Abstract
The lack of biomimetic in vitro models of the human heart has posed a critical barrier to progress in the field of modeling cardiac disease. Human engineered cardiac tissues (hECTs)—autonomous, beating structures that recapitulate key aspects of native cardiac muscle physiology—offer an attractive alternative to traditional in vitro models. Here we describe the use of hECTs to advance our understanding and modeling of cardiac diseases in order to test therapeutic interventions, with a focus on contractile dysfunction in the setting of inherited and acquired forms of cardiomyopathies. Four major procedures are discussed in this chapter: (1) preparation of hECTs from human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) on single-tissue and multitissue bioreactors; (2) data acquisition of hECT contractile function on both of these platforms; (3) hECT modeling of hereditary phospholamban-R14 deletion-dilated cardiomyopathy; and (4) cryo-injury and doxorubicin-induced hECT models of acquired cardiomyopathy.
Keywords: Tissue engineering, Genetic cardiomyopathy, Acquired cardiomyopathy, Contractility, Models of disease, Stem cells
1. Introduction
Nonischemic dilated cardiomyopathy (NIDCM), which is characterized by ventricular dilation and systolic dysfunction in the absence of coronary artery disease, is a major form of heart failure impacting 1 in 20,000 adults per year in the USA [1]. Inotropic support is a strategy of medical management for NIDCM, but it does not treat the underlying cause. This highlights a critical need to develop novel therapeutic strategies for restoring cardiac performance in NIDCM. Stem cell therapy has emerged as a promising approach to address this problem [2].
In our hands, human engineered cardiac tissues (hECTs)— capable of recapitulating key aspects of native cardiac muscle physiology [3]—provide a simple yet effective contractility assay to study therapeutic strategies [4], such as stem cell-based cardiotherapies, in the context of healthy [5] and myocyte-depleted [6] conditions.
Nevertheless, to further progress the translational relevance of such findings (and other therapeutic interventions), it is necessary to continue improving hECT models of both ischemic and nonischemic cardiomyopathy. To this end, in this chapter, we highlight our recent efforts to advance hECT models of NIDCM and ischemic cardiomyopathy.
First, we provide step-by-step instructions on how to create hECTs with our single- and multi-hECT bioreactor platforms using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), and subsequently measure their contractile performance. Next, as one of the first cardiac tissue engineering groups to model familial dilated cardiomyopathy [7], we provide instructions on how to create an hECT model of inherited dilated cardiomyopathy (hereditary phospholamban-R14 deletion-dilated cardiomyopathy hECTs). Finally, motivated by in vivo animal models of acquired ischemic [8] and nonischemic [9] cardiomyopathy, we present instructions and original data on cryoinjury- and doxorubicin-induced hECT cardiomyopathy models, respectively.
Many in vitro disease models are evaluated exclusively using molecular and histology analysis. However, a strength of the hECT system is that it also provides a functional phenotype, which can be further analyzed for structural and molecular characteristics as desired. Altogether, this chapter complements our previous work to further support the use of hECTs as a system to help bridge a gap in traditional experimental models of the heart, in order to provide new opportunities for advancing our understanding of cardiac disease and therapeutics.
2. Materials
2.1. Cell Collection
mTeSR™ 1.
6-well tissue culture treated plates.
+I media: RPMI 1640, B-27 Supplement (50 ), 1% penicillin–streptomycin.
—I media: RPMI 1640, B-27 Supplement Minus Insulin (50×), 1% penicillin–streptomycin.
0.025% trypsin.
DMEM–F-12 Media, 1:1 nutrient mixture.
CHIR99021 (30 mM stock solution).
IWR-1 (10 mM stock solution).
1×phosphate-buffered saline without calcium or magnesium (PBS, pH 7.4), sterile-filtered.
15 mL conical tubes.
1.5 mL Eppendorf tubes.
2.2. Tissue Formation
5 mg/mL type-I collagen.
1 M Sodium hydroxide NaOH.
10× PBS (without calcium or magnesium).
Sterile ultrapure deionized water.
10× Minimum Essential Medium (MEM).
0.2 N HEPES pH 9.
Stem cell-qualified Matrigel.
Petri dishes 60 mm × 15 mm style and 100 mm × 20 mm style.
2.3. Bioreactor Construction
Sylgard 184 silicone elastomer kit (polydimethylsiloxane, or PDMS) custom molds.
Alcohol resistant black marker.
2% bovine serum albumin (BSA).
Silicone vacuum grease.
Tweezers.
2.4. Data Acquisition
Laptop.
GRASS S88X stimulator (Astro-Med, West Warwick, RI).
High-speed monochrome camera, with the ability to capture images at up to 90 frames per second. Such as PixeLINK PL-B741 camera or similar.
Dissecting microscope. Olympus SZ61 or similar.
Carbon rods (for single-tissue data acquisition only).
Carbon plates (for multitissue data acquisition only).
Tungsten wire, gauge 0.01000.
Plate heater (recommended for experiments where physiologic temperature condition is desired).
Boom microscope stand.
Gooseneck lamp.
Vibration isolation table. C-shape table that fits inside the laminar flow hood, this reduces transmission of vibration from the motor/blower to the bioreactor that is being tested.
Laminar flow hood.
Mirror (20 mm enhanced aluminum coated, right angle mirror, Edmund Optics).
Laboratory Jack 2× (for multitissue data acquisition only).
¾00 spacers 4×.
LabVIEW (National Instruments, Austin, TX) and MATLAB (Natick, Massachusetts) software. Custom LabVIEW program used for data acquisition, and MATLAB script used for data analysis are available upon request from the authors.
3. Methods
3.1. Cardiomyocyte Differentiation of hiPSCs
To start the cardiomyocyte differentiation at 80–90% confluency (see Note 3) of hiPSCs in a 6-well plate, replace mTeSR™ 1 maintenance media (see Note 4) with 2 mL I media containing CHIR99021 (10 μM final concentration) per well.
After 24 h, wash with DMEM/F12 (see Note 5) and replace with 2 mL —I media per well.
After 48 h, wash with DMEM/F12 and replace with 2 mL —I media containing IWR-1 (5 μM final concentration) per well.
After 24 h, wash with DMEM/F12 and replace with 2 mL —I media containing IWR-1 (5 μM final concentration) per well.
After 24 h, wash with DMEM/F12 and replace with 2 mL —I media (without IWR-1) per well.
Repeat Subheading 3.1, step 5.
After 24 h, wash with DMEM/F12 and replace with 2 mL +I media per well.
Repeat Subheading 3.1, step 7 (see Note 6) up to days 20–30 of differentiation.
3.2. Preparing the Single-Tissue Bioreactor
Mark the tip of bioreactor posts with alcohol resistant black marker to facilitate later video tracking.
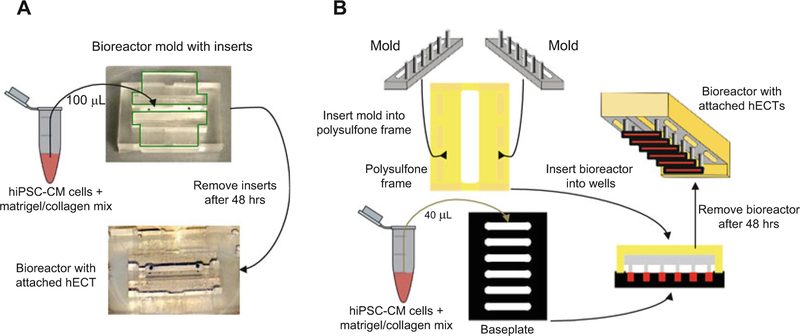
Assemble single-tissue bioreactor with inserts (Fig. 1a).
Sterilize (see Note 7).
In a laminar flow hood, using sterile tweezers, remove the PDMS mold from the autoclave bag; and while holding the mold with the tweezers, apply a spare amount of vacuum grease with the aid of a pipette tip to the bottom of the mold (see Note 8) and then place it on the 60 mm dish applying pressure so the mold adheres to the bottom of the dish.
Pipette approximately 150 μL of 2% BSA into the well of the single-tissue bioreactor, and place in the incubator (37 ○C and 5% CO2) for 1 h.
Remove the BSA, then rinse by serial washes as follows, add approximately 150 μL of 1× PBS, remove and repeat, then add approximately 150 μL of distilled water, remove and repeat. After the second rinse of distilled water aspirate all the liquid from the well and leave the single-tissue bioreactor in the laminar flow hood to air dry.
Fig. 1.
Overview of hECT construction. hECTs are created either with (a) single-tissue or (b) multitissue bioreactors. For single-tissue bioreactors (a), a set volume of the cell–extracellular matrix mix is added to each well of the bioreactor. After 2 h of incubation the bioreactor is submerged in culture media for 48 h. Following hECT compaction, the inserts are slowly removed from the bioreactor. For multitissue bioreactors (b), a set volume of the cell–extracellular matrix mix is added to each well of the baseplate. Six pairs of bioreactor posts are then submerged into matching baseplate wells for 48 h. Following hECT compaction, the bioreactor is slowly lifted out of the baseplate with the tissues suspended between pairs of end-posts
3.3. Collecting hiPSC-CMs from the Monolayer
In our experience an effective time window to harvest the cells for hECT fabrication is between 20 and 30 days of differentiation, as convenient for experimental planning.
On the day of cell harvest, first wash cells once with 1× PBS.
Incubate for 5 min at 37°C and 5% CO2.
Remove cells from each well mechanically using the 0.025% trypsin from each well.
Place trypsin–cell mix into 15 mL conical tube, and neutralize with equal amount of cold (4°C) +I media.
Centrifuge at 300 × g for 5 min.
Aspirate and resuspend with 10 mL +I media.
Count cells using hemocytometer.
Centrifuge at 300 × g for 5 min (see Note 11).
3.4. Preparing the Collagen-Matrigel Mix
Keep all solutions on ice. Keep cells (hiPSC-CM) at room temperature. All volumes listed below are per hECT for the single-tissue bioreactor. For the multitissue bioreactor, multiply each volume by 0.4–0.6 (see Note 12).
Dilute 100.0 μL of 5 mg/mL collagen stock to 3.125 mg/mL with 41.5 μL of sterile ultrapure deionized water, 2.5 μL of1M NaOH, and 16 μL of 10× PBS, avoid bubbles when mixing.
Add 20.0 μL of both 10 MEM and 0.2 N HEPES pH 9 to the dilute collagen mixture (previous step) to create the collagen mix.
Add stem cell-qualified Matrigel to the collagen mix (0.9 mg/mL final concentration).
Store the collagen–Matrigel mix on ice.
3.5. Forming hECTs on the Single-Tissue Bioreactor
For a schematic summarizing these steps, see Fig. 1a.
Aspirate supernatant from hiPSC-CM pellet.
Add 50 μL of collagen–Matrigel mix per million cells (hiPSC- CM) to the cell pellet (see Note 13).
Add 100 μL hiPSC-CMs + collagen–Matrigel mix into the well of the single-tissue bioreactor, avoid bubbles (see Notes 14 and 15).
Discard top lid of 60 mm dish; place 60 mm dish into 100 mm dish (see Note 16).
Add 1 mL of +I media into the edge of the 60 mm dish, being careful not to drop any media onto the bioreactor. This is to ensure the cell–collagen mixture does not get dehydrated dur- ing gel polymerization.
Place into incubator for 2 h at 37 ○C and 5% CO2, this is sufficient time for the collagen to polymerize.
Remove from incubator and place in sterile hood.
Slowly add approximately 14 mL +I media into 60 mm dish so that the entire bioreactor is covered with media (see Note 17).
Return assembly to incubator for 48 h at 37 ○C and 5% CO2.
Remove from incubator and bring to laminar flow hood; using sterile tweezers slowly remove the inserts and exchange media (half volume).
Continue half-media exchanges with +I media daily for tissue maintenance.
3.6. Preparing the Multitissue Bioreactor
For a schematic summary of these steps, see Fig. 1b. Carry out after steps described in Subheading 3.1.
Mark the tip of bioreactor posts with alcohol resistant black marker.
Sterilize (see Note 7).
Insert rack of bioreactor posts into polysulfone frame.
3.7. Forming hECTs Using the Multitissue Bioreactor
For a schematic summary of these steps, see Fig. 1b. Carry out after steps described in Subheadings 3.3 and 3.4.
Aspirate supernatant from hiPSC-CM pellet.
Add 40 μL of collagen–Matrigel mix per million cells (hiPSC- CM) to the cell pellet (see Note 18).
Place baseplate in 60 mm dish.
Add 40 μL hiPSC-CMs + collagen–Matrigel mix into each well of the baseplate (see Note 14).
Insert bioreactor into baseplate (see Note 19).
Discard top lid of 60 mm dish; place 60 mm dish into 100 mm dish (see Note 16).
Add 1 mL of +I media into edge of 60 mm dish to prevent dehydration of the gel.
Place into incubator for 2 h at 37°C and 5% CO2 for gel to polymerize.
Remove from incubator and bring to laminar flow hood.
Slowly add 14 mL +I media into 60 mm dish (see Notes 17 and 20).
Place into incubator for 48 h at 37°C and 5% CO2 (see Note 21).
Remove from incubator; slowly lift bioreactor out of baseplate (see Note 22), and place bioreactor into new 60 mm dish with 14 mL +I media.
3.8. Data Acquisition for Single-hECT and Multi-hECT Bioreactors
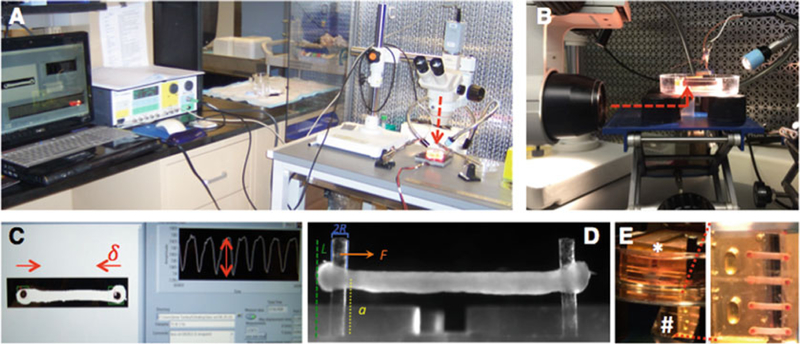
For a schematic summary of these steps, see Fig. 2.
Fig. 2.
Overview of hECT data acquisition. (a) Set up for data acquisition from hECT on single-tissue bioreactor. Camera viewing path (dotted red line) aligned with top view of hECT. (b) Set up for data acquisition from hECT multitissue bioreactor. Camera viewing path (dotted red line) aligned with mirror. (c) Screen view during data acquisition, live tracking of inward post-displacement during hECT contraction (δ), with corresponding measurement of the amplitude of each contraction (double headed arrow). (d) Side view of hECT on single- tissue bioreactor with embedded schematic of the measurements included in the calculation of force. (e) Multitissue bioreactor (*) with tissues submerged facing the bottom of the dish, and reflected image of hECT on mirror (#) to allow for imaging and data collection without manipulation of the hECT
Set up laminar hood with vibration isolation table, gooseneck lamp, boom stand, dissecting microscope and high-speed cam- era. For single-hECT bioreactors, place 60 mm dish with bio- reactor on top of plate heater, and align microscope to view the hECT from the top (Fig. 2a). For multi-hECT bioreactor, include the laboratory jack, place spacers equally distant with mirror in the center, place the 100 mm dish containing the 60 mm dish with the multitissue bioreactor carefully on top of the spacers, and align the microscope to view the reflection of the multitissue bioreactor on the mirror (Fig. 2b, e) (see Note 23). Connect grass stimulator and camera to laptop.
Adjust microscope magnification and limit region of interest to have both posts of one hECT in view. Using an Olympus SZ61 microscope most of the acquisitions are done within the 1–1.5 magnification range (Fig. 2c). Our approach is to use a binary filter of the image to maximize contrast, which is why we use the black marker to mark the top of the posts.
Set the camera to capture at a high frame rate (90 frames per second) by increasing stepwise the speed and light level.
Using a custom LabVIEW program (available upon request), record the displacement of the posts without electrical stimulation to analyze the spontaneous contractile properties of the hECT.
Place carbon rods (or carbon plates) adjacent to the single-tissue (or the multitissue) bioreactor, respectively, and connect them to the grass stimulator electrodes (see Note 24).
Using a custom LabVIEW program, record the displacement of the posts with electrical stimulation (see Note 25).
Measure the post heights and tissue height on the posts by acquiring a side view image of the hECT as in Fig. 2d (see Note 26). For the single tissue bioreactor, place the mirror in the 60 mm dish along a long end of the bioreactor. For the multi-tissue bioreactor acquire side view images with the microscope looking directly through the 60 mm dish.
The data is then processed using a custom MATLAB script (available upon request) to produce the results for the different twitch parameters that are analyzed.
3.9. hECT Model of Inherited Dilated Cardiomyopathy
Carry out steps in Subheadings 3.1 through 3.5 using hECT from distinct groups such as: wild type (hiPSC from healthy donor), mutant (hiPSC from patient carrier of the DCM-associated mutation) or isogenic corrected (using gene editing). Upon comparison of the contractile performance of the different groups, hECT fabricated with hiPSC-CM from the patient shows a phenotype of impaired contractile function when compared to wild type (Fig. 3a) that can be genetically corrected (Fig. 3b, c) (see Note 27).
Fig. 3.
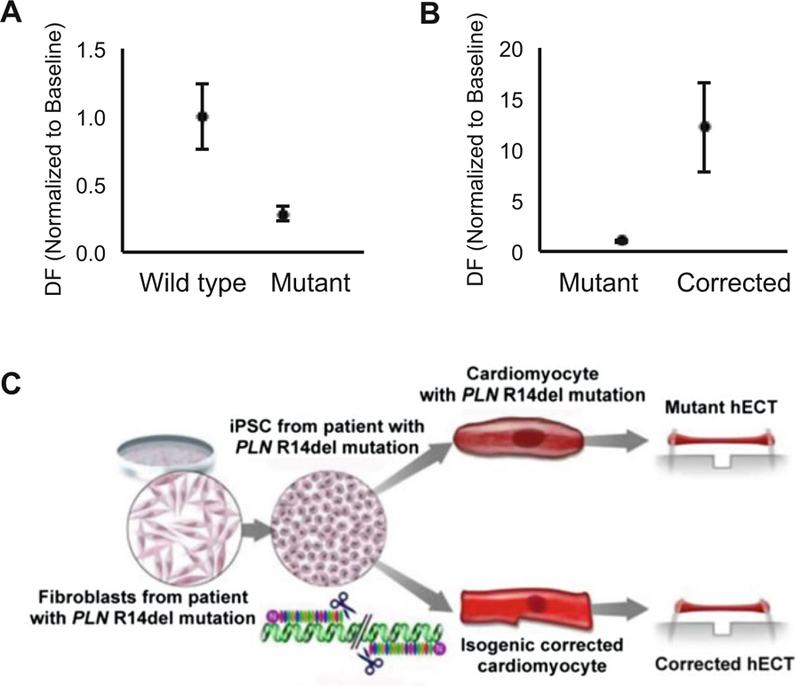
hECT Inherited Models of Cardiac Disease. (a) Effects of model of inherited dilated cardiomyopathy on hECT developed force (DF) (mean ± SEM, n = 4–7). (b) Effects of genetic correction of model of inherited dilated cardiomyopathy on hECT developed force (DF) (mean ± SEM, n = 4–7). (c) Overview of method for inherited dilated cardiomyopathy model and genetic correction of the model; adapted from [7] with permission
Follow steps described above in Subheading 3.5 to fabricate the hECTs.
On day 5 of fabrication, switch to media with serum (composition of the media with serum are described in Note 17).
Upon reaching day 7, pace hECTs using methods described above (see Subheading 3.8).
Rinse with 1× PBS.
Cover hECT in media with serum.
Place in incubator if pacing at additional later time points is desired.
3.10. Cryo-Injury hECT Cardiomyopathy Model
For a schematic summary of the steps, see Fig. 4a. The contractile performance of representative hECTs during this intervention is shown in Fig. 4b, where in acute injury you expect a decrease in function [8].
Fig. 4.
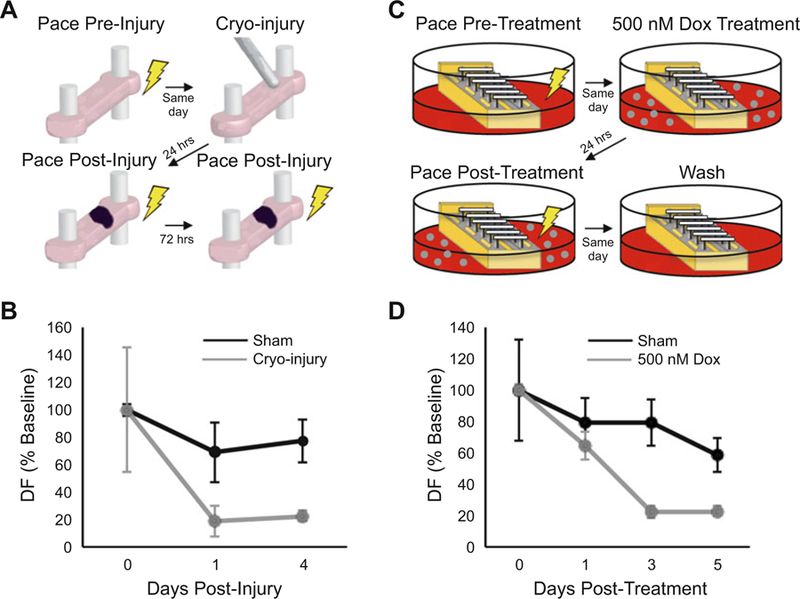
hECT models of acquired cardiac disease. (a) Overview of cryo-injury method for acquired ischemic cardiomyopathy hECT model. (b) Effects of cryo-injury model (grey) on hECT developed force (DF) over time (mean ± SEM, n = 2–3). (c) Overview of doxorubicin (Dox) method for acquired nonischemic cardiomyopathy hECT model. (d) Effects of 500 nM Dox (grey) on hECT DF over time (mean ± SEM, n = 4)
Follow steps described above in Subheadings 3.4 and 3.5 to fabricate the hECTs.
Six days post-tissue formation, pace hECTs using methods described above to measure preinjury baseline function (see Subheading 3.8).
Remove +I media to fully expose the hECT.
Lay a 1.6-mm diameter steel pin frozen in liquid nitrogen on the hECT for 5 s. For sham use a room temperature pin.
Add a few drops of +I media directly at the site where the steel pin is in contact with the hECT; this will thaw the region so the pin can be safely removed. Removing the frozen steel pin from the hECT without previously warming it up with media will result in tearing off the portion of the hECT that is in contact with the frozen steel pin.
Cover hECT in +I media.
Place in incubator for 24 h at 37 ○C and 5% CO2.
Pace hECTs using methods described above (see Subheading 3.8) to measure short-term post-injury contractile function.
Rinse with 1× PBS.
Cover hECT in +I media.
Place in incubator for 72 h (or longer as desired) at 37 ○C and 5% CO2.
Pace hECTs using methods described above (see Subheading 3.8) to monitor long-term post-injury contractile function.
3.11. Doxorubicin- Induced hECT Cardiomyopathy Model
For a schematic summary of these steps, see Fig. 4c. The contractile performance of representative hECTs during this intervention is shown in Fig. 4d, where the doxorubicin treated hECT will show a sharp decrease in function [9] (see Note 28).
Five days post-tissue formation, pace hECTs using methods described above (see Subheading 3.8) to establish pre-doxorubicin baseline contractile function.
Rinse with 1× PBS.
Transfer bioreactor to new 60 mm dish with +I media containing 500 nM doxorubicin.
Place in incubator for 24 h at 37°C and 5% CO2.
Pace hECTs using methods described above (see Subheading 3.8) to measure short-term post-doxorubicin contractile function.
Rinse with 1× PBS.
Transfer bioreactor to new 60 mm dish with +I media.
Place in incubator for 48 h at 37°C and 5% CO2.
Pace hECTs using methods described above (see Subheading 3.8) to monitor long-term post-doxorubicin contractile function.
Repeat Subheading 3.11, steps 6 through step 9 as needed for longer term studies.
Acknowledgments
This work was supported by NIH/NHLBI K01HL133424 (ICT), NIH/NHLBI 1F30HL134283–01A1 (JM), American Heart Association 15POST25090116 (DKC), and NIH/NHLBI R01HL132226 (KDC).
Footnotes
4 Notes
For Subheading 3.9, hiPSCs were generated from a patient with the PLN R14del mutation [7]. For Subheadings 3.10 and 3.11, the SKiPS-31.3 line was used. This line was generated from dermal fibroblasts from a healthy 45-year-old male volunteer with no symptoms of cardiovascular disease [10].
Differentiations work best using hiPSCs between passages 30 and 70.
Confluency greatly affects differentiation efficiency.
E8 media can also be used for maintenance.
Washing away dead cells with DMEM/F12 helps improve differentiation efficiency.
At this stage of differentiation, changing media every 48 h (with 3 mL per well), rather than every 24 h (with 2 mL per well), is also a viable option. After day 10 of differentiation, it is not necessary to wash with DMEM:F12.
The PDMS molds can withstand sterilization cycles in a steam autoclave up to 121°C.
The vacuum grease helps to adhere the mold to the bottom of the culture dish, so that in later steps, when the dish is filled with media, the single-tissue bioreactor will remain submerged. Avoid applying vacuum grease on the center of the mold because that will interfere with visualization of the tissue using an inverted microscope.
TrypLE Express can also be used for dissociation.
For poor differentiations, it is possible to improve hiPSC-CM purity by mechanically removing only the top layer of cells with the dissociation reagent and without incubation proceed to Subheading 3.3, step 5.
Working with 1.5-mL Eppendorf tubes is preferred when making hECTs; cells should be resuspended in 1 mL of +I media post-centrifugation/aspiration and transferred to an Eppendorf tube. Centrifugation is then repeated at 300 g for 5 min.
Using 0.4–0.6 scaling factors have proven successful in our hands. Scaling factor is dependent on desired amount of Matrigel–collagen mix to be used per tissue (see Note 18).
On average, one well from a six-well plate yields enough hiPSC-CM to fabricate one tissue using a single-tissue bioreactor.
To account for pipette error (and to help avoid bubbles), add 10% more of the cell–extracellular matrix mix into the pipette.
In the event of bubbles, aspirate the bubble using a 200 μL pipette tip. Let the bubble slowly rise to the top of the tip, and then return back into the well the cell–extracellular matrix that was aspirated with care to not return the bubble to the well.
Be sure that if you have relevant information on the lid, tran- scribe that information to the lid of the 100 mm dish. It also helps to label the bottom of the dish to keep record of the bioreactor inside the dish.
To improve compaction, add DMEM containing 10% neonatal bovine serum, 1% penicillin–streptomycin and 0.2% Amphotericin B instead of +I for the first 24–48 h.
Using 40–60 μL Matrigel–collagen mix per tissue has proven successful in our hands.
Confirm that bioreactor posts are submerged into the wells, but are not bent from touching the bottom or edges of the well.
May need to add more +I media to confirm that all hECTs are fully submerged into media.
Check on compaction; if compaction is progressing slower than expected it is okay to leave bioreactor in baseplate for an additional 24 h, on the other hand remove earlier if tissue compaction appears accelerated.
This method has been updated since [11]. While holding the baseplate at the bottom of the 60 mm dish, the bioreactor is slowly lifted vertically out of the baseplate into a new 60 mm dish with 14 mL +I media.
The multitissue bioreactor is maintained in culture and tested with the hECT facing the bottom of the dish; the reflected image on the mirror allows visualizing the tissue without requiring any direct manipulation.
To aid in connecting the carbon rods/plates to the grass stimulator electrodes, the electrodes can be fitted with alligator clips soldered to the end of the electrodes; tungsten wire can be looped tightly around the carbon rods/plates; lastly, clasp loose end of each tungsten wire with the alligator clips.
The protocol to follow for electrical stimulation should be determined per the experiment. We typically pace the hECT starting at low frequency (0.25 Hz), and then record hECT contractions (post displacement) at different frequencies using 0.25-Hz increments; bipolar field stimulation, 5 ms pulse duration, using a 12-V pulse wave (545 mV/mm and 480 mV/mm for single and multitissue bioreactor respectively).
The beam bending equation is used to calculate the force (F) (Fig. 2c, d). Post length (L ) and tissue height along the posts (a) are measured using a side view image of the hECT; the post displacement (δ) is measured during data acquisition using LabVIEW. The radius (R) and Young’s modulus (E) are only required to be measured once upon fabrication of a bioreactor, and thereafter the values remain constant. Developed Force (DF) is the difference between the maximum and minimum force during each twitch.
Furthermore, correction of the phenotype can be tested by using the isogenic control cell line. This has been previously described [7], demonstrating that by using genomic correction of the hiPSC-CM by a TALEN method of targeted gene editing, the corrected hECT showed enhanced contractile force compared to the isogenic mutant tissues (Fig. 3c).
In our experience, the adverse effect of doxorubicin on hECT contractile function is dependent on the initial developed force. For desired level of force reduction titrate doxorubicin between 0.25 and 2.0 μM, administered for no longer than 48 h.
References
- 1.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE (2006) Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 296 (15):1867–1876. 10.1001/jama.296.15.1867 [DOI] [PubMed] [Google Scholar]
- 2.Mushtaq M, DiFede DL, Golpanian S, Khan A, Gomes SA, Mendizabal A, Heldman AW, Hare JM (2014) Rationale and design of the percutaneous stem cell injection delivery effects on neomyogenesis in dilated cardiomyopathy (the POSEIDON-DCM study): a phase I/II, randomized pilot study of the comparative safety and efficacy of transendocardial injection of autologous mesenchymal stem cell vs. allogeneic mesenchymal stem cells in patients with non-ischemic dilated cardiomyopathy. J Cardiovasc Transl Res 7 (9):769–780. 10.1007/s12265-014-9594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, Hajjar RJ, Hulot JS, Costa KD (2014) Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J 28 (2):644–654. 10.1096/fj.13-228007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayourian J, Ceholski DK, Gonzalez DM, Cashman TJ, Sahoo S, Hajjar RJ, Costa KD (2018) Physiologic, pathologic, and therapeutic paracrine modulation of cardiac excitationcontraction coupling. Circ Res 122 (1):167–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayourian J, Cashman TJ, Ceholski DK, John- son BV, Sachs D, Kaji DA, Sahoo S, Hare JM, Hajjar RJ, Sobie EA, Costa KD (2017) Experimental and computational insight into human mesenchymal stem cell paracrine signaling and heterocellular coupling effects on cardiac contractility and arrhythmogenicity. Circ Res 121 (4):411–423. 10.1161/CIRCRESAHA.117.310796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serrao GW, Turnbull IC, Ancukiewicz D, Kim DE, Kao E, Cashman TJ, Hadri L, Hajjar RJ, Costa KD (2012) Myocyte-depleted engineered cardiac tissues support therapeutic potential of mesenchymal stem cells. Tissue Eng Part A 18(13–14):1322–1333. 10.1089/ten.TEA.2011.0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stillitano F, Turnbull IC, Karakikes I, Nonnenmacher M, Backeris P, Hulot JS, Kranias EG, Hajjar RJ, Costa KD (2016) Genomic correction of familial cardiomyopathy in human engineered cardiac tissues. Eur Heart J 37(43):3282–3284. 10.1093/eurheartj/ehw307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strungs EG, Ongstad EL, O’Quinn MP, Palatinus JA, Jourdan LJ, Gourdie RG (2013) Cryoinjury models of the adult and neonatal mouse heart for studies of scarring and regen- eration. Methods Mol Biol 1037:343–353. 10.1007/978-1-62703-505-7_20 [DOI] [PubMed] [Google Scholar]
- 9.Toyoda Y, Okada M, Kashem MA (1998) A canine model of dilated cardiomyopathy induced by repetitive intracoronary doxorubicin administration. J Thorac Cardiovasc Surg 115(6):1367–1373. 10.1016/S0022-5223(98)70221-1 [DOI] [PubMed] [Google Scholar]
- 10.Karakikes I, Senyei GD, Hansen J, Kong CW, Azeloglu EU, Stillitano F, Lieu DK, Wang J, Ren L, Hulot JS, Iyengar R, Li RA, Hajjar RJ (2014) Small molecule-mediated directed dif- ferentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Transl Med 3(1):18–31. 10.5966/sctm.2013-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cashman TJ, Josowitz R, Gelb BD, Li RA, Dubois NC, Costa KD (2016) Construction of defined human engineered cardiac tissues to study mechanisms of cardiac cell therapy. J Vis Exp 109:e53447 10.3791/53447 [DOI] [PMC free article] [PubMed] [Google Scholar]