Abstract
Clear cell renal cell carcinoma (ccRCC) is a malignancy characterized by deregulated hypoxia-inducible factor signaling, mutation of several key chromatin modifying enzymes, and numerous alterations in cellular metabolism. Pre-clinical studies have historically been limited to cell culture models, however, the identification of critical tumor suppressors and oncogenes from large-scale patient sequencing data has led to several new genetically engineered mouse models with phenotypes reminiscent of ccRCC. In this review, we summarize recent literature on these topics and discuss how they inform targeted therapeutic approaches for the treatment of ccRCC.
Keywords: Cancer, hypoxia-inducible factors, genetics, mouse models, lipid metabolism, therapy
1. Introduction
Kidney cancer is the 8th most prevalent cancer diagnosed each year in the United States, with an estimated 64,000 new cases in 2017 [1]. The median age at diagnosis for both sexes is 64, and total overall incidence rate has been increasing over the past decade. Clear cell renal cell carcinoma (ccRCC) accounts for 70–80% of kidney cancer diagnoses, and represents the most aggressive subtype of this disease [2]. Risk factors for ccRCC include smoking, obesity, von Hippel-Lindau (VHL) disease (defined below), and hypertension. If disease is detected early and still localized within the kidney, surgical resection or nephrectomy is performed with a 5-year survival rate of ~92%. However, if the tumor has spread locally or systemically, 5-year survival rates drop to 67% or 12%, respectively, due to limitations in current standard-of-care therapies for metastatic disease [1].
A defining morphological hallmark of ccRCC is robust lipid and glycogen accumulation in the cytoplasm of tumor cells, giving rise to the name “clear cell”. Widespread metabolic reprogramming in glucose, lipid, and amino acid metabolism broadly contributes to the clear cell phenotype [3], although the advantages conferred unto tumor cells harboring these adaptations are incompletely understood. Compared with other solid tumors driven by loss of classical tumor suppressors or activation of oncogenes (e.g., those encoding p53 and K-Ras), ccRCC has been labeled as a “metabolic disease” supported by alterations in myriad bioenergetic pathways [4], [5]. Genetic hallmarks include biallelic inactivation of the VHL tumor suppressor gene, the negative regulator of hypoxia-inducible factor (HIF) proteins, as well as copy number alterations of chromosome 3p, 5q, and 14q genes, and high frequency of mutation in chromatin modifying enzymes (e.g., PBRM1, SETD2, and BAP1). Here, we discuss recent advances in ccRCC research related to elucidating the underlying genetic drivers of human disease, progress on obtaining an autochthonous, genetically engineered mouse model (GEMM) for pre-clinical studies, metabolic reprogramming, and therapeutic strategies moving forward.
2. Genetics of ccRCC
2.1. VHL loss and HIF-α stabilization
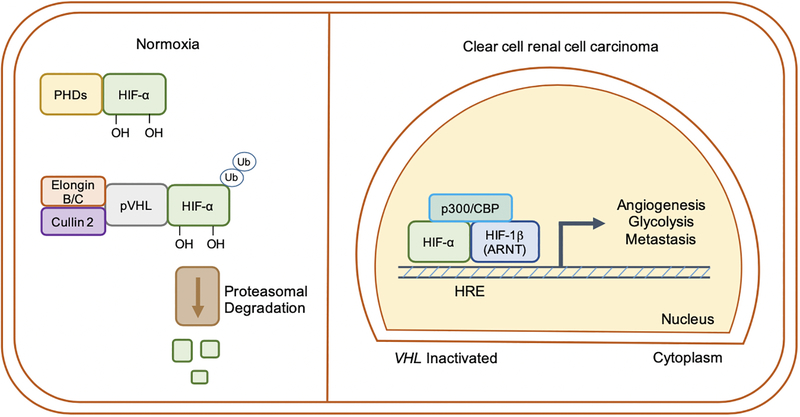
The most common genetic event of ccRCC is copy number deletion, inactivating mutation, and/or epigenetic silencing of VHL (encoding pVHL), the recognition component of an E3-ubiquitin ligase complex responsible for targeting HIF-1α and HIF-2α for proteasomal degradation under normoxic conditions (Figure 1). VHL disease is a familial disorder marked by increased propensity for ccRCC, retinal and cerebellar hemangioblastoma, and pheochromocytoma development. In hereditary disease, patients inherit only one functional copy of the VHL gene, and subsequent loss of heterozygosity (LOH) results in disease [6]. pVHL loss results in constitutive stabilization of HIF-α subunits even under oxygen-replete conditions, which translocate to the nucleus and heterodimerize with HIF-1β (ARNT) [7]. HIFs transcriptionally activate numerous genes involved in cellular processes including glycolysis, angiogenesis, and metastasis of cancer cells [8]. Notably, while VHL inactivation occurs in upwards of 90% of all patients, its loss alone is insufficient to generate ccRCC tumors [9], [10]. Characterization of HIF-α protein accumulation in ccRCC has revealed distinct patterns; ~10% retain wildtype VHL and express neither HIF-1α nor HIF-2α, ~60% of tumors express both HIF-1α and HIF-2α, and ~30% express HIF-2α alone [11]. This can occur as a result of loss of chromosome 14q as disease advances, on which the HIF1A gene resides [12], [13]. Indeed, HIF-1α has been linked with tumor suppressive functions in ccRCC [13], [14], while HIF-2α has been established as a dominant oncogenic driver of disease progression [15], [16], [17], [18]. These observations have supported efforts to develop small molecule antagonists of HIF-2α, which are currently being tested in clinical trials for the treatment of metastatic ccRCC [19], and will be discussed later.
Figure 1. Regulation of hypoxia-inducible factor (HIF) signaling by the von Hippel-Lindau (VHL) tumor suppressor.
Under oxygen-replete conditions, HIF-α subunits are hydroxylated by prolyl hydroxylases (PHDs) and then ubiquitinated by an E3-ubiquitin ligase complex containing pVHL, tagging them for proteasomal degradation. In hypoxia, or when VHL is inactivated (such as in ccRCC), HIF-α subunits escape degradation, translocate to the nucleus, and |heterodimerize with HIF-1β (ARNT). HIFs generally promote a transcriptional program favoring increased angiogenesis, glycolysis, and metastatic capabilities of ccRCC tumors. HRE = hypoxia response element.
2.2. Copy number and single nucleotide variation
Recent sequencing studies involving large cohorts of ccRCC patients have revealed signatures of copy number amplification and deletion across the tumor genome [20], [21]. A 43 megabase region of chromosome 3p contains multiple bona fide or putative tumor suppressor genes including VHL, PBRM1, BAP1, and SETD2 (discussed below) [22]. Mechanistically, this gene inactivation occurs on one allele through intergenic point mutation, and on the second allele through LOH [23], [24]. On a genome-wide scale, copy number variation of the following regions are most abundant in ccRCC tumors: chromosome 3p loss (91%), 5q gain (67%), and 14q loss (49%) [25].
While chromosome 3p and 14q genes are generally associated with having tumor suppressive functions in ccRCC, copy number amplification of a ~60 gene region of chromosome 5q35 harbors candidate oncogenes [21], [25]. SQSTM1, which encodes the p62 protein involved in activation of NRF2 and resistance to oxidative stress, was shown to be a chromosome 5q gene frequently amplified in ccRCC patient samples and cell lines [26]. Suppression of p62 decreased resistance to redox stress and soft agar colony formation in vitro and xenograft tumor growth in vivo. Additionally, siRNA-mediated suppression of SQSTM1 reduced mechanistic target of rapamycin (mTOR) signaling, a key nutrient sensing pathway involved in the regulation of cell proliferation, protein synthesis, and autophagy, suggesting multiple mechanisms by which p62 promotes ccRCC progression [27]. Other work has implicated the chromosome 5q genes EZH2, STC2, and VCAN, which were all copy number gained and overexpressed at the mRNA level, as having oncogenic functions in ccRCC [21], [28]. These data suggest that the chromosome 5q hotspot of gene duplication may contain several clinically relevant targets.
Genome-wide association studies (GWAS) have also been conducted in ccRCC patients to illuminate single nucleotide variants predisposing individuals to the disease. A 2017 study identified seven new susceptibility loci, including a single nucleotide polymorphism (SNP) within a predicted intronic enhancer of DPF3, a histone acetylation and methylation reader protein of the BRG1-associated factor (BAF) 180 and polybromo-BAF (PBAF) chromatin remodeling complexes [29]. Interestingly, the location of this SNP (14q24) is deleted in one quarter to half of patients [30], and dysregulation of other components of BAF and PBAF complexes are common features of ccRCC. The same group also identified BHLHE41, a gene encoding a basic helix-loop-helix protein located in the 12p12.1 susceptibility locus, from GWAS [31]. The ccRCC risk allele associated with this gene increased its expression, and the authors provided functional evidence that ectopic expression of BHLHE41 increased xenograft tumor growth.
An earlier GWAS identified two SNPs associated with RCC susceptibility within a 4.2 kb region of the first intron of EPAS1 (encoding HIF-2α) as well as another at 11q13.3, which is not localized intergenically but flanks MYEOV and CCND1 (encoding cyclin D1) [32]. Additional work demonstrated that the risk SNP at this CCND1 enhancer promoted HIF-2α binding, thereby increasing the mRNA expression of this oncogenic cell cycle regulator [33]. The variants within EPAS1 are also notable as HIF-2α inhibition has been repeatedly demonstrated to reduce ccRCC growth [17], [18], [34]). However, the functional relationship between intronic EPAS1 SNPs and its mRNA expression was not determined. A study of familial renal cell carcinoma identified mutations in the CDKN2B gene through exon sequencing as predisposing individuals to tumor development [35]. CDKN2B encodes the p15INK4B protein, which normally functions as a tumor suppressor by binding and inhibiting cyclin-dependent kinases 4 and 6 to prevent cell cycle progression [36]. These mutations, which germline-inactivated CDKN2B in 5% of patients, were predicted to destabilize the interaction between p15INK4B and the CDKs. Furthermore, expression of wildtype p15INK4B in vitro was shown to suppress colony formation relative to the mutant isoforms, providing support for its ability to act as a tumor suppressor in ccRCC.
Perhaps not unexpectedly, subclonal heterogeneity is a common feature within ccRCC tumors [24]. Such variation manifests as spatially separated mutational patterns and chromosomal imbalances, which create a range of intratumoral phenotypes. For example, gene expression patterns associated with both good and poor prognoses can be found within the same tumor, and roughly 2/3rd of all somatic mutations are not detectable in every sequenced region [23]. The degree to which clonal heterogeneity affects disease progression and informs treatment strategies will be an important consideration for both researchers and clinicians moving forward. Nevertheless, additional studies on copy number and single nucleotide variation may help elucidate the etiology of ccRCC, as well as identify new therapeutic targets of interest.
2.3. Histone and chromatin modifying enzyme mutations
After mutation of VHL (53% of patients), PBRM1, SETD2, and BAP1 are the most commonly mutated genes in ccRCC at 40%, 13%, and 10% of patients, respectively [37]. PBRM1 encodes BAF180, which is the defining subunit of the PBAF switch/sucrose non-fermentable (SWI/SNF) chromatin remodeling complex which uses ATP for nucleosome repositioning [38]. Varela, et al. demonstrated that suppression of PBRM1 through siRNA resulted in enhanced proliferation, colony formation, and migration of several ccRCC cell lines in vitro, consistent with a tumor suppressive function in renal carcinoma [38]. Interestingly, Gao, et al. did not recapitulate these in vitro findings, but found that PBRM1 suppression by CRISPR did promote subcutaneous tumor growth in an on-target fashion [39]. Inactivating or truncating mutations in JARID1C (also known as KDM5C), a histone H3 lysine 4 demethylase, and UTX (KMD6A) a histone H3 lysine 27 demethylase were identified in another study as positively selected for in ccRCC compared to other cancers [40], [41]. Depletion of SETD2, the histone H3 lysine 36 tri-methyltransferase catalyzing methylation at sites of active transcription, was associated with DNA replication stress and reduced loading of nucleosome components onto chromatin [42]. The authors speculated that SETD2 loss of function during disease progression could be a source of genomic instability and heterogeneity within ccRCC. BAP1, a deubiquitinating enzyme involved in transcriptional repression of genes as a polycomb-group protein, exhibits mutations that are largely mutually exclusive with PBRM1 [43]. How hypermethylation of enhancer and promoter CpG regions affects ccRCC progression is an ongoing area of investigation in the field [44].
3. Genetic mouse models of renal cell carcinoma
3.1. Tumor suppressor deletion
As VHL deletion is insufficient to drive ccRCC tumorigenesis [45], conditional knockout mice have been generated in several laboratories combining deletion of candidate tumor suppressor genes based on large-scale patient sequencing data [25], [46]. A recent report combined Vhl and Pbrm1 deletion throughout renal tubules, collecting ducts, and thick ascending limbs using Ksp-Cre [47], and found that 67% of these mice first developed polycystic kidney disease (PKD) within 10–14 months of age [48]. While deletion of either gene alone did not result in PKD or ccRCC, 50% of the double knockout (Vhl−/−, Pbrm1−/−) mice showed signs of tumor incidence after 10 months of age, with clear cell morphology, activated mTOR signaling, and reduction in oxidative phosphorylation gene signatures reported. Notably, while the primary tumors were orthotopically transplantable into NSG mice, metastatic tumor burden was not detected in Vhl−/−, Pbrm1−/− mice.
Though TP53 deletion or mutation is relatively uncommon in human ccRCC tumors, another study examined the effect of conditional Vhl, Trp53, and Rb1 loss in the renal epithelium (utilizing Ksp-Cre), as changes in regulators of the p53 pathway and the G1/S cell cycle transition are frequently copy number modified according to The Cancer Genome Atlas (TCGA) data [49]. The authors found evidence of tumor onset as early as 7.5 months following triple knockout in a subset of mice, which were characterized by HIF-α and mTORC1 pathway activation by carbonic anhydrase 9 and phospho-4E-BP1 immunostaining, respectively. Further analyses of a larger cohort suggested 82% of Vhl−/−, Trp53−/−, Rb1−/− mice developed tumors within 15 months of gene deletion, the majority of which were classified as grade 3 or 4 tumors growing in acinar or pseudo-papillary patterns. These renal tumors had clear or weakly stained cytoplasm, and displayed gene expression and mutational signatures similar to human ccRCC. While some Vhl−/−, Trp53−/−, Rb1−/− tumors were sensitive to 1st and 2nd line chemotherapies sunitinib and everolimus, nearly all were resistant to 3rd line treatment with acriflavine, the pan-HIF-α inhibitor [50]. These results mimic the variability in drug responsiveness typical of the human disease. Similar to Vhl and Pbrm1 co-deletion, Vhl−/−, Trp53−/−, Rb1−/− mice developed tumors which remained localized within the kidney.
Consistent with chromosome 3p genes displaying tumor suppressive functions in ccRCC, the effect of Bap1 deletion in combination with either Vhl or Pbrm1 loss has been examined in several publications from the Brugarolas group. Under the control of Six2-Cre driver, which is expressed in multipotent nephron progenitor cells of the kidney during development, the authors first generated Vhl−/−, Bap+/− mice (deletion of both alleles of Vhl and one of Bap1) [51]. These mice developed kidneys with atypical cysts and neoplasia resembling those of patients with VHL syndrome. Some of the lesions observed were similar to early-stage ccRCC tumors by cytoplasmic clearing, Ki67 expression, and phospho-S6 positivity as a marker of mTOR activity, yet tumors remained small overall. Again, tumors from this mouse model did not metastasize, and Vhl−/−, Bap1+/− mice died after 8 months from renal failure, suggesting that additional events are required for ccRCC progression.
A more recent study from this group used additional Cre drivers to modulate Pbrm1 and Bap1 levels in different lineages within the kidney. Pax8-Cre was used to delete Vhl and Bap1 or Pbrm1 within the proximal and distal renal tubules, loops of Henle, collecting ducts, and parietal cells of the Bowman capsule [52]. Sglt2- and Villin-Cre were also used for more specific deletion within proximal tubule epithelial cells. Tumors generated with Pax8-Cre were larger than those generated with the Six2-Cre model investigated previously. Interestingly, deletion of both alleles of Vhl along with a single allele of either Pbrm1 or Bap1 using Sglt2- and Villin-Cre drivers did not result in ccRCC formation. Based on these data, the authors speculated that ccRCC may be derived from the parietal epithelial cells of the Bowman capsule, rather than proximal tubule cells, as previous histologic and gene expression data would suggest [53], [54]. The authors also found that activation of mTORC1 signaling through inactivation of one allele of Tsc2 caused the formation of higher grade ccRCC specifically in Pbrm1-deficient kidneys. Notably, patients with PBRM1 and BAP1 mutations have distinct gene expression patterns and clinical outcomes, and these alterations are largely mutually exclusive [55]. While BAP1-deficient tumors are higher in grade and portend worse prognosis than PBRM1-deficient tumors, it is believed that BAP1 mutations are secondary to PBRM1 loss in the pathogenesis of ccRCC [56], [57].
3.2. Oncogene activation
An early mouse model of ccRCC was generated using a constitutively active mutant of HIF-1α, termed TRACK (transgenic model of cancer of the kidney) [58]. HIF-1α protein is found in VHL-deficient renal epithelial cells and in early tubular lesions, cysts, and ccRCC, suggesting it is important for carcinogenesis [45]. TRACK mice expressed a triple-mutant HIF-1α, which was insensitive to hydroxylation and therefore degradation by the PHD/pVHL system, under the control of the γ-glutamyl transferase (GGT) proximal tubule-specific promoter. Phenotypes included abnormal vascularization, renal cysts, cleared cells, and high carbonic anhydrase 9 staining. A small percentage of cleared cells accumulated γH2AX, a marker of DNA double-strand breaks, although the mechanism by which HIF-1α led to genomic instability was not described. Even though HIF-1α exerts tumor suppressive functions in established ccRCC, this study demonstrates it is a critical downstream mediator of VHL loss early in disease progression. Accordingly, neither late-stage nor metastatic disease was detected in TRACK mice.
Another two studies investigated the role of MYC overexpression in the kidney and its role in promoting renal tumorigenesis. According to TCGA data, genomic amplification of MYC only occurs in about 5–10% of patients, however, the MYC/MAX transcriptional network has been predicted to promote glycolysis, de-differentiation, and growth in a majority of ccRCC [59]. MYC pathway activation is also associated with a hereditary RCC characterized by translocation of chromosomes 3 and 8 [60]. Conditional overexpression of MYC, but not KRAS, under the GGT gene promoter gave rise to a highly aggressive RCC that most resembled collecting-duct carcinoma [61]. Combined gene expression and metabolite analysis, along with small molecule inhibition of glutaminase, revealed a dependency on glutamine for sustained growth of these tumors, similar to the human disease.
In another report, the combination of MYC overexpression along with further deletion of Vhl and/or Cdkn2a (encoding Ink4/Arf) was investigated for the ability to induce papillary or clear cell renal cell carcinoma [62]. This study utilized the Ksp promoter to drive expression of a doxycycline-inducible Myc transgene. When MYC was overexpressed without deletion of either Vhl or Cdkn2a, the mice developed tumors histologically consistent with papillary RCC. However, Vhl deletion in combination with MYC overexpression (“VM”) mice generated tumors more similar to ccRCC based on cytoplasmic clearing, necrosis, and hemorrhage. Addition of Cdkn2a deletion to this model (“VIM”) further potentiated ccRCC formation compared with Vhl deletion alone, increasing tumor volume and decreasing median survival time. VIM mice had a median survival of 29.5 weeks following gene inactivation/induction, whereas, VM mice had a median survival of 57 weeks. Importantly, 2 out of 6 VIM mice developed metastasis to the liver, and in vitro characterization of a cell line derived from this mouse model showed increased expression of epithelial-mesenchymal transition genes as well as increased matrigel invasion relative to a VM line.
ccRCC research is currently limited by the lack of a GEMM that accurately represents the complete spectrum of phenotypes characteristic of human disease. As evidenced by the publications described above, the various methods used to either delete tumor suppressors and/or express oncogenes in distinct kidney lineages result in different propensities for ccRCC development. Furthermore, the long latency of tumor formation in GEMMs currently available, as well as lack of metastatic spread typical of human ccRCC, poses challenges for researchers studying the molecular mechanisms governing these events. In the meantime, identification of subpopulations of established patient-derived cell lines having metastatic capacity [63] and incorporation of patient-derived xenografts will allow the field to investigate these phenomena in greater detail. Additional GEMMs mimicking the initiation and progression of ccRCC will ultimately be beneficial for the pre-clinical study of targeted therapy. A deeper understanding of the genetic, epigenetic, and metabolic changes that synergize with VHL loss to generate ccRCC, and the order in which they occur, will ultimately facilitate the development of such tools.
4. Metabolic reprogramming in ccRCC
4.1. Glucose utilization
Aerobic glycolysis, commonly known as the “Warburg effect,” is a hallmark of many cancers regardless of cell-of-origin [64], and this phenomenon is amplified in VHL-deficient ccRCC due to HIF activation. A primary consequence of constitutive HIF activation is decreased pyruvate entry into the TCA cycle and increased glycolytic flux towards lactate due to the actions of pyruvate dehydrogenase kinase 1 (PDK1) and lactate dehydrogenase (LDHA), respectively [3]. It is believed that enhanced glycolysis benefits tumors through several mechanisms, one being the generation of building blocks for nucleotides, proteins, and lipids through pathways branching from glycolytic intermediates [65]. The pentose phosphate pathway, beginning with glucose-6-phosphate and generating NADPH and ribose 5-phosphate, is important for lipid and nucleotide production. Additionally, NADPH produced from these reactions can be used to reduce oxidized glutathione, helping to maintain antioxidant capacity in rapidly proliferating cells. The hexosamine pathway, beginning with fructose 6-phosphate, is important for the generation of precursors used in N-glycosylation of proteins. These modifications are important for the proper folding of growth factor and nutrient receptors, which feeds back to regulate cellular metabolism more broadly [66]. Although an inefficient means of ATP generation relative to oxidative phosphorylation, aerobic glycolysis in cancer cells occurs at a rate much higher than in non-proliferative cells [67]. An equivalent rate of ATP synthesis along with the additional generation of anabolic precursors and reducing equivalents is a proposed explanation for why tumors rewire metabolism to promote the Warburg effect.
In addition to their canonical, catalytic functions, several reports demonstrate that certain glucose metabolic enzymes exhibit dual functions through physical association with HIFs [68], [69]. The HIF-1α target gene pyruvate kinase (PKM) encodes isoforms PKM1 and PKM2, of which PKM2 is a key regulator of glycolytic flux. The Semenza group showed that in addition to its catalytic function, PKM2 can bind to HIF-1α as a co-activator and enhance p300 recruitment and localization to hypoxia response elements [68]. Additionally, our group demonstrated that the gene encoding the gluconeogenic enzyme fructose-1,6-bisphosphatase 1, FBP1, is uniformly silenced or deleted in ccRCC patients [70]. Loss of FBP1 promotes ccRCC progression by two distinct mechanisms; first, by promoting glycolytic flux in renal epithelial cells, and secondly, by dissociation with the HIF-α inhibitory domain, facilitating constitutive HIF activity at the chromatin-level. Further research exploring the relationship between HIFs and their cell type-specific transcriptional targets will provide insight into the complex regulation of metabolism in ccRCC.
4.2. PI3K-Akt-mTOR pathway
The phosphoinositide 3-kinase (PI3K) signaling pathway is activated by copy number variation or mutation of its positive effectors PIK3CA, MTOR, and negative regulators PTEN and TSC1/2 in 28% of patients in ccRCC [25], [37]. PI3K activation activates Akt, also known as protein kinase B, by phosphorylation at the cell surface. One of the major downstream targets of activated Akt is mTORC1, which increases anabolic pathways such as protein, lipid, and nucleotide synthesis [71]. Inactivation of the negative regulators of mTORC1, TSC1 and TSC2, has been associated with increased risk of ccRCC development, which presents at an earlier age than sporadic disease [72]. Collectively, these effects render the pathway constitutively active even in the absence of growth factors. The effects of altered glucose and amino acid metabolism on ccRCC pathogenesis was comprehensively reviewed in 2017 [3], as such, we will focus on reprogrammed lipid metabolism in the remainder of this section.
4.3. Lipid metabolism
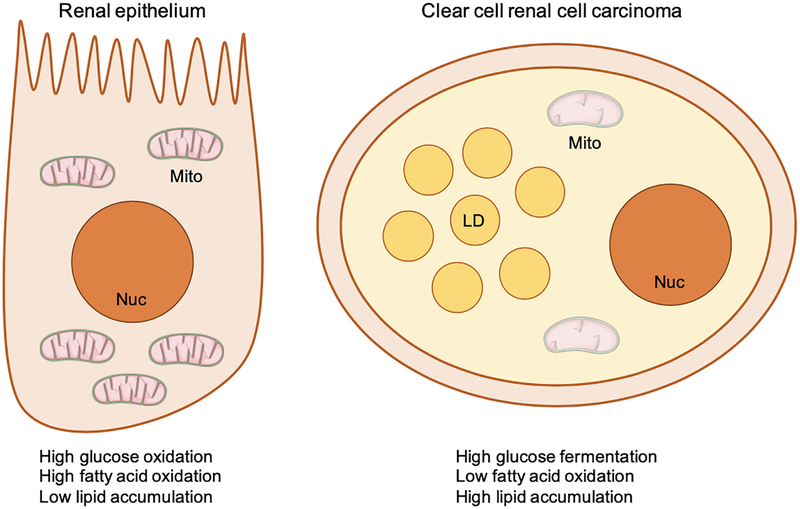
As renal proximal tubule epithelial cells (RPTEC) transition into ccRCC, they undergo dramatic rewiring of various metabolic pathways including lipid utilization, synthesis, and storage (Figure 2). Healthy RPTEC are characterized by high rates of beta-oxidation to fuel their primary role in ion exchange and secretion [73]. Beta-oxidation is the process by which lipids are broken down into acetyl-CoA, generating the reducing equivalents NADH and FADH2 to produce ATP through oxidative phosphorylation. Compared to RPTEC, ccRCC have reduced mitochondrial content and defective mitochondrial structure and activity [74], [75]. At the gene expression level, transcriptional regulators of mitochondrial homeostasis and oxidative phosphorylation are uniformly suppressed in ccRCC relative to adjacent healthy kidney tissue [76]. These adaptations are largely due to constitutive HIF signaling, although additional factors regulating these pathways remain to be explored. One study examined the relationship between HIF-α and PGC-1α, a peroxisome proliferator-activated receptor gamma (PPARγ) transcriptional co-activator involved in the process of mitochondrial biogenesis and respiration [77]. VHL-deficient ccRCC cells exhibit elevated levels of Dec1, a transcriptional repressor and HIF-α target gene, which blocks expression of PGC-1α [78]. Ectopic expression of PGC-1α increased oxidative stress and slowed tumor growth in a subcutaneous xenograft model. Furthermore, lower levels of PPARGC1A (PGC-1α) mRNA were correlated with worse patient survival, and total mitochondrial content was correlated inversely with tumor grade [79], consistent with its role as a tumor suppressor in ccRCC.
Figure 2. Comparison of the metabolic state of renal epithelium and clear cell renal cell carcinoma (ccRCC).
Renal epithelial cells (of the proximal tubule, a proposed cell-of-origin of ccRCC) are characterized by high levels of glucose and fatty acid oxidation to serve their energetic needs. As these cells transition into ccRCC, they begin to store triglyceride and cholesterol ester in cytoplasmic lipid droplets. Furthermore, ccRCC have low levels of fatty acid oxidation and compromised mitochondrial architecture relative to healthy renal epithelium. Nuc = nucleus. Mito = mitochondria. LD = lipid droplet.
Transcriptional repression of carnitine palmitoyltransferase (CPT1A), the rate limiting enzyme involved in fatty acid transport into the mitochondria for beta-oxidation, also occurs in ccRCC via a HIF-α dependent mechanism [80]. A recent report found that pVHL reconstitution in several ccRCC cell lines reduced lipid droplet (LD) accumulation in a CPT1A dependent manner (i.e., knockdown of CPT1A in pVHL-reconstituted cells partially increased lipid accumulation). Interestingly, pVHL status had no effect on lipid uptake rates, as measured through the uptake of BODIPY, an unsaturated, fluorescently labeled fatty acid molecule. Ectopic expression of CPT1A increased oxygen consumption rate, decreased LD abundance, and decreased xenograft tumor growth. Clinically, CPT1A levels and activity are reduced in ccRCC compared with healthy kidney tissue, and patients with reduced CPT1A expression have worse prognosis.
Concomitant with decreased beta-oxidation in ccRCC tumors is increased lipid synthesis and storage. Fatty acid synthesis occurs when cytosolic citrate is carboxylated by the enzyme ATP citrate lyase, or ACLY, to produce acetyl-CoA. Carboxylation of acetyl-CoA to malonyl-CoA by acetyl-CoA carboxylase (ACC) generates the building blocks for long-chain fatty acid formation which can go on to be desaturated and further modified to produce more complex lipid species. Two key enzymes in this process are fatty acid synthase (FASN), which produces palmitic acid (C16:0), and stearoyl-CoA desaturase 1 (SCD1), which primarily produces oleic acid (C18:1) from the desaturation of stearic acid. In ccRCC, metabolism of glutamine-derived α-ketoglutarate to citrate by reductive carboxylation fuels lipid synthesis rather than glucose-derived citrate generated by oxidation [81], [82]. In addition to increasing anabolic lipid metabolism for the production of membranes needed during rapid cell proliferation, new evidence suggests additional roles for de novo lipogenesis (DNL) and lipid uptake in carcinogenesis. FASN levels correlate positively with tumor aggressiveness and poor survival in ccRCC [83] [84], and genetic and pharmacologic inhibition of SCD1 induces apoptosis in ccRCC cell lines both in vitro and in vivo [85]. Treatment with ACC inhibitor reduces total phospholipid and triglyceride content, as predicted, and also increases sensitivity to oxidative stress-induced cell death [86]. Other work has demonstrated that lipid uptake protects against endoplasmic reticulum (ER) stress in ccRCC and other cancers, particularly under hypoxia when cells cannot desaturate fatty acids due to the inactivation of SCD1 [87], [88].
The protective effect of LD accumulation in cancer cells is incompletely understood, although recent studies suggest several mechanisms by which LDs can enhance tumorigenesis and cell viability. LDs consist of a core of neutral lipids including triglyceride and cholesterol esters surrounded by a monolayer of membrane from the endoplasmic reticulum and coat proteins including the perilipin family [89]. Inhibition of the LD coat protein perilipin 2 in ccRCC, a highly-expressed HIF-2α target gene, reduced neutral lipid accumulation, tumor growth, and increased ER stress both in vitro and in vivo [34]. In glioblastoma and breast cancer, it was recently demonstrated that lipid accumulation following hypoxia-reoxygenation protects cells from reactive oxygen species (ROS)-induced cytotoxicity [90]. In another hypoxic microenvironment, the Drosophila neural stem cell niche, LDs that form during oxidative stress reduce ROS levels and inhibit the oxidation of polyunsaturated fatty acids (PUFA) [91]. When PUFA are stored in LDs rather than in membranes, they are less vulnerable to peroxidation, which inhibits proliferation through damaging macromolecules. In theory, these responses are even more critical to maintain in ccRCC, where lipid accumulation is a defining molecular and morphological hallmark of disease. Taken together, these results suggest pathways involving anabolic lipid metabolism could one day be targeted for therapeutic benefit in ccRCC.
In a recently published lipidomic analysis of 49 patients [92], elevated levels of ether-type phospholipids, cholesterol esters, and triacylglycerols were reported in ccRCC, consistent with previously published metabolomic analyses [93], [94], [95]. These metabolic changes were also correlated with gene expression levels of lipogenic genes. While it is clear that suppression of either HIF-1α or HIF-2α markedly reduces LD formation in ccRCC [96], [34], [80], the target genes and mechanisms responsible for the clear cell phenotype have not been completely defined. Additionally, the role of other lipogenic transcription factors such as sterol regulatory element-binding protein 1 or 2 (SREBP1/2), PPARγ, and carbohydrate-responsive element-binding protein (ChREBP) remain to be fully elucidated in ccRCC.
5. Therapeutic strategies for ccRCC
5.1. Anti-angiogenics and mTOR inhibitors
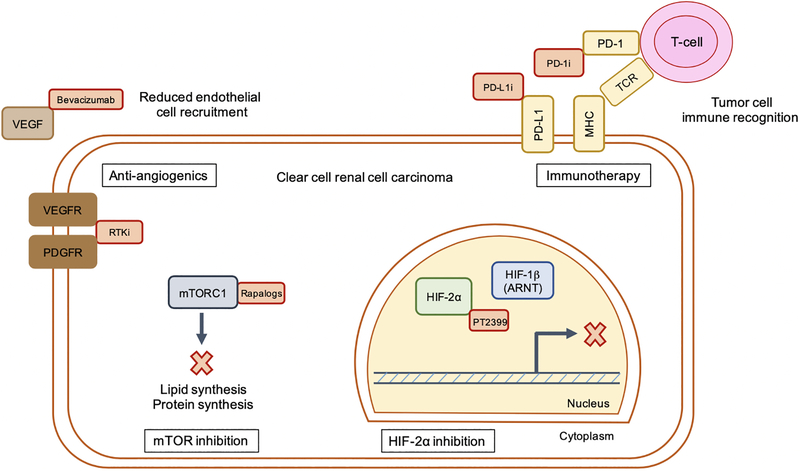
Anti-angiogenic therapy targeting receptor tyrosine kinases (RTKs) such as vascular endothelial growth factor receptor (sunitinib, sorafenib, etc) or VEGF ligand by monoclonal antibody (bevacizumab) has traditionally been a first-line standard-of-care for ccRCC patients [97] (a graphical representation of the therapies reviewed in this section is presented in Figure 3). Although these agents are being investigated for several malignancies including colorectal and lung cancers, anti-angiogenics are theoretically well-suited for the treatment of ccRCC due to constitutive HIF activity and exceedingly high VEGF accumulation. However, clinical trials indicate that at least as single-therapy, anti-angiogenic drugs are only modestly effective for the treatment of metastatic ccRCC. In a randomized, double-blind trial of bevacizumab, the probability of being progression-free after eight months was significantly extended by 30% (high-dose), 14% (low-dose), and 5% (placebo) [98]. However, there was no significant difference in overall survival between placebo, low, or high-dose bevacizumab. A phase III study of sunitinib in patients with high risk of tumor recurrence after nephrectomy revealed that 64.9% of patients were disease-free at three years after adjuvant treatment with sunitinib compared with 59.5% in the placebo group [99]. At five years, the proportions fell to 59.3% (sunitinib) and 51.3% (placebo). Side effects from sunitinib treatment included diarrhea, hypertension, fatigue, and nausea.
Figure 3. Select pharmacological approaches for the treatment of metastatic ccRCC.
Current and future therapeutic strategies for ccRCC include inhibition (denoted by red boxes) of mTOR signaling, angiogenesis, HIF-2α signaling, and immune checkpoint blockade. RTKi = receptor tyrosine kinase inhibitors. Rapalogs = rapamycin analogs. PD-1i = PD-1 inhibitor. PD- L1i = PD-L1 inhibitor.
Rapamycin analogs, or rapalogs (everolimus, temsirolimus), are used for the treatment of advanced ccRCC after patients have been treated with a RTK inhibitor. A phase III study in patients whose disease progressed after receiving either RTK- or VEGF-targeted therapy found that everolimus was effective in prolonging progression-free survival compared with placebo control [100]. Side effects from everolimus included hyperglycemia, hyperlipidemia, and hypercholesterolemia due to effects on mTOR-regulated glucose and lipid metabolism, as well as diarrhea and rash. Yet overall, the risk of severe effects was low and no detrimental effects on health-related quality-of-life was observed in everolimus treated individuals compared with placebo. The clinical trials reported above led to FDA approval for angiogenesis and mTOR inhibitors nearly a decade ago, and current investigation has now shifted toward targeted therapy of HIF-2α as well as immune checkpoint blocade.
5.2. Targeting HIF-2α
HIF-2α is the primary oncogenic driver of ccRCC progression downstream of VHL loss. Pre-clinical work from several labs has examined the efficacy of a HIF-2α inhibitors utilizing a panel of ccRCC cell lines in orthotopic xenograft assays as well as patient-derived xenografts grown subcutaneously [101], [102], [103]. PT2399 blocks the interaction between HIF-2α and HIF-1β/ARNT by binding to a pocket within the PAS-B domain of HIF-2α, thereby eliminating DNA binding. Importantly, PT2399 reduces HIF-2α target gene expression without affecting HIF-1α targets [102]. When tested alongside sunitinib, PT2399 decreased tumorgraft growth by 60% across all samples tested compared with 40% for sunitinib treatment, without causing a reduction in body weight as seen with the latter drug [103]. Resistance mechanisms were observed in tumors initially sensitive to PT2399, which were characterized by increased tumor vascularity and VEGF production despite dissociation between HIF-2α and ARNT. However, they took nearly double the amount of time to develop as tumors treated with sunitinib. Based on these results, a derivative of PT2399, PT2385, is currently being tested in clinical trials for ccRCC [104].
5.3. Targeting glutaminase
Since being reviewed by Wettersten, et al. [3], two phase II clinical trials have been initiated to examine the effect of CB-839, a small molecule inhibitor of glutaminase, along with either cabozantinib, everolimus, or placebo, on patients with advanced or metastatic RCC [105]. Additionally, a pre-clinical report on the role of glutamine addiction in ccRCC further supports the possibility of therapeutically targeting this axis in kidney cancer [106]. Aboud, et al. demonstrate that ccRCC tumors grown orthotopically show increased uptake of 18F-(2S,4R)4-fluoroglutamine relative to adjacent healthy kidney tissue and are sensitive to glutaminase inhibition by CB-839. These results suggest that PET imaging could be useful to identify ccRCC patients likely to respond to glutaminase inhibition clinically.
5.4. Immunotherapy
Immune checkpoint blockade represents a promising strategy for the treatment of many cancer subtypes including ccRCC. Such therapies remove the “brakes” from a patient’s immune system, allowing T-cells to recognize and destroy cancer cells. While ccRCC does not harbor as many somatic mutations per megabase as melanoma or non-small cell lung cancer, which creates a higher neo-antigen load and is thought to predict response to immunotherapy, positive immune responses can be achieved in a subset of patients [107], [108]. One method of achieving this effect is to antagonize programmed cell death-1 receptor (PD-1), which is found on activated T-cells and interacts with PD-L1 or PD-L2 on tumor cells. This interaction normally allows tumor cells to evade the immune response, but when inhibited restores antitumor immunity [109]. Clinical work comparing nivolumab, a monoclonal antibody targeting PD-1, to everolimus, found that nivolumab extended median survival to 25 months compared with 19.6 months for everolimus treatment in patients with advanced ccRCC [110]. However, durable effects were only seen in 20–25% of patients [108]. Current investigations across cancer subtypes are geared toward defining mechanisms that underlie positive and lasting responses to immune checkpoint blockade. A recent study identified signatures of response to checkpoint therapies in ccRCC [111]. Through whole exome sequencing of 35 tumors of patients with metastatic ccRCC, it was determined that biallelic PBRM1 loss was associated with significantly longer overall survival than patients with PBRM1 intact. It was hypothesized that PBRM1 loss-of-function and subsequent alterations in SWI/SNF signaling networks may underlie this effect. It is the hope that through these analyses, specific patient populations can be identified which would respond best to immunotherapy based on the genetic and mutational landscape of their tumors.
6. Conclusion
In this review, we have discussed recent developments in the generation of a genetically engineered mouse model for ccRCC, how reprogramming of glucose and lipid metabolic pathways facilitate tumor progression, and novel therapeutic strategies informed by these pre-clinical studies. Future research investigating additional epigenetic and metabolic hallmarks of ccRCC will likely identify druggable pathways shared across a large percentage of patients. When inhibited or activated through combinatorial therapy, manipulation of these targets will hopefully be effective in prolonging survival in patients with metastatic ccRCC, which is largely resistant to current standard-of-care therapeutics.
Acknowledgments
This work was supported by National Institutes of Health grants T32HD083185 and F31CA206381 to D.J.S. and P01CA104838 to M.C.S.
References
- [1].Institute NC, SEER Cancer Stat Facts: Kidney and Renal Pelvis Cancer, 2018. [Google Scholar]
- [2].Rini BI, Campbell SC, Escudier B, Renal cell carcinoma, The Lancet, 373 (2009) 1119–1132. [DOI] [PubMed] [Google Scholar]
- [3].Wettersten HI, Aboud OA, Lara PN Jr, Weiss RH, Metabolic reprogramming in clear cell renal cell carcinoma, Nature Reviews Nephrology, 13 (2017) 410. [DOI] [PubMed] [Google Scholar]
- [4].Linehan WM, Srinivasan R, Schmidt LS, The genetic basis of kidney cancer: a metabolic disease, Nature reviews urology, 7 (2010) 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hakimi AA, Pham CG, Hsieh JJ, A clear picture of renal cell carcinoma, Nature genetics, 45 (2013) 849. [DOI] [PubMed] [Google Scholar]
- [6].Kaelin WG Jr, Von hippel-lindau disease, Annu. Rev. Pathol. Mech. Dis, 2 (2007) 145–173. [DOI] [PubMed] [Google Scholar]
- [7].Keith B, Johnson RS, Simon MC, HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression, Nature Reviews Cancer, 12 (2012) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mucaj V, Shay JE, Simon MC, Effects of hypoxia and HIFs on cancer metabolism, International journal of hematology, 95 (2012) 464–470. [DOI] [PubMed] [Google Scholar]
- [9].Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron, Cancer cell, 1 (2002) 459–468. [DOI] [PubMed] [Google Scholar]
- [10].Nickerson ML, Jaeger E, Shi Y, Durocher JA, Mahurkar S, Zaridze D, Matveev V, Janout V, Kollarova H, Bencko V, Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors, Clinical cancer research, 14 (2008) 4726–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gordan JD, Lal P, Dondeti VR, Letrero R, Parekh KN, Oquendo CE, Greenberg RA, Flaherty KT, Rathmell WK, Keith B, HIF-α effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma, Cancer cell, 14 (2008) 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kaku H, Ito S, Ebara S, Ouchida M, Nasu Y, Tsushima T, Kumon H, Shimizu K, Positive correlation between allelic loss at chromosome 14q24–31 and poor prognosis of patients with renal cell carcinoma, Urology, 64 (2004) 176–181. [DOI] [PubMed] [Google Scholar]
- [13].Shen C, Beroukhim R, Schumacher S, Zhou J, Chang M, Signoretti S, Kaelin W, Genetic and functional studies implicate HIF1α as a 14q kidney cancer suppressor gene, Cancer discovery, (2011) CD-11–0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL, HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity, Cancer cell, 11 (2007) 407–420. [DOI] [PubMed] [Google Scholar]
- [15].Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li J-L, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ, Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma, Molecular and cellular biology, 25 (2005) 5675–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gordan JD, Bertout JA, Hu C-J, Diehl JA, Simon MC, HIF-2α promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity, Cancer cell, 11 (2007) 335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG Jr, Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein, Cancer cell, 1 (2002) 237–246. [DOI] [PubMed] [Google Scholar]
- [18].Kondo K, Kim WY, Lechpammer M, Kaelin WG Jr, Inhibition of HIF2α is sufficient to suppress pVHL-defective tumor growth, PLoS biology, 1 (2003) e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martínez-Sáez O, Borau PG, Alonso-Gordoa T, Molina-Cerrillo J, Grande E, Targeting HIF-2 α in clear cell renal cell carcinoma: A promising therapeutic strategy, Critical reviews in oncology/hematology, 111 (2017) 117–123. [DOI] [PubMed] [Google Scholar]
- [20].Beroukhim R, Brunet J-P, Di Napoli A, Mertz KD, Seeley A, Pires MM, Linhart D, Worrell RA, Moch H, Rubin MA, Patterns of gene expression and copy-number alterations in von-hippel lindau disease-associated and sporadic clear cell carcinoma of the kidney, Cancer research, 69 (2009) 4674–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dondeti VR, Wubbenhorst B, Lal P, Gordan JD, D’Andrea K, Attiyeh EF, Simon MC, Nathanson KL, Integrative genomic analyses of sporadic clear cell renal cell carcinoma define disease subtypes and potential new therapeutic targets, Cancer research, 72 (2012) 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Peña-Llopis S, Christie A, Xie X-J, Brugarolas J, Cooperation and antagonism among cancer genes: the renal cancer paradigm, Cancer research, 73 (2013) 4173–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Intratumor heterogeneity and branched evolution revealed by multiregion sequencing, New England journal of medicine, 366 (2012) 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, Fisher R, McGranahan N, Matthews N, Santos CR, Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing, Nature genetics, 46 (2014) 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Network CGAR, Comprehensive molecular characterization of clear cell renal cell carcinoma, Nature, 499 (2013) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li L, Shen C, Nakamura E, Ando K, Signoretti S, Beroukhim R, Cowley GS, Lizotte P, Liberzon E, Bair S, SQSTM1 is a pathogenic target of 5q copy number gains in kidney cancer, Cancer cell, 24 (2013) 738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT, p62 is a key regulator of nutrient sensing in the mTORC1 pathway, Molecular cell, 44 (2011) 134–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu L, Xu Z, Zhong L, Wang H, Jiang S, Long Q, Xu J, Guo J, Enhancer of zeste homolog 2 (EZH2) promotes tumour cell migration and invasion via epigenetic repression of E-cadherin in renal cell carcinoma, BJU international, 117 (2016) 351–362. [DOI] [PubMed] [Google Scholar]
- [29].Scelo G, Purdue MP, Brown KM, Johansson M, Wang Z, Eckel-Passow JE, Ye Y, Hofmann JN, Choi J, Foll M, Genome-wide association study identifies multiple risk loci for renal cell carcinoma, Nature communications, 8 (2017) 15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Scelo G, Riazalhosseini Y, Greger L, Letourneau L, Gonzàlez-Porta M, Wozniak MB, Bourgey M, Harnden P, Egevad L, Jackson SM, Variation in genomic landscape of clear cell renal cell carcinoma across Europe, Nature communications, 5 (2014) 5135. [DOI] [PubMed] [Google Scholar]
- [31].Bigot P, Colli LM, Machiela MJ, Jessop L, Myers TA, Carrouget J, Wagner S, Roberson D, Eymerit C, Henrion D, Functional characterization of the 12p12. 1 renal cancer-susceptibility locus implicates BHLHE41, Nature communications, 7 (2016) 12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Purdue MP, Johansson M, Zelenika D, Toro JR, Scelo G, Moore LE, Prokhortchouk E, Wu X, Kiemeney LA, Gaborieau V, Genome-wide association study of renal cell carcinoma identifies two susceptibility loci on 2p21 and 11q13. 3, Nature genetics, 43 (2011) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schödel J, Bardella C, Sciesielski LK, Brown JM, Pugh CW, Buckle V, Tomlinson IP, Ratcliffe PJ, Mole DR, Common genetic variants at the 11q13. 3 renal cancer susceptibility locus influence binding of HIF to an enhancer of cyclin D1 expression, Nature genetics, 44 (2012) 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Qiu B, Ackerman D, Sanchez DJ, Li B, Ochocki JD, Grazioli A, Bobrovnikova-Marjon E, Diehl JA, Keith B, Simon MC, HIF2α-dependent lipid storage promotes endoplasmic reticulum homeostasis in clear-cell renal cell carcinoma, Cancer discovery, 5 (2015) 652–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Jafri M, Wake NC, Ascher DB, Pires DE, Gentle D, Morris MR, Rattenberry E, Simpson MA, Trembath RC, Weber A, Germline mutations in the CDKN2B tumor suppressor gene predispose to renal cell carcinoma, Cancer discovery, 5 (2015) 723–729. [DOI] [PubMed] [Google Scholar]
- [36].Roussel MF, The INK4 family of cell cycle inhibitors in cancer, Oncogene, 18 (1999) 5311. [DOI] [PubMed] [Google Scholar]
- [37].Turajlic S, Larkin J, Swanton C, SnapShot: renal cell carcinoma, Cell, 163 (2015) 1556–1556. e1551. [DOI] [PubMed] [Google Scholar]
- [38].Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, Davies H, Jones D, Lin M-L, Teague J, Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma, Nature, 469 (2011) 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gao W, Li W, Xiao T, Liu XS, Kaelin WG, Inactivation of the PBRM1 tumor suppressor gene amplifies the HIF-response in VHL−/− clear cell renal carcinoma, Proceedings of the National Academy of Sciences, 114 (2017) 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Haaften G, Dalgliesh GL, Davies H, Chen L, Bignell G, Greenman C, Edkins S, Hardy C, O’meara S, Teague J, Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer, Nature genetics, 41 (2009) 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C, Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes, Nature, 463 (2010) 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kanu N, Grönroos E, Martinez P, Burrell RA, Goh XY, Bartkova J, Maya-Mendoza A, Mistrík M, Rowan AJ, Patel H, SETD2 loss-of-function promotes renal cancer branched evolution through replication stress and impaired DNA repair, Oncogene, 34 (2015) 5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Guo G, Gui Y, Gao S, Tang A, Hu X, Huang Y, Jia W, Li Z, He M, Sun L, Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma, Nature genetics, 44 (2012) 17. [DOI] [PubMed] [Google Scholar]
- [44].Shenoy N, Vallumsetla N, Zou Y, Galeas JN, Shrivastava M, Hu C, Susztak K, Verma A, Role of DNA methylation in renal cell carcinoma, Journal of hematology & oncology, 8 (2015) 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rankin EB, Tomaszewski JE, Haase VH, Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor, Cancer research, 66 (2006) 2576–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, Shimamura T, Sato-Otsubo A, Nagae G, Suzuki H, Integrated molecular analysis of clear-cell renal cell carcinoma, Nature genetics, 45 (2013) 860. [DOI] [PubMed] [Google Scholar]
- [47].Shao X, Somlo S, Igarashi P, Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract, Journal of the American Society of Nephrology, 13 (2002) 1837–1846. [DOI] [PubMed] [Google Scholar]
- [48].Nargund AM, Pham CG, Dong Y, Wang PI, Osmangeyoglu HU, Xie Y, Aras O, Han S, Oyama T, Takeda S, The SWI/SNF protein PBRM1 restrains VHL-loss-driven clear cell renal cell carcinoma, Cell reports, 18 (2017) 2893–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Harlander S, Schönenberger D, Toussaint NC, Prummer M, Catalano A, Brandt L, Moch H, Wild PJ, Frew IJ, Combined mutation in Vhl, Trp53 and Rb1 causes clear cell renal cell carcinoma in mice, Nature medicine, 23 (2017) 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL, Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization, Proceedings of the National Academy of Sciences, 106 (2009) 17910–17915. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [51].Wang S-S, Gu Y-F, Wolff N, Stefanius K, Christie A, Dey A, Hammer RE, Xie X-J, Rakheja D, Pedrosa I, Bap1 is essential for kidney function and cooperates with Vhl in renal tumorigenesis, Proceedings of the National Academy of Sciences, 111 (2014) 16538–16543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gu Y-F, Cohn S, Christie A, McKenzie T, Wolff N, Do QN, Madhuranthakam AJ, Pedrosa I, Wang T, Dey A, Modeling renal cell carcinoma in mice: Bap1 and Pbrm1 inactivation drive tumor grade, Cancer discovery, 7 (2017) 900–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yoshida S, Imam A, Olson C, Taylor C, Proximal renal tubular surface membrane antigens identified in primary and metastatic renal cell carcinomas, Archives of pathology & laboratory medicine, 110 (1986) 825–832. [PubMed] [Google Scholar]
- [54].Chen F, Zhang Y, Şenbabaoğlu Y, Ciriello G, Yang L, Reznik E, Shuch B, Micevic G, De Velasco G, Shinbrot E, Multilevel genomics-based taxonomy of renal cell carcinoma, Cell reports, 14 (2016) 2476–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kapur P, Peña-Llopis S, Christie A, Zhrebker L, Pavía-Jiménez A, Rathmell WK, Xie X-J, Brugarolas J, Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: a retrospective analysis with independent validation, The lancet oncology, 14 (2013) 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L, Sivanand S, Spence P, BAP1 loss defines a new class of renal cell carcinoma, Nature genetics, 44 (2012) 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hakimi AA, Ostrovnaya I, Reva B, Schultz N, Chen Y-B, Gonen M, Liu H, Takeda S, Voss MH, Tickoo SK, Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: a report by MSKCC and the KIRC TCGA research network, Clinical Cancer Research, 19 (2013) 3259–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fu L, Wang G, Shevchuk MM, Nanus DM, Gudas LJ, Generation of a mouse model of von Hippel–Lindau kidney disease leading to renal cancers by expression of a constitutively active mutant of HIF1α, Cancer research, 71 (2011) 6848–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tang S-W, Chang W-H, Su Y-C, Chen Y-C, Lai Y-H, Wu P-T, Hsu C-I, Lin W-C, Lai M-K, Lin J-Y, MYC pathway is activated in clear cell renal cell carcinoma and essential for proliferation of clear cell renal cell carcinoma cells, Cancer letters, 273 (2009) 35–43. [DOI] [PubMed] [Google Scholar]
- [60].Drabkin HA, Bradley C, Hart I, Bleskan J, Li FP, Patterson D, Translocation of c-myc in the hereditary renal cell carcinoma associated with at (3; 8)(p14. 2; q24. 13) chromosomal translocation, Proceedings of the National Academy of Sciences, 82 (1985) 6980–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shroff EH, Eberlin LS, Dang VM, Gouw AM, Gabay M, Adam SJ, Bellovin DI, Tran PT, Philbrick WM, Garcia-Ocana A, MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism, Proceedings of the National Academy of Sciences, 112 (2015) 6539–6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bailey ST, Smith AM, Kardos J, Wobker SE, Wilson HL, Krishnan B, Saito R, Lee HJ, Zhang J, Eaton SC, MYC activation cooperates with Vhl and Ink4a/Arf loss to induce clear cell renal cell carcinoma, Nature communications, 8 (2017) 15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Vanharanta S, Shu W, Brenet F, Hakimi AA, Heguy A, Viale A, Reuter VE, Hsieh JJ, Scandura JM, Massagué J, Epigenetic expansion of VHL-HIF signal output drives multiorgan metastasis in renal cancer, Nature medicine, 19 (2013) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pavlova NN, Thompson CB, The emerging hallmarks of cancer metabolism, Cell metabolism, 23 (2016) 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ward PS, Thompson CB, Metabolic reprogramming: a cancer hallmark even warburg did not anticipate, Cancer cell, 21 (2012) 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wellen KE, Thompson CB, A two-way street: reciprocal regulation of metabolism and signalling, Nature reviews Molecular cell biology, 13 (2012) 270. [DOI] [PubMed] [Google Scholar]
- [67].Liberti MV, Locasale JW, The Warburg effect: how does it benefit cancer cells?, Trends in biochemical sciences, 41 (2016) 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL, Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1, Cell, 145 (2011) 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Xie H, Simon MC, Oxygen availability and metabolic reprogramming in cancer, Journal of Biological Chemistry, 292 (2017) 16825–16832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, Mancuso A, Gade TP, Keith B, Nissim I, Fructose-1, 6-bisphosphatase opposes renal carcinoma progression, Nature, 513 (2014) 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dibble CC, Manning BD, Signal integration by mTORC1 coordinates nutrient input with biosynthetic output, Nature cell biology, 15 (2013) 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bjornsson J, Short MP, Kwiatkowski DJ, Henske E, Tuberous sclerosis-associated renal cell carcinoma. Clinical, pathological, and genetic features, The American journal of pathology, 149 (1996) 1201. [PMC free article] [PubMed] [Google Scholar]
- [73].Kang HM, Ahn SH, Choi P, Ko Y-A, Han SH, Chinga F, Park ASD, Tao J, Sharma K, Pullman J, Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development, Nature medicine, 21 (2015) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Meierhofer D, Mayr JA, Foetschl U, Berger A, Fink K, Schmeller N, Hacker GW, Hauser-Kronberger C, Kofler B, Sperl W, Decrease of mitochondrial DNA content and energy metabolism in renal cell carcinoma, Carcinogenesis, 25 (2004) 1005–1010. [DOI] [PubMed] [Google Scholar]
- [75].Nilsson H, Lindgren D, Forsberg AM, Mulder H, Axelson H, Johansson M, Primary clear cell renal carcinoma cells display minimal mitochondrial respiratory capacity resulting in pronounced sensitivity to glycolytic inhibition by 3-Bromopyruvate, Cell death & disease, 6 (2015) e1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tun HW, Marlow LA, Von Roemeling CA, Cooper SJ, Kreinest P, Wu K, Luxon BA, Sinha M, Anastasiadis PZ, Copland JA, Pathway signature and cellular differentiation in clear cell renal cell carcinoma, PloS one, 5 (2010) e10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM, A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis, Cell, 92 (1998) 829–839. [DOI] [PubMed] [Google Scholar]
- [78].LaGory EL, Wu C, Taniguchi CM, Ding C-KC, Chi J-T, von Eyben R, Scott DA, Richardson AD, Giaccia AJ, Suppression of PGC-1α is critical for reprogramming oxidative metabolism in renal cell carcinoma, Cell reports, 12 (2015) 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Simonnet H, Alazard N, Pfeiffer K, Gallou C, Béroud C, Demont J, Bouvier R, Schägger H, Godinot C, Low mitochondrial respiratory chain content correlates with tumor aggressiveness in renal cell carcinoma, Carcinogenesis, 23 (2002) 759–768. [DOI] [PubMed] [Google Scholar]
- [80].Du W, Zhang L, Brett-Morris A, Aguila B, Kerner J, Hoppel CL, Puchowicz M, Serra D, Herrero L, Rini BI, HIF drives lipid deposition and cancer in ccRCC via repression of fatty acid metabolism, Nature communications, 8 (2017) 1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia, Nature, 481 (2012) 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gameiro PA, Yang J, Metelo AM, Pérez-Carro R, Baker R, Wang Z, Arreola A, Rathmell WK, Olumi A, López-Larrubia P, In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation, Cell metabolism, 17 (2013) 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Horiguchi A, Asano T, Asano T, Ito K, Sumitomo M, Hayakawa M, Fatty acid synthase over expression is an indicator of tumor aggressiveness and poor prognosis in renal cell carcinoma, The Journal of urology, 180 (2008) 1137–1140. [DOI] [PubMed] [Google Scholar]
- [84].Hakimi AA, Reznik E, Lee C-H, Creighton CJ, Brannon AR, Luna A, Aksoy BA, Liu EM, Shen R, Lee W, An integrated metabolic atlas of clear cell renal cell carcinoma, Cancer cell, 29 (2016) 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Von Roemeling CA, Marlow LA, Wei JJ, Cooper SJ, Caulfield TR, Wu K, Tan WW, Tun HW, Copland JA, Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma, Clinical Cancer Research, 19 (2013) 2368–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniëls VW, Machiels J, De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation, Cancer research, 70 (2010) 8117–8126. [DOI] [PubMed] [Google Scholar]
- [87].Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP, Thompson CB, Rabinowitz JD, Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids, Proceedings of the National Academy of Sciences, 110 (2013) 8882–8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Young RM, Ackerman D, Quinn ZL, Mancuso A, Gruber M, Liu L, Giannoukos DN, Bobrovnikova-Marjon E, Diehl JA, Keith B, Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress, Genes & development, 27 (2013) 1115–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Walther TC, Farese RV Jr, Lipid droplets and cellular lipid metabolism, Annual review of biochemistry, 81 (2012) 687–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, Pinnick KE, Wigfield S, Buffa FM, Li J-L, Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation, Cell reports, 9 (2014) 349–365. [DOI] [PubMed] [Google Scholar]
- [91].Bailey AP, Koster G, Guillermier C, Hirst EM, MacRae JI, Lechene CP, Postle AD, Gould AP, Antioxidant role for lipid droplets in a stem cell niche of Drosophila, Cell, 163 (2015) 340–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Saito K, Arai E, Maekawa K, Ishikawa M, Fujimoto H, Taguchi R, Matsumoto K, Kanai Y, Saito Y, Lipidomic signatures and associated transcriptomic profiles of clear cell renal cell carcinoma, Scientific reports, 6 (2016) 28932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gebhard R, Clayman R, Prigge W, Figenshau R, Staley N, Reesey C, Bear A, Abnormal cholesterol metabolism in renal clear cell carcinoma, Journal of lipid research, 28 (1987) 1177–1184. [PubMed] [Google Scholar]
- [94].Hoffmann K, Blaudszun J, Brunken C, Höpker WW, Tauber R, Steinhart H, Lipid class distribution of fatty acids including conjugated linoleic acids in healthy and cancerous parts of human kidneys, Lipids, 40 (2005) 1057–1062. [DOI] [PubMed] [Google Scholar]
- [95].Yoshimura K, Chen LC, Mandal MK, Nakazawa T, Yu Z, Uchiyama T, Hori H, Tanabe K, Kubota T, Fujii H, Analysis of renal cell carcinoma as a first step for developing mass spectrometry-based diagnostics, Journal of The American Society for Mass Spectrometry, 23 (2012) 1741–1749. [DOI] [PubMed] [Google Scholar]
- [96].Sundelin JP, Ståhlman M, Lundqvist A, Levin M, Parini P, Johansson ME, Borén J, Increased expression of the very low-density lipoprotein receptor mediates lipid accumulation in clear-cell renal cell carcinoma, PloS one, 7 (2012) e48694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ricketts CJ, Crooks DR, Linehan WM, Targeting HIF2α in Clear-Cell Renal Cell Carcinoma, Cancer cell, 30 (2016) 515–517. [DOI] [PubMed] [Google Scholar]
- [98].Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA, A randomized trial of bevacizumab, an anti–vascular endothelial growth factor antibody, for metastatic renal cancer, New England Journal of Medicine, 349 (2003) 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, Chang Y-H, Escudier B, Donskov F, Magheli A, Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy, New England Journal of Medicine, 375 (2016) 2246–2254. [DOI] [PubMed] [Google Scholar]
- [100].Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial, The Lancet, 372 (2008) 449–456. [DOI] [PubMed] [Google Scholar]
- [101].Wallace EM, Rizzi JP, Han G, Wehn PM, Cao Z, Du X, Cheng T, Czerwinski RM, Dixon DD, Goggin BS, A small-molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinoma, Cancer research, 76 (2016) 5491–5500. [DOI] [PubMed] [Google Scholar]
- [102].Cho H, Du X, Rizzi JP, Liberzon E, Chakraborty AA, Gao W, Carvo I, Signoretti S, Bruick RK, Josey JA, On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models, Nature, 539 (2016) 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, Targeting renal cell carcinoma with a HIF-2 antagonist, Nature, 539 (2016) 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].U.S.N.L.o. Medicine, Renal Carcinoma Clinical Trials, 2018.
- [105].Tannir NM, Fan AC, Lee RJ, Carthon BC, Iliopoulos O, Mier JW, Patel MR, Meric-Bernstam F, DeMichele A, Voss MH, Phase 1 study of glutaminase (GLS) inhibitor CB-839 combined with either everolimus (E) or cabozantinib (Cabo) in patients (pts) with clear cell (cc) and papillary (pap) metastatic renal cell cancer (mRCC), American Society of Clinical Oncology, 2018. [Google Scholar]
- [106].Aboud OA, Habib SL, Trott J, Stewart B, Liang S, Chaudhari AJ, Sutcliffe J, Weiss RH, Glutamine addiction in kidney cancer suppresses oxidative stress and can be exploited for real-time imaging, Cancer research, 77 (2017) 6746–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Schumacher TN, Schreiber RD, Neoantigens in cancer immunotherapy, Science, 348 (2015) 69–74. [DOI] [PubMed] [Google Scholar]
- [108].De Velasco G, Miao D, Voss MH, Hakimi AA, Hsieh JJ, Tannir NM, Tamboli P, Appleman LJ, Rathmell WK, Van Allen EM, Tumor mutational load and immune parameters across metastatic renal cell carcinoma risk groups, Cancer immunology research, 4 (2016) 820–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Parikh M, Lara J, Primo N, Modern Systemic Therapy for Metastatic Renal Cell Carcinoma of the Clear Cell Type, Annual review of medicine, (2017). [DOI] [PubMed] [Google Scholar]
- [110].Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Nivolumab versus everolimus in advanced renal-cell carcinoma, New England Journal of Medicine, 373 (2015) 1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Miao D, Margolis CA, Gao W, Voss MH, Li W, Martini DJ, Norton C, Bossé D, Wankowicz SM, Cullen D, Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma, Science, (2018) eaan5951. [DOI] [PMC free article] [PubMed] [Google Scholar]