,486 participants screened, 1,146 (575 intervention and 571 usual care) were eligible and randomized. Median follow-up (and range) was 28 (22 to 36) months and 1,027 participants completed EOS visits (89.6% retention: 516 intervention, 511 usual care). Of those, 467 intervention and 463 usual care participants attended the clinic for full questionnaire, clinical, and biochemical assessments while 49 and 48 participants, respectively, completed the EOS visit at home or by phone (Figure 1).
Figure 1:
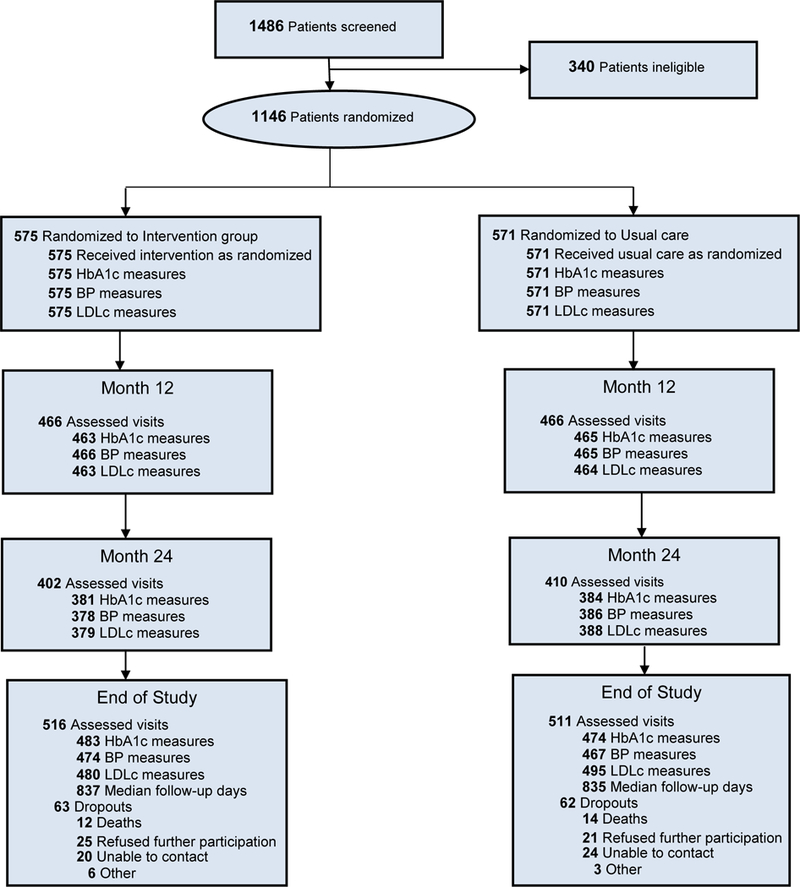
Registration, randomization and follow-up of study participants (CONSORT diagram)
Abbreviations: HbA1c (glycated haemoglobin), BP (Blood pressure), LDLc (low density lipoprotein cholesterol)
At enrollment, participant characteristics between study groups were similar (Table 1), except a higher proportion of intervention group participants were insulin users (46.3 vs. 39.8%; p=0.026). Participants’ mean age was 54.2+/−9.2 years, 45.9% were male, 70.1% had completed some high school education, and 33.6% were considered high-income (monthly income >US$400). Participants’ baseline HbA1c, BP, and LDLc were: 9.9%, 143.3/81.7mmHg, and 122.4mg/dl, respectively. Most participants (70.9%) had HbA1c≥8.0% and SBP≥140mmHg; 47.1% had HbA1c≥8.0% and LDLc≥130mg/dl; and 18.1% patients had HbA1c≥8.0%, SBP≥140mmHg, and LDLc≥130mg/dl. Mean BMI was 27.5kg/m2, mean waist circumference was 96.1cm, and 2.8% were current smokers. Median duration of diabetes was 7.0 years, 6.8% had pre-existing CVD, and 39.4% had previous microvascular complications.
Table 1:
Baseline characteristics by treatment group
| Baseline characteristic | Intervention | Usual care |
|---|---|---|
| N | 575 | 571 |
| Age (years), mean (SD) | 54.2 (9.2) | 54.2 (9.2) |
| Male, (%) | 44.9 | 47.1 |
| Education, (%) | ||
| Up to primary school | 29.4 | 29.4 |
| Secondary school | 44.9 | 42.2 |
| College graduate & above | 25.0 | 27.9 |
| Unknown | 0.7 | 0.5 |
| Income (Rs.), (%) | ||
| <10,000 (US$ 200) | 35.5 | 39.9 |
| 10,000–20,000 | 17.6 | 18.4 |
| >20,000 (US$ 400) | 35.3 | 31.9 |
| Unknown | 11.7 | 9.8 |
| Duration of diabetes, median (IQR) | 7(3–13) | 7 (3–12) |
| Current smoker, (%) | 2.4 | 3.5 |
| Co-morbidities, (%) | ||
| Previous CVD | 7.0 | 6.7 |
| Myocardial infarction | 3.1 | 3 |
| Coronary heart disease | 6.9 | 6.7 |
| Stroke | 2.3 | 1.8 |
| Previous PVD | 6.1 | 5.4 |
| Microvascular complications | 39.0 | 39.8 |
| Retinopathy | 8.5 | 8.9 |
| Neuropathy | 33.9 | 33.4 |
| Nephropathy | 9.0 | 10.5 |
| Depression | 2.1 | 2.3 |
| Examinations, mean (SD) | ||
| Waist circumference (cm) | 96.1 (11.8) | 96.1 (10.9) |
| Weight (kg) | 69.4 (12.6) | 68.9 (13.3) |
| BMI (kg/m2) | 27.5 (4.6) | 27.4 (5.4) |
| Heart rate (bpm) | 84.4 (11.9) | 83.4 (13.0) |
| Metabolic parameters, mean (SD) | ||
| HbA1c (%) | 9.9 (1.5) | 9.9 (1.7) |
| Fasting blood glucose (mg/dl) | 178.9 (61.8) | 176.4 (66.3) |
| LDLc (mg/dl) | 121.5 (36.1) | 123.2 (37.7) |
| HDLc (mg/dl) | 44.1 (8.7) | 43.9 (9.1) |
| Triglycerides (mg/dl), median (IQR) | 138.0 (107–187) | 142.0 (104–198) |
| Total cholesterol (mg/dl) | 194.7 (45.3) | 195.7 (44.3) |
| Systolic blood pressure (mmHg) | 144.2 (18.8) | 142.4 (20.0) |
| Diastolic blood pressure (mmHg) | 82.3 (11.0) | 81.0 (10.8) |
| Creatinine (mg/dl) | 0.94 (0.4) | 1.01 (0.9) |
| Urine albumin-to-creatinine ratio,median, IQR (mg/g) | 7.6 (0.16–58) | 8.1 (0.12–63) |
| Medications use, % | ||
| OHAs | 95.0 | 94.1 |
| Insulin | 46.3 | 39.8 |
| OHAs & insulin | 28.4 | 25.4 |
| BP-lowering drugs | 64.2 | 60.4 |
| Lipid-lowering drugs | 59.1 | 59.0 |
| EQ5D VAS score, mean (SD) | 69.8 (20.1) | 70.2 (19.3) |
| DTSQ score, mean (SD) | 28.4 (6.4) | 28.1 (6.7) |
All data values expressed as mean (SD) unless otherwise indicated
Footnote: All baseline characteristics were similar between treatment groups except insulin use.
Abbreviations: BP (blood pressure), CHD (Coronary Heart Disease), CVD (Cardiovascular Disease), PVD (Peripheral Vascular Disease), DTSQ (Diabetes Treatment Satisfaction Questionnaire), EQ5D VAS (European Quality of life – 5 dimensions Visual Analogue Scale), BMI (body mass index), HbA1c (glycated haemoglobin), LDLc (low-density lipoprotein cholesterol), HDLc (high-density lipoprotein cholesterol), IQR (inter quartile range), Rs (Indian Rupees), US$ (United States dollar), OHA (oral hypoglycaemic agent), bpm (beats per minute), cm (centimetres), SD (standard deviation), kg/m2 (kilogram per metre square), mg/dl (milligrams per decilitre), mg/g (milligram per gram), mmHg (millimetre of mercury)
Definitions: 1. Current smoker refers to cigarette, beedi and cigar smoking (self-reported – participants were asked about current regular use of cigarette/beedi/cigar); 2. Co-morbidities: self-reported by patients and confirmed by a physician; 2.a. Previous CVD includes acute coronary syndrome (myocardial infarction or unstable angina), angioplasty (Percutaneous coronary intervention) or Open heart surgery (coronary artery by-pass graft surgery), chronic stable angina, stroke (ischemic or hemorrhagic) or Transient Ischemic Attack, arrhythmia requiring medical intervention, heart failure. 2.b. Previous PVD includes intermittent claudication or rest pain in legs, 2.c. Neuropathy includes loss of sensation in hands/feet or sexual (erectile dysfunction), 3. Urine albumin-to-creatinine ratio: The ratio is based on measurement of albumin in milligrams and creatinine in grams.
Six CCs had undergraduate degrees in life sciences, two were dieticians, one was trained in computer applications, and one was a nurse. Across sites, CCs managed between 50 and 80 intervention arm patients each. The CCs’ primary responsibilities were to motivate diabetes selfmanagement among patients, facilitate follow-up and preventive screening visits, and with the assistance of the DS-EHR, they encouraged proactive follow-up and treatment modification(s) by physicians. CCs individualized their follow-up frequency and interactions with patients based on risk level and motivation. There was also variation in how intensively sites implemented the intervention. A detailed process evaluation across and within sites was outside the scope of this analysis.
During the study, more than twice as many intervention as usual care participants achieved the primary outcome (18.2% vs 8.1%; RR=2.24 [95%CI: 1.71, 2.92])(Figure 2). Higher proportions of intervention participants achieved HbA1c<7.0% (21.5% vs. 11.1%; RR=1.93 [95%CI: 1.52, 2.45]), SBP<130mmHg (51.0% vs. 45.0%; RR=1.14 [95%CI: 1.04, 1.26]), and LDLc targets (<100 and <70mg/dl for those without and with previous CVD)(56.4% vs. 47.1%; RR=1.23 [95%CI: 1.13, 1.34]). Of the participants with a prior history of CVD (6.5%), there was no significant between-group difference in participants achieving LDLc<70 mg/dl.
Figure 2:
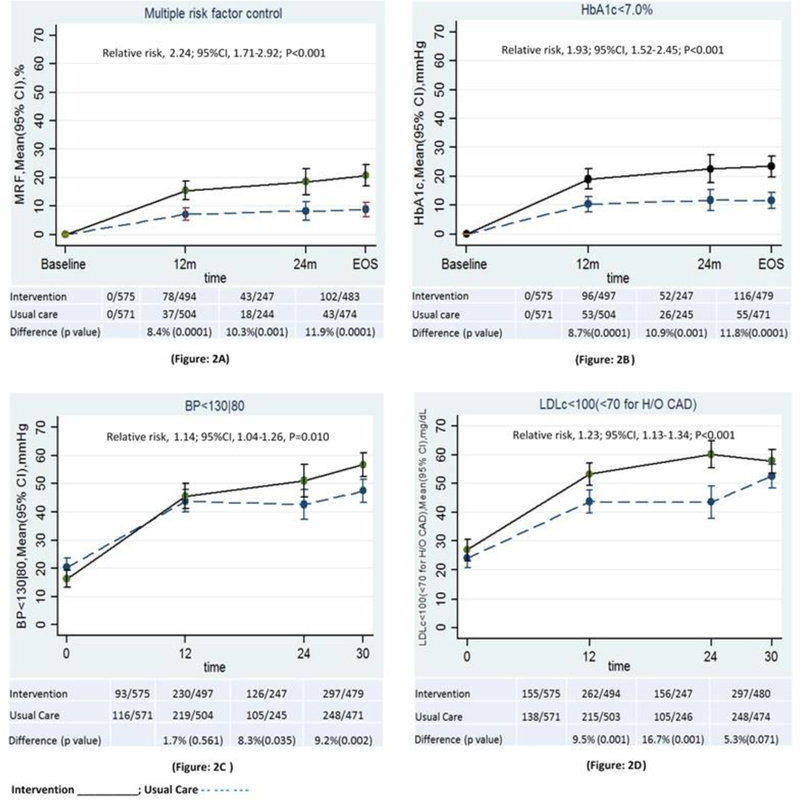
Multiple and single risk factor control by Treatment group during study follow-up
Figure 2.A Proportion change in primary study outcome (multiple risk factor control)
Figure 2.B-2.D: Proportion change in secondary outcomes (single risk factor control: HbA1c<7.0%, BP<130/80 mmHg, LDLc<100 mg/dl, respectively) by treatment group during follow-up.
Overall relative risk was obtained via log binomial models using generalised estimating equations. Estimates combine all non-missing values collected at baseline, months 12, 24, and end-of-study. Model terms included treatment, time, treatment*time interaction, baseline value, and site.
95% CΙ: indicates 95% confidence interval
Difference (p value): the difference (p value) between Intervention and Usual care at each time point
Study time points: baseline, 12m (12 month), 24m (24 month), EOS (End of Study)
Abbreviations: HbA1c (glycated haemoglobin), BP (blood pressure), LDL-c (low density lipoprotein cholesterol)
Compared to usual care, intervention participants achieved greater reductions for cardio-metabolic parameters over the study (Online Figure A): 0.50% point lower HbA1c (95%CI: −0.69, −0.32), 4.04mmHg lower SBP (95%CI: −5.85, −2.22), 2.03mmHg lower DBP (95%CI: −3.00, −1.05), and 7.86mg/dl lower LDLc (95%CI: −10.90, −4.81). Intervention participants also reported greater baseline-to-EOS improvements in HRQL, treatment satisfaction scores and medications use, but not in weight, BMI, or WC, and albumin to creatinine ratio (Online Table A).
In sensitivity analyses to address missing data (Online Table B), we noted no relative or absolute differences in the between-group likelihood of achieving the primary outcome. Achievement of the primary outcome was not statistically significantly different across sites (Online Table C) nor by age, sex, baseline metabolic profile, duration of diabetes, insulin use, or between public and private clinic settings (Figure 3). However, compared to those with up to primary schooling and lowest-income earners, college-educated or high-income participants were 2–3 times more likely to achieve the primary outcome (P=0.006 and P=0.007), respectively.
Figure 3:
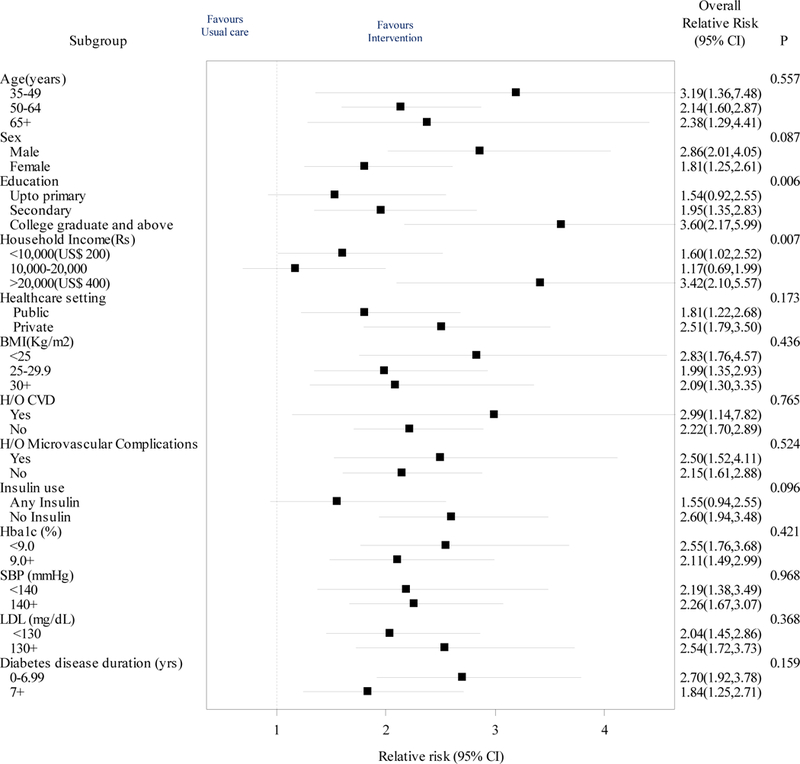
Primary outcome of achieving multiple risk factor targets by subgroups
Abbreviations: HbA1c (glycated hemoglobin), SBP (Systolic blood pressure), LDL (low density lipoprotein cholesterol), H/O (history of), CVD (cardiovascular disease), Microvascular complications (retinopathy, neuropathy and renal failure), BMI (body mass index), Rs (Indian rupees), US$ (United States dollar), CI (confidence intervals)
The primary outcome of multiple risk factor control is shown by prespecified baseline subgroups. Error bars indicate 95%CIs; P values are for the test of homogeneity for each subgroup.
There was no statistically significant between-group difference in occurrence of serious adverse events: hypoglycemic episodes requiring hospitalizations (4 vs. 1; p=0.205), new microvascular complications (163 vs. 190; p=0.080), or new macrovascular events (33 vs. 35; p=0.770), though intervention participants reported more mild hypoglycemic episodes (133 vs. 21; p<0.001) (Online Table D).
DISCUSSION
In this multi-center trial among patients with diabetes and poor cardio-metabolic profiles in India and Pakistan, a multi-component QI was associated with twice the probability of achieving combined diabetes care goals and larger baseline-to-EOS reductions for each risk factor compared to usual care. Similar effects were observed in public or private clinics, and the intervention was not associated with excess serious adverse events. This study adds to the literature in that the intervention purposefully addressed multiple interacting barriers to care and was implemented in diverse and resource-challenged healthcare settings. More importantly, the diabetes QI intervention was associated with sustained health benefits among patients at 2.5 years post-randomization. Furthermore, unlike trials where sponsors provide medications, visit costs, and self-care accessories, CARRS participants –except for annual study visits– were responsible for all of their treatment and follow-up costs and did not receive any additional incentives for their participation, increasing the generalizability of our findings. Given recent population-level improvements in cardio-metabolic profiles of people with diabetes and lower incidence rates of complications in the US (25, 26) that were likely related to QI initiatives, these data suggest that such positive trends may be replicable in LMICs. If scaled, this may have implications for population health and healthcare costs in these countries.
Few trials have evaluated comprehensive cardio-metabolic improvements in patients with diabetes.(15, 38, 39) The Steno-2 Study –based in Denmark, with universal healthcare access–enrolled 160 patients with diabetes aged 55 years with pre-existing microalbuminuria, 25% of whom also had pre-existing CVD. Over 8 years, the intervention arm achieved greater reductions in HbA1c (−0.7%), BP (−10/5mmHg), LDLc (−34mg/dl), leading to remarkable 50–70% reductions in micro- and macro-vascular disease and mortality.(15, 16) Compared to Steno-2 (baseline HbA1c~8.6%, BP~148/86mmHg, LDLc~135mg/dl), CARRS Trial participants in both arms experienced large within-group pre-post changes in HbA1c (−1.6 vs. −1.2% points), BP (−18.3 / −7.9 vs. −12.9 / −4.9 mmHg), and LDLc (−28.5 vs. −21.3 mg/dl) (Online Table A). The sizeable spillover effects in usual care participants reflect the real-life nature of QI and practice change where the same physicians treated patients in both study groups; our reported between-group effect size was, therefore, quite conservative.
The Anglo-Danish-Dutch Study of Intensive Treatment In People with Screen Detected Diabetes in Primary Care (ADDITION) offers additional context for our findings. In the study, clinics were randomized to a provider-focused intervention (educating and auditing clinic staff to better manage glycemia, BP, and lipids) or control.(40) Among 3,057 individuals aged 60 years, 9% of whom had previous CVD, and a lower-risk baseline profile (HbA1c~7.0%, BP~149/86mmHg, LDLc~135mg/dl), both arms achieved within-group improvements, with slightly larger reductions in intervention participants for mean HbA1c (−0.08%), BP (−2.9/1.4mmHg), and LDLc (−7.7mg/dl). These between-group differences were associated with more modest (5–17%) and statistically non-significant reductions in cardiovascular and microvascular outcomes over 5 years.(38, 41)
Our between-group findings fall between the Steno-2 and ADDITION findings. After 2.5 years of follow up, it is yet to be seen whether and by how much the CARRS patient and provider-focused intervention and related metabolic improvements will translate into micro- or macrovascular health benefits. This would be an informative contribution. The higher incidence of mild hypoglycemic episodes observed in CARRS participants to date are reflective of target-driven management; this undesirable effect may be mitigated through shared decision-making between patients and providers, an aspect that is increasingly integrated into QI strategies.
Our findings also offer optimism for sustained effectiveness. In a meta-analysis of 48 cluster and 94 individual randomized controlled trials testing diabetes QI interventions, the longest follow-up period was just 12 months and the largest individual-focused trial included just 206 participants.(27) The aggregate cardio-metabolic improvements reported in this meta-analysis of studies largely from high-income settings (between-group differences of −0.37% [HbA1c], −3.1/1.6 mmHg [BP], −3.9mg/dl [LDLc]), were smaller than our larger low-resource country sample at 2.5 years follow-up.(27)
In terms of acceptability, it was encouraging that intervention participants demonstrated improvements in HRQL and treatment satisfaction scores, suggesting that they did not mind the intervention’s intensity and extra follow-up. Indeed, since both intervention and usual care groups experienced 4–6 point and 0.5–2.0 increases in overall well-being and treatment satisfaction, respectively, it appears that the patients may have appreciated the extra attention provided by the study. It is hard to judge the clinical significance of these increases in PRO scores as they were not anchored to clinical outcomes or stratified by achievement of care goals,(42) but further investigation may provide more insights. Lastly, the PRO scores reported are “global” average scores by arm and may overshadow or hide specific domain changes –be they physical, mental, or emotional– that were more prominent.
Several limitations should also be acknowledged. First, we were unable to examine the relative effectiveness of individual intervention components. However, the individual components included in our QI intervention are already proven and appear to yield modest benefits on their own.(23, 24, 27) Second, although the intervention had a decisive effect in doubling achievement of the primary outcome, in absolute terms, this only amounted to 20% and 50% of intervention participants achieving combined and individual care goals, respectively. Though much improvement is possible, achieving generic dichotomous targets (e.g., HbA1c<7.0%) are increasingly less relevant in contemporary practice;(43) instead, there is strong emphasis on individualized, patient-centered goals.(44, 45) Third, our findings were confined to specialist diabetes clinics in urban South Asia, and it remains to be seen whether similar effects would be observed in primary care or rural settings. Lastly, while contamination or spillover may have limited the observed between-group differences, this pragmatic trial aimed to replicate real-life settings. Thus, our effect size estimates are conservative and these findings should be considered a proof of concept for other low-resource settings.
Diabetes prevalence and related morbidity and mortality have progressively escalated globally in the past three decades,(47) and more so in LMICs. Our findings offer an encouraging demonstration of implementing comprehensive diabetes management in LMIC settings. Broader implementation of QI strategies, as were used in CARRS, offer the potential for achieving diabetes care goals and preserving quality of life for many millions.
Supplementary Material
ACKNOWLEDGEMENTS
The Writing Group and Steering Committee of the Center for cArdio-metabolic Risk Reduction in South Asia (CARRS) Trial wish to thank all the participants and the following groups of contributors:
§CARRS Trial Group
-
(1)
Steering Committee: D Prabhakaran, K M Venkat Narayan, K Srinath Reddy, Nikhil Tandon, V Mohan, Mohammed Masood Kadir, Mohammed K Ali, Vamadevan S Ajay
-
(2)
Coordinating Center (Delhi): Nikhil Tandon, Dorairaj Prabhakaran, Kavita Singh, Raji Devarajan, Roopa Shivashankar
-
(3)
Data Management and Statistical team: Dimple Kondal, Kavita Singh, Raji Devarajan, Shivam Pandey, Ramesh CV
-
(4)
Development of Decision-support Electronic Health Record software: Nikhil Tandon, Mohammed K Ali, K M Venkat Narayan, Dorairaj Prabhakaran, Seema Shah, Roopa Shivashankar, Kavita Singh, Prashant Tandon, Ajeet Khushwaha, Sameer Maheshwari
-
(5)
Randomization Website: Ramanathan K, Ganashekaran
-
(6)
DSMB members: Dr. Anushka Patel (Chair), Dr. Ravindra Mohan Pandey, Dr. Sanjay Kalra
-
(7)
Endpoint adjudication committee: Dr V Mohan (chair), Dr Mark Huffman, Dr Pradeep Venkatesh, Dr Sanjay Kumar Agarwal, Dr Rohit Bhatia
-
(8)
Site Investigators and research staff:
Publicly-funded Clinics
-
a.
TNM College & BYL Nair Charity Hospital, Mumbai
Principal Investigator (PI): Dr. Premlata K Varthakavi; Co-Investigators (Co-I): Dr. Manoj D Chadha, Dr. Nikhil M Bhagwat; Care Coordinator(s): Ms. Roshan D’Britto, Ms.Vaibhavi Mungekar; Research Officer(s): Dr. Rohini Gajare, Mr. Abhishek Matkar, Ms. Charul Arora, Dr. Isha Verma, Dr. Yogesh Varge
-
b.
Osmania General Hospital, Hyderabad:
PI: Dr. Rakesh Kumar Sahay; Co-I(s): Dr. Neelaveni; Care Coordinator(s): Ms. Prashanthi, Ms. Priyanka Parvatini; Research Officer(s): Mr. Ramachandra Reddy
-
c.
Goa Medical College, Bambolim:
PI: Dr. Ankush Desai; Co-I(s): Dr. Kedareshwar Narvencar; Dr Vivek Naik; Care Coordinator(s): Mr. Prashant Ramesh Navelkar; Research Officer(s): Dr. Praciya Gaonkar, Ms. Rupali Naik, Dr. Santoshi Malkarnekar, Dr. Aparna Pai and Dr. Nandini Menon
-
d.
All India Institute of Medical Sciences, Delhi:
PI: Dr. Rajesh Khadgawat; Care Coordinator(s): Ms. Prerna Gupta, Ms. Kanika Aggarwal, Ms. Mansi Chopra; Research Officer(s): Dr. Samita Ambekar, Dr. Manish Sachdeva, Ms. Bhanvi Arora, Dr. Prashant Singh
Semi-private Clinics
-
e.
St. John’s Medical College & Hospital, Bangalore:
PI: Dr. Ganapati Bantwal; Co-I(s): Dr. Prem Pais, Dr. Vaggesh Aiyyar, Dr. Anantharaman, Dr. Vivek Mathew; Study Coordinator: Dr. Sudha Suresh; Care Coordinator(s): Ms. Jomia Michael; Research Officer(s): Ms. Silambarasi, Ms. Praveena Simha, Mr. Ritish Colleru, Ms. Tanmayi Bollina, Ms. Vidhya Lakshmi, Ms. Krithika Rajkumar
Private Clinics
-
f.
Amrita Institute of Medical Sciences, Kochi
PI(s): Dr. A.G. Unnikrishnan (former), Dr. V Usha Menon (current); Co-I(s): Dr. Praveen VP, Dr. Nisha Bhavani, Dr. Nithya Abraham; Care Coordinator(s): Ms. Akhila Ghosh, Ms. Nimmi P.V; Research Officer(s): Mr. Kamaljith
-
g.
CARE Hospital, Hyderabad
PI: Dr.Bipin Sethi; Co-I: Dr. Mazher Ali; Care Coordinator(s): Mr. Pandurang Balaji; Research Officer(s): Dr. Mazher Ali
-
h.
h. MV Hospital, Chennai
PI: Dr. Vijay Vishwanathan; Co-I(s): Dr. M. Jai Ganesh; Study Coordinator: Mr. M. Anand Kumar; Care Coordinator(s): Ms. Anitha; Research Officer(s): Mr.M. Anand Kumar
-
i.
i. Bangalore Endocrinology & Diabetes Research Centre, Bangalore:
PI: Dr. Mala Dharmalingam; Co-I(s): Dr. Priti Shankar; Care Coordinator(s): Ms. Nalinakshi Reddy, Ms. Asmath Unnisa, Ms. Poonam, Ms. Shweta; Research Officer(s): Ms. Nandini, Ms. Kavya
-
j.
j. Aga Khan University, Karachi:
PI: Dr. Muhammad Qamar Masood; Co-I(s): Dr. Abdul Jabbar, Dr. Imran Naeem, Dr. Adeel Khan; Study Coordinator: Dr. Hassan Daudzai; Care Coordinator(s): Ms. Sabahat Naz; Research Officer(s): Ms. Nida Zaidi
-
a.
FUNDING SOURCES:
The CARRS Trial has been funded in part by the National Heart, Lung, and Blood Institute, National Institutes of Health (NIH), Department of Health and Human Services, under Contract No.HHSN268200900026C, and the United Health Group, Minneapolis, MN, USA. Several members of the research team at Public Health Foundation of India (PHFI) and Emory University were/are supported by the Fogarty International Clinical Research Scholars – Fellows program (FICRS-F) through Grant Number 5R24TW007988 from NIH, Fogarty International Center (FIC) through Vanderbilt University, Emory’s Global Health Institute, and D43 NCDs in India Training Program through Award Number 1D43HD05249 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) and FIC. Kavita Singh is supported by the Fogarty International Centre, National Institutes of Health, under Award Number: D43TW008332 (ASCEND Research Network). The contents of this publication is solely the responsibility of the author/s and does not necessarily represent the official views of the National Institutes of Health or the ASCEND Research Network.
FUNDING SOURCE: The National Heart, Lung, Blood Institute, National Institutes of Health, and UnitedHealth Group, USA
Footnotes
REPRODUCIBILITY OF RESEARCH STATEMENT
Protocol: available upon request
Statistical Code: available upon request
Data: available upon request
Contributor Information
Mohammed K. Ali, Emory University, Rollins School of Public Health,1518 Clifton Road, Rm CNR 701, Atlanta, Georgia, 30322, USA.
Kavita Singh, All India Institute of Medical Sciences, Department of Endocrinology & Metabolism,Biotechnology Block, 3rd Floor, Ansari Nagar, New Delhi - 110 029, India and Centre for Chronic Disease Control, India.
Dimple Kondal, Center of Excellence - Center for CArdio-metabolic Risk Reduction in South Asia,Public Health Foundation of India, 4th Floor, Plot No. 47, Sector 44, Institutional Area, Gurgaon - 122 002, Haryana.
Raji Devarajan, Center of Excellence - Center for CArdio-metabolic Risk Reduction in South Asia,Public Health Foundation of India, 4th Floor, Plot No. 47, Sector 44, Institutional Area, Gurgaon - 122 002, Haryana.
Shivani A Patel, Emory University, Rollins School of Public Health,1518 Clifton Road, CNR 6th Floor, Atlanta, Georgia, 30322, USA.
Roopa Shivashankar, Center of Excellence - Center for CArdio-metabolic Risk Reduction in South Asia,Public Health Foundation of India, 4th Floor, Plot No. 47, Sector 44, Institutional Area, Gurgaon - 122 002, Haryana.
Vamadevan S Ajay, Center of Excellence - Center for CArdio-metabolic Risk Reduction in South Asia,Public Health Foundation of India, 4th Floor, Plot No. 47, Sector 44, Institutional Area, Gurgaon - 122 002, Haryana.
A.G. Unnikrishnan, Chellaram Diabetes Institute, Lalani Quantum, Pune-Bangalore National Highway 4, Bavdhan, (Budruk), Pune, Maharashtra 411021.
V Usha Menon, Amrita Institute of Medical Sciences, Department of Endocrinology & Diabetes, AIMS Ponekkara P.O., Kochi - 682 041, Kerala, India.
Premlata K. Varthakavi, TNM College & BYL Nair Charity Hospital, Department of Endocrinology, Dr. A. L. Nair Road, Mumbai Central, Mumbai - 400 008, Maharashtra, India.
Vijay Vishwanathan, MV Hospital for Diabetes & Diabetes Research Centre, No 4, West Madha Church Street, Royapuram, Chennai - 600 013, Tamil Nadu, India.
Mala Dharmalingam, Bangalore Endocrinology & Diabetes Research Centre, #35, 5th Cross, Malleswaram Circle, Bangalore - 560 003, Karantaka, India.
Ganapati Bantwal, St. John’s Medical College & Hospital, Department of Endocrinology, Sarjapur Road, Koramangala, Bangalore - 560 034, Karantaka, India.
Rakesh Kumar Sahay, Osmania General Hospital, Department of Endocrinology, 2nd Floor, Golden Jubilee Block, Afzalgunj, Hyderabad - 500 012, Telangana, India.
Muhammad Qamar Masood, Aga Khan University, Department of Medicine, Section of Endocrinology and Diabetes, Stadium Road Karachi 74800, Pakistan.
Rajesh Khadgawat, All India Institute of Medical Sciences, Department of Endocrinology & Metabolism, Biotechnology Block, 3rd Floor, Ansari Nagar, New Delhi - 110 029, India.
Ankush Desai, Goa Medical College, Endocrine Unit, Department of Medicine, Bambolim, Goa 403202, India,.
Bipin Sethi, CARE Hospital, Department of Endocrinology, Road No 1, Banjara Hills, Hyderabad - 500 034, Telangana, India.
Dorairaj Prabhakaran, Centre for Control of Chronic Conditions, Public Health Foundation of India, 4th Floor, Plot No. 47, Sector 44, Institutional Area, Gurgaon - 122 002, Haryana.
K.M. Venkat Narayan, Emory University, Rollins School of Public Health, 1518 Clifton Road, Rm CNR 7049, Atlanta, Georgia, 30322, USA.
Nikhil Tandon, All India Institute of Medical Sciences, Department of Endocrinology & Metabolism, Biotechnology Block, 3rd Floor, Rm #312, Ansari Nagar, New Delhi - 110 029, India.
REFERENCES
- 1.International Diabetes Federation. Diabetes Atlas (2014 Update). 2014. [Google Scholar]
- 2.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 3.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89. [DOI] [PubMed] [Google Scholar]
- 6.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–40. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165(12):1410–9. [DOI] [PubMed] [Google Scholar]
- 9.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–16. [DOI] [PubMed] [Google Scholar]
- 10.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78. [DOI] [PubMed] [Google Scholar]
- 11.Mohiuddin SM, Mooss AN, Hunter CB, Grollmes TL, Cloutier DA, Hilleman DE. Intensive Smoking Cessation Intervention Reduces Mortality in High-Risk Smokers With Cardiovascular Disease. Chest. 2007;131(2):446–52. [DOI] [PubMed] [Google Scholar]
- 12.Critchley JA, Capewell S. Mortality Risk Reduction Associated With Smoking Cessation in Patients With Coronary Heart Disease: A Systematic Review. JAMA. 2003;290(1):86–97. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. 8. Cardiovascular Disease and Risk Management. Diabetes Care. 2016;39(Supplement 1):S60–S71. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. 3. Foundations of Care and Comprehensive Medical Evaluation. Diabetes Care. 2016;39(Supplement 1):S23–S35. [DOI] [PubMed] [Google Scholar]
- 15.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–93. [DOI] [PubMed] [Google Scholar]
- 16.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–91. [DOI] [PubMed] [Google Scholar]
- 17.Stone MA, Charpentier G, Doggen K, Kuss O, Lindblad U, Kellner C, et al. Quality of care of people with type 2 diabetes in eight European countries: findings from the Guideline Adherence to Enhance Care (GUIDANCE) study. Diabetes Care. 2013;36(9):2628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zabetian A, Keli HM, Echouffo-Tcheugui JB, Narayan KM, Ali MK. Diabetes in the Middle East and North Africa. Diabetes Res Clin Pract. 2013;101(2):106–22. [DOI] [PubMed] [Google Scholar]
- 19.Ali MK, Bullard KM, Gregg EW, Del Rio C. A cascade of care for diabetes in the United States: visualizing the gaps. Ann Intern Med. 2014;161(10):681–9. [DOI] [PubMed] [Google Scholar]
- 20.Mudaliar U, Kim WC, Kirk K, Rouse C, Narayan KM, Ali M. Are recommended standards for diabetes care met in Central and South America? A systematic review. Diabetes Res Clin Pract. 2013. [DOI] [PubMed] [Google Scholar]
- 21.Shivashankar R, Kirk K, Kim WC, Rouse C, Tandon N, Narayan KM, et al. Quality of diabetes care in low- and middle-income Asian and Middle Eastern countries (1993–2012)−−20-year systematic review. Diabetes Res Clin Pract. 2015;107(2):203–23. [DOI] [PubMed] [Google Scholar]
- 22.Kerr EA, Gerzoff RB, Krein SL, Selby JV, Piette JD, Curb JD, et al. Diabetes Care Quality in the Veterans Affairs Health Care System and Commercial Managed Care: The TRIAD Study. Annals of Internal Medicine. 2004;141(4):272–81. [DOI] [PubMed] [Google Scholar]
- 23.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001;24(10):1821–33. [DOI] [PubMed] [Google Scholar]
- 24.Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA. 2006;296(4):427–40. [DOI] [PubMed] [Google Scholar]
- 25.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368(17):1613–24. [DOI] [PubMed] [Google Scholar]
- 26.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514–23. [DOI] [PubMed] [Google Scholar]
- 27.Tricco AC, Ivers NM, Grimshaw JM, Moher D, Turner L, Galipeau J, et al. Effectiveness of quality improvement strategies on the management of diabetes: a systematic review and meta-analysis. Lancet. 2012;379(9833):2252–61. [DOI] [PubMed] [Google Scholar]
- 28.Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27(7):1535–40. [DOI] [PubMed] [Google Scholar]
- 29.Schmittdiel JA, Uratsu CS, Karter AJ, Heisler M, Subramanian U, Mangione CM, et al. Why don’t diabetes patients achieve recommended risk factor targets? Poor adherence versus lack of treatment intensification. J Gen Intern Med. 2008;23(5):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah S, Singh K, Ali MK, Mohan V, Kadir MM, Unnikrishnan AG, et al. Improving diabetes care: multi-component cardiovascular disease risk reduction strategies for people with diabetes in South Asia--the CARRS multi-center translation trial. Diabetes Res Clin Pract. 2012;98(2):285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Standards of Medical Care in Diabetes—2010. Diabetes Care. 2010;33(Supplement 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229–32. [PubMed] [Google Scholar]
- 33.International Diabetes Federation. IDF Diabetes Atlas, 6th Edition Brussels, Belgium: International Diabetes Federation; 2013. [Google Scholar]
- 34.Little RJA. A Test of Missing Completely at Random for Multivariate Data with Missing Values. Journal of the American Statistical Association. 1988;83(404):1198–202. [Google Scholar]
- 35.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–95. [DOI] [PubMed] [Google Scholar]
- 36.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchenko YV, Eddings W. A note on how to perform multiple-imputation diagnostics in Stata. 2011. [Google Scholar]
- 38.Griffin SJ, Borch-Johnsen K, Davies MJ, Khunti K, Rutten GE, Sandbaek A, et al. Effect of early intensive multifactorial therapy on 5-year cardiovascular outcomes in individuals with type 2 diabetes detected by screening (ADDITION-Europe): a cluster-randomised trial. Lancet. 2011;378(9786):156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bittner V, Bertolet M, Barraza Felix R, Farkouh ME, Goldberg S, Ramanathan KB, et al. Comprehensive Cardiovascular Risk Factor Control Improves Survival: The BARI 2D Trial. Journal of the American College of Cardiology. 2015;66(7):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G, et al. The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. Int J Obes Relat Metab Disord. 2000;24 Suppl 3:S6–11. [DOI] [PubMed] [Google Scholar]
- 41.Sandbaek A, Griffin SJ, Sharp SJ, Simmons RK, Borch-Johnsen K, Rutten GE, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial: the ADDITION-Europe Study. Diabetes Care. 2014;37(7):2015–23. [DOI] [PubMed] [Google Scholar]
- 42.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. Journal of Clinical Epidemiology. 2003;56(5):395–407. [DOI] [PubMed] [Google Scholar]
- 43.Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013. [Google Scholar]
- 44.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9. [DOI] [PubMed] [Google Scholar]
- 45.Ismail-Beigi F, Moghissi E, Tiktin M, Hirsch IB, Inzucchi SE, Genuth S. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011;154(8):554–9. [DOI] [PubMed] [Google Scholar]
- 46.Alshurafa M, Briel M, Akl EA, Haines T, Moayyedi P, Gentles SJ, et al. Inconsistent definitions for intention-to-treat in relation to missing outcome data: systematic review of the methods literature. PLoS One. 2012;7(11):e49163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali MK, Jaacks LM, Kowalski AJ, Siegel KR, Ezzati M. Noncommunicable Diseases: Three Decades Of Global Data Show A Mixture Of Increases And Decreases In Mortality Rates. Health Affairs. 2015;34(9):1444–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


