Abstract
Cell delivery reagents often exploit the endocytic pathway as a route of cell entry. Once endocytosed, these reagents must overcome endosomal entrapment to insure the release of their macromolecular cargos into the cytosol of cells. In this review, we describe several examples of prototypical synthetic reagents that are capable of endosomal escape and examine their mechanisms of action, their efficiencies, and effects on cells. Although these delivery systems are chemically distinct, some commonalities in how they interact with cellular membranes can be inferred. This, in turn, sheds some light on the process of endosomal escape, and may help guide the development and optimization of next-generation delivery tools.
Graphical Abstract
Introduction
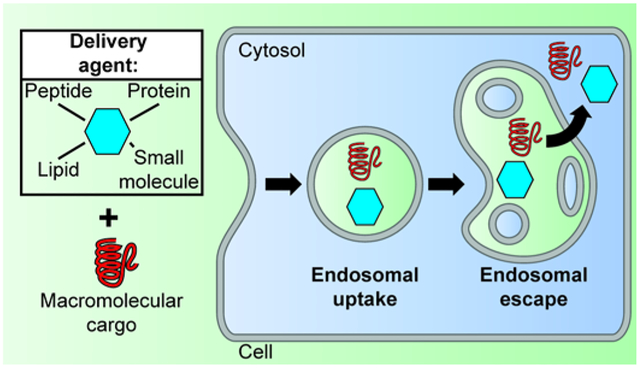
Delivering macromolecules such as nucleic acids or proteins (subsequently referred to as cargos) into live cells requires traversing cellular membranes. The plasma membrane of human cells is a biological barrier that prevents access into the cell’s interior, and, consequently, numerous delivery strategies have focused on facilitating the transport of molecular cargos across this membrane. However, reagents that can permeabilize the plasma membrane, and thereby allow the passage of large molecules, are often toxic. In particular, extensive bilayer damage, as may be necessary to accommodate large cargos, can lead to immediate necrosis. Conversely, cells can repair membrane defects and sustain some level of disruption in the integrity of their plasma membrane (1). Repair processes are typically initiated by the influx of calcium into the cell, a process that may accompany cargo membrane translocation (2). Yet, calcium influx may also initiate the apoptotic cascade and lead to cell death several hours after the membrane translocation events have taken place (3, 4). Overall, it is therefore clear that achieving direct plasma translocation of large molecules often constitute a perilous balancing act between delivery efficiency and cell death.
To enter cells, membrane systems other than the plasma membrane can be exploited. In particular, human cells internalize extracellularly administered cargos by endocytosis. This can be mediated by specific cell surface interactions, or simply involve non-specific engulfment of molecules present in the extracellular fluid (5, 6). Upon internalization, cargos are sequestered within membrane-bound endosomes. Through fusion and fission events, endosomes exchange some of their luminal content, and cargos can subsequently distribute throughout a complex endosomal pathway that includes early endosomes, recycling endosomes, multivesicular bodies, late endosomes, and lysosomes (7). Cargos trapped in this pathway may either be recycled to the cell surface or undergo degradation by exposure to lysosomal enzymes (7). Notably, while conceptually in the cell, endosomally-trapped cargos do not have access to the cell interior, that is the cytosolic space and organelles such as the nucleus. Delivery vectors that simply promote endocytosis of cargos are therefore not sufficient to permit successful cytosolic penetration. Instead, cargos have to cross the membrane of endosomes and delivery agents need to mediate this process. Therefore, delivery remains a membrane translocation challenge and some of the issues previously described in the context of crossing the plasma membrane apply. Yet, the endosomal pathway includes membrane systems that are different from the plasma membrane. Consequently, the mechanisms by which translocation is mediated, the efficiencies with which this is achieved, and the cellular responses that accompany delivery may all be distinct.
The idea of promoting endosomal escape so as to achieve cytosolic delivery has been pursued for several decades (8). How this can be done efficiently and what accompanies this process, on both a molecular or cellular level, have remained challenging questions. In this review, we described a survey of reagents that have been used to enhance endosomal escape. We focus on several prototypical examples that span various areas of the chemical space, including small molecules, peptides, lipids, and polymers. Using cell-based studies as a primary source of information, we highlight how these reagents escape from endosomes, how well they mediate this process, and what cellular responses are engaged upon membrane permeation. These insights reveal future directions that may be exploited for the development of optimized delivery tools.
1. What are the molecules that induce endosomal escape?
Several molecules have been identified that have the ability to undergo endocytic uptake followed by endosomal escape. While chemically distinct, several of these species share some common features, highlighting how some fundamental chemical rule may underlie their activity. All reagents typically improve delivery outcomes for a variety of cargo. Herein, we do not describe applications in which they have been found to be useful. Instead, we focus on what these reagents are and how they are thought to mediate escape of macromolecular cargos.
1.1. Cationic lipid/cationic polymers
Cationic lipids spontaneously form liposomes in aqueous media. These liposomes form complexes with RNA or DNA, referred to as lipoplexes, by coating the phosphate backbone of the polynucleotides. The complexes formed are typically highly positively charged as the total number of cations contributed by the liposomes exceeds the number of anionic phosphates contributed by the nucleic acids. Alternatively, cationic lipids are common constituents of lipid nanoparticles (LNP), one of the most advanced delivery systems for siRNAs (9). LNPs encapsulate their nucleic acid cargos within their core, thereby yielding structures distinct from lipoplexes.
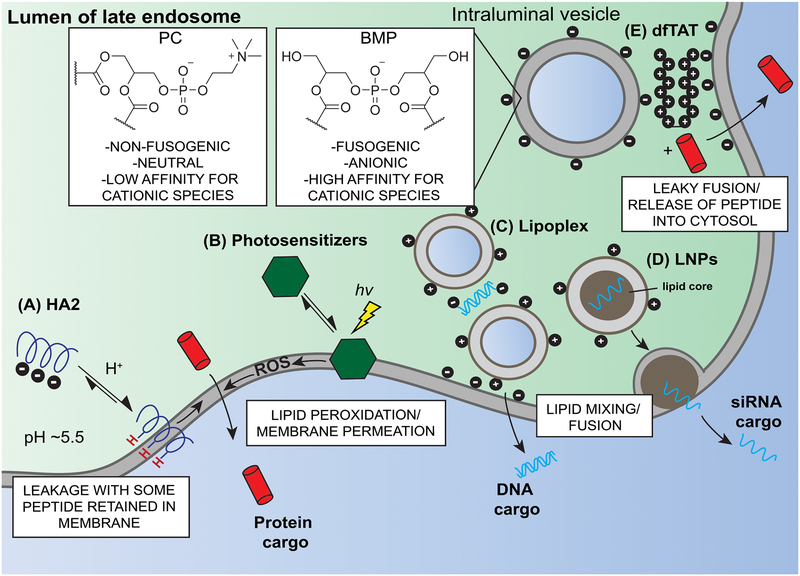
Fluorescently-labeled lipoplexes and LNPs have been shown to enter cells through endocytosis (10, 11). Once endocytosed, the weakening of electrostatic interactions within the lipoplex is thought to contribute to leakage. In this model, negatively-charged phospholipids, which are typically located on the cytoplasmic leaflet of endosomal membrane (e.g. phosphatidylserine, PS), would flip across the bilayer to the luminal leaflet. Electrostatic interactions between the cationic lipids and the anionic phospholipids may then form, thereby promoting lipid mixing and translocation (Figure 1) (12). Alternatively, endosomal escape may involve the zwitterionic lipid phosphatidylethanolamine (PE), an endogenous constituent of human membranes. In particular, lipoplexes fuse in vitro with bilayers containing DOPE (dioleoyl PE), an activity attributed to the inherent fusogenicity of PE (13). In this model, the bilayers of the lipoplexes would mix lipids with PE-containing endosomal membranes and DNA would escape through a hemifusion pore (Figure 1) (14, 15). Notably, a similar in vitro fusogenic process was observed between lipoplexes and bilayers containing bis(monoacylglycero)phosphate (BMP, also referred to as LBPA). Unlike PE, which represents approximately 20% of the lipids present in human cells. BMP is of relative low abundance overall (16, 17). Yet, BMP is highly enriched in the membrane of late endosomes and lysosomes. For instance, BMP represents up to 77% of total lipids in the membrane of some of the vesicles endogenously present in the lumen of late endosomes.(16–18). Notably, BMP is both anionic and fusogenic (17, 19, 20). It therefore combines the two important features highlighted in the two models described. One may therefore speculate that late endosomes or lysosomes may represent cellular compartments favorable for endosomal escape. It is also possible that cationic lipids are engaged in similar endosomal escape mechanisms regardless of whether they are incorporated into lipoplexes or LNPs. A notable difference, however, is that LNPs also often include ionizable lipids, species that are neutral during LNP formation but that become cationic within the acidic lumen of endosomes (21). Unlike their constitutively cationic counterparts, ionizable lipids are less likely to be involved in direct binding to anionic siRNAs. Lipid-RNA binding, which is necessary for entrapping the nucleic acid cargo, may conceptually inhibit the lipids involved from interacting with membranes. In turn, this means that ionizable lipids may then be better able to mediate endosomal escape, as observed by their improved delivery efficacies (22).
Figure 1.
Examples of delivery agents and of their modes of action. A) The acidic pH of the late endosome protonates the amphiphilic peptide HA2, triggering a conformational change that promotes membrane insertion and membrane permeabilization. B) In the presence of light, photosensitizers generate reactive oxygen species (ROS) that oxidize membranes, leading to membrane permeabilization. C) Lipoplex- and LNP-mediated escape is triggered by electrostatic interactions between the cationic lipids of the lipoplex and the anionic phospholipids present in endosomes. In addition, lipid mixing with fusogenic cellular lipids enable the cytosolic penetration of nucleic acid cargo. D) Electrostatic interactions between the arginine-rich CPP dfTAT and anionic intraluminal vesicles trigger bilayer contact among late endosomal membranes. This, in turn, promotes leaky fusion and cytosolic release. For all examples, the endosomal escape events are shown as taking place in late endosomes. These organelles are not necessarily the only site of escape for these reagents (e.g. HA2, photosensitizers). Yet, the presence of BMP, a lipid that is both anionic and fusogenic, makes the membranes of late endosomes, and not the PC-enriched plasma membrane, likely targets of cationic delivery reagents.
Polyethylenimine (PEI) is a cationic polymer composed of repeating amine groups that can complex with RNA or DNA. Like lipoplexes, PEI/nucleic acid polyplexes enter cells through endocytosis (23). A commonly proposed mechanism for PEI-mediated endosomal escape is based on the “proton sponge effect” (24). In this model, as polyplexes traffic through endosomes of increasing luminal acidity (pH~6.5, 5.5, and 4.5 for early endosomes, late endosomes, lysosomes, respectively), PEI becomes increasingly protonated. In particular, upon reaching lysosomes, the protonation of PEI continues and results in an influx of protons, Cl− ions and water. Subsequent osmotic pressure and repulsions between the protonated polyplexes result in the swelling of the organelles and subsequent leakage (25). Yet, using a pH-sensor to monitor lysosomal pH, Andresen and coworkers have detected no change in lysosomal pH post PEI uptake. These results are therefore in contradiction with the “proton sponge” model (26). Alternatively, as with lipoplexes, cationic polyplexes may disrupt membranes by coming into contact with the endosomal membrane (27).
1.2. Cationic peptides/cationic synthetic proteins
Several classes of peptides can permeabilize endosomal membranes. They include arginine rich cationic peptides and amphiphilic peptides. A prototypical arginine-rich cell-penetrating peptide (CPP) is TAT. Derived from the HIV-1 transactivator of transcription, the TAT peptide (RKKRRQRRR) enters cells through macropinocytosis (28–30). From within the endocytic pathway, TAT is capable of delivering a variety of cargos into the cytosol of cells (31, 32). Quantitative measurements of how much material escapes endosomes have however indicated that the endosomal escape activity of TAT is poor (see section 2). This low activity is in turn challenging to monitor and gaining mechanistic insights has been challenging. Nonetheless, by blocking the endocytic pathway at various steps and by monitoring cytosolic access with sensitive assays, Shepartz and coworkers have established that the late endosomes are sites of escape within the pathway (33). Melikov and co-workers corroborated these results by demonstrating that TAT can cause the leakage of bilayers of a composition similar to that expected for late endosomal membranes (i.e. containing BMP) (Figure 1) (34).
Several studies have highlighted how linking several TAT peptides to one another can improved endosomal escape. Multimeric TAT constructs can be constructed by linking multiple repeats of the peptide tag at the termini of a protein, or by attaching the peptides onto synthetic scaffold (35–37). Recently, constructs containing 2 of 3 TAT branches (e.g. dfTAT or 2TAT have 2 branches, 3TAT has 3) have been shown to be significantly more prone to escaping endosomes than constructs with a single TAT unit (1TAT) (38, 39). Here again, late endosomes were found to be the sites of endosomal leakage and cytosolic egress. In addition, the endosomolytic species 2TAT and 3TAT mediate the leakage of bilayers containing BMP. Notably, leakage is abolished when BMP is substituted to other anionic lipids. This in turn suggests that electrostatic interactions alone are not sufficient to explain membrane leakage. In addition, 2TAT or 3TAT do not cause the leakage of single liposomes. Instead, these reagents bring multiple liposomes into contact and induce fusion between liposomes. It is during these fusion events that leakage may then take place. Notably, the BMP-specific leakage-inducing activity of the reagents increases with the number of TAT repeats (3TAT>2TAT>>1TAT), as do their cell penetration activity. Finally, an anti-BMP antibody, which blocks the contact and fusion of BMP-containing bilayers in vitro, also blocks cell penetration in live cells (39). Overall, these results suggest that the polycationic peptides interact with anionic BMP, thereby promoting the contact, fusion, and leakage of late endosomal membranes.
1.3. Amphiphilic peptides
Amphiphilic peptides that bind lipid bilayers because of their partial hydrophobicity can be used to disrupt endosomal membrane. An example includes 6His-CM18-PTD4, a chimera of the 6His tag, PTD4 (YARAAAARQARA), and CM18 (KWKLFKKIGAVLKVLTTG). Because its interaction with membranes is presumably primarily driven by its hydrophobicity, this peptide permeabilizes membranes in a nonselective manner. It can therefore destabilize the plasma membrane of cells and be rather toxic. However, by limiting the time of peptide exposure, cells can survive treatment. Similarly, low concentrations of peptides may be tolerated. In both situations, endocytosis may take place before surface damage occurs. Subsequently, the peptide may accumulate inside the lumen of endosomes and reach a threshold concentration above which membrane leakage is achieved (40). Gene-editing CRISPR ribonucleoproteins have been successfully delivered into cells using this approach (41).
In order to make membrane-disrupting peptides specific towards endosomal membrane, a strategy is to make them pH-responsive. Using such peptides, one can take advantage of the fact that the lumen of endosomes is acidified, and use low pH as a trigger for membrane insertion and leakage (42). One example of a pH-responsive peptide is the fusogenic HA2 peptide, which is derived from the hemagglutinin (HA2, GLFGAIAGFIENGWEGMIDGWYG) glycoprotein of the influenza virus (43). The protonation of several aspartate and glutamate residues present in the peptide results in an overall increase in the hydrophobicity of the peptide. In turn, the protonated peptide inserts into lipid bilayer, resulting in disruption of the membrane (44, 45). HA2-containing constructs have improved the endosomal escape activity of several delivery agents (46, 47). HA2 has for instance been attached to TAT, TAT being designed to mediate endocytosis while HA2 is designed to mediate escape. Based on hemolysis-based studies, HA2-TAT analogs behave as species with a single pKa (e.g. despite multiple protonation sites) (48). Membrane disruption is exclusively mediated by the protonated form of the peptide and hemolysis is correlated to how much protonated peptide is present on the surface of a bilayer (Figure 1). Membrane leakage is therefore more efficient at low pH because more protonated peptide is present in the protonated/deprotonated equilibrium. Yet, the peptides are not inactive at pH 7. Instead, while the equilibrium is shifted towards the deprotonated and inactive form of the peptides, some protonated peptides are present at this pH (the concentration can be approximated using the Henderson-Hasselbach equation and the peptide apparent pKa) (44). In other words, the membrane disruption obtained with such peptides at low pH can also be obtained at high pH, as long as the overall concentration of peptide is increased. Overall, this means that the peptide disrupts the plasma membrane of live cells above a threshold concentration (see Section 3). Nonetheless, below this cytotoxic threshold concentration, HA2-TAT analogs can remain relatively innocuous to cells, be internalized by cells, mediate endosomal leakage, and release cargos trapped inside endosomes. However, HA2-like moieties tend to remain bound to the membrane of endosomes post-leakage (48, 49). Consequently, cargos that are directly linked to HA2-like moieties can remain tethered to endosomes even after the endosomes have been permeabilized.
Recently, Akishiba et al. synthesized analogs of the cationic and amphipathic peptide M-lycotoxin and found that a single leucine to glutamic acid substitution (L17E) prompted the selective disruption of endosomal membranes. In vitro liposome assays showed that the peptide requires an acidic pH and protonation of E17 for membrane leakage. However, unlike HA2, the protonated L17E-lycotoxin peptide does not disrupt all bilayers. Instead, it displays a propensity for membranes enriched in negatively-charged phospholipids. Because anionic lipids are relatively absent from the outer leaflet of a cellular plasma membrane, reagents such as L17E-lycotoxin may have limited impact at the cell surface and display reduced toxicities (50).
1.4. Small molecules
Small molecules, often referred to as lysosomotropic agents, can disrupt late endosomal or lysosomal membranes. For instance, L-leucyl-L-leucine O-methyl ester (LLOME) is believed to accumulate in lysosomes and lead to osmotic swelling followed by leakage (51). Additionally, cathepsin C may mediate the formation of a membrane-lytic LLOME polymer within these organelles (52). Similar to LLOME, the antimalarial drug chloroquine accumulates in acidic vesicles (51). Chloroquine is a weak base that gets protonated in late endosomes and lysosomes. As with LLOME, its lysosomal accumulation is thought to result in osmotic swelling (53). Both LLOME and chloroquine are rather toxic. Yet, lysosomotropic agents can be used to enhance the cytosolic penetration of various delivery approaches. (54, 55).
In an effort to increase the cytosolic delivery of antisense and siRNA oligonucleotides, Juliano and coworkers screened compound libraries to identify small molecules that enhance the delivery of oligonucleotides. The authors found one candidate in particular, UNC7938, that could deliver oligonucleotide cargo at low concentrations, in cell cultures and in vivo. Colocalization between UNC7938 and GFP-Rab7, but not with GFP-LAMP1, suggests that UNC7938-mediated endosomal escape likely occurs at the late endosome (56). The mechanism of action of UNC7938 has not been described. However, UNC7938 contains a pyridopyrazine core and amine substituent that presumably confers a cationic charge to the molecule. It is therefore possible that UNC7938 may interact with anionic bilayers.
Photosensitizers are small molecule chromophores that can generate reactive oxygen species when irradiated with visible light. Photosensitizers typically consist of conjugated ring structures and these molecules are often hydrophobic. They therefore tend to partition indiscriminately in the membranes of cells. Irradiation then leads to lipid peroxidation, membrane rupture and cell death (4, 57). Historically, photosensitizers have therefore been used as cell killing agents in the context of photodynamic therapy applications. Interestingly, linking hydrophilic groups to the hydrophobic core of photosensitizers, whether by addition of charged moieties or peptides sequences, render these molecules more soluble (58). When incubated with cells, they then tend to accumulate within the endocytic pathway without partitioning in the plasma membrane (59–61). Such reagents can then be irradiated while trapped within endosomes and selectively photo-oxidize the membrane of these organelles (Figure 1) (62). Macromolecular cargos loaded in the lumen of endosomes by prior incubation with cells can then be released in the cytosol of cells. This approach is referred to as Photo-Chemical Internalization (PCI) (63).
1.5. Conclusions
All reagents described herein are chemically quite distinct. They may therefore display unique membrane disruption behaviors. Yet, when combined, it is clear that many share some similar features: positive charges combined with hydrophobicity. One exception, at least at first glance, may be arginine-rich CPPs, which are often cationic but not hydrophobic. Yet, it has been observed that the addition of short hydrophobic sequences can enhance their activity (64, 65). Moreover, such peptides are typically conjugated to fluorophores for cellular tracking. These fluorophores, by contributing some hydrophobicity, may themselves be involved in some aspects of the membrane disruption process. This has been clearly demonstrated in the context of monomeric CPPs, where the fluorophore used for peptide labeling impacts cell viability, peptide uptake, and CPP binding to bilayers (39, 66–68). Similarly, fluorophores also impact multivalent systems such as 3TAT, as suggested by the fact that a non-labeled 3TAT construct is noticeably less prone to inducing membrane leakage that an analog labeled with tetramethylrhodamine (the relationship 3TAT>2TAT>1TAT in regard to membrane penetration is however true when comparing labeled or unlabeled analogs) (39).
The commonalities observed here may also apply to how these reagents disrupt membranes. In particular, it is becoming apparent that late endosomes represent a unique membrane system that is utilized for escape. In particular, the lipid BMP appears to provide fusogenic and anionic properties that uniquely respond to cationic/hydrophobic reagents. Notably, similar observations have recently been made with cationic viral components, late endosomes being a site of viral entry for the blue tongue or dengue viruses (69, 70). Overall, it is therefore likely that defining the rules that govern the interplay between current reagents and late endosomes may reveal how to better exploit these organelles as gateways into cells.
2. How efficient is endosomal escape?
The issue of endosomal escape efficiency is complicated. In particular, it includes several intertwined questions. To what extent do delivery agents and macromolecules escape from endosomes? To what extent does the endocytosed material remain trapped within the endosomal lumen? How many endosomes undergo leakage in a given cell? How does this process vary from cell to cell within the same experiment? These questions remain unanswered in many instances. Herein, we highlight several reports that address these questions quantitatively.
2.1. Reagents with low apparent endosomal escape activity
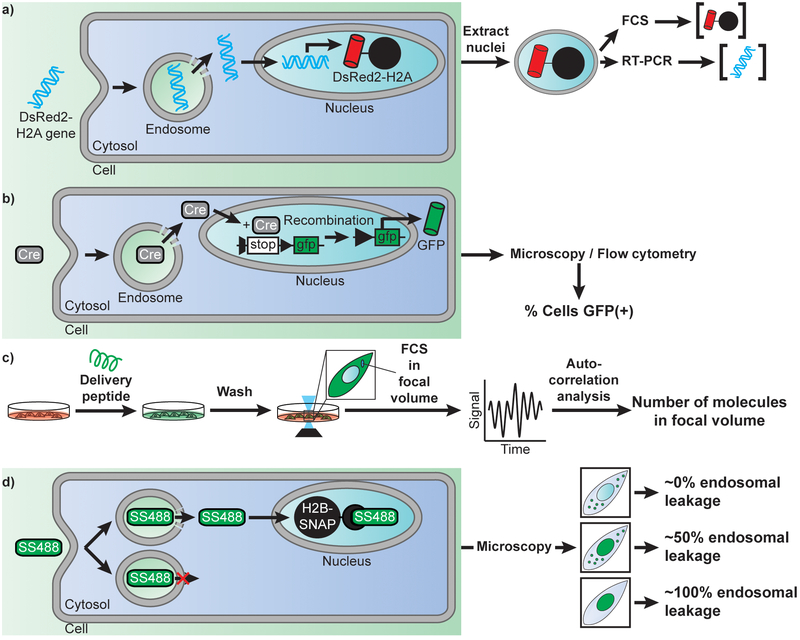
The efficiency of plasmid DNA transfection by LipofectAMINE2000 (LFA), a cationic lipid formulation, and PEI have been assessed by Glover et al (71). The goal of their study was not to measure endosomal escape per se, but instead to assess how many plasmids reach the nucleus of cells. Furthermore, the authors were interested in establishing how nuclear access correlates with expression of a gene encoded in the delivered plasmid. To address these questions, cells were transfected with a plasmid encoding DsRed2-H2A, a fluorescently-tagged histone that is incorporated into chromatin upon expression (Figure 2a). The nuclei of cells containing both the delivered plasmid and its protein product were subsequently isolated (the viability of cells is not directly discussed in this report; yet, one can infer that cells capable of expressing DsRed2-H2A are likely alive prior to nuclear isolation). Real-time PCR was then used to quantitatively measure the amount of plasmid DNA present while flow cytometry was used to determine the amount of protein expressed based on its fluorescence signal. This analysis reveals that cells exposed to 4μg of plasmid (2.2×106 plasmids per cell) for 24h accumulate 350 plasmids/h in the first 8h of exposure, this rate subsequently accelerating. At the end of the 24h incubation, PEI could deliver 1.8×104 plasmids per nucleus while LFA delivered 8.3×103, representing overall yields (nuclear plasmid per cell/total DNA administered per cell) of 0.8% and 0.4%, respectively. Notably, while LFA delivers less plasmid into the nucleus of cells than PEI, it leads to equivalent levels of DsRed-H2A expression per cell, while also transfecting a higher percentage of cells overall. These results are in agreement with the notion that cationic lipids dissociate from their DNA cargo upon endosomal escape and cytosolic egress (72), leaving a naked DNA that may enter the nucleus only inefficiently. In contrast, PEI remains associated with DNA after endosomal escape and subsequently promotes the nuclear delivery of the cargo (73). While this may be an advantage for delivery, it is possible that the PEI that remains bound to DNA upon reaching the nuclear destination may then interfere with transcription. Overall, these results indicate that the multi-step process of DNA transfection is of relatively poor efficiency. Yet, they do not reveal directly whether endosomal escape is itself a bottleneck. However, cells incubated with fluorescently-labeled polyplexes or lipoplexes typically show a punctate distribution of fluorescence signal, as observed by high-magnification microscopy. This punctate signal corresponds to the accumulation of fluorescent material within endosomes. By contrast, no signal is typically detectable in the cytosol or nucleus of cells. Overall, this indicates that the vast majority of endocytosed complexes stay trapped within the endocytic pathway and that endosomal escape is a limiting step.
Figure 2.
Examples of approaches used to detect the efficiency of cellular delivery and of endosomal escape. A) Measuring the efficiency of DNA nuclear delivery by using a DsRed2-H2A reporter. Once endocytosed, a plasmid encoding the histone H2A labeled with the fluorescent protein DsRed, escapes endosome, penetrate the cytosol, and translocate into the nucleus. Expression of the delivered plasmid leads to a fluorescent DsRed signal sequestered in the nuclei of cells by incorporation of H2A into chromatin. Nuclei are extracted and analyzed by flow cytometry to establish protein expression level. Nuclei of various intensities are then sorted, and their plasmid content is quantified by real-time PCR. This analysis can therefore reveal how many plasmids entered the nucleus of cells for a given transfection reagent and relate delivery efficiency to gene expression outcomes. B) Measuring the efficiency of enzyme delivery by using the Cre recombinase as a model. Cells transfected with a GFP gene under a loxP split promoter are treated with Cre recombinase and a delivery agent. Upon successful delivery of Cre recombinase, the split promoter is recombined allowing downstream expression of GFP. Cells are then scored for delivery based on the presence or absence of cytosolic GFP fluorescence. C) Quantitative measurement of the concentration of a peptide or protein delivered into the cytosol of cells. Cells are treated with a fluorescently labeled cell-penetrating species. Cells are then washed to remove all traces of fluorescent signal outside cells and imaged by confocal microscopy. A focal volume within the cytoplasmic area of a cell is chosen and Fluorescence Correlation Spectroscopy is performed. Autocorrelation analysis is performed, and the y-intercept of the autocorrelative curve generated is used to determine the cytosolic concentration of fluorescent molecules (76). D) Quantitative determination of how leaky endosomes are upon treatment with a delivery agent. Cells are transfected with a gene encoding a fusion construct of the histone H2B labeled with a SNAP-tag. Cells are then treated with the delivery agent and the cell-impermeable fluorophore SNAP-Surface 488 (SS488). Depending on the efficiency of the delivery agent and of its membrane disruption activity, endocytosed SS488 is either entrapped in the endosome or released in the cytosol of cells. Once in the cytosol, SS488 is sequestered to the nucleus via an irreversible reaction with the SNAP-tag. As a result, the fluorescence of SS488 is either punctate (trapped inside endosomes), or nuclear (bond to SNAP-H2B). More specifically, the nuclear capture depletes the cytosolic signal, thereby revealing more clearly the signal left inside endosomes. In turn, this approach can be used to estimate the efficiency of endosomal leakage, that is, how much signal is in the nucleus vs how much is left trapped inside endosomes.
The TAT peptide is a ubiquitous delivery agent that has been used in with a variety of cargos in many applications. Despite its popularity, how well TAT works remains often unclear. In order to assess the extent to which TAT is capable of delivering enzymes into cells, Dowdy and co-workers have used Cre recombinase (Figure 2b) as a model (74). In this assay, cells are transfected with a loxP-STOP-LoxP-eGFP. Upon introduction of Cre recombinase into the cytosol and nucleus of cells, the enzyme excises the STOP signal present in the reporter DNA, leading to expression of eGFP. The expression of eGFP was quantitated via flow cytometry, excluding dead cells stained with propidium iodide (PI) from analysis. The authors showed that incubation of cells with the fusion TAT-Cre for 1 h led to a majority (~80%) of cells expressing eGFP 18 h later. On one hand, these results clearly highlight that TAT can successfully bring cargos into cells. However, as described with lipoplexes, microscopy observation of a fluorescently labeled TAT-Cre shows exclusive retention of the protein inside endosomes. It is therefore likely that, while the endosomal escape activity of TAT is limited. In particular, TAT may be capable to deliver a few copies of TAT-Cre per cells, and given the catalytic properties of Cre, these few copies may be sufficient to activate the reporter plasmids present in the cell (as few as 4 Cre molecules, 4 Cre-bound sites being required for excision). Moreover, this assay is binary: there is enough Cre recombinase that enters cells to activate eGFP expression or there isn’t. Therefore, above a given Cre recombinase threshold, eGFP is expressed regardless of how many enzymes are delivered. This, in turn, does not allow the testing of cell-to-cell variability.
In order to assess how much peptides may enter the cytosol of cells, two groups have recently reported the use of fluorescence correlation spectroscopy (Figure 2c) (75, 76). In these assays, fluorescently-labeled peptides are incubated with cells. In one instance, live cells are isolated by FACS (based on size and granularity) and lysed. Cell lysates are then subjected to ultracentrifugation so as to isolate a cytosolic fraction. Samples are subsequently analyzed by bulk Fluorescence Correlation Spectroscopy (FCS) and the concentration of fluorophore present is extracted from autocorrelation curves, using standards of known concentration as calibration. In another instance, FCS is directly performed in the focal volume contained in the cytoplasmic space of cells (cells observed are determined to be viable based on their ability to remain adherent after a brief treatment with trypsin). Autocorrelation analysis yields an estimate of the number of molecules present in this volume. Using these alternative approaches, TAT was found to enter the cytosol of cells with an efficient of 2% ([TAT]cytosol vs [TAT]outside cells). More specifically, a 30 min incubation with 500 nM of peptide yields to a cytosolic concentration of 10 nM (76). Similarly, Antp, a cationic CPP (RQIKIWFQNRRMKWKK) also used for delivery applications, was found to enter at very low level (75). In particular, incubation of 1×106 cells with 1 μM peptide for 2 h (~1.2×108 molecules/cell), yields approximately 9.0×105 molecules/cell of Antp internalized. Moreover, only 1.8×104 molecules/cell are present in the cytosol, the remainder being trapped inside endosomes. Overall, this represents efficiencies of 2% (cytosol/endosome) and 0.015% (cytosol/total outside cells). Notably, the authors observed that addition of PAS (GKPILFF), a hydrophobic peptide previously shown to enhance endosomal escape by Futaki and co-workers (64), showed an increase in both total internalization (i.e. endocytosis, up to 1.5×107 molecules/cell) and cytosolic release (up to 4.2×106 molecules/cell), corresponding in yields of 28% (cytosol/endosome) and 3.5% (cytosol/total). Notably, despite this higher endosomal escape activity, the distribution of fluorescence signal remains punctate in microscopy images. Because endosomal escape remains relatively low, it is unknown whether the cytosolic delivery achieved involves just a few molecules escaping many endosomes in a cell, or conversely, many molecules escaping a single endosome among hundreds of organelles.
2.2. Reagents with high apparent endosomal escape activity.
Several reports have highlighted how some reagents are efficient enough that the molecules they deliver can readily be observed in the cytosol of cells by fluorescence microscopy (30, 56, 71). For instance, when PCI is used as a delivery method, molecules trapped inside endosomes can be seen bursting out into the cytoplasm upon irradiation of photosensitizers (77). Similarly, dfTAT, a disulfide-bonded dimer of TAT labeled with two tetramethylrhodamine fluorophores, can release high levels of molecules in the cytosol of cells, albeit in the absence of light activation (78). Herein, we use dfTAT as an example to illustrate how efficient endosomal escape can be. Like monomeric TAT, at low incubation concentration (<2 μM, 1h), dfTAT accumulates within endosomes without any apparent access to the cytosol. However, as incubation concentration is increased, a majority of cells display a diffuse fluorescence signal throughout the cell, with noticeable staining of nucleolar compartments. This staining is, in turn, confirmation that some of the signal detected is indeed intracellular, and not simply caused by out-of-focus fluorescence from peptide bound to the exterior of the cell. This staining is detectable in close to 100% of cells when 5 μM of peptide or more is used (dead cells, identified by SYTOX nuclear staining, represent less than 5% of the total population and are excluded from quantitation). In contrast, monomeric TAT remains trapped inside endosomes, even when the amount of TAT internalized in endosomes exceed that of dfTAT by more than 2-fold (50 μM TAT vs 5 μM dfTAT incubation).
The cytosolic entry of dfTAT is such that microscopy images typically show little to no fluorescence left inside endosomes. Inhibitors of endocytosis and of endosomal trafficking nonetheless, all confirm that the peptide enters the cytosol by escaping from endosomes (79). Endosomal escape therefore appears highly efficiently. However, one may envision how, above a certain level of cytosolic entry, the cytosolic fluorescence may mask the signal left trapped inside endosomes. To address this question, an assay based on the cell-impermeable fluorophore SNAP-Surface was developed (Figure 2d). In this assay, cells are transfected with SNAP-H2B, a histone protein fused to the SNAP-tag. Upon cell entry, as mediated by endocytosis and dfTAT-induced endosomal escape, SNAP-Surface diffuses freely into the cytosol of cells. However, upon nuclear entry, SNAP-H2B covalently captures the fluorophore. This leads to the retention of the fluorophore in the nuclear compartments and to a depletion of the signal in the cytosol. Importantly, this means that the signal that remains trapped inside endosomes, that is signal from failed endosomal escape, is better revealed. Based on this assay, up to 90% of the fluorophore molecules reach the nucleus of cells in the presence of dfTAT (0% in its absence). The number of observable endosomes loaded with fluorophore is as low as a dozen, while hundreds are present when dfTAT is absent. Overall, this suggests that dfTAT can release a majority of internalized molecules into the cytosol, and that this activity involves the leakage of many organelles in a cell. Notably, because dfTAT can stimulate an increase in cellular fluid-phase uptake (as reported for TAT and other arginine-rich peptides), the concentration of macromolecules that reach the interior of cells can exceed the extracellular concentration. Nonetheless, when comparing these results to the previous studies described, it is important to note that the number of moles of molecules that enter cells remain an overall small percentage of the number of molecules extracellularly administered. Specifically, with cells exposed to 1.5×1010 molecules/cell of GFP, dfTAT-mediated delivery yields to 4.2×108 molecules/cell of protein internalized overall (leading to a 0.28% yield). As with SNAP-surface, the fluorescence of the protein is almost exclusively in the cytosol of cells, indicating that the cytosol/endosome yield of release is close to 100%. This notion is further supported by the delivery of the transcription factor HOXB4: while TAT can deliver HOXB4 and induce the expression of a luciferase gene-reporter, dfTAT can improve this activity by more than 60-fold (80).
2.3. Conclusions
The efficiency of endosomal escape of various delivery agents appears to vary widely. It is important to note that, in many instances, high efficiency is not required. As pointed out earlier with the example of DNA plasmid or Cre recombinase, low levels of cell entry are adequate if few macromolecules delivered are sufficient to execute a biological function. For other applications, this may however not be satisfactory and more robust delivery tools are then required. Additionally, more efficient endosomal escape may, in principle provide, other advantages. They may include lower variability and higher percentage of cells with successfully delivered cargos. Moreover, by improving endosomal escape, lower levels of externally administered cargo may be required. While this may only provide added convenience in cell cultures by reducing the loss of reagents often challenging and costly to prepare, this may prove absolutely necessary for in vivo applications where delivery agents and their cargos cannot be introduced in high concentrations. Yet, as desirable as it may be to develop delivery reagents with improved endosomal activity, this raises the question of whether or not cells can tolerate high levels of endosomal membrane disruption. This question is addressed in the next section.
3. Toxicity associated with delivery tools
The toxicity of delivery agents tends to generally increase with concentration and often correlates with whether or not cytosolic entry has taken place. Thus, is it possible that the endosomal escape event is in itself causing cell death? Moreover, if the cell survives cytosolic penetration, what cellular processes may then be impacted?
3.1. Evidence of toxicity associated directly with endosomal escape
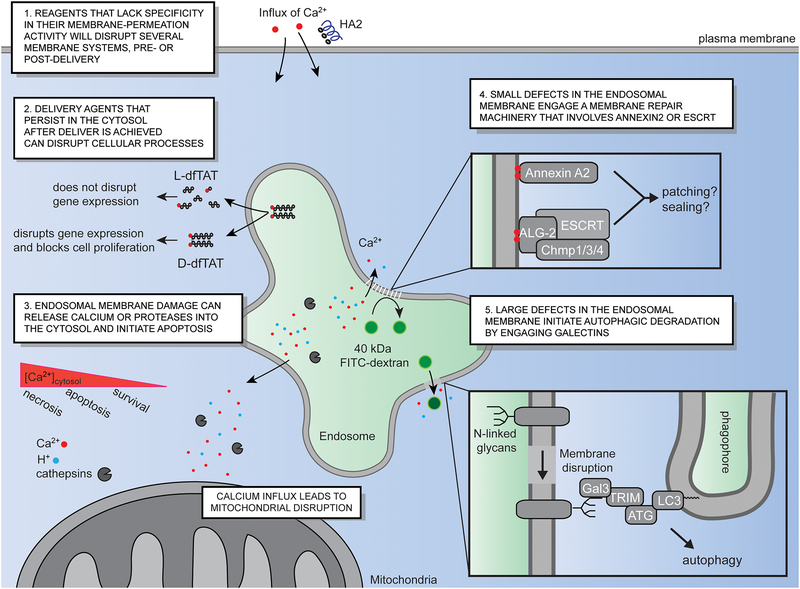
As previously described, PCI can be very efficient in mediating endosomal escape. Unfortunately, PCI can also be very toxic. The plasma membrane of cells often bleb and become permeable within seconds of endosomes rupturing upon light activation (4). This is indicative of rapid necrosis (62). Because endosomal leakage can release the photosensitizers used for PCI into various areas of the cell, one possibility may be that these photosensitizers act upon the membranes of organelles other than endosomes (e.g. mitochondria) as irradiation is prolonged. However, when directly microinjected and irradiated in the cytoplasm of cells, photosensitizers do not necessarily reproduce the necrotic response observed during light-induced endosomal leakage (4). It is therefore the disruption of the endosomal membrane itself that may induce necrosis (Figure 3). Notably, the rapid endosomal membrane disruption achieved in this photochemical process is accompanied by a burst of calcium release into the cytosol of cells (4). Indeed, while the concentration of calcium is relatively high outside cells and within the endocytic pathway, it is low in the cytoplasm (81). Endosomal membrane disruption therefore yields an increase in how much calcium enters the cytosol of cells. This excess of cytoplasmic calcium then enters mitochondria, triggering opening of the mitochondrial permeability transition pore and necrosis (82). Conversely, performing PCI in the absence of calcium, in the presence of calcium chelators, or in the presence of inhibitors of calcium mitochondrial transport, abolish cell death (4). Therefore, calcium alone accounts for the cell-death observed in this example. The release of late endosomal/lysosomal proteases, such as cathepsins, into the cytosol can also trigger cell death. Cathepsin B activates pro-apoptotic factors Bid and Bax, leading to subsequent mitochondrial outer membrane permeabilization and caspase activation (83–85). The toxicity associated with cathepsin release may be more pronounced when lysosomes are permeabilized, as these organelles may be more concentrated in these hydrolases than other compartments of the endosomal pathway (86).
Figure 3.
Delivery agents trigger various intracellular responses, related or unrelated to their endosomal escape activity. (1) Delivery agents that display low membrane selectivity may promote endosomal leakage while disrupting other cellular membranes. In particular, they may disrupt the plasma membrane of cells before cytosolic delivery is achieved, and that of intracellular organelles after successful endosomal escape. (2) Non-degradable reagents, such as D-dfTAT, exert deleterious effects on cells that its degradable counterpart dfTAT does not elicit. In particular, D-dfTAT inhibits cell proliferation and alters gene expression. This is independent of endosomal escape as both dfTAT and D-dfTAT mediate the leakage of late endosomal membranes with equivalent efficiencies. (3) Endosomal membrane disruption can lead to the release of luminal calcium and cathepsins into the cytosol of cells. This can in turn trigger apoptotic pathways. In the case of PCI, if endosomal escape is rapid, the rapid burst of calcium released triggers simultaneous mitochondrial membrane permeabilization necrosis. 4) Small membrane defects, that is, defects that cause the release of small molecules such as calcium but not of large species such as a 40 kDa Dextran, initiate the recruitment of annexin 2A and of the ESCRT machinery. This then initiates membrane repair, as detected by the rescue of endosomal acidification and function. (5) Endosomes exposed to large membrane disruptions, that is, disruptions that may accommodate large cargos such as a 40 kDa Dextran, are targeted for degradation by autophagy. In particular, upon membrane disruption, luminal N-linked glycans become exposed and accessible to intracellular lectins such galectin-3. This triggers the assembly of autophagy machinery bound to a phagophore, eventually leading to the engulfment and degradation of the damaged organelles.
3.2. Evidence that endosomal escape is not necessarily toxic
dfTAT-mediated endosomal escape is nontoxic: ~100% of cells are viable even when exposed to a peptide concentration that exceeds that required for achieving efficient endosomal release (i.e. 25 μM dfTAT, 5 μM being sufficient for cytosolic delivery in >90% cells). Moreover, dfTAT-mediated escape does not affect proliferation rates, indicating an absence of metabolic stress. In addition, dfTAT does not induce a noticeable transcriptional response. In particular, only 11 mRNA transcripts, out of 47000 surveyed, were found to be up or down-regulated immediately after a 1h incubation with the peptide. Treated and untreated cells were virtually indistinguishable 1 and 24h later (38). This is in marked contrast with cationic lipids, as similar analyses reveal that the expression of hundreds of genes are modified when cells are exposed to Lipofectamine (87). Obviously, this is also in stark contrast to PCI, as described above. To explain such differences, it may be worth noting that dfTAT-mediated endosomal release is rather specific, dfTAT apparently acting upon the membrane of late endosomes and not on that of other organelles (in particular lysosomes; PCI may not discriminate which organelles are disrupted). In addition, endosomal release is progressive. The number of cells containing successfully delivered cytosolic cargo increases steadily over a period of 30 to 45 min, consistent with the progressive transport of dfTAT/cargos from the cell surface to the late endosomes (88). Once in the late endosomes, it is unclear how rapidly dfTAT will mediate membrane disruption. Yet, one can envision that a threshold concentration of peptide must first accumulate. Endosomal leakage may not happen in all endocytic organelles at almost the same time, as can be achieved with PCI, but instead one late endosome at a time. If so, calcium may be released steadily, and not in a large and sudden concentration burst. Cells may then be better capable of maintaining homeostasis via calcium pumps and channels.
3.3. Delivery agents can be toxic independently of endosomal escape
As pointed out above, the gene expression responses induced by dfTAT and Lipofectamine do not match the efficiency with which these reagents escape endosomes. It is therefore unclear that the paradigm “high membrane disruption efficiency = high level of deleterious effects on cells” is true. On one hand, it is possible that delivery agents impact cells negatively by interactions that take place on the cell surface. This is likely the case for reagents such as HA2, which, while tuned to disrupt endosomal membrane, can also act upon the plasma membrane (Figure 3) (89). It is also possible that delivery agents may modify cells from within the endosomal pathway. In particular, it is unclear how cells respond to the accumulation of cationic lipids within the endosomal pathway, as these species may incorporate within bilayers throughout the cell, and significantly change the biophysical behavior of membrane in general (i.e. there are no natural cationic lipids in human cells). Finally, a delivery agent may have deleterious effects not outside the cell, not from within the endocytic pathway, but once it enters the cytosol of cells (Figure 3). An example is D-dfTAT, an analog of dfTAT synthesized with D, instead of L, amino acids. D-dfTAT displays a similar endosomal escape activity to dfTAT. However, unlike dfTAT, D-dfTAT is not rapidly degraded during delivery because of its inherent resistance to proteolytic cleavage. For instance, while dfTAT is rapidly degraded within cells (all peptide being cleared in less than 3h once in the cell), D-dfTAT remains intact in the cytosol and nucleus of cells for a period of over 3 days. In contrast to dfTAT, D-dfTAT dramatically impacts gene expression, and, while non-toxic, reduces cellular proliferation rates (90). Given that dfTAT and D-dfTAT both mediate membrane-disruption and endosomal escape, the differences in cellular responses may therefore arise from what D-dfTAT does once it stably resides in the cell. In turn, this example may serve as a cautionary tale. In particular, while high endosomal escape efficiency is desirable to deliver high levels of cargos inside cells, it is also likely to be accompanied by the cytosolic egress of a relatively high amount of delivery agents. How this delivery agent impacts the cell, especially if it stays in the cell for a prolonged period of time, then becomes an important consideration.
3.4. Membrane repair and degradation processes may mask the damage produced by delivery agents
Depending on the extent of membrane damage, cells may respond by either degrading damaged membranes through autophagy or repairing them. The clearance of damaged organelles is characterized by the initial recruitment of galectins, specifically galectin-3 and galectin-8, that bind β-galactoside moieties exposed on the cytosol-exposed leaflet of vesicular membranes (Figure 3) (91–93). Galectin recruitment followed by the subsequent induction of autophagy has been characterized for a number of systems, including siRNA-lipid nanoparticles, calcium phosphate precipitates and cationic lipids (94–96). Recently, Skowyra et al. found that galectin-3 recruitment is characteristic of large membrane disruptions. In contrast, smaller disruptions (i.e. small enough so as to not lead to the cytosolic release of a 40kDa Dextran) trigger the recruitment of ESCRT proteins (97). In turn, ESCRT repairs damaged membranes, as observed by the reacidification and recovery of damaged organelles. Alternatively, the annexin A2/S100A10 complex may also be recruited to damaged endosomal membranes (98). It is speculated that ESCRT and annexin A2/S100A10 complex repair membranes by either sealing sites of permeation, or by recruiting nearby vesicles for bilayer patching (Figure 3).
4. Conclusions and Future challenges
The endocytic pathway provides numerous opportunities to achieve the delivery of macromolecules into cells. As highlighted in this review, it is becoming increasing clear that a long-standing bottleneck, endosomal entrapment, is a problem that can be circumvented. With new delivery agents being developed, and as issues of delivery efficiencies are being progressively solved with continuous optimization of these systems, new challenges are emerging. In particular, a major limitation in exploiting the endocytic pathway for delivery is the exposure of cargos to the highly degradative environment of the endolysosomal lumen. Specifically, nucleases and proteases can degrade cargo as it traffics through the endocytic pathway, before it may reach its destination. Endosomal escape may then lead to the release of no cargo at all, or of partially-degraded products with unexpected and off-target effects (i.e. partial cleavage of proteins may lead to the generation of dominant-negative domains). As a result, it is often challenging to measure the exact concentrations of macromolecular cargo once it enters the cytosol. It is therefore likely that some applications will require the development of approaches that protect cargos during delivery. In addition, unless the purpose of achieving delivery is to kill cells, delivery should in principle be as traceless as possible. A future challenge therefore consists of designing delivery reagents that do not trigger any deleterious effects in the cell. Finally, different applications may require distinct features. Some may benefit from reagents that work in all cell types, while others may necessitate cell-specific delivery. Some reagents may also need to be optimal for protein delivery (i.e. cell reprogramming with extracellularly administered transcription factors), for nucleic acids (i.e. gene knock-down by RNA interference), or both (i.e. gene editing with Cas9-gRNA RNP complexes). The chances that a single delivery tool can fulfill all of these expectations are obviously low. Instead it is likely that developing a wide array of synthetic delivery reagents, while learning from their respective advantages and limitations, will be necessary to push the field forward.
Acknowledgements
This work was supported by award R01GM110137 from the US National Institute of General Medical Sciences. This work was also supported by award RP100819 from the Cancer Prevention Research Institute of Texas.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References:
- (1).Andrews NW, and Corrotte M (2018) Plasma membrane repair. Current Biology 28, R392–R397. [DOI] [PubMed] [Google Scholar]
- (2).Palm-Apergi C, Lorents A, Padari K, Pooga M, and Hällbrink M (2008) The membrane repair response masks membrane disturbances caused by cell-penetrating peptide uptake. The FASEB Journal 23, 214–223. [DOI] [PubMed] [Google Scholar]
- (3).Lorents A, Kodavali PK, Oskolkov N, Langel Ü, Hällbrink M, and Pooga M (2012) Cell-penetrating peptides split into two groups based on modulation of intracellular calcium concentration. The Journal of biological chemistry 287, 16880–16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Muthukrishnan N, Johnson GA, Lim J, Simanek EE, and Pellois JP (2012) TAT-mediated photochemical internalization results in cell killing by causing the release of calcium into the cytosol of cells. Biochimica et biophysica acta 1820, 1734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Mettlen M, Chen P-H, Srinivasan S, Danuser G, and Schmid SL (2018) Regulation of Clathrin-Mediated Endocytosis. Annual Review of Biochemistry 87, 871–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sandvig K, Kavaliauskiene S, and Skotland T (2018) Clathrin-independent endocytosis: an increasing degree of complexity. Histochemistry and Cell Biology 150, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Mayor S, Presley JF, and Maxfield FR (1993) Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. The Journal of cell biology 121, 1257–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Wagner E (1999) Application of membrane-active peptides for nonviral gene delivery. Advanced Drug Delivery Reviews 38, 279–289. [DOI] [PubMed] [Google Scholar]
- (9).Juliano RL, and Carver K (2015) Cellular uptake and intracellular trafficking of oligonucleotides. Adv Drug Deliv Rev 87, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Zuhorn IS, Kalicharan R, and Hoekstra D (2002) Lipoplex-mediated transfection of mammalian cells occurs through the cholesterol-dependent clathrin-mediated pathway of endocytosis. J Biol Chem 277, 18021–8. [DOI] [PubMed] [Google Scholar]
- (11).Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, Manygoats K, Seifert S, Andree C, Stöter M, Epstein-Barash H, Zhang L, Koteliansky V, Fitzgerald K, Fava E, Bickle M, Kalaidzidis Y, Akinc A, Maier M, and Zerial M (2013) Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nature Biotechnology 31, 638. [DOI] [PubMed] [Google Scholar]
- (12).Xu Y, and Szoka FC Jr. (1996) Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 35, 5616–23. [DOI] [PubMed] [Google Scholar]
- (13).Koltover I, Salditt T, Radler JO, and Safinya CR (1998) An inverted hexagonal phase of cationic liposome-DNA complexes related to DNA release and delivery. Science (New York, N.Y.) 281, 78–81. [DOI] [PubMed] [Google Scholar]
- (14).Koynova R, Tarahovsky YS, Wang L, and MacDonald RC (2007) Lipoplex formulation of superior efficacy exhibits high surface activity and fusogenicity, and readily releases DNA. Biochimica et biophysica acta 1768, 375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pantazatos DP, and MacDonald RC J. T. J. o. M. B (1999) Directly Observed Membrane Fusion Between Oppositely Charged Phospholipid Bilayers. 170, 27–38. [DOI] [PubMed] [Google Scholar]
- (16).Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, and Gruenberg J (1998) A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 392, 193–7. [DOI] [PubMed] [Google Scholar]
- (17).Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, Escola JM, Lebrand C, Cosson P, Kobayashi T, and Gruenberg J (2002) Separation and characterization of late endosomal membrane domains. J Biol Chem 277, 32157–64. [DOI] [PubMed] [Google Scholar]
- (18).Kobayashi T, Startchev K, Whitney AJ, and Gruenber J (2001) Localization of lysobisphosphatidic acid-rich membrane domains in late endosomes. Biological chemistry 382, 483–5. [DOI] [PubMed] [Google Scholar]
- (19).Chevallier J, Chamoun Z, Jiang G, Prestwich G, Sakai N, Matile S, Parton RG, and Gruenberg J (2008) Lysobisphosphatidic Acid Controls Endosomal Cholesterol Levels. 283, 27871–27880. [DOI] [PubMed] [Google Scholar]
- (20).Hafez IM, Maurer N, and Cullis PR (2001) On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Therapy 8, 1188. [DOI] [PubMed] [Google Scholar]
- (21).Kanasty R, Dorkin JR, Vegas A, and Anderson D (2013) Delivery materials for siRNA therapeutics. Nature Materials 12, 967. [DOI] [PubMed] [Google Scholar]
- (22).Sato Y, Hatakeyama H, Sakurai Y, Hyodo M, Akita H, and Harashima H (2012) A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. Journal of controlled release : official journal of the Controlled Release Society 163, 267–76. [DOI] [PubMed] [Google Scholar]
- (23).Rejman J, Conese M, and Hoekstra D (2006) Gene transfer by means of lipo- and polyplexes: role of clathrin and caveolae-mediated endocytosis. Journal of liposome research 16, 237–47. [DOI] [PubMed] [Google Scholar]
- (24).Cho YW, Kim JD, and Park K (2003) Polycation gene delivery systems: escape from endosomes to cytosol. The Journal of pharmacy and pharmacology 55, 721–34. [DOI] [PubMed] [Google Scholar]
- (25).Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, and Behr JP (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America 92, 7297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, and Andresen TL (2013) The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Molecular therapy : the journal of the American Society of Gene Therapy 21, 149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Stepanenko AA, and Heng HH (2017) Transient and stable vector transfection: Pitfalls, off-target effects, artifacts. Mutation research 773, 91–103. [DOI] [PubMed] [Google Scholar]
- (28).Green M, and Loewenstein PM (1988) Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55, 1179–1188. [DOI] [PubMed] [Google Scholar]
- (29).Frankel AD, and Pabo CO (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55, 1189–93. [DOI] [PubMed] [Google Scholar]
- (30).Kaplan IM, Wadia JS, and Dowdy SF (2005) Cationic TAT peptide transduction domain enters cells by macropinocytosis. Journal of controlled release : official journal of the Controlled Release Society 102, 247–53. [DOI] [PubMed] [Google Scholar]
- (31).Eguchi A, and Dowdy SF (2009) siRNA delivery using peptide transduction domains. Trends in pharmacological sciences 30, 341–5. [DOI] [PubMed] [Google Scholar]
- (32).Zhao M, and Weissleder R (2004) Intracellular cargo delivery using tat peptide and derivatives. Medicinal research reviews 24, 1–12. [DOI] [PubMed] [Google Scholar]
- (33).Appelbaum JS, LaRochelle JR, Smith BA, Balkin DM, Holub JM, and Schepartz A (2012) Arginine topology controls escape of minimally cationic proteins from early endosomes to the cytoplasm. Chemistry & biology 19, 819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Yang S-T, Zaitseva E, Chernomordik LV, and Melikov K (2010) Cell-penetrating peptide induces leaky fusion of liposomes containing late endosome-specific anionic lipid. Biophysical journal 99, 2525–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Angeles-Boza AM, Erazo-Oliveras A, Lee Y-J, and Pellois J-P (2010) Generation of Endosomolytic Reagents by Branching of Cell-Penetrating Peptides: Tools for the Delivery of Bioactive Compounds to Live Cells in Cis or Trans. Bioconjugate Chemistry 21, 2164–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Becker-Hapak M, McAllister SS, and Dowdy SF (2001) TAT-mediated protein transduction into mammalian cells. Methods (San Diego, Calif.) 24, 247–56. [DOI] [PubMed] [Google Scholar]
- (37).Kawamura KS, Sung M, Bolewska-Pedyczak E, and Gariépy J (2006) Probing the Impact of Valency on the Routing of Arginine-Rich Peptides into Eukaryotic Cells. Biochemistry 45, 1116–1127. [DOI] [PubMed] [Google Scholar]
- (38).Erazo-Oliveras A, Najjar K, Dayani L, Wang T-Y, Johnson GA, and Pellois J-P (2014) Protein delivery into live cells by incubation with an endosomolytic agent. Nat Meth 11, 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Brock DJ, Kustigian L, Jiang M, Graham K, Wang TY, Erazo-Oliveras A, Najjar K, Zhang J, Rye H, and Pellois JP (2018) Efficient cell delivery mediated by lipid-specific endosomal escape of supercharged branched peptides. Traffic (Copenhagen, Denmark) 19, 421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Salomone F, Cardarelli F, Di Luca M, Boccardi C, Nifosi R, Bardi G, Di Bari L, Serresi M, and Beltram F (2012) A novel chimeric cell-penetrating peptide with membrane-disruptive properties for efficient endosomal escape. Journal of controlled release : official journal of the Controlled Release Society 163, 293–303. [DOI] [PubMed] [Google Scholar]
- (41).Del’Guidice T, Lepetit-Stoffaes JP, Bordeleau LJ, Roberge J, Theberge V, Lauvaux C, Barbeau X, Trottier J, Dave V, Roy DC, Gaillet B, Garnier A, and Guay D (2018) Membrane permeabilizing amphiphilic peptide delivers recombinant transcription factor and CRISPR-Cas9/Cpf1 ribonucleoproteins in hard-to-modify cells. PloS one 13, e0195558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Meyer M, Philipp A, Oskuee R, Schmidt C, and Wagner E (2008) Breathing Life into Polycations: Functionalization with pH-Responsive Endosomolytic Peptides and Polyethylene Glycol Enables siRNA Delivery. Journal of the American Chemical Society 130, 3272–3273. [DOI] [PubMed] [Google Scholar]
- (43).Wharton SA, Martin SR, Ruigrok RW, Skehel JJ, and Wiley DC (1988) Membrane fusion by peptide analogues of influenza virus haemagglutinin. The Journal of general virology 69 (Pt 8), 1847–57. [DOI] [PubMed] [Google Scholar]
- (44).Zhelev DV, Stoicheva N, Scherrer P, and Needham D (2001) Interaction of synthetic HA2 influenza fusion peptide analog with model membranes. Biophys J 81, 285–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Korte T, Ludwig K, and Herrmann A (1992) ph-dependent hydrophobicity profile of hemagglutinin of influenza virus and its possible relevance in virus fusion. Bioscience reports 12, 397–406. [DOI] [PubMed] [Google Scholar]
- (46).Subramanian A, Ma H, Dahl KN, Zhu J, and Diamond SL (2002) Adenovirus or HA-2 fusogenic peptide-assisted lipofection increases cytoplasmic levels of plasmid in nondividing endothelium with little enhancement of transgene expression. 4, 75–83. [DOI] [PubMed] [Google Scholar]
- (47).Ye SF, Tian MM, Wang TX, Ren L, Wang D, Shen LH, and Shang T (2012) Synergistic effects of cell-penetrating peptide Tat and fusogenic peptide HA2-enhanced cellular internalization and gene transduction of organosilica nanoparticles. Nanomedicine : nanotechnology, biology, and medicine 8, 833–41. [DOI] [PubMed] [Google Scholar]
- (48).Lee Y-J, Johnson G, and Pellois J-P (2010) Modeling of the endosomolytic activity of HA2-TAT peptides with red blood cells and ghosts. Biochemistry 49, 7854–7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Lee Y-J, Johnson G, Peltier GC, and Pellois J-P (2011) A HA2-Fusion tag limits the endosomal release of its protein cargo despite causing endosomal lysis. Biochimica et Biophysica Acta (BBA) - General Subjects 1810, 752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Akishiba M, Takeuchi T, Kawaguchi Y, Sakamoto K, Yu HH, Nakase I, Takatani-Nakase T, Madani F, Graslund A, and Futaki S (2017) Cytosolic antibody delivery by lipid-sensitive endosomolytic peptide. Nature chemistry 9, 751–761. [DOI] [PubMed] [Google Scholar]
- (51).Ohkuma S, and Poole B (1981) Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J Cell Biol 90, 656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Thiele DL, and Lipsky PE (1990) Mechanism of L-leucyl-L-leucine methyl ester-mediated killing of cytotoxic lymphocytes: dependence on a lysosomal thiol protease, dipeptidyl peptidase I, that is enriched in these cells. Proceedings of the National Academy of Sciences of the United States of America 87, 83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Wibo M, and Poole B (1974) Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. The Journal of cell biology 63, 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Abes S, Williams D, Prevot P, Thierry A, Gait MJ, and Lebleu B (2006) Endosome trapping limits the efficiency of splicing correction by PNA-oligolysine conjugates. Journal of controlled release : official journal of the Controlled Release Society 110, 595–604. [DOI] [PubMed] [Google Scholar]
- (55).Soundara Manickam D, Bisht HS, Wan L, Mao G, and Oupicky D (2005) Influence of TAT-peptide polymerization on properties and transfection activity of TAT/DNA polyplexes. Journal of controlled release : official journal of the Controlled Release Society 102, 293–306. [DOI] [PubMed] [Google Scholar]
- (56).Yang B, Ming X, Cao C, Laing B, Yuan A, Porter MA, Hull-Ryde EA, Maddry J, Suto M, Janzen WP, and Juliano RL (2015) High-throughput screening identifies small molecules that enhance the pharmacological effects of oligonucleotides. Nucleic acids research 43, 1987–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Wang TY, Libardo MDJ, Angeles-Boza AM, and Pellois JP (2017) Membrane Oxidation in Cell Delivery and Cell Killing Applications. ACS chemical biology 12, 1170–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Choi Y, McCarthy JR, Weissleder R, and Tung CH (2006) Conjugation of a photosensitizer to an oligoarginine-based cell-penetrating peptide increases the efficacy of photodynamic therapy. ChemMedChem 1, 458–63. [DOI] [PubMed] [Google Scholar]
- (59).Maiolo JR, Ottinger EA, and Ferrer M (2004) Specific Redistribution of Cell-Penetrating Peptides from Endosomes to the Cytoplasm and Nucleus upon Laser Illumination. Journal of the American Chemical Society 126, 15376–15377. [DOI] [PubMed] [Google Scholar]
- (60).Matsushita M, Noguchi H, Lu YF, Tomizawa K, Michiue H, Li ST, Hirose K, Bonner-Weir S, and Matsui H (2004) Photo-acceleration of protein release from endosome in the protein transduction system. FEBS letters 572, 221–6. [DOI] [PubMed] [Google Scholar]
- (61).Endoh T, Sisido M, and Ohtsuki T (2009) Spatial regulation of specific gene expression through photoactivation of RNAi. Journal of controlled release : official journal of the Controlled Release Society 137, 241–5. [DOI] [PubMed] [Google Scholar]
- (62).Srinivasan D, Muthukrishnan N, Johnson GA, Erazo-Oliveras A, Lim J, Simanek EE, and Pellois JP (2011) Conjugation to the cell-penetrating peptide TAT potentiates the photodynamic effect of carboxytetramethylrhodamine. PloS one 6, e17732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K, Moan J, Gaudernack G, Fodstad O, Kjolsrud S, Anholt H, Rodal GH, Rodal SK, and Hogset A (1999) Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer research 59, 1180–3. [PubMed] [Google Scholar]
- (64).Takayama K, Nakase I, Michiue H, Takeuchi T, Tomizawa K, Matsui H, and Futaki S (2009) Enhanced intracellular delivery using arginine-rich peptides by the addition of penetration accelerating sequences (Pas). Journal of Controlled Release 138, 128–133. [DOI] [PubMed] [Google Scholar]
- (65).Lönn P, Kacsinta AD, Cui X-S, Hamil AS, Kaulich M, Gogoi K, and Dowdy SF (2016) Enhancing Endosomal Escape for Intracellular Delivery of Macromolecular Biologic Therapeutics. Scientific Reports 6, 32301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Brunner J, and Barton JK (2006) Targeting DNA mismatches with rhodium intercalators functionalized with a cell-penetrating peptide. Biochemistry 45, 12295–302. [DOI] [PubMed] [Google Scholar]
- (67).Birch D, Christensen MV, Staerk D, Franzyk H, and Nielsen HM (2017) Fluorophore labeling of a cell-penetrating peptide induces differential effects on its cellular distribution and affects cell viability. Biochimica et Biophysica Acta (BBA) - Biomembranes 1859, 2483–2494. [DOI] [PubMed] [Google Scholar]
- (68).Hedegaard SF, Derbas MS, Lind TK, Kasimova MR, Christensen MV, Michaelsen MH, Campbell RA, Jorgensen L, Franzyk H, Cárdenas M, and Nielsen HM (2018) Fluorophore labeling of a cell-penetrating peptide significantly alters the mode and degree of biomembrane interaction. Scientific Reports 8, 6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Patel A, Mohl B-P, and Roy P (2016) Entry of Bluetongue virus capsid requires the late endosomal specific lipid lysobisphosphatidic acid. Journal of Biological Chemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Zaitseva E, Yang ST, Melikov K, Pourmal S, and Chernomordik LV (2010) Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS pathogens 6, e1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Glover DJ, Leyton DL, Moseley GW, and Jans DA (2010) The efficiency of nuclear plasmid DNA delivery is a critical determinant of transgene expression at the single cell level. 12, 77–85. [DOI] [PubMed] [Google Scholar]
- (72).Xu Y, and Szoka FC (1996) Mechanism of DNA Release from Cationic Liposome/DNA Complexes Used in Cell Transfection. Biochemistry 35, 5616–5623. [DOI] [PubMed] [Google Scholar]
- (73).Pollard H, Remy JS, Loussouarn G, Demolombe S, Behr JP, and Escande D (1998) Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. Journal of Biological Chemistry 273, 7507–7511. [DOI] [PubMed] [Google Scholar]
- (74).Wadia JS, Stan RV, and Dowdy SF (2004) Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nature Medicine 10, 310. [DOI] [PubMed] [Google Scholar]
- (75).Rezgui R, Blumer K, Yeoh-Tan G, Trexler AJ, and Magzoub M (2016) Precise quantification of cellular uptake of cell-penetrating peptides using fluorescence-activated cell sorting and fluorescence correlation spectroscopy. Biochimica et Biophysica Acta (BBA) - Biomembranes 1858, 1499–1506. [DOI] [PubMed] [Google Scholar]
- (76).LaRochelle JR, Cobb GB, Steinauer A, Rhoades E, and Schepartz A (2015) Fluorescence Correlation Spectroscopy Reveals Highly Efficient Cytosolic Delivery of Certain Penta-Arg Proteins and Stapled Peptides. Journal of the American Chemical Society 137, 2536–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Selbo PK, Weyergang A, Høgset A, Norum O-J, Berstad MB, Vikdal M, and Berg K (2010) Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. Journal of Controlled Release 148, 2–12. [DOI] [PubMed] [Google Scholar]
- (78).Erazo-Oliveras A, Najjar K, Truong D, Wang T-Y, Brock Dakota J., Prater Austin R., and Pellois J-P (2016) The Late Endosome and Its Lipid BMP Act as Gateways for Efficient Cytosolic Access of the Delivery Agent dfTAT and Its Macromolecular Cargos. Cell Chemical Biology 23, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Erazo-Oliveras A, Najjar K, Dayani L, Wang T-Y, Johnson GA, and Pellois J-P (2014) Protein delivery into live cells by incubation with an endosomolytic agent. Nature methods 11, 861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Huang C-H, Chen P-M, Lu T-C, Kung W-M, Chiou T-J, Yang M-H, Kao J-Y, and Wu K-J (2010) Purified Recombinant TAT-Homeobox B4 Expands CD34+ Umbilical Cord Blood and Peripheral Blood Progenitor Cells Ex Vivo. 16, 487–496. [DOI] [PubMed] [Google Scholar]
- (81).Orrenius S, Zhivotovsky B, and Nicotera P (2003) Regulation of cell death: the calcium–apoptosis link. Nature Reviews Molecular Cell Biology 4, 552. [DOI] [PubMed] [Google Scholar]
- (82).Golstein P, and Kroemer G (2007) Cell death by necrosis: towards a molecular definition. Trends in biochemical sciences 32, 37–43. [DOI] [PubMed] [Google Scholar]
- (83).Bidère N, Lorenzo HK, Carmona S, Laforge M, Harper F, Dumont C, and Senik A (2003) Cathepsin D Triggers Bax Activation, Resulting in Selective Apoptosis-inducing Factor (AIF) Relocation in T Lymphocytes Entering the Early Commitment Phase to Apoptosis. Journal of Biological Chemistry 278, 31401–31411. [DOI] [PubMed] [Google Scholar]
- (84).de Castro MAG, Bunt G, and Wouters FS (2016) Cathepsin B launches an apoptotic exit effort upon cell death-associated disruption of lysosomes. Cell Death Discovery 2, 16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Wang F, Gomez-Sintes R, and Boya P (2018) Lysosomal membrane permeabilization and cell death. Traffic (Copenhagen, Denmark). [DOI] [PubMed] [Google Scholar]
- (86).Bright NA, Davis LJ, and Luzio JP (2016) Endolysosomes Are the Principal Intracellular Sites of Acid Hydrolase Activity. Current biology : CB 26, 2233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Jacobsen L, Calvin S, and Lobenhofer E (2009) Transcriptional effects of transfection: the potential for misinterpretation of gene expression data generated from transiently transfected cells. BioTechniques 47, 617–24. [DOI] [PubMed] [Google Scholar]
- (88).Erazo-Oliveras A, Najjar K, Truong D, Wang TY, Brock DJ, Prater AR, and Pellois JP (2016) The Late Endosome and Its Lipid BMP Act as Gateways for Efficient Cytosolic Access of the Delivery Agent dfTAT and Its Macromolecular Cargos. Cell Chem Biol 23, 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Pichon C, Freulon I, Midoux P, Mayer R, Monsigny M, and Roche AC (1997) Cytosolic and nuclear delivery of oligonucleotides mediated by an amphiphilic anionic peptide. Antisense & nucleic acid drug development 7, 335–43. [DOI] [PubMed] [Google Scholar]
- (90).Najjar K, Erazo-Oliveras A, Brock DJ, Wang TY, and Pellois JP (2017) An l- to d-Amino Acid Conversion in an Endosomolytic Analog of the Cell-penetrating Peptide TAT Influences Proteolytic Stability, Endocytic Uptake, and Endosomal Escape. J Biol Chem 292, 847–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Paz I, Sachse M, Dupont N, Mounier J, Cederfur C, Enninga J, Leffler H, Poirier F, Prevost MC, Lafont F, and Sansonetti P (2010) Galectin-3, a marker for vacuole lysis by invasive pathogens. Cellular microbiology 12, 530–44. [DOI] [PubMed] [Google Scholar]
- (92).Thurston TL, Wandel MP, von Muhlinen N, Foeglein A, and Randow F (2012) Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482, 414–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Kreibich S, Emmenlauer M, Fredlund J, Rämö P, Münz C, Dehio C, Enninga J, and Hardt W-D Autophagy Proteins Promote Repair of Endosomal Membranes Damaged by the Salmonella Type Three Secretion System 1. Cell Host & Microbe 18, 527–537. [DOI] [PubMed] [Google Scholar]
- (94).Wittrup A, Ai A, Liu X, Hamar P, Trifonova R, Charisse K, Manoharan M, Kirchhausen T, and Lieberman J (2015) Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat Biotechnol 33, 870–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Chen X, Khambu B, Zhang H, Gao W, Li M, Chen X, Yoshimori T, and Yin XM (2014) Autophagy induced by calcium phosphate precipitates targets damaged endosomes. J Biol Chem 289, 11162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Mo RH, Zaro JL, Ou JH, and Shen WC (2012) Effects of Lipofectamine 2000/siRNA complexes on autophagy in hepatoma cells. Molecular biotechnology 51, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Skowyra ML, Schlesinger PH, Naismith TV, and Hanson PI (2018) Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science (New York, N.Y.) 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Scharf B, Clement CC, Wu XX, Morozova K, Zanolini D, Follenzi A, Larocca JN, Levon K, Sutterwala FS, Rand J, Cobelli N, Purdue E, Hajjar KA, and Santambrogio L (2012) Annexin A2 binds to endosomes following organelle destabilization by particulate wear debris. Nature communications 3, 755. [DOI] [PMC free article] [PubMed] [Google Scholar]