Abstract
Background:
Pulmonary arterial hypertension (PAH) results in right ventricular (RV) failure, electro-mechanical dysfunction and heightened risk of sudden cardiac death (SCD), although exact mechanisms and predisposing factors remain unclear. Because impaired chronotropic response to exercise is a strong predictor of early mortality in patients with PAH, we hypothesized that progressive elevation in heart rate can unmask ventricular tachyarrhythmias (VT) in a rodent model of monocrotaline (MCT)-induced PAH. We further hypothesized that intra-tracheal gene delivery of aerosolized AAV1.SERCA2a (AAV1.S2a), an approach which improves pulmonary vascular remodeling in PAH, can suppress VT in this model.
Objective:
To determine the efficacy of pulmonary AAV1.S2a in reversing electrophysiological (EP) remodeling and suppressing VT in PAH.
Methods:
Male rats received subcutaneous injection of MCT (60 mg/kg) leading to advanced PAH. Three weeks following MCT, rats underwent intra-tracheal delivery of aerosolized AAV1.S2a (MCT+S2a, N=8) or saline (MCT, N=9). Age-matched rats served as controls (CTRL, N=7). The EP substrate and risk of VT were determined using high-resolution optical action potential (AP) mapping ex vivo. The expression levels of key ion channel subunits, fibrosis markers and hypertrophy indices were measured by RT-PCR and histochemical analyses.
Results:
Over 80% of MCT but none of the CTRL hearts were prone to sustained VT by rapid pacing (P<0.01). Aerosolized gene delivery of AAV1.S2a to the lung suppressed the incidence of VT to <15% (P<0.05). Investigation of the EP substrate revealed marked prolongation of AP duration (APD), increased APD heterogeneity, a reversal in the trans-epicardial APD gradient, and marked conduction slowing in untreated MCT compared to CTRL hearts. These myocardial EP changes coincided with major remodeling in the expression of K and Ca channel subunits, decreased expression of Cx43 and increased expression of pro-fibrotic and pro-hypertrophic markers. Intra-tracheal gene delivery of aerosolized AAV1 carrying S2a but not luciferase resulted in selective upregulation of the human isoform of SERCA2a in the lung but not the heart. This pulmonary intervention, in turn, ameliorated MCT-induced APD prolongation, reversed spatial APD heterogeneity, normalized myocardial conduction, and suppressed the incidence of pacing-induced VT. Comparison of the minimal conduction velocity (CV) generated at the fastest pacing rate before onset of VT or at the end of the protocol revealed significantly lower values in untreated compared to AAV1.S2a treated PAH and CTRL hearts. Reversal of EP remodeling by pulmonary AAV1.S2a gene delivery was accompanied by restored expression of key ion channel transcripts. Restored expression of Cx43 and collagen but not the pore-forming Na channel subunit Nav1.5 likely ameliorated VT by improving CV at rapid rates in PAH.
Conclusion:
Aerosolized AAV1.S2a gene delivery selectively to the lungs ameliorates myocardial EP remodeling and VT susceptibility at rapid heart rates. Our findings highlight for the first time the utility of a non-cardiac gene therapy approach for arrhythmia suppression.
Introduction
Pulmonary arterial hypertension (PAH) is a chronic disease of the pulmonary vasculature that increases right ventricular (RV) loading. This, in turn, culminates in RV failure with a 5-year mortality rate of >40%.1 PAH patients who exhibit a heightened risk of sudden cardiac death (SCD) are prone to ventricular tachyarrhythmias (VT),2 although the exact mechanisms and predisposing factors remain unclear. This, in large part, is due to a poorer understanding of the electrophysiological (EP) substrate that is formed in the context of RV compared to left ventricular (LV) failure. Moreover, existing treatment options for PAH patients are highly inadequate, highlighting an urgent need for developing novel mechanism-based approaches that are both effective and safe. Considering the relatively high incidence of SCD in PAH, translation of such treatments to the clinic requires a comprehensive understanding of their potential pro-arrhythmic risks or anti-arrhythmic benefits.
We and others have shown that PAH is characterized by dysregulated proliferation of pulmonary artery smooth muscle cells leading to maladaptive vascular remodeling as schematized in Supplemental Fig 1A.3, 4 In the systemic circulation, vascular injury is associated with downregulation of the sarcoplasmic reticulum Ca(2+)-ATPase 2a (SERCA2a) and alterations in Ca(2+) homeostasis in vascular smooth muscle cells that stimulate proliferation, migration, and dedifferentiation, all of which contribute to adverse vascular remodeling.5 Because SERCA2a expression in the pulmonary artery is markedly down-regulated in animal models and humans with PAH, we developed an intra-tracheal gene delivery approach of aerosolized adeno-associated virus 1 carrying SERCA2a (AAV1.S2a) selectively to the lung for the purpose of restoring pulmonary SERCA2a levels. Using this approach, we reported improved pulmonary function in both PAH rats3 and pigs.6, 7 The impact of this therapeutic strategy on EP function, however, remained unknown. The major objectives of the current study were to: 1) perform a comprehensive evaluation of the EP substrate and risk of ventricular arrhythmias in a standard rodent model of monocrotaline (MCT)-induced PAH that causes RV failure; and 2) determine the efficacy of our aerosolized pulmonary gene delivery approach in reversing pathological EP remodeling and suppressing arrhythmias in PAH rats.
Methods
Rat model of MCT-induced PAH
All procedures involving animal handling were approved by the Animal Care and Use Committee of the Icahn School of Medicine at Mount Sinai and adhered to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health. All animals were purchased from the Charles River Laboratory. Adult male Sprague Dawley rats (300-350 g) were subcutaneously injected with MCT (60 mg/kg) as a standard model of PAH, or 0.9% saline as a control (CTRL) group. This model recapitulates hallmark features of the clinical disease, including increased pulmonary vascular resistance, elevated right ventricular (RV) afterload, and RV failure.8 Three weeks following MCT injection, rats were treated with AAV1.S2a (3.5X1011 vg/ml) via intra-tracheal delivery as previously described (MCT+S2a),3 or were left untreated for an additional 3-4 weeks (MCT). A total of 16 MCT and 11 MCT+S2a rats entered the study, of which 4/16 MCT and 1/11 MCT+S2a died prematurely, before the experimental endpoint. Of the remaining 12 MCT and 10 MCT+S2a rats, a full ex vivo experimental protocol yielding high-quality optical action potential signals with adequate signal-to-noise characteristics for automated analyses was achieved in 9 MCT and 8 MCT+S2a hearts. An additional set of normal rats that were not injected with MCT received intra-tracheal delivery of aerosolized AAV1.S2a (CTRL+S2a, N=6). At the terminal time-point (6-7 weeks after MCT or sham injection), rats were sacrificed for electrophysiological, hemodynamic, molecular, and histological analyses.
Gene vector and delivery
Human AAV1.S2a was produced as previously described.3 Treatments were aerosolized in a 250μL volume for single-dose intra-tracheal delivery using an IA-1C Microsprayer (PennCentury, Wyndmoore, PA).
Optical action potential mapping of ex vivo perfused hearts
Rats were injected intraperitoneally with heparin and high-dose sodium pentobarbital. Hearts were rapidly excised and Langendorff perfused with Tyrode’s solution at 37°C. Atria were excised in order to suppress competitive stimulation of the ventricles by the sinoatrial node allowing for reliable steady-state pacing over a wide range of stimulation frequencies. Perfusion pressure was maintained at 60-70 mmHg by regulating coronary perfusion flow. Preparations were placed in a tissue bath, and the anterior surface was gently pressed against an imaging glass window by a stabilizing piston.9-13 Motion artifact was minimized by inducing electromechanical dissociation using 10μM blebbistatin delivered over a 10min interval. To avoid surface cooling, the preparations were immersed in the coronary effluent, which was maintained at a physiological temperature by a heat exchanger assembly. Cardiac rhythm was continuously monitored via silver electrodes connected to an ECG amplifier. Cardiac rhythm, perfusion pressure, and flow were continuously monitored during each experiment. Hearts were stained with the voltage-sensitive dye di-4-ANEPPS for 10min as previously reported9, 10 Measurement of optical action potentials was performed using an 80×80 pixel CCD camera (Scimedia) coupled to an imaging macroscope containing a high numerical aperture lens, a dichroic mirror, excitation and emission filters, a beam splitter, and a light-collimating tube. Excitation light was directed in an epifluorescence configuration onto the heart through a side-port of the macroscope in a manner that minimized heterogeneity of incident light across the mapped 9×9-mm2 region. Emitted light was collected by the front lens of the macroscope, filtered and directed onto the CCD detector. High-resolution (1-ms temporal, 0.1-mm spatial) optical action potentials were measured simultaneously from 6400 sites during each recording. To improve signal quality, 4×4 pixel spatial binning was performed yielding an array of 400 (20×20) high-fidelity optical action potentials that were amenable for accurate automated analyses.
Electrophysiological Measurements
Hearts from CTRL (N=7), CTRL+S2a (N=6), MCT (N=8), MCT+S2a (N=8), and MCT+Luciferase (N=3) rats underwent pacing at progressively shorter pacing cycle lengths (PCLs) ranging from 300 to 60ms in 10-20ms decrements or until sustained ventricular tachycardia (VT) was generated and maintained for at least 30 seconds.
Conduction Velocity:
Conduction delays were assessed by recording action potentials during steady-state pacing. Local activation time at each site was defined as the maximum first derivative during the upstroke of the action potential. Velocity vectors (magnitude and direction) were derived from the activation times of each pixel relative to those of its neighbors. Conduction velocity (CV) was measured by averaging the magnitude of the velocity vectors along the transverse axis of impulse propagation. The critical conduction velocity (CVc) was defined as the last measurable CV at the fastest rate before induction of VT or the end of the experimental protocol if VT could not be induced.
Action Potential Duration:
Repolarization times at 75% relative to the action potential amplitude were quantified. Action potential duration (APD75) was defined as the temporal difference between the repolarization and activation times at each site.
Real Time Polymerase Chain Reaction
Total RNA was isolated using RNeasy Mini kits (Qiagen), and 1000ng were reverse transcribed using a standard protocol provided by the vendor (High-Capacity cDNA Reverse Transcription Kit, Thermofisher Scientific). Quantitative real time polymerase chain reaction (RT-PCR) amplification of cDNA was performed with primers listed below, using the PerfeCTa SYBR Green FastMix. The specificity of each primer set was examined by analyzing the dissociation curve. The 10μl sample volume consisted of 1X SYBR Green master mix, 400nM gene-specific primers and 2μl template. In order to assess the specificity of AAV1.S2a delivery in the lung, RT-PCR was performed with a sense primer located on the CMV promoter (AGACCCAAGCTGGCTAGCGTTTA) and an antisense primer positioned in the human SERCA2a sequence (TTCTTCAGCCGGTAACTCGTTGGA). Primers used in our study are listed below:
| Gene | Forward Primer (5′→ 3′) | Reverse Primer (5′℩ 3′) |
| KCND2 | CCACTGCACATCACCTCCAT | GTAGCTCAGGAGATGCGGTC |
| KCND3 | GCTGTCTCGGTCATCACCAA | GTGAAGATCATGACGCACGC |
| KCNJ2 | CATACCCGACAACAGTGCAG | GTCCGCCAGGTACCTCTGT |
| CACNA1G | ACTGACTGTGCGGAAGTCTG | CCTGACTGAGCTTTGGGGAG |
| CACNA1C | TCTGCTCTGCCTGACTCTGA | CACACAATTGGCAAAAATCG |
| GJA1 | TATTCGTGTCTGTGCCCACC | CTGCTTCAGGTGCATCTCCA |
| COL1A1 | AATGGTGCTCCTGGTATTGC | GGTTCACCACTGTTGCCTTT |
| COL1A2 | CGAGACCCTTCTCACTCCTG | GCATCCTTGGTTAGGGTCAA |
| COL3A1 | GGGATCCAATGAGGGAGAAT | GGCCTTGCGTGTTTGATATT |
| MYH6 | GCTTTGGGAAGTTCATCAG | GCCTTTAGCTGGAAGATCAC |
| NPPA | CCCGACCCACGCCAGCATGG | CAACTGCTTTCTGAAAGGGGT |
| NPPB | ACAATCCACGATGCAGAAGCT | GGGCCTTGGTCCTTTGAGA |
| GAPDH | TGACAACTCCCTCAAGATTGTCA | GGCATGGACTGTGGTCATGA |
| MYH7 | CTAGGAGGCGGAGGAACAG | CTTGGCGCCAATGTCACG |
| SCN5a | GTCTTCAAGCTGGCCAAGTC | CCGAGTAGTTCTTGCCGAAG |
| SERCA2a | TATTGGCTGGTGAAGGAGGT | GACAATGTCTGCTGGCTCAA |
| GAPDH | TGACAACTCCCTCAAGATTGTCA | GGCATGGACTGTGGTCATGA |
| Human Serca2a | AGCGGTTACTCCAGTATTGCAG | CTGTCCATGTCACTCCACTTCC |
Immunofluorescence
Paraffin-embedded rat RV cardiac sections (6μm) were deparaffinized followed by epitope retrieval. The sections were blocked in DAKO solution and 10% goat serum before labeling with primary antibodies against SERCA2a (1:300, 21stCentury Biochemicals, Marlborough, MA), α-actinin (1:100; Abcam) and Cx43 (1:100; Sigma-Aldrich). Secondary antibodies were used for fluorescent labeling of the sections (Jackson ImmunoResearch Laboratories). Images were captured using a Zeiss Slide Scanner Axio Scan.
Lung tissue histology
Rat lungs were inflated with OCT/PBS (50/50), embedded in OCT and sectioned. Sections stained using hematoxylin and eosin were examined by light microscopy. Pulmonary arterioles located distal to terminal bronchioles were identified. The external diameter and the cross-sectional medial wall thickness were measured. The relative vessel wall thickness was calculated as [(outer vessel wall circumference) - (inner vessel wall circumference)] / (outer vessel wall circumference).
Hemodynamic Studies
Rats were anesthetized with 3-4% isoflurane, intubated via a tracheotomy, and mechanically ventilated. The thoracic cavity was opened and a Scisense catheter was inserted into the pulmonary artery. The pulmonary artery pressures were then measured directly. Hemodynamic data were recorded using an ADVantage P–V Control Unit (Transonic Systems).
Right ventricular weight
The hearts were dissected immediately after sacrifice. The weight ratio of the right ventricle (RV) to the left ventricle (LV) plus septum [RV/(LV+S)] was calculated as an index of right ventricular hypertrophy.
Statistical Analyses
Data are presented as mean ± SEM. Fisher’s exact test was used to compare differences in VT propensity. Differences among multiple means of electrophysiological parameters and mRNA expression levels (assuming normal Gaussian distribution) were assessed by one-way ANOVA followed by Tukey's post hoc correction analysis. If the data did not pass the D’Agostino-Pearson normality test, the non-parametric equivalent (Kruskal-Wallis followed by the Dunn's post hoc multiple comparisons test analysis) was used. Pearson’s Correlation Coefficient and its corresponding P value were calculated to determine a relationship between conduction velocity and upstroke velocity.
Results
As expected, MCT injection caused marked pulmonary vessel thickening and lumen narrowing in both large and small pulmonary arterioles (Supplementary Figure 1B & C). Consequently, systolic, diastolic, and mean arterial pressures increased in MCT animals (Supplemental Figure 1D), leading to RV hypertrophy as shown by the increase in the Fulton Index (Supplemental Figure 1E). Importantly, pulmonary AAV1.S2a normalized the pulmonary vascular pathology, resulting in a corresponding restoration of PA pressures and RV hypertrophy (Supplemental Figures 1A-1E). These structural differences occurred without a change in in vivo heart rate (Figure 1F).
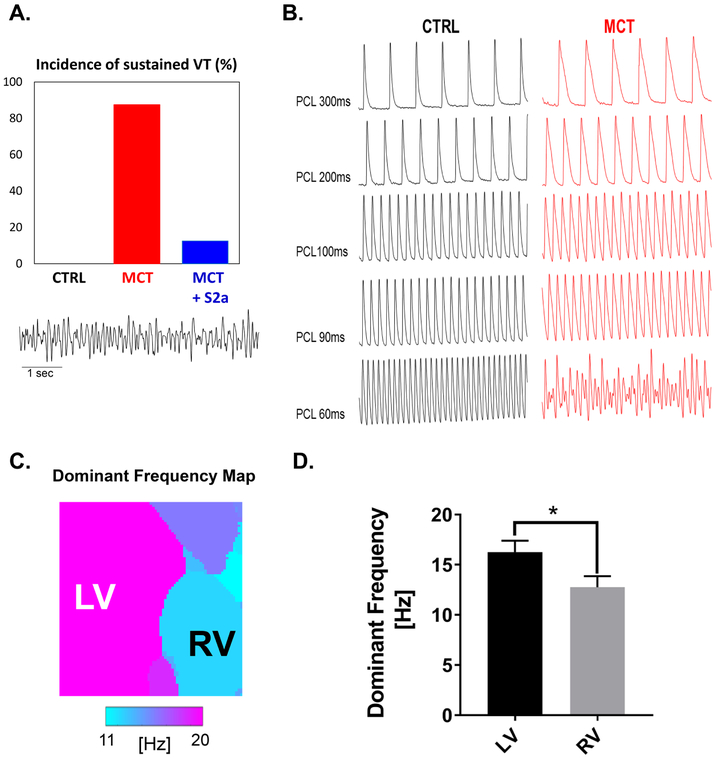
Figure 1. Incidence, characteristics, mode-of-induction, and reversibility of VT.
A. Bar graph showing the incidence of pacing-induced sustained VT in CTRL, MCT, and MCT + S2a hearts. B. Mode of induction of VT by progressive elevation of pacing rate. Representative action potential traces recorded from CTRL (black) and MCT (red) hearts during steady-state pacing at progressively shorter pacing cycle lengths leading up to the initiation of sustained VT in MCT but not CTRL. C. A contour map showing the distribution of the frequency of ventricular activation during VT in a representative MCT heart. D. Bar graph representing the average dominant frequency in the RV and LV of MCT hearts during VT.
Intra-tracheal gene delivery of aerosolized AAV1.S2a suppresses pacing-induced VT in PAH
We next assessed potential differences in the susceptibility to sustained VT of untreated (MCT) and AAV1.S2a-treated (MCT+S2a) hearts relative to CTRL. Specifically, we challenged CTRL, MCT, and MCT+S2a hearts with steady-state pacing at progressively faster rates down to a pacing cycle length (PCL) of 60ms. Using this pacing protocol, none of the CTRL (0/7) hearts exhibited VT. In sharp contrast, 80% of MCT hearts were prone to pacing-induced sustained VT (Figure 1A and B). Aerosolized AAV1.S2a treatment markedly suppressed VT susceptibility as only 1/8 MCT+S2a hearts exhibited pacing-induced arrhythmias using the identical protocol (Figure 1A). The average pacing cycle length required for VT initiation in MCT hearts was 85.7ms. Dominant frequency analysis during VT showed a significantly higher frequency of activation in the LV compared to the RV (Figure 1 C and D) in this model.
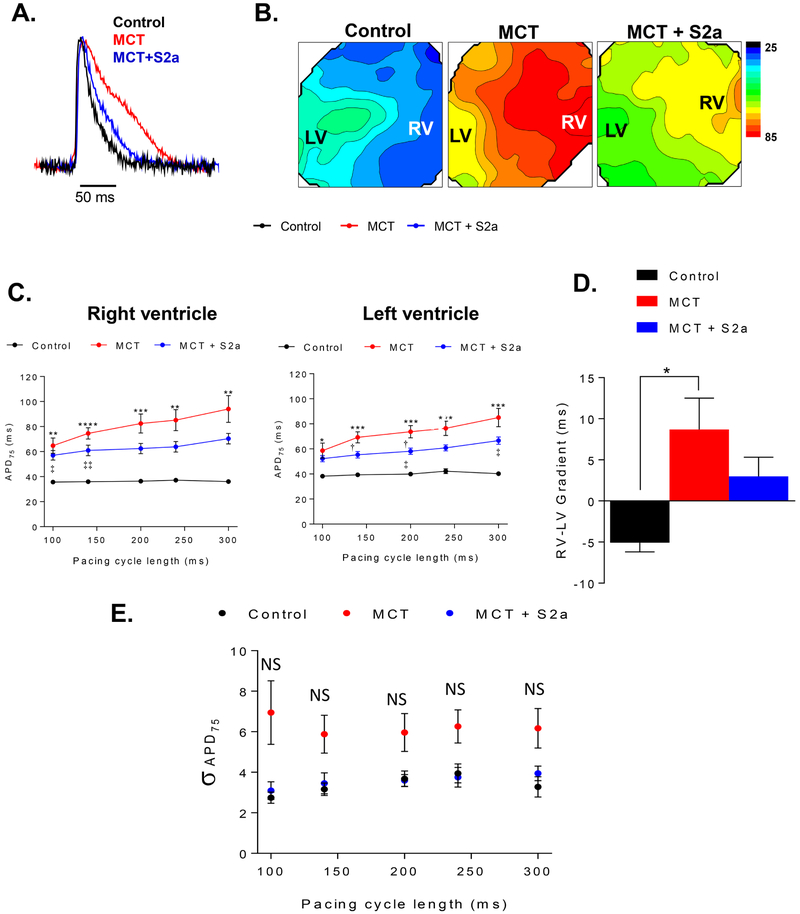
Intra-tracheal gene delivery of aerosolized AAV1.S2a partially reverses pathological APD prolongation in PAH
We proceeded to investigate the EP substrate in order to determine the underlying arrhythmia mechanisms. We first assessed the extent of APD prolongation at baseline, a standard index of EP remodeling in hypertrophy and failure. Shown in Figure 2A are superimposed action potential (AP) traces recorded during steady-state pacing at PCL 300ms from representative CTRL (black), untreated MCT (red), and AAV1. S2a-treated MCT (MCT+S2a, blue) hearts. Also shown (Figure 2B) are representative APD contour maps depicting the distribution of APD levels across the anterior epicardium of CTRL, MCT, and MCT+S2a hearts. Clearly, action potentials recorded from untreated (red) MCT hearts were markedly prolonged compared to CTRL at this baseline pacing frequency. To gain further insight into the nature of APD prolongation caused by RV failure, we compared the rate-dependence of APD in both the RV and LV of CTRL, MCT, and MCT+S2a hearts (Figure 2C). While both ventricles exhibited significant APD prolongation in PAH (i.e. MCT, red), the extent of this prolongation was typically greater in the RV compared to the LV especially at slow pacing rates (i.e. PCL 300ms). This, in turn, resulted in the reversal of the basal APD gradient across the epicardium in MCT (RV > LV) compared to CTRL (LV > RV) hearts (Figure 2B). Quantification of the RV-to-LV APD gradient for all hearts is shown in Figure 2D. Interestingly, MCT+S2a hearts exhibited a blunted RV-to-LV APD gradient (Figure 2D) consistent with minimal transepicardial APD heterogeneity. While APD in both LV and RV tended to be shorter in MCT+S2a (blue) compared to untreated MCT (red) hearts at most pacing cycle lengths, differences were generally not significant (Figure 2C). More importantly, at fast rates leading up to the initiation of VT (i.e. PCL 100ms), APD was almost identical in untreated and AAV1.S2a treated MCT hearts (Figure 2C).
Figure 2. Action potential remodeling in untreated and S2a-treated MCT hearts relative to control.
A. Superimposed AP traces from representative CTRL (black), MCT (red), and MCT+S2a (blue) treated hearts during baseline steady-state pacing. Average epicardial APD measured at 75% of repolarization (APD75) in CTRL (black), MCT (red), and MCT+S2a (blue).B. Contour maps representing APD75 across the anterior epicardial surface. C. Average RV and LV APD75 in CTRL (black), MCT (red), and MCT+S2a (blue). D. Average APD75 gradient from the RV to the LV. N =4 CTRL, 9 MCT, and 5 MCT+S2a. APD = action potential duration; APD75 = APD measured at 75% repolarization; PCL = pacing cycle length. E. APD heterogeneity indexed by the standard deviation of APD75 values measured across all pixels in CTRL (black), MCT (red), and MCT+S2a (blue). N=4 control, 9 MCT, and 5 MCT+S2a. *P <0.05, **P <0.005, ***P <0.0005, ****P <0.0001 by one-way ANOVA and Tukey post-test. † P MCT vs MCT+S2a <0.05, †† P MCT vs MCT+S2a <0.005. ‡ P MCT+S2a vs CTRL <0.05, ‡‡ P MCT+S2a vs CTRL <0.005.
We next determined changes in APD heterogeneity as indexed by the standard deviation (σ) of APD75 values measured simultaneously from 400 voxels (each voxel consisting of a 4×4 pixel bin) across the epicardium of each CTRL, MCT, and MCT+S2a heart. Consistent with an adverse EP substrate, σ in MCT hearts exhibited a strong trend towards increased levels at any given pacing cycle length compared to CTRL hearts, albeit differences did not reach statistical significance (Figure 2E). Treatment of MCT animals with aerosolized AAV1.S2a (MCT+S2a) normalized σ to CTRL values (Figure 2E).
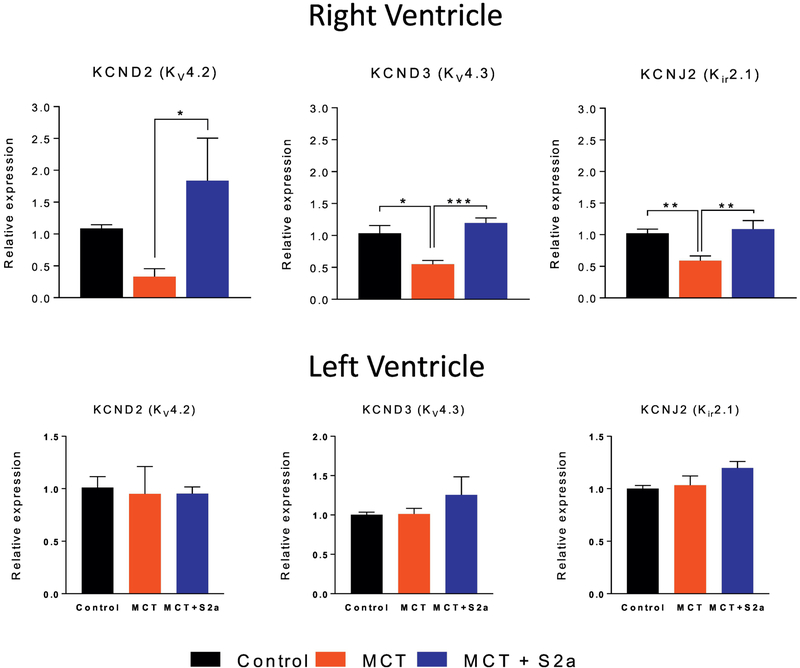
To determine if changes in APD were related to remodeling of repolarizing ion channels, we measured the mRNA expression levels of key K channel pore-forming subunits in CTRL (black), MCT (red), and MCT+S2a (blue) hearts (Figure 3). Consistent with APD prolongation, we found marked downregulation in Kv4.3 and Kir2.1 transcript levels in the RV of untreated MCT rats. These changes in K channel expression were restricted to the RV as no differences were found in LV samples from the same hearts (Figure 3, bottom).
Figure 3. mRNA expression of key K channel subunits in the RV and LV of CTRL, MCT and MCT+S2a hearts.
Top row mRNA expression of the Kv4.2 (N= 6 for all groups), Kv4.3 (N= 5 CTRL, 4 MCT, 7 MCT+S2a), and Kir2.1 (N=10 CTRL, 8 MCT, 7 MCT+S2a) K channels in the RV; Bottom row mRNA expression of Kv4.2, Kv4.3, and Kir2.1 in the LV of each group. *P <0.05, **P <0.005, ***P <0.0005 by one-way ANOVA and Tukey post-test.
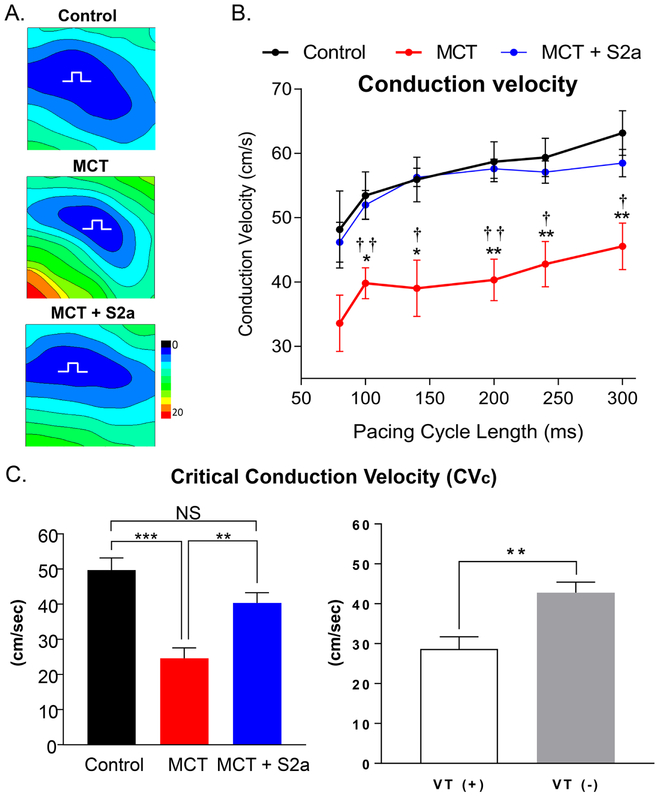
Myocardial conduction slowing in advanced pulmonary arterial hypertension is fully reversed by aerosolized S2a gene therapy to the lung
CV slowing is a hallmark feature of structurally-remodeled hearts that predisposes to arrhythmias.9, 11, 12 Shown in Figure 4A are representative isochrones from CTRL, MCT, and MCT+S2a hearts. Also shown (Figure 4B) are the average transverse CV values measured at multiple pacing cycle lengths in all groups. While untreated MCT hearts exhibited a 28% reduction in CV compared to CTRL, treatment with AAV1.S2a fully restored CV to CTRL levels at all tested pacing cycle lengths (Figure 4B).
Figure 4. Conduction slowing and its Restoration by intra-tracheal delivery of aerosolized AAV1.S2a.
A. Representative isochrone maps for Ctrl, MCT and MCT+S2a hearts. B. Rate-dependence of CV in CTRL (black, N=4), MCT (red, N=8), and MCT+S2a (blue, N=6). C. Comparison of the mean critical conduction velocity (CVc) across groups (left, N=4 CTRL, 8 MCT, 6 MCT+S2a), and between VT (+) and VT (−) hearts (right) (N=8 and N=10, respectively). *P <0.05, **P <0.005, ***P <0.0005, one-way ANOVA and Tukey post-test, † P MCT vs MCT+S2a <0.05, †† P MCT vs MCT+S2a <0.005.
To determine the functional significance of conduction slowing, we compared the so-called “critical” CV (CVc) measured at the fastest pacing rate before VT induction or at the end of the protocol (PCL 60ms) if VT could not be induced. As shown in Figure 4C, MCT hearts exhibited >50% reduction in CVc compared to CTRL hearts. AAV1.S2a treatment reversed this deficit. Comparison of CVc in VT (+) versus VT (−) hearts (Figure 4C, right) demonstrated significantly slower conduction in hearts that were prone to VT compared to those that were not.
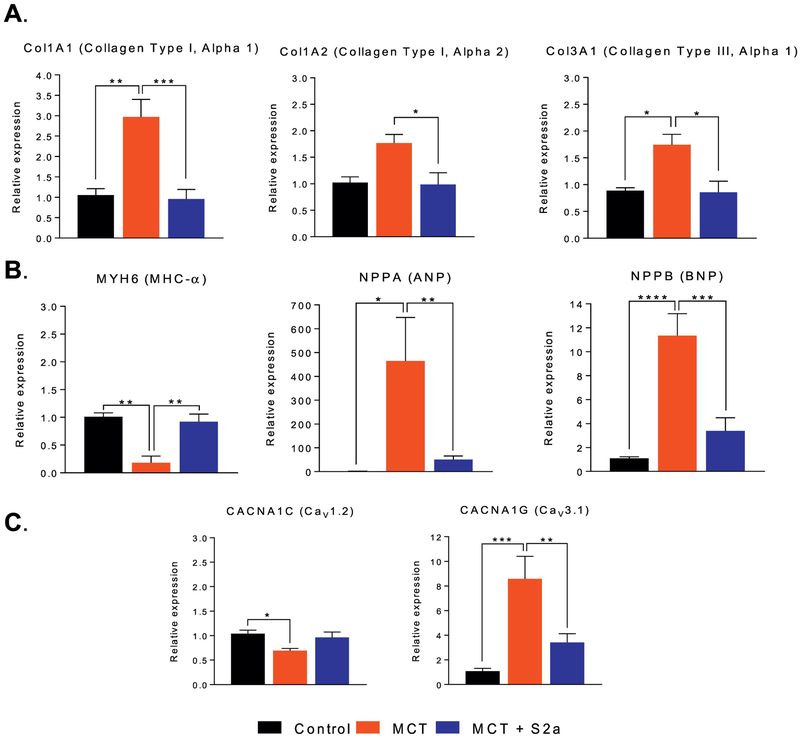
Conduction abnormalities arise as a consequence of structural and/or gap junctional remodeling or changes in myocardial excitability. We assessed the extent of structural remodeling as indexed by pro-fibrotic and pro-hypertrophic markers. Consistent with advanced structural heart disease, MCT animals exhibited 2-3 fold increase in Col1A1, Col1A2, and Col3A1 mRNA expression (Figure 5A), a 10-fold reduction in MHC-α along with increased ANP and BNP levels compared to CTRL hearts (Figure 5B). Interestingly, this pro-fibrotic and pro-hypertrophic gene expression program was reversed by AAV1.S2a treatment, which also corrected hypertrophy associated changes in calcium channel isoforms. Specifically, decreased Cav1.2 and increased Cav3.1 expression in MCT were either fully (in the case of Cav1.2) or partially (in the case of Cav3.1) reversed by intra-tracheal S2a treatment (Figure 5C).
Figure 5. Changes in the expression of the molecular correlates of myocardial conduction.
A. mRNA expression of fibrosis markers Col1A1 (N=5 CTRL, 4 MCT, 7 MCT+S2a), Col1A2 (N= 5 CTRL, 4 MCT, 5 MCT+S2a), and Col3A1 (N= 4 CTRL, 4 MCT, 7 MCT+S2a), measured by RT-PCR in CTRL, MCT, and MCT+S2a hearts. B. mRNA expression of myosin heavy chain α (MHC-α) (N= 4 CTRL, 4 MCT, 7 MCT+S2a), atrial natriuretic peptide (ANP) (N= 4 CTRL, 4 MCT, 7 MCT+Sa), and brain natriuretic peptide (BNP) (N= 6 CTRL, 6 MCT, 7 MCT+S2a). C. mRNA expression of the pore-forming L- and T-type calcium channels, Cav1.2 and Cav3.1 in CTRL, MCT, and MCT+S2a RV samples. *P <0.05, **P <0.005, ***P <0.0005 by one-way ANOVA and Tukey post-test.
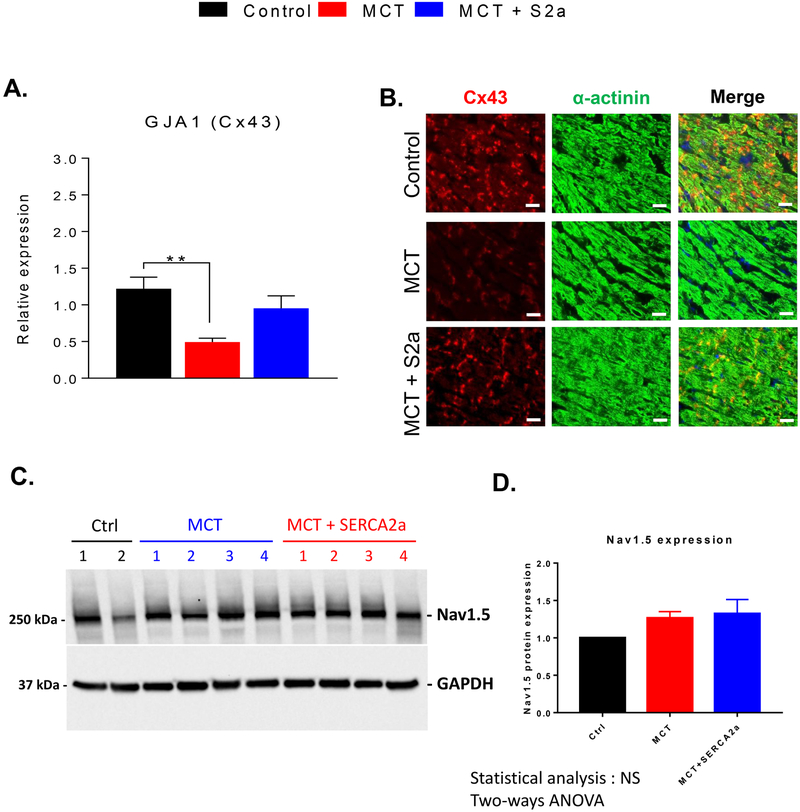
We then measured the expression of the main ventricular gap junction protein Cx43 in CTRL, MCT, and MCT+S2a hearts using RT-PCR (Figure 6A) as well as immunostaining and confocal microscopy (Figure 6B). As expected, Cx43 expression was decreased by >60% in the RV of untreated MCT hearts at both the mRNA and protein levels. This deficit was effectively reversed by intra-tracheal AAV1.S2a treatment (Figure 6 A&B). In contrast, the expression of the sodium channel alpha subunit Nav1.5 was not altered in MCT or MCT+S2a relative to CTRL (Figure 6 C&D).
Figure 6. Cx43 expression in the RV of CTRL, MCT and MCT+S2a rats.
A. Cx43 expression (N=8 CTRL, 8 MCT, 5 MCT+S2a) measured by RT-PCR in CTRL, MCT, and MCT+S2a hearts. B. Representative images of Cx43 (red) and α-actinin (green) immunostaining in RV tissue sections from CTRL, MCT, and MCT+S2a hearts. C. Representative western blot showing the expression of Nav1.5 in CTRL, MCT, and MCT+S2a hearts. D. Average Nav1.5 expression in the 3 groups. **P <0.005 by one-way ANOVA and Tukey post-test.
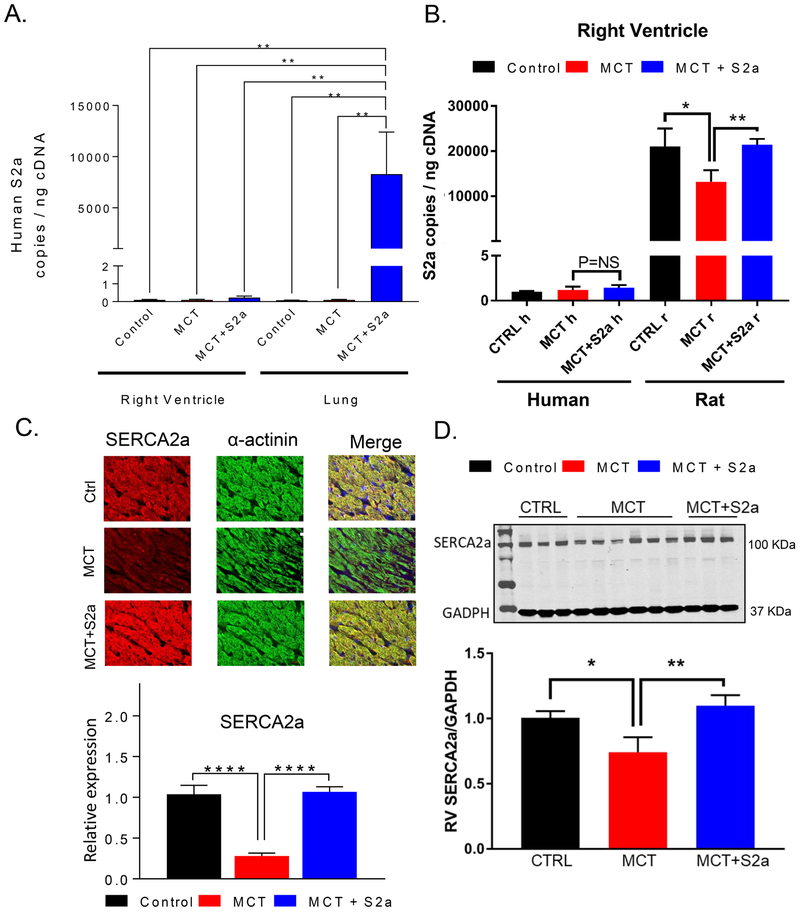
The overarching hypothesis of the present work was that upregulation of SERCA2a in the lung would restore the electrical dysfunction of the heart. To that end, we examined the specificity of our extra-cardiac gene delivery approach by measuring the viral genome copies in tissue samples from the lung and the RV of CTRL, MCT, and MCT+S2a rats. The number of exogenous human SERCA2a genome copies (specific to SERCA2a in the AAV1 vector) was markedly increased in the lung but not RV tissue samples in AAV1.S2a-treated MCT rats in comparison to their CTRL and untreated MCT counterparts (Figure 7A). Similarly, measurement of the human and rat isoforms of SERCA2a in the RV of CTRL, MCT, and MCT+S2a rats revealed negligible expression of the former compared to that of the latter in all 3 groups (Figure 7B).
Figure 7: Efficiency and selectivity of AAV1.S2a gene delivery to the lung.
A. Transgene copies were assessed by RT-PCR in RV and lung tissue samples to determine the efficiency and specificity of AAV1.S2a gene transfer to the lung. N=5-6 rats per group. ** P<0.01. B. Human and rat SERCA2a expression measured by RT-PCR in RV samples from CTRL, MCT, and MCT+S2a hearts. C. Representative immunofluorescence images and expression of SERCA2a (red) relative to α-actinin (green) in RV tissue sections from CTRL, MCT, and MCT+S2a hearts. D. Representative western blots of SERCA2a and GAPDH as a loading control, and quantification of relative SERCA2a protein expression in the RV of CTRL, MCT, and MCT+S2a hearts.
Moreover, decreased arrhythmia propensity by our pulmonary gene therapy approach was most likely caused by overall reversal of PAH symptoms rather than a direct effect on the myocardium. In support of this, we performed intra-tracheal S2a gene transfer in an additional set (N=6) of normal rats for the purpose of determining if the pulmonary-specific gene transfer somehow altered myocardial electrophysiological properties in healthy controls. As shown in Supplemental Figure 2, we found no differences in EP properties or arrhythmia vulnerability between our untreated (CTRL) and AAV1.S2a-treated normal (CTRL+S2a) rats. Finally, regression of EP remodeling was indeed caused by upregulation of pulmonary SERCA2a levels since treatment of MCT rats with a control vector (AAV1.luc) failed to impact VT propensity or key EP parameters (Supplemental Figure 3).
As a molecular correlate of electro-mechanical dysfunction, we measured the native expression of the rat isoform of SERCA2a in RV samples from CTRL, MCT, and MCT+S2a hearts. We found markedly reduced (by 75%) rat SERCA2a levels in the RV of MCT hearts that were fully restored by the pulmonary gene transfer of the human SERCA2a isoform at both the mRNA and protein levels (Figure 7C&D).
Discussion
PAH leading to RV failure is a major cause of mortality, in large part, due to SCD.2 While RV failure promotes the incidence of arrhythmias, the exact mechanisms and predisposing factors remain unclear. Since the pathophysiology of RV failure is relatively understudied compared to its LV counterpart, treatment options for RV failure along with its arrhythmic component are very limited. In the present study, we performed a comprehensive evaluation of the EP substrate both at baseline and during challenge with rapid pacing leading up to the onset of VT in a well-established rodent model of MCT-induced PAH. We further tested the efficacy of an intratracheal gene delivery approach targeting aerosolized AAV1.S2a to the lung for the reversal of pathological EP remodeling and arrhythmia propensity in the heart. Our major findings are as follows:
The rodent model of MCT-induced PAH is associated with adverse EP remodeling involving both ventricles. This includes rate-dependent APD prolongation, increased APD heterogeneity, reversal of the trans-epicardial APD gradient, and reduced myocardial conduction velocity.
EP changes in untreated MCT rats culminate in a substrate that is amenable for the initiation and perpetuation of VT during challenge with rapid pacing.
Investigation of the EP substrate immediately prior to the induction of VT highlights the importance of conduction slowing in the pathogenesis of VT.
Aerosolized AAV1.S2a gene delivery to the lung improves myocardial EP function by partially or completely restoring the molecular correlates of EP, mechanical and structural remodeling at the transcriptional level.
Arrhythmia propensity in the rat model of MCT-induced PAH
SCD accounts for approximately 30% of deaths in patients with PAH.14 While the exact incidence and nature of arrhythmic events remain unclear, recent clinical evidence suggests that ventricular arrhythmias are likely to be under-estimated in this patient population15, which ultimately develops mechanical dyssynchrony and bi-ventricular dysfunction driven by RV failure. As such, there is a compelling need to closely monitor electrocardiograhic indices as well as the incidence of VT in PAH patients.2, 15 Because impaired chronotropic response of PAH patients to exercise has been shown to predict adverse outcomes,16 including early mortality, we hypothesized that elevated heart rates mimicking vigorous exercise or stress would unmask an otherwise silent arrhythmogenic phenotype. Indeed, we found that elevation of pacing rate was sufficient to induce sustained VT in the rat MCT model but in none of the CTRL hearts. These findings of enhanced arrhythmia risk are consistent with a previous study in which VT in the same rat model was readily induced by burst stimulation17 or aconitine exposure.18 In our study, the average ex vivo pacing rate required to induce VT was approximately 1.5-2 fold the in vivo heart rate of rats. This is qualitatively similar to the rise in heart rate achieved during vigorous exercise. A previous study demonstrated that ventricular fibrillation was initiated in the rat model of MCT-induced PAH exclusively from RV sources, and was subsequently supported by focal and reentrant activity.19 In our study, we found activation patterns consistent with focal activity as well as reentry emanating from both chambers (not shown). Interestingly, dominant frequency analysis revealed significantly faster rates in the LV than the RV, possibly suggesting breakdown of wavefronts in the region with the greatest refractoriness (i.e. the RV). Consistent with this finding, Benoist et al17 also reported a higher VT activation frequency in the LV than the RV in a similar model. Our finding of higher activation frequency in the LV than RV argues against triggers identified solely in RV myocytes as being sufficient for VT maintenance as dominant frequency was higher, not lower, in the LV.
Adverse EP remodeling in PAH
Our experimental design allowed us to observe the dynamically changing EP substrate from baseline (low-stress) pacing conditions through high-stress conditions that culminated in the initiation of sustained VT. We also examined a treatment group in which VT was markedly suppressed by a novel pulmonary-targeted gene therapy approach. This, in turn, enabled us to identify the specific EP parameters that are likely to be causally implicated in the pathogenesis of VT versus those that are either bystanders or that may simply act as modulatory factors.
As expected, hearts from MCT-induced PAH rats exhibited marked prolongation of APD, a well-established index of electrical remodeling in hypertrophy and heart failure. In fact, the extent of APD prolongation that we report in the current study (approx. 2-fold at PCL 300ms) is consistent with our previous findings in the rat model of LV hypertrophy caused by ascending aortic banding9 as well as with other reports on MCT-induced PAH.18, 20 The relevance of APD prolongation to human pathophysiology is underscored by the fact that PAH patients are known to exhibit significant QT (and corrected QT) interval prolongation even when undergoing treatment for their PAH symptoms.21 A notable finding of the present report, however, was that APD prolongation involved both ventricles in a model in which mechanical dysfunction is largely restricted to the RV. This suggests that adverse EP remodeling in PAH likely precedes and may contribute to LV mechanical dysfunction and bi-ventricular failure. Of note, EP remodeling in the LV of PAH hearts is not likely explained by intrinsic changes in cellular properties, since isolated myocytes from the LV did not exhibit APD prolongation or propensity for triggered activity19 in previous studies. The lack of intrinsic changes in action potentials recorded from isolated LV myocytes is supported by our finding of preserved K channel transcripts in that chamber despite marked downregulation in its RV counterpart (Figure 3). Factors contributing to EP remodeling of the left ventricle at the tissue but not isolated myocyte levels likely involve changes in mechanical synchrony or ventricular activation patterns which have been identified in PAH patients and animal models using echocardiography and magnetic resonance imaging. Of note, Temple et al.22 reported a high incidence of atrioventricular node dysfunction and heart block in the rat MCT model. These changes are expected to contribute to chronic mechanical dyssynchrony, which we have found to alter EP parameters, especially in the late-activated regions.23 Interestingly, a recent report indicates that RV pacing improves cardiac function in PAH likely by improving ventricular synchrony.24 The impact of chronic RV pacing on EP remodeling in PAH is unknown and warrants direct investigation in future studies.
While our findings regarding APD prolongation at baseline are consistent with previous reports, our interpretation of their relative contribution to pacing-induced VT in PAH differ as we were able to demonstrate comparable prolongation in untreated and AAV1.S2a-treated MCT hearts at the relevant rates that precipitated VT. Specifically, despite our finding of APD prolongation in both ventricles of MCT hearts at basal rates, the difference in APD between MCT and MCT+S2a hearts was ultimately negated at cycle lengths leading up to VT. This suggests that APD prolongation is not required for VT initiation. Instead, there must be other factors that mediate both the pro-arrhythmic state in PAH and the antiarrhythmic benefit of aerosolized AAV1.S2a gene delivery.
Conduction slowing is another hallmark feature of advanced structural heart disease that predisposes to VT.11 In the present study we report a 28% reduction in average transverse CV in MCT compared to CTRL hearts. Unlike changes in APD, CV slowing was even more pronounced (>50%) at elevated rates leading up to the initiation of VT. Our finding of CV slowing in this model is consistent with some previous reports25 but not others.26, 27 Specifically, we found homogenous CV slowing in this model across the entire epicardium and along both transverse and longitudinal directions, with no major change in anisotropy. CV slowing in our model coincided with marked downregulation in Cx43 at the mRNA and protein levels and a pro-fibrotic gene expression program. Remarkably, conduction defects which likely gave rise to pacing-induced VT in this model were fully reversed by AAV1.S2a gene therapy to the lungs. Along with the functional improvement in myocardial conduction, pro-fibrotic markers were downregulated in AAV1.S2a-treated compared to untreated MCT hearts.
Limitations
There are major differences in heart rate and the action potential profile between rodents and humans. The unique morphology and relatively short duration of the rat action potential limit the formation of large voltage gradients during final repolarization, which can cause functional conduction block and reentry. Terminal repolarization is sculpted, in large part, by the transient outward K current (/to) in rats but not humans. As such, our results regarding the direct relationship between APD75 and K channel remodeling should be extrapolated with caution to humans.
Our ex vivo approach for evaluating arrhythmia susceptibility was based on challenge of preparations with rapid pacing. Indeed, the rapid rates that elicited VT in langendorf perfused rat hearts do not reflect the typical average heart rates in humans. Nonetheless, the percent increase in pacing rate that was required for VT initiation over the basal rate is similar to the percent rise in heart rate achieved in humans during vigorous exercise. Moreover, since our experimental design consisted of continuous steady-state pacing at progressively more rapid pacing rates, our optical recordings taken at fixed intervals following a change in pacing rate did not capture the initiation of VT episodes. Future studies are needed to examine the location and properties of the arrhythmia triggering events in this model and their modification by intratracheal gene delivery.
Another key limitation is the absence of adrenergic signaling in our ex vivo perfused preparations. Indeed, adrenergic signaling in diseases such as PAH can modulate electrical remodeling and arrhythmia propensity.
Moreover, our focus in the present study was on ventricular arrhythmias and the ventricular electrophysiological substrate. Future studies are needed to carefully examine issues related to AV node conduction, AV block in response to rapid atrial pacing, and supraventricular arrhythmias, which were not be accounted for by our experimental design.
Summary
In the present work, we demonstrated the safety and anti-arrhythmic efficacy of an extracardiac gene therapy approach targeting the lungs in PAH. Specifically, we found that intratracheal gene delivery of aerosolized AAV1.S2a suppresses conduction-dependent VT by normalizing the molecular correlates of EP, mechanical, and structural remodeling. These results are particularly important in light of the dearth of therapeutic approaches, antiarrhythmic or otherwise, for PAH.
Supplementary Material
Supplemental Figure 1. MCT causes pulmonary vessel thickening that leads to RV remodeling, which is reversed with pulmonary AAV1.S2a. A. Schematic illustrating the mechanism by which SERCA2a downregulation in the pulmonary vasculature causes pulmonary arterial hypertension. SERCA2a downregulation causes defects in calcium sequestration, leading to the accumulation of cytosolic calcium in vascular smooth muscle cells (SMC). This, in turn, causes the activation of calcineurin (CaN), which dephosphorylates, and therefore activates (NFATC). Activated NFATC subsequently translocates to the nucleus and upregulates the expression of inflammatory mediators and vascular SMC proliferation. As such, SERCA2a overexpression in SMCs restores calcium homeostasis, leading to reduced CaN activation of NFATC, a reversal of SMC proliferation, and restoration of the inner pulmonary artery vessel diameter. B. Lung sections were stained with hematoxylin and eosin and distal pulmonary arteries were examined by microscopy for morphometric analysis. Representative images of lung sections from CTRL, MCT and MCT+S2a animals. Scale bar represents 100μm. C. Morphometric analysis of the distal pulmonary arteries: Vessels were divided into two categories according to their external diameter (<50 μm and >50 μm) and the medial wall thickness was determined for each category and referenced against the control group (N=5-7 rats per group). D. Systolic pulmonary arterial pressure (SPAP), Diastolic pulmonary arterial pressure (DPAP), and Mean pulmonary arterial pressure (MPAP) in CTRL, MCT, and MCT+S2a groups. E. The Fulton index was calculated by excising the RV, and dividing its weight by the weight of the LV and septum (N= 6 CTRL, 8 MCT, 8 MCT+S2a). F. Average in vivo heart rate at the time of pulmonary arterial pressure measurements in all groups. *P <0.05, **P <0.001, ***P <0.0005, ****p <0.0001 by one-way ANOVA and Tukey post-test.
Supplemental Figure 2. Electrophysiological effects of intra-tracheal delivery of aerosolized AAV1.S2a to normal rats. A. Representative action potential traces recorded at increasing pacing rates in CTRL and CTRL+S2a hearts. Neither group was prone to pacing-induced VT (0/7 CTRL vs 0/6 CTRL+S2a). B. Average APD75 was comparable between CTRL and CTRL+S2a hearts. C. Rate-dependence of CV was similar in CTRL and CTRL+S2a groups (P=NS at all pacing rates). Representative isochrones maps recorded at PCL 140ms in CTRL and CTRL+S2a hearts. D. Mean critical conduction velocity measured at the fastest pacing rate was comparable in CTRL and CTRL+S2a hearts (P=NS).
Supplemental Figure 3. Comparison of the untreated MCT group with MCT rats treated with intra-tracheal delivery of a control AAV vector carrying luciferase (AAV1.luc). A. Unlike AAV1.S2a treatment, AAV1.luc failed to suppress the susceptibility of MCT hearts to VT. B. Untreated and AAV1.luc-treated MCT hearts exhibited comparable electrophysiological properties.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health and the American Heart Association: R01 HL091923 (FGA), R21AG054211 (FGA), R01HL133554 (LH), K01 HL133424 (ICT) and 17SDG33370112 (YS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
Disclosures: None of the authors have any relationships to disclose
References
- 1.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the reveal registry. Chest. 2012;142:448–456 [DOI] [PubMed] [Google Scholar]
- 2.Bandorski D, Bogossian H, Ecke A, Wiedenroth C, Gruenig E, Benjamin N, Arlt M, Seeger W, Mayer E, Ghofrani A, Hoeltgen R, Gall H. Evaluation of the prognostic value of electrocardiography parameters and heart rhythm in patients with pulmonary hypertension. Cardiol J. 2016;23:465–472 [DOI] [PubMed] [Google Scholar]
- 3.Hadri L, Kratlian RG, Benard L, Maron BA, Dorfmuller P, Ladage D, Guignabert C, Ishikawa K, Aguero J, Ibanez B, Turnbull IC, Kohlbrenner E, Liang L, Zsebo K, Humbert M, Hulot JS, Kawase Y, Hajjar RJ, Leopold JA. Therapeutic efficacy of aav1.Serca2a in monocrotaline-induced pulmonary arterial hypertension. Circulation. 2013;128:512–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghamohammadzadeh R, Zhang YY, Stephens TE, Arons E, Zaman P, Polach KJ, Matar M, Yung LM, Yu PB, Bowman FP, Opotowsky AR, Waxman AB, Loscalzo J, Leopold JA, Maron BA. Up-regulation of the mammalian target of rapamycin complex 1 subunit raptor by aldosterone induces abnormal pulmonary artery smooth muscle cell survival patterns to promote pulmonary arterial hypertension. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2016;30:2511–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, Hajjar RJ, Lompre AM. Sarco/endoplasmic reticulum ca2+-atpase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circulation research. 2005;97:488–495 [DOI] [PubMed] [Google Scholar]
- 6.Aguero J, Ishikawa K, Hadri L, Santos-Gallego C, Fish K, Hammoudi N, Chaanine A, Torquato S, Naim C, Ibanez B, Pereda D, Garcia-Alvarez A, Fuster V, Sengupta PP, Leopold JA, Hajjar RJ. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. American journal of physiology. Heart and circulatory physiology. 2014;307:H1204–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguero J, Ishikawa K, Hadri L, Santos-Gallego CG, Fish KM, Kohlbrenner E, Hammoudi N, Kho C, Lee A, Ibanez B, Garcia-Alvarez A, Zsebo K, Maron BA, Plataki M, Fuster V, Leopold JA, Hajjar RJ. Intratracheal gene delivery of serca2a ameliorates chronic post-capillary pulmonary hypertension: A large animal model. Journal of the American College of Cardiology. 2016;67:2032–2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Arroyo JG, Farkas L, Alhussaini AA, Farkas D, Kraskauskas D, Voelkel NF, Bogaard HJ. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302:L363–369 [DOI] [PubMed] [Google Scholar]
- 9.Jin H, Chemaly ER, Lee A, Kho C, Hadri L, Hajjar RJ, Akar FG. Mechanoelectrical remodeling and arrhythmias during progression of hypertrophy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:451–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie C, Hu J, Motloch LJ, Karam BS, Akar FG. The classically cardioprotective agent diazoxide elicits arrhythmias in type 2 diabetes mellitus. Journal of the American College of Cardiology. 2015;66:1144–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circulation research. 2004;95:717–725 [DOI] [PubMed] [Google Scholar]
- 12.Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. American journal of physiology. Heart and circulatory physiology. 2007;293:H1223–1230 [DOI] [PubMed] [Google Scholar]
- 13.Akar FG, Aon MA, Tomaselli GF, O'Rourke B. The mitochondrial origin of postischemic arrhythmias. J Clin Invest. 2005;115:3527–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Annals of internal medicine. 1991;115:343–349 [DOI] [PubMed] [Google Scholar]
- 15.Bandorski D, Erkapic D, Stempfl J, Holtgen R, Grunig E, Schmitt J, Chasan R, Grimminger J, Neumann T, Hamm CW, Seeger W, Ghofrani HA, Gall H. Ventricular tachycardias in patients with pulmonary hypertension: An underestimated prevalence? A prospective clinical study. Herzschrittmachertherapie & Elektrophysiologie. 2015;26:155–162 [DOI] [PubMed] [Google Scholar]
- 16.Holland AE, Hill CJ, Glaspole I, Goh N, Dowman L, McDonald CF. Impaired chronotropic response to 6-min walk test and reduced survival in interstitial lung disease. Respir Med. 2013;107:1066–1072 [DOI] [PubMed] [Google Scholar]
- 17.Benoist D, Stones R, Drinkhill M, Bernus O, White E. Arrhythmogenic substrate in hearts of rats with monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. American journal of physiology. Heart and circulatory physiology. 2011;300:H2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharin SN, Krandycheva VV, Tsvetkova AS, Shumikhin KV. Remodeling of ventricular repolarization in experimental right ventricular hypertrophy. J Electrocardiol. 2017;50:626–633 [DOI] [PubMed] [Google Scholar]
- 19.Umar S, Lee JH, de Lange E, Iorga A, Partow-Navid R, Bapat A, van der Laarse A, Saggar R, Saggar R, Ypey DL, Karagueuzian HS, Eghbali M. Spontaneous ventricular fibrillation in right ventricular failure secondary to chronic pulmonary hypertension. Circ Arrhythm Electrophysiol. 2012;5:181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka Y, Takase B, Yao T, Ishihara M. Right ventricular electrical remodeling and arrhythmogenic substrate in rat pulmonary hypertension. Am J Respir Cell Mol Biol. 2013;49:426–436 [DOI] [PubMed] [Google Scholar]
- 21.Rich JD, Thenappan T, Freed B, Patel AR, Thisted RA, Childers R, Archer SL. Qtc prolongation is associated with impaired right ventricular function and predicts mortality in pulmonary hypertension. International journal of cardiology. 2013;167:669–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Temple IP, Logantha SJ, Absi M, Zhang Y, Pervolaraki E, Yanni J, Atkinson A, Petkova M, Quigley GM, Castro S, Drinkhill M, Schneider H, Monfredi O, Cartwright E, Zi M, Yamanushi TT, Mahadevan VS, Gurney AM, White E, Zhang H, Hart G, Boyett MR, Dobrzynski H. Atrioventricular node dysfunction and ion channel transcriptome in pulmonary hypertension. Circ Arrhythm Electrophysiol. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiba T, Hesketh GG, Barth AS, Liu T, Daya S, Chakir K, Dimaano VL, Abraham TP, O'Rourke B, Akar FG, Kass DA, Tomaselli GF. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 2009;119:1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Handoko ML, Lamberts RR, Redout EM, de Man FS, Boer C, Simonides WS, Paulus WJ, Westerhof N, Allaart CP, Vonk-Noordegraaf A. Right ventricular pacing improves right heart function in experimental pulmonary arterial hypertension: A study in the isolated heart. American journal of physiology. Heart and circulatory physiology. 2009;297:H1752–1759 [DOI] [PubMed] [Google Scholar]
- 25.Benoist D, Stones R, Drinkhill MJ, Benson AP, Yang Z, Cassan C, Gilbert SH, Saint DA, Cazorla O, Steele DS, Bernus O, White E. Cardiac arrhythmia mechanisms in rats with heart failure induced by pulmonary hypertension. American journal of physiology. Heart and circulatory physiology. 2012;302:H2381–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardziyenka M, Campian ME, de Bruin-Bon HA, Michel MC, Tan HL. Sequence of echocardiographic changes during development of right ventricular failure in rat. J Am Soc Echocardiogr. 2006;19:1272–1279 [DOI] [PubMed] [Google Scholar]
- 27.Uzzaman M, Honjo H, Takagishi Y, Emdad L, Magee AI, Severs NJ, Kodama I. Remodeling of gap junctional coupling in hypertrophied right ventricles of rats with monocrotaline-induced pulmonary hypertension. Circulation research. 2000;86:871–878 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. MCT causes pulmonary vessel thickening that leads to RV remodeling, which is reversed with pulmonary AAV1.S2a. A. Schematic illustrating the mechanism by which SERCA2a downregulation in the pulmonary vasculature causes pulmonary arterial hypertension. SERCA2a downregulation causes defects in calcium sequestration, leading to the accumulation of cytosolic calcium in vascular smooth muscle cells (SMC). This, in turn, causes the activation of calcineurin (CaN), which dephosphorylates, and therefore activates (NFATC). Activated NFATC subsequently translocates to the nucleus and upregulates the expression of inflammatory mediators and vascular SMC proliferation. As such, SERCA2a overexpression in SMCs restores calcium homeostasis, leading to reduced CaN activation of NFATC, a reversal of SMC proliferation, and restoration of the inner pulmonary artery vessel diameter. B. Lung sections were stained with hematoxylin and eosin and distal pulmonary arteries were examined by microscopy for morphometric analysis. Representative images of lung sections from CTRL, MCT and MCT+S2a animals. Scale bar represents 100μm. C. Morphometric analysis of the distal pulmonary arteries: Vessels were divided into two categories according to their external diameter (<50 μm and >50 μm) and the medial wall thickness was determined for each category and referenced against the control group (N=5-7 rats per group). D. Systolic pulmonary arterial pressure (SPAP), Diastolic pulmonary arterial pressure (DPAP), and Mean pulmonary arterial pressure (MPAP) in CTRL, MCT, and MCT+S2a groups. E. The Fulton index was calculated by excising the RV, and dividing its weight by the weight of the LV and septum (N= 6 CTRL, 8 MCT, 8 MCT+S2a). F. Average in vivo heart rate at the time of pulmonary arterial pressure measurements in all groups. *P <0.05, **P <0.001, ***P <0.0005, ****p <0.0001 by one-way ANOVA and Tukey post-test.
Supplemental Figure 2. Electrophysiological effects of intra-tracheal delivery of aerosolized AAV1.S2a to normal rats. A. Representative action potential traces recorded at increasing pacing rates in CTRL and CTRL+S2a hearts. Neither group was prone to pacing-induced VT (0/7 CTRL vs 0/6 CTRL+S2a). B. Average APD75 was comparable between CTRL and CTRL+S2a hearts. C. Rate-dependence of CV was similar in CTRL and CTRL+S2a groups (P=NS at all pacing rates). Representative isochrones maps recorded at PCL 140ms in CTRL and CTRL+S2a hearts. D. Mean critical conduction velocity measured at the fastest pacing rate was comparable in CTRL and CTRL+S2a hearts (P=NS).
Supplemental Figure 3. Comparison of the untreated MCT group with MCT rats treated with intra-tracheal delivery of a control AAV vector carrying luciferase (AAV1.luc). A. Unlike AAV1.S2a treatment, AAV1.luc failed to suppress the susceptibility of MCT hearts to VT. B. Untreated and AAV1.luc-treated MCT hearts exhibited comparable electrophysiological properties.