Abstract
Objective
To estimate the prevalence and describe the patterns of concurrent HPV and sexually transmitted infections (STIs) and associated factors among HIV-negative young Western Cape, South African women participating in the Efficacy of HPV Vaccine to Reduce HIV Infection (EVRI) Trial.
Methods
HIV-negative women aged 16–24 years old were enrolled in the EVRI trial (NCT01489527) and randomized to receive the licensed 4-valent HPV vaccine or placebo. At study entry, participants were clinically evaluated for five STIs: HSV-2, chlamydia, gonorrhea, syphilis and disease causing HPV genotypes (6/11/16/18/31/33/35/39/45/51/52/56/58/59/68). Demographic and sexual history characteristics were compared among women with STI coinfections, single infection, and no infection using Pearson Chi-square and Mann-Whitney tests. Odds ratios were calculated to evaluate factors associated with STI coinfection prevalence.
Results
Among 388 young women, STI coinfection prevalence was high: 47% had ≥2 concurrent STIs, 36% had a single STI, and 17% had none of the five evaluated STIs. HPV/HSV-2 (26%) was the most prevalent coinfection detected followed by HPV/HSV-2/CT (17%) and HPV/CT (15%). Coinfection prevalence was independently associated with alcohol use (adjusted OR=2.01, 95% CI=1.00–4.06) and having a sexual partner with an STI (adjusted OR = 6.96, 95% CI = 1.53–30.08).
Conclusions
Among high-risk young women from underserved communities such as in Southern Africa, a multicomponent prevention strategy that integrates medical and behavioral interventions targeting both men and women is essential to prevent acquisition of concurrent STI infections and consequent disease.
Keywords: HPV, chlamydia, gonorrhea, syphilis, HSV-2
INTRODUCTION
As one of the most common sexually transmitted infections (STIs), human papillomavirus (HPV) infection accounts for nearly a third of infection-related cancers worldwide.1 The African continent, particularly sub-Saharan Africa, experiences a disproportionate burden of these HPV-associated cancers.2,3 Sub-Saharan Africa also has a high burden of HIV4 and other STIs such as chlamydia, gonorrhea, trichomoniasis, herpes simplex virus type 2 (HSV-2) and syphilis;5–7 STIs implicated in HIV acquisition and transmission.8–10 Similar to the other STIs there is growing evidence that HPV may also increase susceptibility to HIV acquisition.11–13 These and other published reports of STIs in Africa indicate that both urban and rural Sub-Saharan African men and women are at high risk for acquiring concurrent STIs.14,15 In such resource-constrained settings, a host of factors likely converge to influence STI coinfection risk including early age of sexual debut, lower education, migration, higher number of lifetime sex partners, concurrent sexual relationships and previous infection with an STI.6,7
Often, STIs such as chlamydia and gonorrhea are asymptomatic16,17 and in the absence of screening and appropriate treatment, these STIs could have severe consequences for the sexual and reproductive health of women. Untreated STIs accelerate women’s risk for pelvic inflammatory disease leading to chronic pelvic pain and infertility.18,19 Pregnant women with untreated STIs are at high risk for obstetric complications as well as fetal and neonatal morbidity and mortality.19 Given these long-term sequelae, epidemiological data on the prevalence and types of STI coinfections among high-risk, HIV-negative young women remain critical for targeted STI prevention and intervention programs that include routine screening, referral for appropriate treatment, and HPV vaccination.
The current analysis estimates the prevalence and describes patterns of concurrent STIs among HIV-negative, young South African women with any of the following five STIs: chlamydia, gonorrhea, syphilis, HSV-2 and disease causing HPV genotypes in the EVRI study. A secondary aim was to assess factors associated with prevalent concurrent STIs.
MATERIALS AND METHODS
Study Population
Young female residents of the Western Cape, South Africa were enrolled from November 2012 to July 2013 in a Phase II randomized placebo controlled double blind trial, the Efficacy of HPV Vaccine to Reduce HIV Infection (EVRI) Trial (NCT01489527) that utilized the licensed 4-valent HPV (4vHPV) vaccine (Gardasil).20 Women were recruited from the Bloekombos primary health care clinic and Kraaifontein Day Hospital and were enrolled if they met the following eligibility criteria: (1) aged 16–24 years, (2) no history of abnormal Pap smear, (3) reported having vaginal intercourse, (4) not currently pregnant or breastfeeding, (5) HIV negative, (6) no autoimmune disease requiring steroid use, (7) not had a splenectomy, (8) not currently enrolled in an HIV prevention trial, (9) no IV drug use in the past 6 months, (10) no serious allergic reactions history requiring medical attention, (11) no allergies to aluminum, yeast, or benzonase, (12) no previous HPV vaccination, (13) willingness to comply with 4 scheduled visits within 7 months of enrollment; and (14) agreed to use effective contraception during sexual intercourse for the vaccination period.
Women were randomized 1:1 to receive the 4vHPV vaccine or placebo (saline) at enrollment, month 2 and month 6. Participants were followed for one month after the final dose when individual unblinding occurred and women from the placebo arm were offered the Gardasil vaccine. This analysis focuses on STI coinfections at the enrollment visit, prior to vaccine allocation.
The study protocol was approved by the Institutional Review Boards of the University of South Florida, USA and Stellenbosch University, South Africa. Additionally, the protocol followed South African policies and ethical guidelines concerning parental permission for children to participate in research studies.
Study procedures
At enrollment, participants completed a tablet-based questionnaire using a computer-assisted self-interview available in English, Xhosa and Afrikaans that assessed their sexual history, health and sociodemographic characteristics. Urine specimens for chlamydia and gonorrhea testing were collected and sera for syphilis testing and HSV-2 antibody status assessment were obtained. A study physician performed a physical exam including a speculum exam of the vagina and cervix. Specimens for HPV detection were collected from the vulva/ labia and from the endocervix/ectocervix followed by a digital vaginal exam. Samples for HPV detection were obtained using a prewetted Dacron cotton swab placed in STM (Digene Hybrid Capture test kit; Digene Corporation).
Laboratory analyses
Chlamydia and gonorrhea were detected from urine specimens with the Amplex CT/NG real- time detection method (Seegene, South Korea). Syphilis was detected using the Captia™ Syphilis (Treponema pallidum)-G assay (Trinity Biotech, Jamestown, NY). All specimens positive for syphilis were confirmed positive by a repeat test with the same assay.21 The Captia™ Syphilis (Treponema pallidum)-G test may test positive in patients with active or inactive disease and those with previously treated infection.21 HSV-2 antibody status was measured by the Captia HSV-2 type specific IgG enzyme-linked immunoassay (EIA, Trinity Biotech). A primary HSV-2 infection or clinical recurrence cannot be determined from a serological test. However, HSV-2 is a lifelong infection with potential for recurrent episodes in the absence of chronic suppressive therapy, and intermittent viral shedding is common even in the absence of clinical symptoms.22 Thus, for this analysis, an HSV-2 antibody positive test was considered a current infection.
HPV DNA was detected by polymerase chain reaction (PCR). The Qiagen Media Kit was utilized to extract genomic DNA from cervical specimens and then amplified by PCR with the PGMY09/11 L1 consensus primer system and AmpliTaq Gold polymerase (Perkin-Elmer, Norwalk, CT). HPV DNA genotyping was performed on all cervical specimens using the Linear Array HPV Genotyping Test (Roche Diagnostics) which detects 37 HPV genotypes (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53–55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69–73, 81–84, 89, IS39). The Linear Array test is unable to detect HPV 52 coinfections in the presence of HPV 33, 35, or 58, so prevalence of HPV 52 infection might be underestimated. Although the assay provides estimates for all 37 HPV types, this analysis focuses on the HPV genotypes known to cause disease in women, specifically cervical cancer (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), and genital warts (HPV 6 and 11).23
Statistical Analysis
Data were analyzed using SAS ® 9.4 software (SAS Institute Inc., Cary, NC, USA). A p-value of less than 0.05 was considered statistically significant. Women were categorized into three groups by their STI status: no infection, single infection, or coinfection. No infection was defined as testing negative for all five STIs, specifically, chlamydia, gonorrhea, syphilis, HSV-2, or disease causing HPV genotypes; single infection was defined as having any one of the five STIs, and coinfection was defined as having two or more of the five STIs concurrently. Enrollment sociodemographic characteristics and sexual behavior were compared between the three groups using Mann-Whitney test for continuous variables, and Fisher’s exact test or Pearson chi-square test for categorical variables. Demographic variables were chosen based on associations with STIs shown in previous studies. To assess factors associated with STI status, a multinomial logistic regression model compared single infection vs. no infection, and coinfection vs. no infection. Odds ratios (OR) and 95% confidence intervals (CI) were computed as a measure of the association. Backwards elimination was performed to assess factors independently associated with single infection or coinfection with p value ≤0.20 chosen as the a priori cutoff for retention in the multivariable model.24 The final models were adjusted for age as it was associated with HPV and STI prevalence in previous EVRI analyses.20
RESULTS
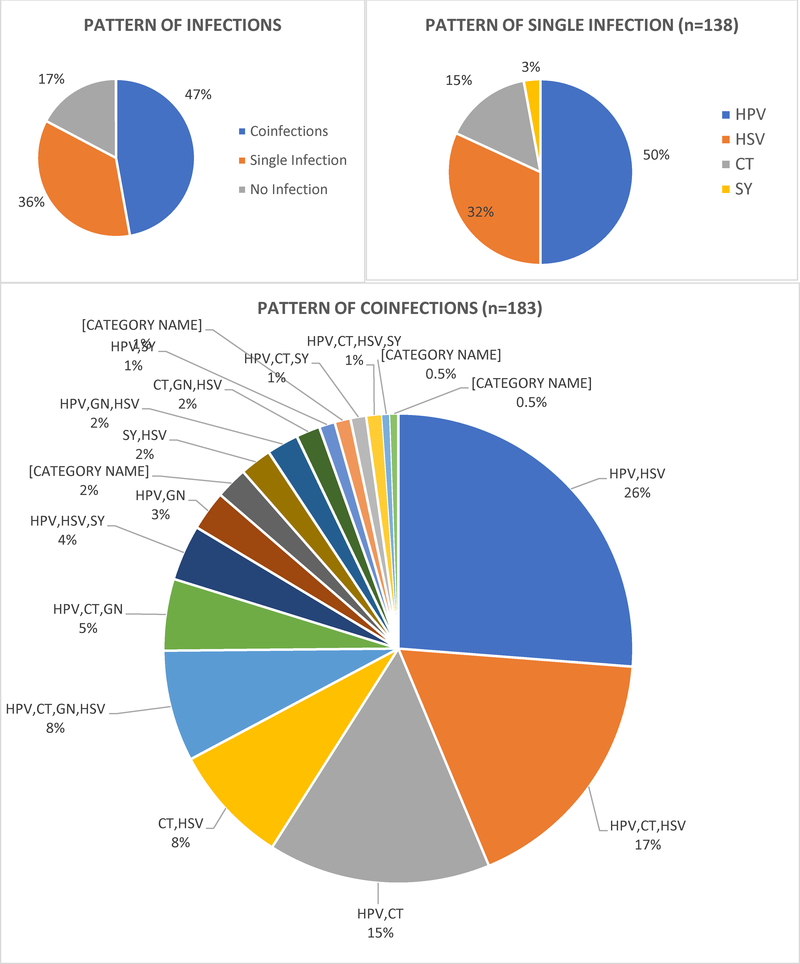
Of the 402 enrolled women, three with false negative results for HIV, five with inadequate samples for HPV detection and six with specimens that were β-globin negative and HPV DNA negative were excluded, resulting in a total of 388 women included in the final analysis. Similar to previously reported EVRI analyses,20 STI prevalence was high in these women: HPV (57.5%), HSV-2 antibodies (46%), chlamydia (33.5)%, gonorrhea (11.1%) and syphilis (5.9%) (See Supplementary Table S1). STI coinfection prevalence was also high Among the 388 women, 183 (47%) had coinfections, 138 (36%) had a single infection, and 67 (17%) had no infections (Figure 1A). Socio-demographic and sexual health characteristics were similar between the three groups with regard to age, education, marital status, median age at first vaginal sex, birth control use, and history of pregnancy (Table 1). Although the median number of lifetime male sexual partners was low, there was a wide range of reported sex partners in all three groups: “no infection” (median 2: range, 1–22); “single infection” (median 3: range, 1–21); and “coinfections” (median 3: range, 1–24) respectively. A higher proportion of women with coinfections (56.2%) reported three or more lifetime number of male sexual partners compared to women with a single infection (50.4%) or women with no infections (40%), however the number of partners was not significantly different between the three groups. Alcohol use (p=0.02), and having a sexual partner with an STI (p=0.01) were significantly different across the three groups of women (Table 1). Supplementary Tables S2 – S6 describe socio-demographic and sexual health characteristics associated with individual STIs.
Figure 1:
STI coinfection patterns among HIV-negative young South African women in the EVRI trial with one or more STIs (CT=Chlamydia, GC=Gonorrhea, SY=Syphilis, HSV=Herpes Simplex-2, HPV=disease causing HPV genotypes, specifically genital warts (HPV 6, 11) and cervical cancer (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). A, Pattern of infections: no infection, single infection and coinfections; B, Pattern of single infections among women with one of the five evaluated STIs; C, Coinfection patterns among women with concurrent STIs
Table 1:
Comparison of selected demographic and sexual health characteristics among HIV-negative young South African women (n=388) with no STIs, one STI and ≥2 STIs (HPVa, chlamydia, gonorrhea, syphilis, HSV-2)
| No Infection | Single Infection | Coinfection | p valueb | |
|---|---|---|---|---|
| (n=67) | (n=138) | (n=183) | ||
| no. (%) | no. (%) | no. (%) | ||
| Median age in years (range) | 21 (16–24) | 20 (16–24) | 20 (16–24) | 0.85 |
| 16–18 | 14 (20.9) | 27 (19.5) | 41 (22.4) | |
| 19–21 | 30 (44.7) | 69 (50.0) | 92 (50.2) | |
| 22–24 | 23 (34.3) | 42 (30.4) | 50 (27.3) | |
| Marital status | ||||
| Single/Widowed | 62 (92.5) | 132 (95.6) | 177 (96.7) | 0.35 |
| Married/Living Together | 5 (7.4) | 6 (4.3) | 6 (3.2) | |
| Education | 0.09 | |||
| ≤ Grade 7 | 7 (10.4) | 5 (3.6) | 7 (3.8) | |
| Grade 8–12 | 27 (40.3) | 76 (55.0) | 109 (59.5) | |
| Passed Grade 12 | 16 (23.8) | 30 (21.7) | 32 (17.4) | |
| Some college/tech | 17 (25.3) | 27 (19.5) | 35 (19.1) | |
| ≥1 alcohol drink in past month | 31 (46.2) | 79 (57.2) | 119 (65.0) | 0.02 |
| Ever used tobaccoc | 9 (13.4) | 26 (18.8) | 36 (19.6) | 0.51 |
| Median age of first vaginal sex (range) | 16 (13–19) | 17 (13–21) | 17 (1–21) | 0.46 |
| Prior history of STI | 0.86 | |||
| Yes | 13 (19.4) | 30 (21.7) | 45 (24.5) | |
| No | 39 (58.2) | 79 (57.2) | 96 (52.4) | |
| Don’t Know | 15 (22.3) | 29 (21.0) | 42 (22.9) | |
| Sexual partner with an STId | 4 (5.9) | 16 (11.5) | 35 (19.1) | 0.01 |
| Currently using birth controle | 30 (44.7) | 66 (47.8) | 88 (48.0) | 0.97 |
| Current birth control use | ||||
| Oral contraceptives | 1 (1.4) | 7 (5.0) | 6(3.2) | 0.41 |
| IUD/loop/coil | 0 (0.0) | 2 (1.4) | 0(0.0) | 0.16 |
| Depo Provera | 19 (28.3) | 37 (26.8) | 43 (23.5) | 0.67 |
| Condoms | 15 (22.3) | 31 (22.4) | 46 (25.1) | 0.82 |
| Ever been pregnant | 31 (46.2) | 70 (50.7) | 91 (49.7) | 0.83 |
| Lifetime no. of male sexual partnersf; median (range) | 2 (1–22) | 3 (1–21) | 3 (1–24) | 0.26 |
| 1 | 11 (24.4) | 20 (19.0) | 24 (16.6) | |
| 2 | 16 (35.5) | 32 (30.4) | 39 (27.0) | |
| 3+ | 18(40.0) | 53 (50.4) | 81 (56.2) | |
| Number of sexual partners in the past 6 monthsg; median (range) | 1 (0–3) | 1 (0–5) | 1(0–5) | 0.64 |
| 0 | 2 (5.8) | 7 (8.5) | 7 (6.4) | |
| 1 | 28 (82.3) | 61 (74.3) | 77 (71.3) | |
| 2+ | 4 (11.7) | 14 (17.0) | 24 (22.2) | |
| Ever received money/drugs/ presents for sex | 4 (5.9) | 4 (2.9) | 5 (2.7) | 0.42 |
Included HPV types known to cause disease, specifically genital warts (HPV 6, 11) and cervical cancer (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68)
Pearson Chi square test for categorical variables and Mann-Whitney test for continuous variables
Tobacco- cigarettes, roll-ups, pipes, cigars, chew, snuff
Data based on self-report
Sample size reduced to n=254 due to missing values: no infection (n=43), single infection (n=92), coinfection (n=119)
Sample size reduced to n=294 due to missing values: no infection (n=45), single infection (n=105), coinfection (n=144)
Sample size reduced to n=224 due to missing values: no infection (n=34), single infection (n=82), coinfection (n=108)
Participants misreported not having vaginal intercourse in the enrollment questionnaire because of confusion related to the term “vaginal”
Prevalence and patterns of concurrent sexually transmitted infections
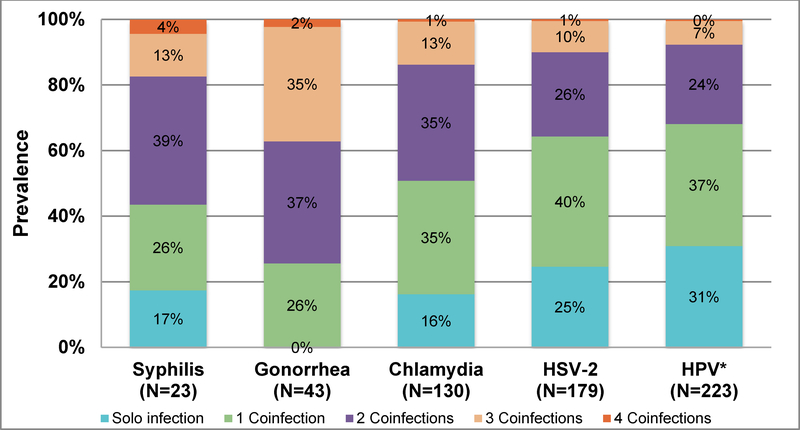
Figure 2 shows the number of women that tested positive for each of the five STIs and the proportion that were positive for single or multiple concurrent infections for each STI. Nearly 83% of women with syphilis, 100% of women with gonorrhea, 84% of women with chlamydia, 75% of women with HSV-2 and 69% of women with disease causing HPV genotypes were co-infected with at least one other STI (Figure 2). Although disease causing HPV types had the highest overall prevalence among the five STIs (57.5%), HPV-infected women had the lowest prevalence of coinfections, with nearly a third of the women (31%) having no other concurrent STIs. In contrast, the highest prevalence of coinfections was among women with gonorrhea with all women having at least one coinfection, 37% co-infected with two STIs and 37% co-infected with ≥3 STIs.
Figure 2.
Prevalence of coinfectionsa among South African young women with at least one of the five STIs (HPVb, chlamydia, gonorrhea, syphilis, HSV-2) (n=321).
aFor example, among the 23 women that had syphilis detected, 4 (17%) had a single infection, 6 (26%) were coinfected with syphilis and another STI, 9 (39%) were coinfected with syphilis and two other STIs, 3 (13%) were coinfected with syphilis and three other STIs and 1(4%) was coinfected with syphilis and all four other STIs.
bHPV genotypes known to cause disease specifically genital warts (HPV 6, 11) and cervical cancer (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68)
Among women with a coinfection (n=183) (Figure 1C), HPV/HSV-2 (26%) was the most prevalent coinfection detected. An additional 33% of women (n=60) tested positive for HPV/HSV-2 coinfections concurrently with other STIs: HPV, HSV-2, chlamydia (17%); HPV, HSV-2, chlamydia, gonorrhea (8%); HPV, HSV-2, syphilis (4%); HPV, HSV-2, gonorrhea (2%); HPV, HSV-2, chlamydia, syphilis (1%); (Figure 1C). Concurrent HPV and chlamydia infections were detected in 48% of women with STI coinfections. Of these, 33% were detected in the presence of other STIs: HPV, chlamydia, HSV-2 (17%); HPV, chlamydia, gonorrhea, HSV-2 (8%); HPV, chlamydia, gonorrhea (5%); HPV, chlamydia, syphilis (1%); and HPV, chlamydia, HSV-2, syphilis (1%). One young woman had all five STIs detected.
Factors associated with prevalent single infection and coinfections
Coinfection with STIs was independently associated with alcohol use (aOR = 2.01, 95% CI = 1.00–4.06) and having a sexual partner with an STI (aOR = 6.79, 95% CI = 1.53–30.08) compared to women with no STIs. (Table 2). Education, marital status, birth control use, tobacco use, ever been pregnant, and lifetime number of sex partners were not significantly associated with prevalence of coinfections or single infection compared to women with no STIs after adjusting for age.
Table 2:
Factors associated among women with a single STI (HPVa, chlamydia, gonorrhea, syphilis, HSV-2) and women with STI coinfections vs. women with no STIs
| Single Infection | Coinfections | |||
|---|---|---|---|---|
| uORb(95% CI) | aORc(95% CI) | uORb(95% CI) | aORc(95% CI) | |
| Age in years | ||||
| 22–24 | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) |
| 19–21 | 1.26(0.64–2.44) | 1.70(0.75–3.81) | 1.41(0.74–2.68) | 1.44(0.66–3.22) |
| 16–18 | 1.05(0.46–2.40) | 0.76(0.27–2.12) | 1.34(0.61–2.94) | 0.99(0.37–2.58) |
| Marital status | ||||
| Single/Widowed | 1(Ref) | 1(Ref) | ||
| Married/Living Together | 0.56(0.16–1.91) | 0.42(0.12–1.42) | ||
| Education | ||||
| Some college/tech | 1(Ref) | 1(Ref) | ||
| ≤ Grade 7 | 0.45(0.12–1.64) | 0.48(0.14–1.60) | ||
| Grade 8–12 | 1.77(0.83–3.74) | 1.96(0.95–4.01) | ||
| Passed Grade 12 | 1.18(0.50–2.78) | 0.97(0.42–2.23) | ||
| ≥1 alcohol drink in past month | ||||
| No | 1(Ref) | 1(Ref) | ||
| Yes | 1.55(0.86–2.79) | 1.51(0.73–3.10) | 2.16(1.22–3.81) | 2.01(1.00–4.06) |
| Ever used tobacco | ||||
| No | 1(Ref) | 1(Ref) | ||
| Yes | 1.49(0.65–3.40) | 1.57(0.71–3.47) | ||
| History of STI | ||||
| No | 1(Ref) | 1(Ref) | ||
| Yes | 1.13(0.53–2.42) | 1.40(0.68–2.89) | ||
| Partner with an STId | ||||
| No | 1(Ref) | 1(Ref) | 1(Ref) | 1(Ref) |
| Yes | 2.06(0.66–6.43) | 3.31(0.70–15.69) | 3.72(1.27–10.91) | 6.96(1.53–30.08) |
| Currently using birth controle | ||||
| Yes | 1(Ref) | 1(Ref) | ||
| No | 0.90(0.41–2.01) | 0.81(0.37–1.75) | ||
| Ever been pregnant | ||||
| Never | 1(Ref) | 1(Ref) | ||
| Ever | 0.83(0.46–1.50) | 0.87(0.49–1.52) | ||
| Lifetime no. of male sexual partnersf | ||||
| 1 | 1(Ref) | 1(Ref) | ||
| 2 | 1.10(0.42–2.84) | 1.11(0.44–2.80) | ||
| 3+ | 1.62(0.65–4.02) | 2.06(0.85–4.96) | ||
Abbreviations: HPV: human papillomavirus; 95% CI: 95% Confidence Interval; Ref: reference group
Included HPVgenotypes known to cause disease, specifically genital warts (HPV 6, 11) and cervical cancer (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68)
uOR: unadjusted Odds Ratio was estimated by multinomial logistics regression model/link=glogit function; no infection (n=67); single infection (n=138); coinfection (n=183)
aOR: adjusted Odds Ratio; was estimated by multinomial logistics regression model using /link=glogit function and using a backward elimination procedure with a stay p-value of 0.20. Variable age was forced in the adjusted model. no infection (n=67); single infection (n=138); coinfection (n=183)
Data based on self-report
Sample size for uOR reduced to n=254 due to missing values: no infection (n=43); single infection (n=92); coinfection (n=119)
Sample size for uOR reduced to n=224 due to missing values: no infection (n=45); single infection (n=105); coinfection (n=144)
DISCUSSION
In this cohort of young HIV-negative Western Cape South African women, we detected a high prevalence (47%) of two or more concurrent STIs, specifically chlamydia, gonorrhea, syphilis, HSV-2 and disease causing HPV types. The most prevalent coinfection detected was HPV/HSV-2 and this combination also frequently occurred with the other three STIs. There were no significant differences between women with a single infection compared to women with no infection in terms of their sociodemographic and sexual health characteristics. However, coinfection prevalence was independently associated with alcohol use and having a sexual partner with an STI. Although these behavioral data were self-reported, the high prevalence of STI coinfections suggests that young Western Cape women are rapidly acquiring not just a single STI but multiple concurrent infections after sexual debut.
Coinfections with chlamydia, gonorrhea, HSV-2 and syphilis have been reported among HIV-negative women in sub Saharan Africa but the pattern varies geographically with higher prevalence of HSV-2, gonorrhea and chlamydia coinfections in South Africa 7,14,25 versus gonorrhea and syphilis coinfections in Tanzania and Zambia.7 Women in all three studies were slightly older with a mean age ≥25 years7,14,25 compared to a median of 20 years in our study; however, younger women (< 25 years old) in these studies were more likely to have prevalent STIs and acquire STI coinfections.7,14,25,26
Almost all women with coinfections (99%) in our study harbored either an HPV or HSV-2 infection, possibly a reflection of the high prevalence of these two STIs globally. Another explanation is that these two STIs are more likely to be detected because HSV-2 is a lifelong infection and HPV infection can persist for years. It is plausible that HPV and HSV-2 infections may elevate the risk of acquiring other bacterial STIs (chlamydia, gonorrhea and syphilis). Previous reports indicate that African women with a prevalent HSV-2 infection are at risk to acquire chlamydia,25 gonorrhea,25,27 and syphilis.27 Similarly, these bacterial and viral STIs are known to increase HIV acquisition and transmission.8–13 Some biological explanations proposed are that STIs may amplify inflammatory processes changing the genital tract milieu which increases susceptibility to other STIs.28 Among adolescent females, an immature cervix29 and variability in the composition of vaginal microbiota after puberty30 are additional biologic factors that could influence STI acquisition.29,30 However, in the current prevalence study temporality cannot be established limiting our ability to determine which STI was acquired first.
Of concern, among HPV-infected women, concurrent infections with chlamydia, gonorrhea and HSV-2 have been linked to HPV persistence and increased risk of cervical neoplasia.31–34 We have previously reported a high prevalence of high-risk HPV genotypes (56%) in this population.20 Thus, given the high prevalence of coinfections (69%) among women with disease causing HPV types in this study, estimating factors that influence this coinfection prevalence in women was critical. In our adjusted analyses, coinfection prevalence was increased in women reporting alcohol use and a male sexual partner with a history of an STI. The low median lifetime number of male sexual partners in our study suggests that in such high-risk populations, a male sexual partner’s STI history may be more important than a woman’s lifetime number of sexual partners. Thus, to reduce STI coinfection prevalence in young women, medical and behavioral interventions that include STI screening and treatment programs, condom use and HPV vaccination must target both women and men.
Although the EVRI trial was prospective, because of its short follow-up period of seven months, we were unable to assess incidence of STI coinfections. Future studies are needed to clarify the relationship between bacterial and viral STI coinfection incidence and evaluation of younger women’s risk of acquiring these infections, either concurrently or sequentially. Our study findings may not be generalizable to all South African young women and may not reflect the STI and risk conditions in other sub-Saharan settings. However, a major strength of our study is its relatively large sample size that allowed estimation of single and concurrent STI prevalence as well as STI coinfection patterns. The demographic and behavioral data were based on self-report that could be susceptible to recall and social-desirability biases. To minimize these biases, we administered a tablet-based questionnaire in the local languages rather than an interviewer administered survey. For those women reporting multiple sexual partners, there was no way to determine concurrent sexual relationships which may have influenced the observed high prevalence of coinfections. It is difficult to establish whether women who tested positive for HSV-2 and syphilis have active disease. However, HSV-2 is a chronic infection with recurrent episodes and viral shedding in the absence of continual suppressive therapy. Finally, the prevalence of other genital tract infections such as Trichomonas vaginalis and Mycoplasma genitalium was not assessed.
STI coinfection prevalence was high in this cohort of young HIV-negative Western Cape women despite a low median lifetime number of male sexual partners. Also, STI coinfection prevalence was independently associated with alcohol use and having a male sex partner with an STI. These data suggest the need for a multicomponent STI prevention strategy for both men and women that incorporates medical and behavioral interventions including integration of HIV, STI and cervical screening, referral to appropriate treatment and HPV vaccination.
Supplementary Material
Key Messages.
STI coinfection prevalence is high with 47% of HIV-negative young South African women having two or more concurrent STIs.
STIs can have severe consequences on the sexual and reproductive health of women and may increase the likelihood of HIV acquisition.
A multicomponent STI prevention strategy is essential to reduce STI coinfections and should include both genders
ACKNOWLEDGEMENTS
This research was supported in part by research funding from Merck Sharp & Dohme Corp. The opinions expressed in this paper are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp.
The authors acknowledge the contributions of Charlotte Lawn, Wendy Adendorff, Zukiswa Gloria Ncume, Kayoko Kennedy, Dale Barrios, Jeannie Vaughn, David Jackson, Shahieda Isaacs, Nafiisah Chotun, and all study participants, without whom this study would not have been possible.
FUNDING SOURCE
Merck (IISP39582) was the main sponsor of this trial and provided the study product. This work was also supported by the National Cancer Institute at the National Institutes of Health (Cancer Prevention Fellowship R25T CA147832 to S.L.S.).
Footnotes
CONFLICTS OF INTEREST
A.R.G. is a member of Merck research advisory boards. A.R.G and S.L.S received research funding from Merck. M.F.S.v.d.L. received research funding from Sanofi-Pasteur MSD; he is a co-investigator in a Sanofi-Pasteur-MSD HPV vaccine trial; he sat on a vaccine advisory board of GSK. For the remaining authors, no conflicts of interest were declared.
REFERENCES
- 1.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012;13(6):607–15. doi: S1470-2045(12)70137-7 [pii] 10.1016/S1470-2045(12)70137-7 [doi] [published Online First: 2012/05/12] [DOI] [PubMed] [Google Scholar]
- 2.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine 2012;30 Suppl 5:F12–23. doi: S0264-410X(12)01080-8 [pii] 10.1016/j.vaccine.2012.07.055 [doi] [published Online First: 2012/12/05] [DOI] [PubMed] [Google Scholar]
- 3.Denny L, Adewole I, Anorlu R, et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer 2014;134(6):1389–98. doi: 10.1002/ijc.28425 [published Online First: 2013/08/10] [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). HIV/AIDS: WHO; 2016 [updated July 2016. Available from: http://www.who.int/mediacentre/factsheets/fs360/en/ accessed July 27 2016.
- 5.Kenyon C, Buyze J, Colebunders R. Classification of incidence and prevalence of certain sexually transmitted infections by world regions. Int J Infect Dis 2014;18:73–80. doi: 10.1016/j.ijid.2013.09.014 [published Online First: 2013/11/12] [DOI] [PubMed] [Google Scholar]
- 6.Johnson LF, Coetzee DJ, Dorrington RE. Sentinel surveillance of sexually transmitted infections in South Africa: a review. Sexually transmitted infections 2005;81(4):287–93. doi: 10.1136/sti.2004.013904 [published Online First: 2005/08/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapiga S, Kelly C, Weiss S, et al. Risk factors for incidence of sexually transmitted infections among women in South Africa, Tanzania, and Zambia: results from HPTN 055 study. Sexually transmitted diseases 2009;36(4):199–206. doi: 10.1097/OLQ.0b013e318191ba01 [published Online First: 2009/03/07] [DOI] [PubMed] [Google Scholar]
- 8.Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS (London, England) 2006;20(1):73–83. [published Online First: 2005/12/06] [DOI] [PubMed] [Google Scholar]
- 9.Rottingen JA, Cameron DW, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sexually transmitted diseases 2001;28(10):579–97. [published Online First: 2001/11/02] [DOI] [PubMed] [Google Scholar]
- 10.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sexually transmitted diseases 2008;35(11):946–59. doi: 10.1097/OLQ.0b013e3181812d15 [published Online First: 2008/08/08] [DOI] [PubMed] [Google Scholar]
- 11.Houlihan CF, Larke NL, Watson-Jones D, et al. Human papillomavirus infection and increased risk of HIV acquisition. A systematic review and meta-analysis. AIDS (London, England) 2012;26(17):2211–22. doi: 10.1097/QAD.0b013e328358d908 [published Online First: 2012/08/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rositch AF, Gravitt PE, Tobian AA, et al. Frequent detection of HPV before and after initiation of antiretroviral therapy among HIV/HSV-2 co-infected women in Uganda. PloS one 2013;8(1):e55383. doi: 10.1371/journal.pone.0055383 [published Online First: 2013/02/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith-McCune KK, Shiboski S, Chirenje MZ, et al. Type-specific cervico-vaginal human papillomavirus infection increases risk of HIV acquisition independent of other sexually transmitted infections. PloS one 2010;5(4):e10094. doi: 10.1371/journal.pone.0010094 [published Online First: 2010/04/14] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldblum PJ, Lie CC, Weaver MA, et al. Baseline factors associated with incident HIV and STI in four microbicide trials. Sexually transmitted diseases 2010;37(10):594–601. [published Online First: 2010/09/30] [PubMed] [Google Scholar]
- 15.Mhlongo S, Magooa P, Muller EE, et al. Etiology and STI/HIV coinfections among patients with urethral and vaginal discharge syndromes in South Africa. Sexually transmitted diseases 2010;37(9):566–70. doi: 10.1097/OLQ.0b013e3181d877b7 [published Online First: 2010/05/27] [DOI] [PubMed] [Google Scholar]
- 16.Moodley D, Moodley P, Sebitloane M, et al. High prevalence and incidence of asymptomatic sexually transmitted infections during pregnancy and postdelivery in KwaZulu Natal, South Africa. Sexually transmitted diseases 2015;42(1):43–7. doi: 10.1097/olq.0000000000000219 [published Online First: 2014/12/17] [DOI] [PubMed] [Google Scholar]
- 17.Mlisana K, Naicker N, Werner L, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis 2012;206(1):6–14. doi: 10.1093/infdis/jis298 [published Online First: 2012/04/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westrom LMD, Joesoef RM, Reynolds GP, et al. Pelvic Inflammatory Disease and Fertility: A Cohort Study of 1,844 Women with Laparoscopically Verified Disease and 657 Control Women with Normal Laparoscopic Results. [Article]. Sexually transmitted diseases 1992;19(4):185–92. [PubMed] [Google Scholar]
- 19.Moodley P, Sturm AW. Sexually transmitted infections, adverse pregnancy outcome and neonatal infection. Semin Neonatol 2000;5(3):255–69. doi: 10.1053/siny.2000.0026 [DOI] [PubMed] [Google Scholar]
- 20.Giuliano AR, Botha MH, Zeier M, et al. High HIV, HPV, and STI prevalence among young Western Cape, South African women: EVRI HIV prevention preparedness trial. Journal of acquired immune deficiency syndromes (1999) 2015;68(2):227–35. doi: 10.1097/qai.0000000000000425 [published Online First: 2014/1½2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halling VW, Jones MF, Bestrom JE, et al. Clinical comparison of the Treponema pallidum CAPTIA syphilis-G enzyme immunoassay with the fluorescent treponemal antibody absorption immunoglobulin G assay for syphilis testing. J Clin Microbiol 1999;37(10):3233–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta R, Warren T, Wald A. Genital herpes. Lancet (London, England) 2007;370(9605):2127–37. doi: 10.1016/s0140-6736(07)61908-4 [published Online First: 2007/12/25] [DOI] [PubMed] [Google Scholar]
- 23.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol 2009;10(4):321–2. [DOI] [PubMed] [Google Scholar]
- 24.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. American journal of epidemiology 1989;129(1):125–37. [published Online First: 1989/01/01] [DOI] [PubMed] [Google Scholar]
- 25.Venkatesh KK, van der Straten A, Mayer KH, et al. African women recently infected with HIV-1 and HSV-2 have increased risk of acquiring Neisseria gonorrhoeae and Chlamydia trachomatis in the Methods for Improving Reproductive Health in Africa trial. Sexually transmitted diseases 2011;38(6):562–70. doi: 10.1097/OLQ.0b013e31820a8c2c [published Online First: 2011/02/01] [DOI] [PubMed] [Google Scholar]
- 26.Naidoo S, Wand H, Abbai NS, et al. High prevalence and incidence of sexually transmitted infections among women living in Kwazulu-Natal, South Africa. AIDS research and therapy 2014;11:31. doi: 10.1186/1742-6405-11-31 [published Online First: 2014/09/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaul R, Nagelkerke NJ, Kimani J, et al. Prevalent herpes simplex virus type 2 infection is associated with altered vaginal flora and an increased susceptibility to multiple sexually transmitted infections. J Infect Dis 2007;196(11):1692–7. doi: 10.1086/522006 [published Online First: 2007/11/17] [DOI] [PubMed] [Google Scholar]
- 28.Mayer KH, Venkatesh KK. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. American journal of reproductive immunology (New York, NY : 1989) 2011;65(3):308–16. doi: 10.1111/j.1600-0897.2010.00942.x [published Online First: 2011/01/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moscicki AB, Ma Y, Holland C, et al. Cervical ectopy in adolescent girls with and without human immunodeficiency virus infection. J Infect Dis 2001;183(6):865–70. doi: 10.1086/319261 [published Online First: 2001/03/10] [DOI] [PubMed] [Google Scholar]
- 30.Hickey RJ, Zhou X, Settles ML, et al. Vaginal microbiota of adolescent girls prior to the onset of menarche resemble those of reproductive-age women. mBio 2015;6(2) doi: 10.1128/mBio.00097-15 [published Online First: 2015/03/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paba P, Bonifacio D, Di Bonito L, et al. Co-expression of HSV2 and Chlamydia trachomatis in HPV-positive cervical cancer and cervical intraepithelial neoplasia lesions is associated with aberrations in key intracellular pathways. Intervirology 2008;51(4):230–4. doi: 10.1159/000156481 [published Online First: 2008/09/25] [DOI] [PubMed] [Google Scholar]
- 32.Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. The New England journal of medicine 1992;327(18):1272–8. doi: 10.1056/nejm199210293271804 [published Online First: 1992/10/29] [DOI] [PubMed] [Google Scholar]
- 33.Silva J, Cerqueira F, Medeiros R. Chlamydia trachomatis infection: implications for HPV status and cervical cancer. Archives of gynecology and obstetrics 2014;289(4):715–23. doi: 10.1007/s00404-013-3122-3 [published Online First: 2013/12/19] [DOI] [PubMed] [Google Scholar]
- 34.Smith JS, Herrero R, Bosetti C, et al. Herpes simplex virus-2 as a human papillomavirus cofactor in the etiology of invasive cervical cancer. Journal of the National Cancer Institute 2002;94(21):1604–13. [published Online First: 2002/11/07] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.